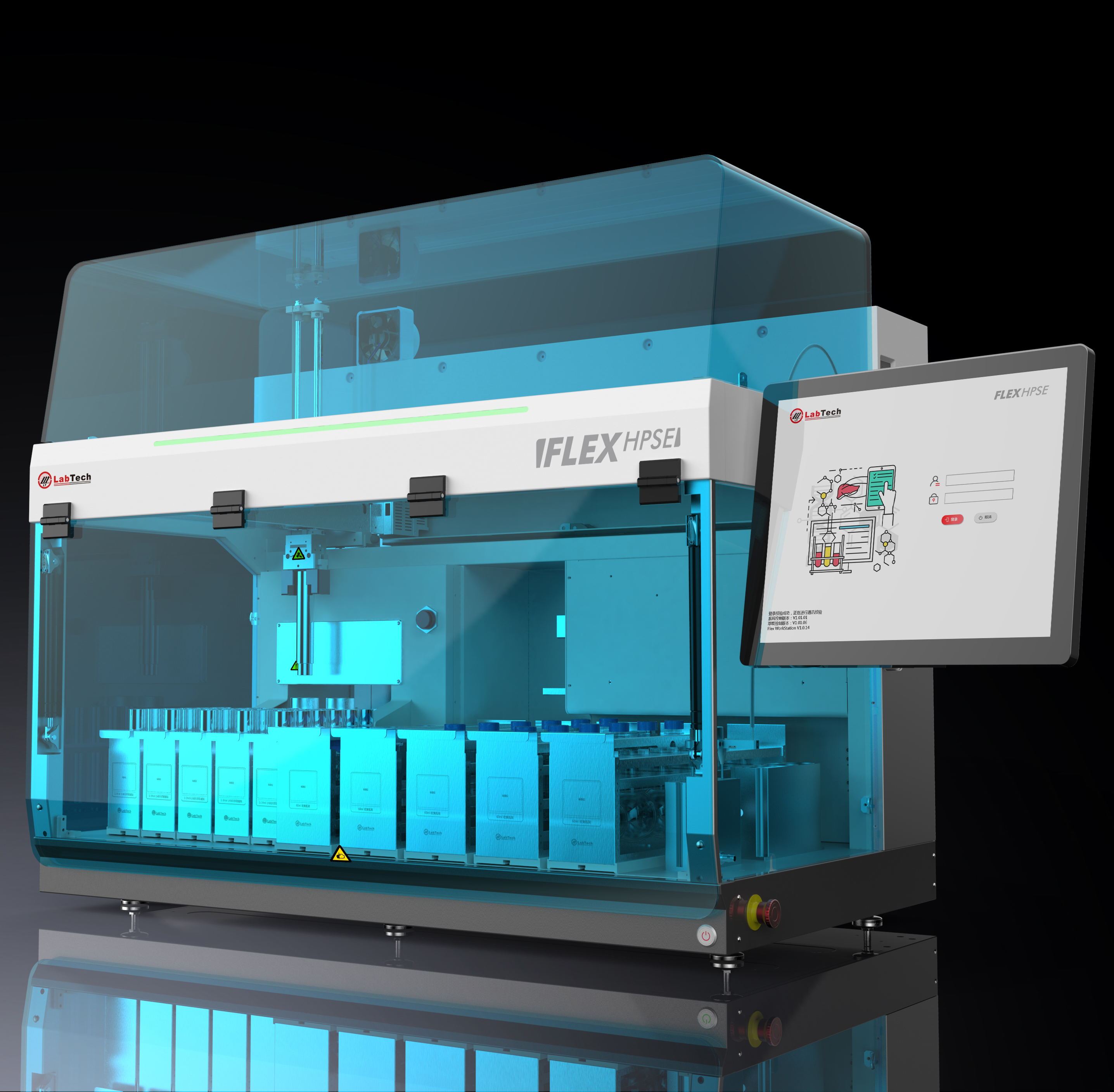
方案详情
文
添加生物炭后土壤中扑热息痛的降解途径Paracetamol degradation pathways in soil after biochar addition
方案详情

添加生物炭后土壤中扑热息痛的降解途径Paracetamol degradation pathways in soil after biochar additionEnvironmentalPollution307(2022)119546Contents lists available at ScienceDirect F.J. Chac.on et al.Environmental Pollution 307 (2022)119546 Environmental Pollution journal homepage: www.elsevier.com/locate/envpol 添加生物炭后土壤中扑热息痛的降解途径 Paracetamol degradation pathways in soil after biochar addition* Francisco J. Chacnon *, Maria L. Cayuela, Miguel A. Sáanchez-Monedero Department of Soil and Water Conservation and Organic Waste Management, CEBAS-CSIC, Box 164 Espinardo, 30100, Murcia, Spain 西班牙国家研究委员会塞古拉应用土壤生物学研究所水土保持及有机废物管理部 ARTICLE INFO ABSTRACT Keywords: Paracetamol Soil Redox Conductivity Degradation Contaminant Little is known about the effect of biochar on the degradation of paracetamol in soil, considering the ubiquity of this pollutant in the environment. Given the importance of the electrochemical properties of biochar for contaminant remediation, we investigated the influence of raw and designer redox-active biochars on paracet-amol degradation in soil. Metabolite quantifciation indicated that a minimum of 53% of the spiked paracetamol was transformed in biochar-amended soil, resulting in the accumulation of different degradation products. The identifciation of these products allowed us to chart paracetamol degradation pathways in soil with and without biochar amendment. Some of the major degradation routes were observed to proceed via catechol and phenol,despite being previously described as having only a minor role in paracetamol metabolism. Additionally, a new transformation route from paracetamol to NAPQI was discovered in anaerobic soil originating from direct redox reactions on the surface of the designer biochars. These results may contribute to change our understanding of the environmental fate of paracetamol in soil and the role of biochar in its biodegradation. 1. Introduction The increasing abundance and variety of emerging contaminants represents an environmental threat. Paracetamol, also known as acet-aminophen (ACT), is one of the most consumed medications around the world (Hider-Mlynarz et al., 2018; Lalic et al., 2018). It is also present in combination with other pharmaceuticals in more than 600 different prescription medicines and commonly used in veterinary practices (FDA, 2020; Livingston, 2010). Despite the toughening environmental and waste management regulations in the pharmaceutical and agro-food industry, ACT is frequently detected in water, sediments, sewage sludge and soil (Wu et al., 2012). Water concentrations are highly variable,with reported values ranging from a few micrograms up to 461 ng LL1(Kleywegt et al., 2019; Masoner et al., 2014; Phong Vo et al., 2019). Data on the presence of ACT in contaminated soil is scarce (Peerez Solsona, S.,Montemurro, N., Chiron, S., Barcel,o, 2021), although the typical con-centrations found are lower than 100 ug kg-1 (Ashfaq et al., 2017; Ek Henning et al., 2020). However, the initial concentration of ACT at the moment of contamination is probably much higher, given its relatively fast binding to soil and degradation (Li et al., 2014). Furthermore, ACT can accumulate in soils irrigated with wastewater (Kinney et al., 2006a ).A common agricultural practice in many areas, especially arid and semi-arid agroecosystems (Singh, 2021). The application of biosolids and manure to soil is another common source of ACT pollution (Ek Henning et al., 2020). ACT has been found in biosolid samples in con-centrations up to 1400 ug kg-1 (Kinney et al., 2006b ), while contami-nated animal manure derived from veterinary practices in animal farms is also frequently detected (Ek Henning et al., 2020). Although studies about ACT toxicity to soil organisms and micro-biota are severely lacking, ACT uptake and toxicity has been extensively researched for plants and aquatic organisms. ACT is known to cause oxidative stress to plants at concentrations as low as 1000 pg kg一1(Bartha et al., 2010; Kummerova et al., 2016; Türkogglu et al., 2017),while reproductive disruption, DNA damage and oxidative stress are some of the most notable effects found in aquatic organisms (Guiloski et al., 2017; Oliveira et al., 2015; Solee et al., 2009). Furthermore, some ACT metabolites present a higher toxicity than the parent compound (Macherey and Dansette, 2008), making its remediation a priority.Despite the high removal effciiencies of many municipal wastewater and biosolid treatment plants (ZZur et al., 2018), the continuous presence of ACT in the environment proves them insuffciient, supporting the need for in-site remediation strategies (Yang et al., 2021b ). Biochar, the product of the pyrolysis of biomass, is increasingly being considered as a promising material for soil and water remediation (Qiu et al., 2022; Yang et al., 2021a; Zama et al., 2018). It has been suc-cessfully applied for both organic and inorganic contaminant removal E-mail address: fjchacon@cebas.csic.es (F.J. Chacoon). https://doi.org/10.1016/j.envpol.2022.119546 Received 28 February 2022; Received in revised form 6 May 2022; Accepted 24 May 2022 Availableonline26May2022 0269-7491/©2022TheAuthors.PublishedbyElsevierLtd.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/). (Ko n g e t al., 2018; Ra o et al ., 2017; Zh a n g et a l., 2013), and i t is (Kong et al., 2018; Rao et al., 2017; Zhang et al., 2013), and it is considered a sustainable alternative to other remediation methods. Its considered a sustainable alternative to other remediation methods. Its ability for organic contaminant remediation arises from a combination of distinctive biochar properties like its heterogeneous aromatic struc-ture, high surface area, surface functional groups, pH and high porosity,ture, high surface area, surface functional groups, pH and high porosity,among others (Liu et al., 2018). The electrochemical properties of bio-char are one of these key contributo char rs to contaminant are one of these key contributors to contaminant r r e e m m e e d d i i a a t t i i o o n n ,,consisting of its redox capacity (its ability to exchange electrons through consisting of its redox capacity (its ability to exchange electrons through reduction-oxidation reactions) and conductivity (its capacity to trans-reduction-oxidation reactions) and conductivity (its capacity to trans-port electrons from one part of the material to another). These properties are known to increase contaminant degradation through a variety of mechanisms, including direct interspecies electron transfer, microbial mechanisms, including direct interspecies electron transfer, microbial electron shuttling, the production of radicals or redox reactions between electron shuttling, the production of radicals or redox reactions between the contaminant and biochar (Wu et al., 2017; Yu et al., 2015; Zhang et al., 2018). For example, biochar can act as an intermediate between cells and contaminants thanks to its conductive aromatic network. This can accelerate the electron transfer between them and enhance their remediation (Chen et al., 2013). It can also affect the solubility and bioavailability of many toxic metals by changing their oxidation state bioavailability of many toxic metals by changing their oxidation state through redox reactions (Cho p p a l a e t a l ., 2016; Xu e t al., 2019through redox reactions (Choppala et al., 2016; Xu et al., 2019))..Furthermore, many organic contaminants can be directly transformed Furthermore, many organic contaminants can be directly transformed on the surface of biochar by reactions with its redox-active functional groups and persistent free radicals. Fortunately, simple biochar modif i cations allow for a tailored Fortunately, simple biochar modifciations allow for a tailored enhancement of its electrochemical properties, promoting its contami-nant remediation effect (C h ac o n e t a l ., 2022). This optimization could nant remediation effect (Chacon et al., 2022). This optimization could be essentia l , as the high adsorption exhibited by biochar has been shown be essential, as the high adsorption exhibited by biochar has been shown to inhibit the biodegradation of different po toadation llutants in soil such inhibit the biodegr of different pollutants in soil such a a s s atrazine, PHAs and pentachlorophenol (Loganathan et al., 2009; Ukal-ska-Jaruga et al., 2019; Zhu et al., 2020). The enhancement is achieved by i ncreasing the moieties responsible for the desired electrochemical by increasing the moieties responsible for the desired electrochemical properties. In the case of the redox capacity, this can be accomplished by increasing the number of redox-active functionali t ies on the surface o increasing the number of redox-active functionalities on the surface o f f biochar (mostly quinones and phenolic groups), persistent free radic biochar (mostly quinones and phenolic groups), persistent free radic a a l l s s and redox-active metals (C h acon et al ., 2020). A high pyrolysis tem-and redox-active metals (Chacon et al., 2020). A high pyrolysis tem-perature above 700 C will condense the aromatic structures of biochar,creating an electron-conducting graphitic network able to transfer creating an electron-conducting graphitic network able to transfer electrons (Keiluweit et al., 2010; Sun et al., 2017). Unfortunately, the high temperatures needed for the creation of a conductive network high temperatures needed for the creation of a conductive network usually result in the destruction of redox-active functional groups on the surface of biochar. This makes it dif f icult to direct l y obtain a biochar surface of biochar. This makes it diffciult to directly obtain a biochar presenting both high conductivity and redox capacity.presenting both high conductivity and redox capacity. In this study, we evaluated the influence of biochar amendment on In this study, we evaluated the infulence of biochar amendment on the degradation pathways of ACT in soi l . In order to do this, we iden-the degradation pathways of ACT in soil. In order to do this, we iden-t i fied its main transformation metabolites and proposed a general tifeid its main transformation metabolites and proposed a general degradation pathway. We expect biochar high adsorption to affect ACT bioavailability, which will determine its transformation rate and may modify its degradation routes. Tailored biochars wi t h enhanced elec-modify its degradation routes. Tailored biochars with enhanced elec-trochemical properties were also tested to optimize and promote ACT remediation. Being ACT a redox-active molecule, our hypothesis is that the enhanced redox properties and conductivity of the designer biochars the enhanced redox properties and conductivity of the designer biochars will i ncrease ACT t ransformation either directly or through a microbial will increase ACT transformation either directly or through a microbial electron shuttle mechanism. 2. Materials and methods 2.1. Chemicals 2.1. Chemicals 13C-Acetaminophen (99%) was purchased from Cambridge Isotope Laboratories, USA. 1,4-benzoquinone, 4-aminophenol, ani l ine, ABTS Laboratories, USA. 1,4-benzoquinone, 4-aminophenol, aniline, ABTS (>98%), catechol, hydrochloric acid (HCl , 37%), hydroquinone, iron ( 98%), catechol, hydrochloric acid (HCl, 37%), hydroquinone, iron (III) chloride (FeCl 3 , 97%), maleic acid, methacetin, N-acetyl-p-benzo-quinone imine, neutral red (>90%), nitric acid (HNO3, 70%), p-ami -quinone imine, neutral red ( 90%), nitric acid (HNO , 70%), p-ami-3nophenol, phenol, potassium permanganate (KMnO4, >99%), sodium nophenol, phenol, potassium permanganate (KMnO 4 , 99%), sodium chloride (NaCl, >99%), sodium phosphate dibasic (Na2HPO4,>99%),chloride (NaCl, 99%), sodium phosphate dibasic (Na 2 HPO , 99%), 4sodium phosphate monobasic (NaH2PO4, >99%) and trans-2-hexenoic sodium phosphate monobasic (NaH 2 PO , 99%) and trans-2-hexenoic 4 acid (99%) were obtained from Merck. The acetaminophen dimer was acid (99%) were obtained from Merck. The acetaminophen dimer was purchased from Clearsynth, while 3-hydroxyacetaminophen was ob-purchased from Clearsynth, while 3-hydroxyacetaminophen was ob-tained from Astatech, USA. 2.2. Biochar production Olive tree pruning residues were chosen as a renewable feedstock,since woody biochars have been previously found to carry out both conductive and redox-based electron transfer (C ha c o n et a l ., 2020; S u n conductive and redox-based electron transfer (Chacon et al., 2020; Sun et a l ., 2017). Previous to pyrolysis, the feedstock was dried, milled and et al., 2017). Previous to pyrolysis, the feedstock was dried, milled and sieved below 6 mm. Slow pyrolysis was performed in a RSR-B sieved below 6 mm. Slow pyrolysis was performed in a RSR-B 80/500/11 rotatory quartz tube furnace under Ar fulx. After loading the feedstock, the following heating protocol was applied: (1) from the feedstock, the following heating protocol was applied: (1) from ambient temperature to 105 C at5Cmin-11;(2) 45 min at 105℃;(3)ambient temperature to 105 C at 5 C min ; (2) 45 min at 105 C; (3)linear heating to the intended highest treatment temperature (HTT) at 5 Cmin -1; (4) 120 min at the HTT (5) cool down. Biochars were 5 C min ; (4) 120 min at the HTT (5) cool down. Biochars were washed with methanol to remove its soluble organic fraction, washed again with water and grounded by ball milling for 30 s. The washing steps with methanol and water were performed to remove potential interfering compounds during the quant i fication of metabol i tes with interfering compounds during the quantifciation of metabolites with UPLC-MS. The milling process was selected both to increase the redox capacity of biochar and to remove the wide differences in biochar par-ticle shape and size that result from pyrolyzing the olive tree pruning waste. The characterization of the di f ferent biochars was performed in a waste. The characterization of the different biochars was performed in a previous work (C h a c o n et al., 2020) and the methods are described in previous work (Chacon et al., 2020) and the methods are described in the SI (Text S1). Two raw biochars pyrolyzed at the highest treatment temperatures of 400°C (B-400) and 1000 °C (B-1000) were used as base materials for 400 C (B-400) and 1000 C (B-1000) were used as base materials for modifciation. To increase their redox capacity, 5 g of unmodifeid bio-char were treated with 250 mL of a 0.025 M KMnO solution and stirred4for 24 h. Biochar was then filtered, washed with 50 mL of 5% HCl to for 24 h. Biochar was then flitered, washed with 50 mL of 5% HCl to remove metal impurities, with methanol to remove its soluble organic fraction, again thoroughly with water and dried overnight at 80 C. The obtained modified biochars were labeled B-400-KMnO4 and B-1000-obtained modifeid biochars were labeled B-400-KMnO 4 and B-1000-KMnO4 according to their original pyrolysis temperature. To obtain a KMnO 4 according to their original pyrolysis temperature. To obtain a biochar rich in redox-active metals, 20 g of feedstock material and 2 g of FeCl were mixed in 1 L of water, stirred for 24 h, centrifuged at 45003rpm for 30 min and dried at 80 °C overnight. The biochar obtained after rpm for 30 min and dried at 80 C overnight. The biochar obtained after pyrolysis at 400 °C was labeled B-400-Fe. Pyrolysis at 1000 °C of Fe-pyrolysis at 400 C was labeled B-400-Fe. Pyrolysis at 1000 C of Fe-preloaded biomass was not performed, as ini t ial tests showed this preloaded biomass was not performed, as initial tests showed this combination affected the integrity of the quartz tube. 2.3. Soil incubation 2.3. Soil incubation A loamy soil was taken from the top layer (30 cm) of a feild in Italy (46°08'01.9"N13°13'44.6"E), previously under corn (Zea mays L.). The (46 08 01.9 N 13 13 44.6 E), previously under corn (Zea mays L.). The soil was air-dried, homogenized , sieved at 2 mm and stored i n plastic soil was air-dried, homogenized, sieved at 2 mm and stored in plastic 3bags until use. The soil had a pH of 5.2, a bulk density of 1.32 g cm and a composition of 19.6% clay, 31.9% silt, 48.5% sand and an organic C content of 1.3%. To reactivate soil, dried soil was pre-incubated for 1week to 50% of its water holding capacity (WHC) before the ACT in-cubation experiments. A subsample of 10% of soil was spiked with ACT dissolved in methanol and placed in a fume hood until evaporated and dissolved in methanol and placed in a fume hood until evaporated and then mixed with the rest of the soil for an initial ACT concentration of 11g g . A biochar application rate of 1% w/w was used, corresponding to ~20 MgBiochar Ha -1assuming a soil depth of 15 cm. For t he aerobic ~20 Mg B i o c h a r Ha assuming a soil depth of 15 cm. For the aerobic incubations, samples of 10 g of contaminated soil-biochar mixtures were incubations, samples of 10 g of contaminated soil-biochar mixtures were placed in polypropylene containers and deionized water was added to reach 60% of their WHC, homogenized and maintained for the duration of the experiment for optimal microbial activity. In the anaerobic i n-of the experiment for optimal microbial activity. In the anaerobic in-cubations, water was added to the partitions until a 1:2 w/v soil/water mixture was prepared, the containers f lushed with nitrogen and sealed,mixture was prepared, the containers fulshed with nitrogen and sealed,simulating foloding conditions (OECD, 2002). Samples were then kept in the dark at 20 °C. After 2, 4, 8, 16, 32 and 60 days of incubation, three the dark at 20 C. After 2, 4, 8, 16, 32 and 60 days of incubation, three replicate samples per treatment and day were stored at -20 °C unt replicate samples per treatment and day were stored at 20 C unt i i l l destructive extraction. Table 1Physicochemical characterization of biochars and adsorption coeffciients for ACT. B-400 B-400-KMnO 4 B-400-Fe B-1000 B-1000-KMnO 4 HTT(C)HTT( C) 400 400 400 1000 1000 1Redox capacity (mmole g-)Redox capacity(mmol e g ) 0.413 1 Conductivity (mS cm ) 0.0611 1Radicals (10" spins.g-)Radicals (10spins g ) 7.54 pH (1:20 w/v)pH (1:20w/v) 8.97 2 1N -BET Surf. Area (m g ) 11.02 C –OH (at%) 13.8 C=O(at%)CO(at%) 3.27 COOH (at%)COOH (at%) 1.86 –CC graphitic (at%) 48.2 –C-C aliphatic (at%)CC aliphatic(at%) 12.7 S+B mixB mix pH (1:2 w/v) 5.53 1 Ka(mLg-)K (mL g ) 39.6d 1.12 0.572 0.085 0.418 0.06 0.02 3.74 1.51 1.35 1.64 92.5 1.65 7.55 7.70 11.1 6.06 <2 124 24.3 14.5 15.7 12.5 3.69 10.1 10.2 4.88 1.87 3.26 2.02 2.67 2.75 2.44 46.7 44.9 64.6 52.9 0.97 17.7 2.70 7.28 5.46 5.31 5.81 5.37 212 44.5 59.1 56.8 HTT: Highest treatment temperature of pyrolysis; S + B: Soil + biochar mixture.HTT: Highest treatment temperature of pyrolysis; S B: Soil biochar mixture. Ka: Distribution coefficient for 1 pgAcT/gsoil·K : Distribution coeffciient for 1 g /g .d ACT soil Soil samples after incubation were subjected twice to automated Soxhlet extraction with 150 mL of methano l at 250 °C using a Soxtherm Soxhlet extraction with 150 mL of methanol at 250 C using a Soxtherm system (Gerhart, Germany) for 180 min. Extracted ACT and its trans-formation metabolites were separated by UPLC (Waters I-Class Series)and identified both by matching their retention times to known stan-and identifeid both by matching their retention times to known stan-dards and by QToF-MS/MS analysis (maXis impact Series, Bruker Dal-dards and by QToF-MS/MS analysis (maXis impact Series, Bruker Dal-tonics) equipped with an electrospray ionization source (ESI) in positive ion mode. No transformation metabolites or interference compounds were detected in the negative controls without ACT at day 0, which supports their origin as transformation products from the degradation of paracetamol. The separation was performed on an Acquity UPLC BEH C18 column (2.1×100 mm,1.7 um particle size) with gradient elution.C18 column (2.1 100 mm, 1.7 m particle size) with gradient elution.Flow rate was 0.25 mL min-1 and injection volume 10 uL . Mobile phase Flow rate was 0.25 mL min and injection volume 10 L. Mobile phase A was water 0.1% formic acid and mobile phase B was methanol 0.1% formic acid, with the following gradient used (referencing mo-bile phase B):0 min , 5%; 1.5 min, 25%; 9.5 min, 80%; 11.5 min, 95%; re-bile phase B): 0 min, 5%; 1.5 min, 25%; 9.5 min, 80%; 11.5 min, 95%; re-equilibration with 5% of mobile phase B. Average ACT recovery after day 0 ranged from 86 to 95%((T a bl e S 1), which was considered day 0 ranged from 86 to 95%(Table S1), which was considered satisfactory. 2.5. Adsorption study 2.5. Adsorption study Adsorption was determined with a batch equilibration Adsorption was determined with a batch equilibration t t e e c c h h n n i i q q u u e e following OECD guideline 106 (OEC D, 2000). 2 g of soil (dry weight) or following OECD guideline 106 (OECD, 2000). 2 g of soil (dry weight) or soil-biochar mixture and 0.01 M CaCl2 was added to 50 mL poly-soil-biochar mixture and 0.01 M CaCl 2 was added to 50 mL poly-propylene tubes to reach a 1:25 soil/solution ratio (two replicates). The solution pH is shown in T a bl e S2. Samples were placed in a shaker at solution pH is shown in Table S2. Samples were placed in a shaker at 200 rpm and ACT added to get a concentration 1of 1 ngg=. After 5 h the 200 rpm and ACT added to get a concentration of 1 g g . After 5 h the samples were centrifuged at 3000 rpm for 20 min, after which 2 mL of the supernatant were removed and analyzed by UPLC. The distribution coefficient Ka was calculated using the following equation:coeffciient K d was calculated using the following equation: where Aeq is the equilibrium adsorption percentage; Vo is the initial where A is the equilibrium adsorption percentage; V is the initialeq 0volume in the aqueous phase, and msoil represents dry soil mass (g). The volume in the aqueous phase, and m represents dry soil mass (g). Thesoil adsorption equilibrium time (5 h) was obtained by performing the adsorption equilibrium time (5 h) was obtained by performing the adsorption experiment on the same conditions but different shaking duration (1, 3, 5, 7 and 8h).duration (1, 3, 5, 7 and 8 h). 2.6. Treatment of data and statistics Data from the degradation experiment w Data from the degradation experiment w a a s s fi f t ti t t e e d d t t o o t t h h e e f f o o l l l l o o w w i i n n g g frist-order exponential decay equation to obtain the ACT degradation rate (k): where t is time (in days), Co is the initial detected ACT concentration (in where t is time (in days), C 0 is the initial detected ACT concentration (in gg 1, C i s the ACT concentration at time t and k is the rate constant.g g , C t is the ACT concentration at time t and k is the rate constant.The significance level of the differences in the rate constants was The signifciance level of the differences in the rate constants was calculated by one-way ANOVA, using the Holm-Sídak test and adjusting the p-values for multiple comparisons.the p-values for multiple comparisons. 3. Results and discussion 3.1. Biochar characteristics 3.1. Biochar characteristics The complete characterization of the different biochars was per-The complete characterization of the different biochars was per-formed in a previous work (C haco n et a l ., 2020). formed in a previous work (Chacon et al., 2020). A A l l l l t t h h e e t t r r e e a a t t m m e e n n t t s s w w e e r r e e able to increase the redox capacity of the two unmodified able to increase the redox capacity of the two unmodifeid b b i i o o c c h h a a r r s s ,,B-400 and B-1000 (Table 1), which had the lowest values of all samples (0.413 and 0.085 mmol e-g-1, respectively).(0.413 and 0.085 mmol e g-1, respectively). Thei r treatment with KMnO4 was able to greatly increase the number Their treatment with KMnO 4 was able to greatly increase the number of functional groups on the surface and therefore their redox capacity,especially in the case of the B-400-KMnO4 biochar which tripled its especially in the case of the B-400-KMnO biochar which tripled its4original value (1.12 mmol e g-1). B-400 presents an amorphous original value (1.12 mmol e g ). B-400 presents an amorphous structure more susceptible to oxidation, which allowed for a more structure more susceptible to oxidation, which allowed for a more complete introduction of functional groups. The stable aromatic complete introduction of functional groups. The stable aromatic network of B-1000, produced at a very high treatment temperature that allows the formation of a graphitic structure, resulted in a lower intro-duction of functional groups and only a moderate redox capacity in the duction of functional groups and only a moderate redox capacity in the B-1000-KMnO4 biochar (0.418 mmol e g)1. I n t he case of the Fe-B-1000-KMnO biochar (0.418 mmol e g ). In the case of the Fe-4preloaded biochar, B-400-Fe, the redox capacity increased by 38%preloaded biochar, B-400-Fe, the redox capacity increased by 38%(0.572 mmole g -1) compared to the original B-400. Only ~34% of the (0.572 mmol e g ) compared to the original B-400. Only ~34% of the increase in the redox capacity can be directly linked to the presence of metal on the surface (Table S3), while the rest is probably related to the higher surface area of B-400-Fe. The pyrolysis at 400 °C of B-400 was not enough to produce a The pyrolysis at 400 C of B-400 was not enough to produce a condensed aromatic network able to transport electrons, which resulted in a very low conductivity in all biochars pyrolyzed at this temperature.The i ntroduction of functiona l groups by th The introduction of functional groups by th e e t t r r e e a a t t m m e e n n t t s s d d e e g g r r a a d d e e d d s s o o m m e e of the graphitic fraction of the original biochar, which reduced the of the graphitic fraction of the original biochar, which reduced the already low conductivity even fur t her. B-1000-KMnO4, however,already low conductivity even further. B-1000-KMnO , however,4retained some of the conductivity of the original B-1000 biochar, which retained some of the conductivity of the original B-1000 biochar, which was more than 25 times higher than the rest of biochars. This created an electrochemically hybrid biochar with both a moderate redox capacity and conductivity that might provide a synergistic effect on the degra-dation of ACT.dation of ACT. 3.2. Acetaminophen adsorption 3.2. Acetaminophen adsorption The pKa of ACT is 9.4, which makes it neutral in most of the pH range studied for the different soil-biochar mixtures (5.31-–5.81). Thus, we studied for the different soil-biochar mixtures (5.315.81). Thus, we assume that electrostatic interactions were insignificant for the assume that electrostatic interactions were insignifciant for the adsorption process, while n-t i nteractions and H-bonding to both adsorption process, while - interactions and H-bonding to both phenolic and quinone groups are probably the dominant mechanisms (Patel e t a l ., 2021). All soil-biochar mixtures had a higher adsorption (Patel et al., 2021). All soil-biochar mixtures had a higher adsorption than unamended soi l (Ka=27.1 mLg 1,T ab l e 1). B-400-KMnO4, with than unamended soil (K d 27.1 mL g , Table 1). B-400-KMnO , with 4its high surface area and concentration of oxygen-containing functional groups (for H-bonding) was the biochar with the highest adsorption,groups (for H-bonding) was the biochar with the highest adsorption,followed by B-1000, which had the highest aromaticity (for T-丁 in-followed by B-1000, which had the highest aromaticity (for - in-teractions). From their Ka values and the amount of water added,teractions). From their K the d values and the amount of water added, the percentage of adsorbed ACT in the soil-biochar mixture can be theo-retically calculated (Fi g . S 1; Ta ble S4). > 95% of spiked ACT is expected retically calculated (Fig. S1; Table S4). 95% of spiked ACT is expected to be adsorbed to soil in all samples, with the B-400-KMnO4 amended to be adsorbed to soil in all samples, with the B-400-KMnO 4 amended soil adsorbing >99% of the added ACT.soil adsorbing 99% of the added ACT. 3.3. Acetaminophen degradation After only 8 d of incubation, the aerobic unamended soil was able to degrade 72% of the spiked contaminant and 88% after 16 d (Fig. 1),proving the ability of the soil to biodegrade the pollutant. The presence of oxygen makes ACT susceptible to be oxidized a of oxygen makes ACT susceptible to be oxidized a t t i i t t s s h h y y d d r r o o x x y y l l g g r r o o u u p p ,,either biotically or abiotically (L i et al., 2017; W u e t a l ., 2012). How-either biotically or abiotically (Li et al., 2017; Wu et al., 2012). How-ever, the reducing conditions of the anaerobic incubation are expected to prevent this oxidation substantially, which explains the lower trans-formation in anaerobic soil. The amendment of raw biochars inhibited the degradation of ACT in both incubations, especially in the case of B-400. After 4 d of aerobic incubation, soil amended with B-400 was only able to transform 30% of the contaminant, while unamended soi l only able to transform 30% of the contaminant, while unamended soil already transformed 64%. A similar situation was found in the anaerobic incubation, where after 16 days only 34% of ACT was degraded in the B-400 amended soi l compared to the 56% of the unamended one. For B-400 amended soil compared to the 56% of the unamended one. For some contaminants, adsorption to biochar has been proposed as the mechanism through which their degradation is inhibited, since it could prevent their contact with microorganisms or their permanent bonding to soil organic matter (Loganathan et al., 2009; Ukalska-Jaruga et al.,2019). The conductive network of the B-1000 biochar could solve this bioavailability problem to some extent by transferring electrons from one part of the materia l to another, making its inhibition much lower.one part of the material to another, making its inhibition much lower.This became apparent by the simi l ar ACT degradation kinetics of the This became apparent by the similar ACT degradation kinetics of the B-1000 amended and unamended soils, especially in anaerobic condi-tions. Thus, the absence of conductivity in biochars pyrolyzed at rela-tively low temperatures could result in a higher potential environmental risk during the f irst weeks after contamination .risk during the frist weeks after contamination. Biochar modification greatly increased the rate of A Biochar CT trans- modifciation greatly increased the rate of ACT trans-formation, with the exception of B-400-Fe amendment in anaerobic soil.Despite the potent i al bioavailabi l ity issues, the non-conductive but Despite the potential bioavailability issues, the non-conductive but highly redox-active B-400-KMnO4 had a much faster and complete highly redox-active B-400-KMnO 4 had a much faster and complete remediation than both raw biochars and unamended soil. Moreover, two of the biochars with the higher ACT adsorption (B-400-KMnO4 and B-of the biochars with the higher ACT adsorption (B-400-KMnO 4 and B-1000-KMnO4) showed i ncreased degradation compared to those with a 1000-KMnO 4 ) showed increased degradation compared to those with a lower adsorption (B-400-Fe and B-400). This supports the hypothesis that ACT can be directly transformed on the surface of biochar, for that ACT can be directly transformed on the surface of biochar, for Aerobic incubations Anaerobic incubations Days Days Days Days Fig. 2. Electrochemical analysis of the redox Fig. 2. Electrochemical analysis of the redox reaction between ACT and biochar (B-400-KMnO4). (a) Addition of NAPQI and its KMnO ). (a) Addition of NAPQI and its4reduction to ACT on the electrode surface;(b) Unresponsive addition of biochar (b) Unresponsive addition of biochar revealing no direct redox reaction between biochar and the electrode; (c) Sequential biochar and the electrode; (c) Sequential addition of NAPQI and biochar. The elec-addition of NAPQI and biochar. The elec-trical current appearing after the addition of biochar suggests a redox reaction between biochar and ACT, as there is no reaction biochar and ACT, as there is no reaction between biochar and the electrode. example by redox-active functional groups, metals or even by persistent free radicals formed during pyrolysis, a mechanism that has been pro-free radicals formed during pyrolysis, a mechanism that has been pro-posed for structurally similar contaminants such as p-nitrophenol (Yang et al., 2017, 2016). This is supported by electrochemical analysis, which reveals the existence of electron transfer from ACT to biochar in solution (Fi g . 2). The exceptional redox capacity and surface area of the (Fig. 2). The exceptional redox capacity and surface area of the B-400-KMnO4 biochar seemed to provide a sufficiently ample platform B-400-KMnO biochar seemed to provide a suffciiently ample platform4for ACT transformation, counterac t ing any potential bioavailability for ACT transformation, counteracting any potential bioavailability problems, which was not the case for the less redox-active B-400. The higher conductivity of the hybrid B-1000-KMnO4 increased ACT The higher conductivity of the hybrid B-1000-KMnO 4 increased ACT rate of remediation compared to B-400-KMnO4, even though it resulted rate of remediation compared to B-400-KMnO 4 , even though it resulted in a lower redox capacity. Furthermore, despite having a lower con-ductivity than that of the unmodified B-1000 and a similar redox ca-ductivity than that of the unmodifeid B-1000 and a similar redox ca-pacity than B-400, the result i ng synergy from having a moderate amount pacity than B-400, the resulting synergy from having a moderate amount of both properties led to a much faster ACT remediation. The transfer of electrons from one part of biochar to another through the conductive network could regenerate redox-active moieties involved in the trans-formation of ACT (Pr a d o e t a l ., 2019), while the higher amount of formation of ACT (Prado et al., 2019), while the higher amount of functional groups could facilitate microbial el ectron s functional huttling groups could facilitate microbial electron shuttling b b y y attaching cells to the surface and increasing the electron transfer rate attaching cells to the surface and increasing the electron transfer rate between biochar, cel l s and the contaminant (Li an d Cheng , 2019; L i between biochar, cells and the contaminant (Li and Cheng, 2019; Li et al., 2019), Therefore, biochars with both a high redox capacity (for direct ACT transformation) and a developed conductive network (to avoid bioavailability issues and increase microbial electron shuttling)are ideal for a fast and effective ACT remediation in soil. Although most biochars exhibited a similar behavior in both types of incubation, B-400-Fe showed a drastic decline in the rate and extent of remediation in anaerobic conditions. XRD analysis of B-400-Fe (Fig. S2)reveals the presence of magnetite on its surface, which contains both Fe (II) and Fe(III) forms of the metal. Previous research has proved that ACT can be directly oxidized by Fe(III) to the radical form of the ACT can be directly oxidized by Fe(III) to the radical form of the molecule (Tong et al., 2021). In aerobic conditions, Fe(II) is oxidized to Fe(III), continually renewing the available Fe(III ) to oxidize ACT and Fe(III), continually renewing the available Fe(III) to oxidize ACT and increasing its rate of remediation. In the anaerobic i ncubation with increasing its rate of remediation. In the anaerobic incubation with B-400-Fe, however, Fe(III) is quickly depleted. Having only a slightly better electron exchange capacity than B-400 and no conductivity to offset the bioavai l abil i ty problem, the result is a lower ACT degradation offset the bioavailability problem, the result is a lower ACT degradation than in aerobic conditions and an inhibitory effect similar to the un-than in aerobic conditions and an inhibitory effect similar to the un-modifeid B-400. Although not the objective of this work, this premise could the examined by measuring the dynamic concentration of Fe(II)and Fe(III).and Fe(III). 3.4. ACT degradation pathways 3.4. ACT degradation pathways A more in-depth study of the degradation products of ACT is required A more in-depth study of the degradation products of ACT is required to understand biochar influence on ACT remediation. Additionally, the to understand biochar infulence on ACT remediation. Additionally, the intermediate by-products that are formed after ACT degradation may present different toxicities, some being even more detrimental than the parent compound. Besides acetaminophen itself (M7), eleven additional metabolites were detected (T ab l e S5), including p-aminophenol (M1),metabolites were detected (Table S5), including p-aminophenol (M1),maleic acid (M2), aniline (M3), hydroquinone (M4), 3-hydroxyacetami-nophen (M5), N-acetyl-p-benzoquinone imine (NAPQI, M6), catechol nophen (M5), N-acetyl-p-benzoquinone imine (NAPQI, M6), catechol (M8), the dimer form of acetaminophen (M9), methacetin (M10), 4-(M8), the dimer form of acetaminophen (M9), methacetin (M10), 4-methoxyphenol (M11) and phenol (M12). Four of them (M1, M5, M6methoxyphenol (M11) and phenol (M12). Four of them (M1, M5, M6and M11) appeared earlier than the rest, from which four different ACT transformation pathways can be discerned, mainly hydroxyl oxidation transformation pathways can be discerned, mainly hydroxyl oxidation (I), deacetylation (II), methylation (III) and hydroxylation (IV). The (I), deacetylation (II), methylation (III) and hydroxylation (IV). The higher concentration of NAPQI, p-aminophenol and 3-hydroxyacetami-nophen in the aerobic incubations (Fig. 3) suggests that these conditions favor ACT transformation through hydroxyl oxidation, deacetylation favor ACT transformation through hydroxyl oxidation, deacetylation and hydroxylation. On the other hand, anaerobic condi t ions result i n the and hydroxylation. On the other hand, anaerobic conditions result in the predominance of 4-methoxyphenol, pointing to methylation as the main a 15-古 10- 5 0 0 8 121620 24 28 32 Day s 8642 产 0 一 一 0 4 8 121620 24 28 32 Days 10- 20-a 815-64 2 10- 5 一 三一 0-8中 口 一 0 4 8 12162024 28 32 0 812 16 20 24 28 32 Day s Days 020-86 4 O 15-寸 10- 5- 0084 一——。 0- 一 十 ——一 二 0 8 12162024 28 32 C 0 4 8 121620 24 28 32 Days Days 10- O815-6 04 10 5- 2 0 一 0 8 12162024 28 32 C 8 12162024 28 32 Day s Day s a 20 8615-4 2 10 5 平 0- 0 0 0 8 12162024 28 32 8121620 24 28 32 Days Day s Days Days -C- p-aminophenol (M 1 ) 4-Methoxyphenol (M11) 一 ◇◇一 Methacetin (M10) ◇1Hydroquinone (M4) 一一 3-hydroxyacetaminophen (M5)-- Phenol (M12) NAPQI (M6) 七 一 Catechol (M8) Fig. 3. Evolution in the concentration of transformation metabolites in soil as a % fraction of the spiked ACT . Error bars represent ± SD (n =3).Fig. 3. Evolution in the concentration of transformation metabolites in soil as a % fraction of the spiked ACT. Error bars represent SD (n 3). anaerobic degradation route. The concentration of metabol i tes in this anaerobic degradation route. The concentration of metabolites in this first phase of ACT transformation usually finds its peak around 2-–4 days frist phase of ACT transformation usually fnids its peak around 24 days of incubation. A second group of metabolites composed of hydroqui-none, phenol and catechol takes over after 4 days of incubation, usually finding their peak at 8 days and slowly declining even past 32 days of fniding their peak at 8 days and slowly declining even past 32 days of incubation. These compounds present the highest concentrations found in the extracts, probably accumulating as degradation pools from several ACT transformation pathways. They can also be interconverted one to another depending on the preferred biotransformation route.another depending on the preferred biotransformation route. ACT degradation through p-aminophenol and hydroquinone is the dominant pathway described in most of the literature, being catechol a minor route (W u e t a l., 2012; Zur et al ., 2018). Phenol has only been minor route (Wu et al., 2012; Zur et al., 2018). Phenol has only been detected in non-microbial degradation of ACT (Wa ng e t al., 2016),detected in non-microbial degradation of ACT (Wang et al., 2016),which may be a result of the aerobic nature of most of the studies performed with microorganisms. In our case, however, catechol and phenol were the most abundantly detected metabolites in the extracts of phenol were the most abundantly detected metabolites in the extracts of the aerobic and anaerobic incubations, respectively. Different soils are known to display alternative pathways to contaminant degradation (Li e t a l., 2014; L i a ng et a l ., 2016), probably originating from their different et al., 2014; Liang et al., 2016), probably originating from their different environmental matrices and microbial populations. Alternatively, the lower concentration of some metabolites such as p-aminophenol or hy-droquinone may simply be the result of a higher rate of transformation droquinone may simply be the result of a higher rate of transformation than other intermediates, which would lead to a lower accumulation. As a fnial step before entering the tricarboxylic acid cycle, these compounds will undergo ring cleavage reactions to form acids of cyclic chain such as maleic acid (with a peak between 8 and 16 days, Fi g. S 3). Ani l ine w maleic acid (with a peak between 8 and 16 days, Fig. S3). Aniline w a a s s also detected during the entire incubation but at negligible concentra-also detected during the entire incubation but at negligible concentra-tions below 50 ppb (data not shown), much lower than the level at Fig. 4. Proposed reaction pathways for the degradation of acetaminophen in soi l . Yellow arrows i ndicate routes that are favored by aerobic co Fig. 4. Proposed reaction pathways for the degradation of acetaminophen in soil. Yellow arrows indicate routes that are favored by aerobic co n n d d i i t t i i o o n n s s ,, w w h h i i l l e e b b l l u u e e arrows indicate those more prevalent in anaerobic conditions. Roman numerals refer to hydroxyl oxidation (I), deacetylation (II), methylation (III) and hydroxylation (IV) degradation routes. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)(IV) degradation routes. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.) which i t is expected to be environmentally harmful (Aru o j a e t a l ., 2011;which it is expected to be environmentally harmful (Aruoja et al., 2011;Chen e t a l ., 2013).Chen et al., 2013). The leachate of the soluble organic fraction from biochar is a com-mon phenomenon in soil (Chen et al., 2019; Quilliam et al., 2012).Negative controls without ACT showed that the washing steps after Negative controls without ACT showed that the washing steps after pyrolysis and treatments were enough to remove potential interfering compounds in UPLC-MS analysis. Still, with time the labile organic compounds in UPLC-MS analysis. Still, with time the labile organic fraction of biochar could be degraded in soil and release some of the fraction of biochar could be degraded in soil and release some of the phenolic compounds studied. After 8 days of incubation, only catechol was found in the soil-biochar controls, equivalent to a maximum of 1.2%of the spiked ACT in the B-400-KMnO4 sample (T ab le S 6). The peak of the spiked ACT in the B-400-KMnO sample (Table S6). The peak4concentration of catechol in the ACT-contaminated samples (including concentration of catechol in the ACT-contaminated samples (including unamended soi l) typical l y reached 5-–20% of the spiked contaminant unamended soil) typically reached 520% of the spiked contaminant after 8 days, which along with the absence of this metabolite in the non-contaminated unamended soil supports its main origin as a degra-dation by-product of ACT.dation by-product of ACT. The genera l reaction The general reaction p p a a t t h h w w a a y y f f o o r r t t h h e e d d e e g g r r a a d d a a t t i i o o n n o o f f A A C C T T i i n n s s o o i i l l i i s s proposed in Fig. 4 on the basis of the detected degradation intermediates and previous literature (L i e t a l ., 2014; Wu et a l., 2012; Zur et al., 2018).and previous literature (Li et al., 2014; Wu et al., 2012; Zur et al., 2018). 3.4.1. ACT hydroxyl oxidation (I) As previously mentioned, ACT is very susceptible to be oxidized at the hydroxyl group and is the most l ikely mechanism for its initial the hydroxyl group and is the most likely mechanism for its initial transformation in aerobic soil. The process is initiated by the loss of one electron and one hydrogen, which produces the radical form of the molecule. The phenoxyl radical is able to oligomerize through self-molecule. The phenoxyl radical is able to oligomerize through self-coupling reactions (P e ng et al., 2017; T ong coupling reactions (Peng et al., 2017; Tong e e t t a a l l ..,, 22002211)) a a n n d d i i t t s s h h i i g g h h reactivity also allows for cross-coupling to soil organic matter to form permanent bound residues. Previous research proposed that this binding to soil organic matter could be the main fate in the transformation of ACT i n soi l , ranging f rom 73% to 93% of the i ni ti al spiked ACT (L i et a l .,ACT in soil, ranging from 73% to 93% of the initial spiked ACT (Li et al.,2014). This is expected to reduce its bioavailability and may therefore be considered detoxified. In our case, however, for some of the biochars the considered detoxifeid. In our case, however, for some of the biochars the sum of metabolites at 8 days of incubation (where the peak of catechol sum of metabolites at 8 days of incubation (where the peak of catechol and phenol is usually found) can reach up to a 53% equivalent of the initial spiked ACT, in agreement with studies performed in soil-water systems where degradation rather than permanent binding was the systems where degradation rather than permanent binding was the main fate of ACT (Liang et al., 2016; Lin et al., 2010). The real per-centage of transformed ACT is probably even higher, as the concentra-tions detected in the extracts are just a static value of a complex,tions detected in the extracts are just a static value of a complex,dynamic process. This suggests that some of the bound residues, for dynamic process. This suggests that some of the bound residues, for example those bound by a non-covalent mechanism such as physical example those bound by a non-covalent mechanism such as physical entrapment, could slowly be released from soil organic matter. Alter-natively, the high temperatures reached in the extraction process could have released bound residues that may not do so under normal soil conditions. Even in this case, the presence of degradation products conditions. Even in this case, the presence of degradation products would indicate a previous ACT transformation step before their binding to soil. The second loss of an electron and a hydrogen produces the inter-The second loss of an electron and a hydrogen produces the inter-mediate NAPQI (M6), which is highly reactive and a much more toxic metabolite than ACT (C hun e t a l., 2009). This oxidation can be meta-metabolite than ACT (Chun et al., 2009). This oxidation can be meta-bol i cally performed by different microbia l oxidases such as cytochrome bolically performed by different microbial oxidases such as cytochrome P450 or directly by molecular oxygen (Macherey and Dansette, 2008),which explains the higher NAPQI concentration in the aerobic samples.A direct redox reaction with quinones or Fe on the surface of biochar is another plausible route in the production of NAPQI . While quinones in another plausible route in the production of NAPQI. While quinones in biochar (contributing to its electron accepting capacity) can accept up to two electrons, Fe(III) can only accept one to be reduced to Fe(II). The presence of Fe is therefore expected to promote the formation of oligo presence of Fe is therefore expected to promote the formation of oligo-mers in addi t ion to NAPQI (through the production of the radical form of mers in addition to NAPQI (through the production of the radical form of ACT), whi l e biochar quinones will mainly favor the production of ACT), while biochar quinones will mainly favor the production of NAPQI. Indeed, B-400-Fe amendment showed detectable levels of dimer formation several times higher than the rest of the samples, especia formation several times higher than the rest of the samples, especia l l l l y y i i n n the anaerobic incubation due to the absence of oxygen (Fig. S4). Dimer production was still higher in all biochar amended soils compared to unamended soil , which may originate from an incomplete oxidation of unamended soil, which may originate from an incomplete oxidation of ACT by the quinone groups or from the presence of persistent free rad-ACT by the quinone groups or from the presence of persistent free rad-icals on the surface of biochar. Although radicals have generally a very icals on the surface of biochar. Although radicals have generally a very short life, their presence in biochar is higher than in other materials. Its characteristic aromatic structure is able to stabilize by resonance the unpaired electrons of radicals, increasing their half-life from hours to unpaired electrons of radicals, increasing their half-life from hours to months or even years (Ruan et al., 2019). 3.4.2. ACT deacetylation (II)3.4.2. ACT deacetylation (II) Although contaminant deacetylation is a process present in both Although contaminant deacetylation is a process present in both aerobic and anaerobic conditions, the concentration of p-aminophenol (M1) in our soil was generally higher in aerobic conditions. p-amino-phenol was a transient metabolite that only remained in soil for a few days and at low concentrations, being quickly converted to hydroqui-days and at low concentrations, being quickly converted to hydroqui-none (M4). This transformation to hydroquinone has been described for many different microorganisms (Zh a ng e t a l ., 2012many different microorganisms (Zhang et al., 2012)),, a a f f t t e e r r w w h h i i c c h h m m a a y y b b e e further converted to phenol (M12) under reducing conditions (B ra ck-further converted to phenol (M12) under reducing conditions (Brack-mann and Fuchs, 1993), 1,4-benzoquinone under aerobic conditions (Devillers et al., 1990), or its ring opened to form products such as maleic acid (M2) (F e r rei r o et al .,2021). Hydroquinone and i ts potential maleic acid (M2) (Ferreiro et al., 2021). Hydroquinone and its potential aerobic product 1,4-benzoquinone are much more toxic than ACT both to aquatic and soil microorganisms (En g u it a a nd Leitao,2013). The peak to aquatic and soil microorganisms (Enguita and Leitao, 2013). The peak of hydroquinone appeared sooner in the soil amended with the of hydroquinone appeared sooner in the soil amended with the enhanced biochars (at 4 days instead of 8 days) and, as was the case with NAPQI, i ts overall concentration increased during the first days NAPQI, its overall concentration increased during the frist days compared both to the unamended soil and the raw biochars. This risk seems reduced in anaerobic conditions due to a lower concentration of seems reduced in anaerobic conditions due to a lower concentration of hydroquinone and its probable conversion to the less harmful phenol,hydroquinone and its probable conversion to the less harmful phenol,which will mitigate this potential toxicity. 3.4.3. ACT methylation (III)3.4.3. ACT methylation (III) The conversion of ACT to methacetin (M10) through the methylation The conversion of ACT to methacetin (M10) through the methylation of its hydroxyl group occurred very early in the incubation, a process that has been described before (L i e t al ., 2014). Following its deacety-that has been described before (Li et al., 2014). Following its deacety-lation and deamination, 4-methoxyphenol (M11) lation and deamination, 4-methoxyphenol (M11) w w a a s s a a b b u u n n d d a a n n t t l l y y detected after 4 days, especially in the anaerobic i ncubations. detected This after 4 days, especially in the anaerobic incubations. This higher anaerobic production may simply be the result of the higher disposal of ACT for methylation, as other degradation routes are less available in the absence of oxygen. Different studies show a very low toxicity of this compound (CIR, 1985, EFSA, 2011), whose main risk is the reactivity of its transformation products. This metabolite can be degraded through further methylation of the re degraded through further methylation of the re m m a a i i n n i i n n g g h h y y d d r r o o x x y y l l g g r r o o u u p p to form 1,4-dimethoxybenzene or by O-d to form 1,4-dimethoxybenzene or by O-d e e m m e e t t h h y y l l a a t t i i o o n n t t o o p p r r o o d d u u c c e e h h y y --droquinone. The ring fsision step of 1,4-dimethoxybenzene gives way to 2-hexenoic acid (Li et al., 2014), which was not detected in the extracts.Many methoxylated phenolic compounds usually have to be O-deme-Many methoxylated phenolic compounds usually have to be O-deme-thylated before ring cleavage (Mallinson et al., 2018; Milliken et al.,2004), which suggests that the main transformation pathway of 2004), which suggests that the main transformation pathway of 4-methoxyphenol in our soil iSt through O-demethylation 4-methoxyphenol in our soil is through O-demethylation to 3.4.4. ACT hydroxylation (IV) The f inal ACT t ransformation pathway detected in our soil was its The fnial ACT transformation pathway detected in our soil was its hydroxylation to 3-hydroacetaminophen (M5), a less harmful metabo-hydroxylation to 3-hydroacetaminophen (M5), a less harmful metabo-lite than ACT (Forte et al., 1984). This conversion has been hypothesized to occur directly via microbial oxidases or hydroxyl radical (OH)to occur directly via microbial oxidases or hydroxyl radical ( OH)addition (B org es e t a l ., 2010; Li e t a l ., 2020; Val e r o e t al., 2003).addition (Borges et al., 2010; Li et al., 2020; Valero et al., 2003).Tyrosinase, laccase and CYP450 are some of the enzymes known to produce 3-hydroxyacetaminophen either as an intermediate product or as a final metabolite (Ba et al .,2014; Olicon-He r n a n d ez et a l ., 2020).as a fnial metabolite (Ba et al., 2014; Olicon-Hernandez et al., 2020).Being oxidases, they all require molecular oxygen as cofactor to func-Being oxidases, they all require molecular oxygen as cofactor to func-tion, which explains the higher concentration of the intermediate in the aerobic incubations. As for radical hydroxylation, OH addition at the meta position may be favored due to the presence of the hydroxyl group meta position may be favored due to the presence of the hydroxyl group in ACT, a stronger electron donor than -–NHC in ACT, a stronger electron donor thanNHC O O C C H H 3 ((T T o o r r r r e e s s e e t t a a l l ..,, 2003;32003;Yang et al., 2009). Biochar is known to generate OH radicals (Fang et a l., 2015; Y a ng et al., 2015), for example in the reaction of molecular et al., 2015; Yang et al., 2015), for example in the reaction of molecular oxygen with phenolic groups on the surface of biochar (responsible of its electron donating capacity). The appearance of 3-hydroxyacetamino-phen in the anaerobic incubations could be ascribed to unavoidable residual O2 or to marginal microbial anaerobic hydroxylation (Fuc hs residual O 2 or to marginal microbial anaerobic hydroxylation (Fuchs et a l., 1994; Sc h i n k et a l ., 2000). The abundant ca et al., 1994; Schink et al., 2000). The abundant ca t t e e c c h h o o l l i i s s p p r r o o b b a a b b l l y y t t h h e e main degradation product of this metabolite, followed by ring opening.Whi l e this metabolite has an intermediate toxicity between pheno l and While this metabolite has an intermediate toxicity between phenol and hydroquinone (B oy d e t al ., 1997; Chen e t a l ., 2009), the presence of both hydroquinone (Boyd et al., 1997; Chen et al., 2009), the presence of both catechol and hydroquinone (as is our case) seems to have a synergistic toxic effect that can increase its risk, especially in aerobic conditions (Chen et al., 2010). 4. Conclusions We have shown that transformation to other metabolites is the main We have shown that transformation to other metabolites is the main fate of paracetamol (ACT) in soil, especially in the soil amended with designer redox-active biochars. The presence of several oxidative designer redox-active biochars. The presence of several oxidative transformation pathways in aerobic conditions resulted in a much faster transformation pathways in aerobic conditions resulted in a much faster ACT degradation than in anaerobic soil, with catechol as the main ACT degradation than in anaerobic soil, with catechol as the main degradation metabolite. Although the commonly described hydroqui-none degradation route was also present in our soil, the most abundant metabolites found were catechol and phenol (for the aerobic and metabolites found were catechol and phenol (for the aerobic and anaerobic incubations, respectively). These intermediates have been anaerobic incubations, respectively). These intermediates have been seldom quantified during the identification of ACT transform seldom quantifeid during the identifciation of ACT transform a a t t i i o o n n products, which may explain why they have been previously considered products, which may explain why they have been previously considered as minor products of ACT metabolism. Biochar addition affected ACT transformation pathways and kinetics in soil, with its electrochemical properties having a direct role i n this in soil, with its electrochemical properties having a direct role in this outcome. This was exploited in the designer biochars, whose enhanced electrochemical properties allowed them to overcome the inhibition of the raw biochars derived from their adsorption and immobilization of ACT. Soi l amended with these tailored biochars surpassed the original ACT. Soil amended with these tailored biochars surpassed the original ability of soil to degrade ACT , especially in anaerobic conditions, where ability of soil to degrade ACT, especially in anaerobic conditions, where they provided another route for ACT transformation, i.e. redox-mediated hydroxyl oxidation, not present in unamended soil. This study shows that the complex nature of the i nteractions between soi l , biochar and the that the complex nature of the interactions between soil, biochar and the contaminants require in-depth study and careful planning to ensure that there is no increase in the overall environmental risk after biochar there is no increase in the overall environmental risk after biochar addition. CRediT author statement All authors contributed equally to this work. Declaration of competing interest Declaration of competing interest The authors declare that they have no known competing fniancial interests or personal relation interests or personal relation s s h h i i p p s s t t h h a a t t c c o o u u l l d d h h a a v v e e a a p p p p e e a a r r e e d d t t o o i i n n f f lu ul e e n n c c e e the work reported in this paper.the work reported in this paper. Acknowledgments Financial support from the Spanish Ministry of Economy and Financial support from the Spanish Ministry of Economy and Competitiveness (Projects N° CTM 2015-67200-R) and Spanish Ministry Competitiveness (Projects Nº CTM 2015-67200-R) and Spanish Ministry of Science, Innovation and Universities cofunded with EU FEDER funds (Nº RTI 2018-099417-B-I00) is greatly appreciated. Appendix A. Supplementary data Appendix A. Supplementary data Supplementary data to this article can be found onl Supplementary data to this article can be found onl i i n n e e a a t t h h t t t t p p s s ::////d d o o i i ..org/10.1016/j.envpol.2022.119546. References Aruoja, V., Sihtmae , M., Dubourguier, H.-C., Kahru, A., 2011. Toxicity o f 58 subst i tuted Aruoja, V., Sihtmae, M., Dubourguier, H.-C., Kahru, A., 2011. Toxicity of 58 substituted anilines and phenols to algae Pseudokirchneriella subcapitata and bacteria Vibrio –fsicheri: comparison with published data and QSARs. Chemosphere 84, 13101320. https://doi.org/10.1016/j.chemosphere.2011.05.023. Ashfaq, M., Nawaz Khan, K., Saif Ur Rehman, M., Mustafa, G., Faizan Nazar, M., Sun, Q.,Iqbal, J., Mulla, SikandarI., Yu, C.-P., 2017. Ecological risk assessment of pharmaceuticals in the receiving environment of pharmaceutical wastewater in Pakistan. Ecotoxicol . Environ. Saf. 136,31-–39. h t t ps ://d o i.or g /10.1016/j .Pakistan. Ecotoxicol. Environ. Saf. 136, 3139. https://doi.org/10.1016/j.ecoenv.2016.10.029. Ba, S., Haroune, L., Cruz-Morato, C., Jacquet, C., Touahar, I.E., Bellenger, J.-P.,Legault, C.Y., Jones, J.P., Cabana, H., 2014. Synthesis and characterization of combined cross-linked laccase and tyrosinase aggregates transforming acetaminophen as a model phenolic compound in wastewaters. Sci. Total Environ.–487, 748755. https://doi.org/10.1016/j.scitotenv.2013.10.004. Bartha, B., Huber, C., Harpaintner, R., Schronder, P., 2010. Effects of acetaminophen in Brassica juncea L. Czern.: investigation of uptake, translocation, detoxifciation, and –the induced defense pathways. Environ. Sci. Pollut. Control Ser. 17, 15531562.https://doi.org/10.1007/s11356-010-0342-y . Bedner, M., MacCrehan, W.A., 2006. Transformation of acetaminophen by chlorination produces the toxicants 1,4-benzoquinone and N-Acetyl-p-benzoquinone imine.–Environ. Sci. Technol. 40,Environ. Sci. Technol. 40, 551166-522. ht tps ://d o i.org / 522. https://doi.org/1100..11002211//e e s s 00550099007733.. Borges, R.S., do Nascimento, J.L.M., Alves, C.N., 2010. A theoretical study for oxidative metabolism of acetaminophen. J. Comput. Theor. Nanosci. 7, 1968. https://doi.org/10.1166/jctn.2010.1569, 1972. Boyd, E.M., Meharg, A.A., Wright, J., Killham, K., 1997. Assessment of toxicological interactions of benzene and its primary degradation products (catechol and phenol)using alux-modi f ied bacter i al bioassay. Env i ron. Toxicol. Chem. 16, 849-–856.using alux-modifeid bacterial bioassay. Environ. Toxicol. Chem. 16, 849856.https://doi.org/10.1002/etc.5620160503. Brackmann, R., Fuchs, G., 1993. Enzymes of anaerobic metabolism of phenolic compounds. 4-Hydroxybenzoyl-CoA reductase (dehydroxylating) from a denitrifying Pseudomonas species. Eur. J . Biochem. 213, 563-–571. h t tp s://d o i.o r g /10.1111/Pseudomonas species. Eur. J. Biochem. 213, 563571. https://doi.org/10.1111/j j.1432-1033.1993.tb17795.x . Chacon, F.J., Sanchez-Monedero, M.A., Lezama, L., Cayuela, M.L., 2020. Enhancing biochar redox properties through feedstock selection, metal preloading and post-pyrolysis treatments. Chem. Eng. J. 395, 125100. https://doi.org/10.1016/j. cej.2020.125100. Chacon, F.J., Cayuela, M.L., Cederlund, H., Sagnchez-Monedero, M.A., 2022. Overcoming biochar limitations to remediate pentachlorophenol in soil by modifying its electrochemical properties. J. Hazard Mater. 426, 127805. https://doi.org/10.1016/j.jhazmat.2021.127805. Chen, H., Yao, J., Wang, F., Choi, M.M.F., Bramanti, E., Zaray, G., 2009. Study on the toxic effects of diphenol compounds on soil microbial activity by a combination of methods. J . Hazard Mater. 167, 846-–851. htt ps://d o i .o r g/10.1016/j.methods. J. Hazard Mater. 167, 846851. https://doi.org/10.1016/j.jhazmat.2009.01.066. Chen, H., Yao, J., Wang, F., Zhou, Y., Chen, K., Zhuang, R., Choi, M.M.F., Zaray, G.,2010. Toxicity of three phenolic compounds and their mixtures on the gram-positive bacteria Bacillus subtilis in the aquatic environment. Sci. Total Environ. 408,–10431049. https://doi.org/10.1016/j.scitotenv.2009.11.051. Chen, H., Zhuang, R., Yao, J., Wang, F., Qian, Y., Masakorala, K., Cai, M., Liu, H., 2013.Short-term effect of aniline on soil microbial activity: a combined study by isothermal microcalorimetry, glucose analysis, and enzyme assay techniques.Environ. Sci. Pollut. Control Ser. 21, 674-–683. h t t ps://d o i .or g/10.1007/s11356-Environ. Sci. Pollut. Control Ser. 21, 674683. https://doi.org/10.1007/s11356-013-1955-8. Chen, X., Yang, L., Myneni, S.C.B., Deng, Y., 2019. Leaching of polycyclic aromatic hydrocarbons (PAHs) from sewage sludge-derived biochar. Chem. Eng. J. 373,–840845. https://doi.org/10.1016/j.cej.2019.05.059. Choppala, G., Bolan, N., Kunhikrishnan, A., Bush, R., 2016. Differential effect of biochar upon reduction-induced mobility and bioavailability of arsenate and chromate.Chemosphere 144, 374-–381. h t t p s ://doi .org /10.1016/j.c h e m osph e re.2015.08.043.Chemosphere 144, 374381. https://doi.org/10.1016/j.chemosphere.2015.08.043. Chun, L.J., Tong, M.J., Busuttil, R.W., Hiatt, J.R., 2009. Acetaminophen hepatotoxicity –and acute liver failure. J. Clin. Gastroenterol. 43, 342349. https://doi.org/ 10.1097/mcg.0b013e31818a3854. CIR, 1985. 2 final r eport on the safety assessment of p-hydroxyanisole. J . Am. Col l .CIR, 1985. 2 fnial report on the safety assessment of p-hydroxyanisole. J. Am. Coll.–Toxicol. 4, 3163. https://doi.org/10.3109/10915818509078686. Devillers, J., Boule, P., Vasseur, P., Prevot, P., Steiman, R., Seigle-Murandi, F., Benoit-Guyod, J.L., Nendza, M., Grioni, C., Dive, D., 1990. Environmental and health risks –of hydroquinone. Ecotoxicol. Environ. Saf. 19, 327354. https://doi.org/10.1016/0147-6513(90)90035-4. EFSA, 2011. Scienti f ic opinion on f la a v v o o u u r r i i n n g g g g r r o o u u p p e e v v a a l l u u a a t t i i o o n n 2222,, r r e e v v i i s s i i o o n n 11 EFSA, 2011. Scientifci opinion on fl (FGE.22Rev1): ring-substituted phenolic substances from chemical groups 21 and 25. EFSA J. 9, 1990. https://doi.org/10.2903/j.efsa.2011.1990. Ek He n ni n g, H.,, et al .Pu t na -N imane, I ., Kalin ow sk i , R., P e rkol Ek Henning, H., , et al.Putna-Nımane, I., Kalinowski, R., Perkol a a ,, N N ..,, B B o o g g u u s s z z ,, A A ..,,K ub l i na , A., Hai b a , E ., Ba r d a , I ., Ka rk ovs k a ,Kublina, A., Haiba, E., Barda, I., Karkovska, I I ..,, S S c c h h i i ü t t z z ,, J J ..,, M M e e h h t t o o n n e e n n ,, J J ..,, S S i i i i m m e e s s ,, K K ..,,Nyhle n , K., Dzintare, L ., Aysto, L., Sin i c Nyhlen, K., Dzintare, L.,s, L ., La h t, M., Lehto n en , M ., St a p f, M ., Aysto, L., Sin¸ ics, L., Laht, M., Lehtonen, M., Stapf, M.,Str i d h , P., 2020. P h ar m ac e u t ica l s i n th e Ba l ti c S ea Reg i on-–E miss i ons, Co n s u mp Stridh, P., 2020. Pharmaceuticals in the Baltic Sea Region Emissions, Consump t t i i o o n n a n d E nv i r o n m en ta l R i s ks. L a ns s t yr e l sen Os t e r and Environmental Risks. Lansstyrelsen Oster g g o o t t l l a a n n d d ,, L L i i n n k k o o p p i i n n g g .. Enguita, F.J., Leitao, A.L., 2013. Hydroquinone: environmental pollution, toxic i ty, and Enguita, F.J., Leitao, A.L., 2013. Hydroquinone: environmental pollution, toxicity, and microbial answers. BioMed Res. Int. 2013, e542168 https://doi.org/10.1155/2013/542168. Fang, G., Zhu, C., Dionysiou, D.D., Gao, J., Zhou, D., 2015. Mechanism of hydroxyl radical generation from biochar suspensions: implications to diethyl phthalate degradation. Bioresour. Technol . 176, 210-–217. h t tp s://d o i .o r g /10.1016/j .degradation. Bioresour. Technol. 176, 210217. https://doi.org/10.1016/j. biortech.2014.11.032. FDA, 2020. Don'’t Double up on Acetaminophen [WWW Document ]. US Food and Drug FDA, 2020. Dont Double up on Acetaminophen [WWW Document]. US Food and Drug Administration. URL. https://www.fda.gov/consumers/consumer-updates/dont -double-acetaminophen . Ferreiro, C., Sanz, J., Villota, N., de Luis, A., Lombrarna, J.I., 2021. Kinetic modelling for concentration and toxicity changes during the oxidation of 4-chlorophenol by UV/H2O2. Sci. Rep. 11 https://doi.org/10.1038/s41598-021-95083-7. Forte, A.J., Wilson, J.M., Slattery, J.T., Nelson, S.D., 1984. The formation and toxicity of catechol metabolites of acetaminophen in mice. Drug Metabol. Dispos.: Biol. Fate –Ch em . 12, 484-491.Chem. 12, 484491. Fuchs, G. et al., n.d. Biochemistry of anaerobic biodegradation of aromatic compounds,–in: Biochemistry of Microbial Degradation. Springer, pp. 513553. Guiloski, I.C., Ribas, J.L.C., Piancini, L.D.S., Dagostim, A.C., Cirio, S.M., F,avaro, L.F.,Boschen, S.L., Cestari, M.M., da Cunha, C., Silva de Assis, H.C., 2017. Paracetamol causes endocrine disruption and hepatotoxicity in male fsih Rhamdia quelen after –subchronic exposure. Environ. Toxicol. Pharmacol. 53, 111120. https://doi.org/10.1016/j.etap.2017.05.005. Hider-Mlynarz, K., Cavalie, P., Maison, P., 2018. Trends in analgesic consumption in France over the last 10 years and comparison of patterns across Europe. Br. J. Clin.Pharmacol. 84, 1324-–1334. h t tp s://doi.o r g /10.1111/bc p .13564.Pharmacol. 84, 13241334. https://doi.org/10.1111/bcp.13564. Keiluweit, M., Nico, P.S., Johnson, M.G., Kleber, M., 2010. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 44,–12471253. https://doi.org/10.1021/es9031419. Kinney, C.A., Furlong, E.T., Werner, S.L., Cahill, J.D., 2006a. Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ.Toxicol. Chem. 25, 317. https://doi.org/10.1897/05-187r.1. Kinney, C.A., Furlong, E.T., Zaugg, S.D., Burkhardt, M.R., Werner, S.L., Cahill, J.D.,Jorgensen, G.R., 2006b. Survey of organic wastewater contaminants in biosolids destined for land application. Environ. Sci. Technol. 40, 7207-–7215. ht tp s://doi.destined for land application. Environ. Sci. Technol. 40, 72077215. https://doi.org/10.1021/es0603406. Kleywegt, S., Payne, M., Ng, F., Fletcher, T., 2019. Environmental loadings of active pharmaceutical ingredients from manufacturing facilities in Canada. Sci. Total Environ. 646, 257-–264. h tt ps://do i.o r g/10.1016/j .s ci t ot env.2018.07.240.Environ. 646, 257264. https://doi.org/10.1016/j.scitotenv.2018.07.240. Kong, L., Gao, Y., Zhou, Q., Zhao, X., Sun, Z., 2018. Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. J. Hazard Mater. 343, 276-–284. h t t ps ://doi .or g /10.1016/j.j ha zmat .2017.09.040.Mater. 343, 276284. https://doi.org/10.1016/j.jhazmat.2017.09.040. Kummerova, M., Zezulka, S., Babula, P., Triska, J., 2016. Possible ecological risk of two Kummerova, M., Zezulka, S., Babula, P., Tríska, J., 2016. Possible ecological risk of two pharmaceuticals diclofenac and paracetamol demonstrated on a model plant Lemna minor. J . Hazard Mater. 302, 351-–361. htt ps://d o i .o r g/10.1016/j .minor. J. Hazard Mater. 302, 351361. https://doi.org/10.1016/j. jhazmat.2015.09.057. Lalic, S., Ilomaki , J., Bell, J.S., Korhonen, M.J., Gi s ev, N., 2018. Prevalence and i ncidence Lalic, S., Ilomaki, J., Bell, J.S., Korhonen, M.J., Gisev, N., 2018. Prevalence and incidence –of prescription opioid analgesic use in Australia. Br. J. Clin. Pharmacol. 85, 202215.https://doi.org/10.1111/bcp.13792. Li, C., Cheng, S., 2019. Functional group surface modifications for enhancing the Li, C., Cheng, S., 2019. Functional group surface modifciations for enhancing the formation and pe formation and pe r r f f o o r r m m a a n n c c e e o o f f e e x x o o e e l l e e c c t t r r o o g g e e n n i i c c b b i i o o fi f l li m m s s o o n n t t h h e e a a n n o o d d e e o o f f a a –bioelectrochemical system. Crit. Rev. Biotechnol. 39, 10151030. https://doi.org/10.1080/07388551.2019.1662367. Li, J., Ye, Q., Gan, J., 2014. Degradation and transformation products of acetaminophen –in soil. Water Res. 49, 4452. https://doi.org/10.1016/j.watres.2013.11.008. Li, Y., Pan, Y., Lian, L., Yan, S., Song, W., Yang, X., 2017. Photosensitized degradation of acetaminophen in natural organic matter solutions: the role of triplet states and oxygen. Water Res. 109, 266-–273. h t t p s ://doi .org /10.1016/j.wat r es.2016.11.049.oxygen. Water Res. 109, 266273. https://doi.org/10.1016/j.watres.2016.11.049. Li, H., Wang, B., Deng, S., Dai, J., Shao, S., 2019. Oxygen-containing functional groups on bioelectrode surface enhance expression of c-type cytochromes in bioflim and boost extracellular electron transfer. Bioresour. Technol. 292, 121995. https://doi.org/10.1016/j.biortech.2019.121995. Li, B., Ma, X., Deng, J., Li, Q., Chen, W., Li, G., Chen, G., Wang, J., 2020. Comparison of acetaminophen degradation in UV-LED-based advance oxidation processes: reaction kinetics, radicals contribution, degradation pathways and acute toxicity assessment.Sci. Total Environ. 723, 137993. https://doi.org/10.1016/j.scitotenv.2020.137993. Liang, C., Lan, Z., Zhang, X., Liu, Y., 2016. Mechanism for the primary transformation of acetaminophen in a soil/water system. Water Res. 98, 215–-224. h t t p s ://doi.org /acetaminophen in a soil/water system. Water Res. 98, 215224. https://doi.org/10.1016/j.watres.2016.04.027. Lin, A.Y.-C., Lin, C.-A., Tung, H.-H., Chary, N.S., 2010. Potential for biodegradation and sorption of acetaminophen, caffeine, propranolol and acebutolol in lab-scale aqueous enviro –nments. J. Haza aqueous environments. J. Haza r r d d M M a a t t e e r r .. 118833,, 224422-225500.. h h t t t t p p s s ::////d d o o i i ..o o r r g g //1100..11001166//j j ..jhazmat.2010.07.017. Liu, Y., Lonappan, L., Brar, S.K., Yang, S., 2018. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: a review. Sci . Total Environ. 645, 60-–70. h tt ps ://d o i .o r g/10.1016/j .review. Sci. Total Environ. 645, 6070. https://doi.org/10.1016/j. scitotenv.2018.07.099. Livingston, A., 2010. Pain and analgesia in domestic animals. In: Cunningham, F.,Elliot, J., Lees, P. (Eds.), Comparative and Veterinary Pharmacology. Springer,p p . 159-–189.pp. 159189. Loganathan, V.A., Feng, Y., Sheng, G.D., Clement, T.P., 2009. Crop-residue-derived char infulences sorption, desorption and bioavailability of atrazine in soils. Soil Sci. Soc.–Am. J. 73, 967974. https://doi.org/10.2136/sssaj2008.0208. Macherey, A.C., Dansette, P.M., 2008. Biotransformations leading to toxic metabolites:–chemical aspects. In: The Practice of Medicinal Chemistry. Elsevier Ltd, pp. 674696. Mallinson, S.J.B., Machovina, M.M., Silveira, R.L., Garcia-Borr,as, M., Gallup, N., Johnson, C.W., Allen, M.D., Skaf, M.S., Crowley, M.F., Neidle, E.L., Houk, K.N.,Beckham, G.T., DuBois, J.L., McGeehan, J.E., 2018. A promiscuous cytochrome P450aromatic O-demethylase for lignin bioconversion. Nat. Commun. 9 https://doi.org/10.1038/s41467-018-04878-2. Masoner, J.R., Kolpin, D.W., Furlong, E.T., Cozzarelli, I.M., Gray, J.L., Schwab, E.A.,2014. Contaminants of emerging concern in fresh leachate from landflils in the conterminous United States. Environ. Sci.: Process. Impacts 16, 2335-–2354. h t t ps ://conterminous United States. Environ. Sci.: Process. Impacts 16, 23352354. https://doi.org/10.1039/c4em00124a . Milliken, C.E., Meier, G.P., Watts, J.E.M., Sowers, K.R., May, H.D., 2004. Microbial anaerobic demethylation and dechlorination of chlorinated hydroquinone metabolites synthesized by basidiomycete fungi. Appl. Environ. Microbiol. 70,–385392. https://doi.org/10.1128/aem.70.1.385-392.2004. OECD, 2000. Adsorption/desorption Using a Batch Equilibrium Method, vol. 106. OECD Test Guideline, Paris, France. https://doi.org/10.1787/20745753. OECD, 2002. OECD GUIDELINE for the TESTING of CHEMICALS No. 307: Aerobic and –Anaerobic Transformation in Soil. No. April, 117. Olicon-Hernandez, D.R., Ortuzar, M., Pozo, C., Gonzalez-Lopez, J., Aranda, E., 2020.Olicon-Hernandez, D.R., Ortúzar, M., Pozo, C., Gonzalez-Lopez, J., Aranda, E., 2020.Metabolic capability of Penicillium oxalicum to transform high concentrations of anti-infalmmatory and analgesic drugs. Appl. Sci. 10, 2479. https://doi.org/ 10.3390/app10072479. Oliveira, L.L.D., Antunes, S.C., Gonçalves, F., Rocha, O., Nunes, B., 2015. Evaluation of ecotoxicological effects of drugs on Daphnia magna using different enzymatic –biomarkers. Ecotoxicol. Environ. Saf. 119, 123131. https://doi.org/10.1016/j. ecoenv.2015.04.028. Patel, M., Kumar, R., Pittman, C.U., Mohan, D., 2021. Ciprofloxacin and acetaminophen Patel, M., Kumar, R., Pittman, C.U., Mohan, D., 2021. Ciprofolxacin and acetaminophen sorption onto banana peel biochars: environmental and process parameter infulences. Environ. Res. 201, 111218. https://doi.org/10.1016/j. envres.2021.111218. Peng, A., Huang, M., Chen, Z., Gu, C., 2017. Oxidative coupling of acetaminophen –mediated by Fe3+-saturated montmorillonite. Sci. Total Environ. 595, 673680.https://doi.org/10.1016/j.scitotenv.2017.03.274. erez Solsona, Sandra, Montemurro, N., Serge Chiron, Barcelo, Damia, 2021. Interaction and Fate of Pharmaceuticals in Soil-Crop Systems the Impact of Reclaimed Wastewater. Springer Nature, S.L . Phong Vo, H.N., Le, G.K., Hong Nguyen, T.M., Bui, X.-T., Nguyen, K.H., Rene, E.R., Vo, T.D.H., Thanh Cao, N.-D., Mohan, R., 2019. Acetaminophen micropollutant: historical and current occurrences, toxicity, removal strategies and transformation pathways in different environments. Chemosphere 236, 124391. https://doi.org/10.1016/j.chemosphere.2019.124391. Prado, A., Berenguer, R., Esteve-Núnsez, A., 2019. Electroactive biochar outperforms highly conductive carbon materials for biodegrading pollutants by enhancing –microbial extracellular electron transfer. Carbon 146, 597609. https://doi.org/10.1016/j.carbon.2019.02.038. Qiu, M., Liu, L., Ling, Q., Cai, Y., Yu, S., Wang, S., Fu, D., Hu, B., Wang, X., 2022. Biochar for the removal of contaminants from soil and water: a review. Biochar 4. https://doi.org/10.1007/s42773-022-00146-1. Quilliam, R.S., Rangecroft, S., Emmett, B.A., Deluca, T.H., Jones, D.L., 2012. Is biochar a source or sink for polycyclic aromatic hydrocarbon (PAH) compounds in agricultural soils? GCB Bioenergy 5, 96-–103. h t t ps://doi .org/10.1111/gcbb.12007.soils? GCB Bioenergy 5, 96 103. https://doi.org/10.1111/gcbb.12007. Rao, M.A., Di Rauso Simeone, G., Scelza, R., Conte, P., 2017. Biochar based remediation of water and soil contaminated by phenanthrene and pentachlorophenol.Chemosphere 186,193-–201. h t t p s://doi .or g /10.1016/j.ch e mo s p h e r e.2017.07.125.Chemosphere 186, 193201. https://doi.org/10.1016/j.chemosphere.2017.07.125. Ruan, X., Sun, Y., Du, W., Tang, Y., Liu, Q., Zhang, Z., Doherty, W., Frost, R.L., Qian, G.,Tsang, D.C., 2019. Formation, characteristics, and applications of environmentally –persistent free radicals in biochars: a review. Bioresour. Technol. 281, 457468. Schink, B., Philipp, B., Müller, J., 2000. Anaerobic degradation of phenolic compounds.Naturwissenschaften 87, 12-–23. h t t ps://d oi .o r g /10.1007/s 001140050002.Naturwissenschaften 87, 1223. https://doi.org/10.1007/s001140050002. Singh, A., 2021. A review of wastewater irrigation: environmental implications. Resour.Conserv. Recycl. 168, 105454. https://doi.org/10.1016/j.resconrec.2021.105454. Snyder, R., 2000. Overview of the toxicology of benzene. J. Toxicol. Environ. Health,Part A 61, 339-–346. htt ps://do i .org/10.1080/00984100050166334.Part A 61, 339346. https://doi.org/10.1080/00984100050166334. Sole, M., Shaw, J.P., Frickers, P.E., Readman, J.W., Hutchinson, T.H., 2009. Effects on feeding rate and biomarker responses of marine mussels experimentally exposed to propranolol and acetaminophen. Anal. Bioanal. Chem. 396, 649-–656. h t tp s://d oi .propranolol and acetaminophen. Anal. Bioanal. Chem. 396, 649656. https://doi.org/10.1007/s00216-009-3182-1. Sun, T., Levin, B.D.A., Guzman, J.J.L., Enders, A., Muller, D.A., Angenent, L.T.,Lehmann, J., 2017. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat. Commun. 8 https://doi.org/10.1038/ncomms14873. Tong, Y., Wang, X., Sun, Z., Gao, J., 2021. Two transformation pathways of Acetaminophen with Fe3 saturated clay particles in dark or light. Chemosphere 278, 130399. https://doi.org/10.1016/j.chemosphere.2021.130399. Torres, R.A., Torres, W., Peringer, P., Pulgarin, C., 2003. Electrochemical degradation of p-substituted phenols of industrial interest on Pt electrodes. Chemosphere 50,–97104. https://doi.org/10.1016/s0045-6535(02)00487-3. Türkoglu, E., Osma, E., Elveren, M., 2017. Effects of acetaminophen (paracetamol) and gemfbirozil on seed development and antioxidant enzyme activities in different –wheat varieties. Iran. J. Sci. Technol. Trans. A-Science 43, 20752082. https://doi. org/10.1007/s40995-017-0386-7. Ukalska-Jaruga, A., Debaene, G., Smreczak, B., 2019. Dissipation and sorption processes of polycyclic aromatic hydrocarbons (PAHs) to organic matter in soils amended by –exogenous rich-carbon material. J. Soils Sediments 20, 836849. https://doi.org/10.1007/s11368-019-02455-8. ’Valero, E., Lozano, M.I., Varon, R., Garcia-Carmona, F., 2003. Enzymatic synthesis of 3-–hydroxyacetaminophen catalyzed by tyrosinase. Biotechnol. Prog. 19, 16321638. https://doi.org/10.1021/bp034075t . Wang, H., Liu, Y., Jiang, J.-Q., 2016. Reaction kinetics and oxidation product formation –in the degradation of acetaminophen by ferrate (VI). Chemosphere 155, 583590.https://doi.org/10.1016/j.chemosphere.2016.04.088. Wu, S., Zhang, L., Chen, J., 2012. Paracetamol in the environment and its degradation by microorganisms. Appl. Microbiol. Biotechnol. 96, 875-–884. h tt p s://do i .org /microorganisms. Appl. Microbiol. Biotechnol. 96, 875884. https://doi.org/10.1007/s00253-012-4414-4. Wu, S., Fang, G., Wang, Y., Zheng, Y., Wang, C., Zhao, F., Jaisi, D.P., Zhou, D., 2017.Redox-active oxygen-containing functional groups in activated carbon facilitate microbial reduction of ferrihydrite. Environ. Sc i. Technol . 51, 9709-–9717. htt ps://microbial reduction of ferrihydrite. Environ. Sci. Technol. 51, 97099717. https://doi.org/10.1021/acs.est.7b01854. Yang, L., Yu, L.E., Ray, M.B., 2009. Photocatalytic oxidation of paracetamol: dominant reactants, intermediates, and reaction mechanisms. Environ. Sci. Technol. 43,–460465. https://doi.org/10.1021/es8020099. Xu, Y., Yan, Y., Obadamudalige, N.L., Ok, Y.S., Bolan, N., Li, Q., 2019. Redox-Mediated Biochar-Contaminant Interactions in Soil. In: Biochar from Biomass and Waste –Fundamentals and Applications. Elsevier, pp. 409419. Yang, J., Pan, B., Li, H., Liao, S., Zhang, D., Wu, M., Xing, B., 2015. Degradation of p-nitrophenol on biochars: role of persistent free radicals. Environ. Sci. Technol. 50,–694700. https://doi.org/10.1021/acs.est.5b04042. Yang, J., Pignatello, J.J., Pan, B., Xing, B., 2017. Degradation of p-nitrophenol by lignin and cellulose chars: H2O2-mediated reaction and direct reaction with the char.–Environ. Sci. Technol. 51, 89728980. https://doi.org/10.1021/acs.est.7b01087. Yang, Y., Ye, S., Zhang, C., Zeng, G., Tan, X., Song, B., Zhang, P., Yang, H., Li, M.,Chen, Q., 2021a. Application of biochar for the remediation of polluted sediments.J. Hazard Mater. 404, 124052. https://doi.org/10.1016/j.jhazmat.2020.124052. Yang, Y., Zhang, X., Jiang, J., Han, J., Li, W., Li, X., Yee Leung, K.M., Snyder, S.A.,Alvarez, P.J.J., 2021b. Which micropollutants in water environments deserve more attention globally? Environ. Sci. Technol. 56, 13-–29. htt p s://doi .org/10.1021/acs.attention globally? Environ. Sci. Technol. 56, 1329. https://doi.org/10.1021/acs.est.1c04250. Yu, L., Yuan, Y., Tang, J., Wang, Y., Zhou, S., 2015. Biochar as an electron shuttle for reductive dechlorination of pentachlorophenol by Geobacter sulfurreducens. Sci.Rep. 5 https://doi.org/10.1038/srep16221. Zama, E.F., Reid, B.J., Arp, H.P.H., Sun, G.-X., Yuan, H.-Y., Zhu, Y.-G., 2018. Advances in research on the use of biochar in soil for remediation: a review. J. Soils Sediments –18, 24332450. https://doi.org/10.1007/s11368- 018-2000-9. Zhang, L., Hu, J., Zhu, R., Zhou, Q., Chen, J., 2012. Degradation of paracetamol by pure bacterial cultures and their microbial consortium. Appl. Microbiol. Biotechnol. 97,–36873698. https://doi.org/10.1007/s00253-012-4170-5. Zhang, X., Wang, H., He, L., Lu, K., Sarmah, A., Li, J., Bolan, N.S., Pei, J., Huang, H.,2013. Using biochar for remediation of soils contaminated with heavy metals and –organic pollutants. Environ. Sci. Pollut. Control Ser. 20, 84728483. https://doi.org/10.1007/s11356-013-1659-0. Zhang, K., Sun, P., Faye, M.C.A.S., Zhang, Y., 2018. Characterization of biochar derived from rice husks and its potential in chlorobenzene degradation. Carbon 130,–730740. https://doi.org/10.1016/j.carbon.2018.01.036. Zhu, M., Lv, X., Franks, A.E., Brookes, P.C., Xu, J., He, Y., 2020. Maize straw biochar addition inhibited pentachlorophenol dechlorination by strengthening the predominant soi l reduction processes in f looded soi l . J . Hazard Mater. 386, 122002.predominant soil reduction processes in foloded soil. J. Hazard Mater. 386, 122002.https://doi.org/10.1016/j.jhazmat.2019.122002. Zur, J., Pinski, A., Marchlewicz, A., Hupert-Kocurek, K., Wojcieszy,nska, D., Guzik, U.,2018. Organic micropollut —ants paracetamol and ibup 2018. Organic micropollutants paracetamol and ibup r r o o f f e e n n -t t o o x x i i c c i i t t y y ,,biodegradation, and genetic background of their utilization by bacteria. Environ. Sci.– Pollut. Control Ser. 25, 21498 21524. https://doi.org/10.1007/s11356-018-2517-x .
确定



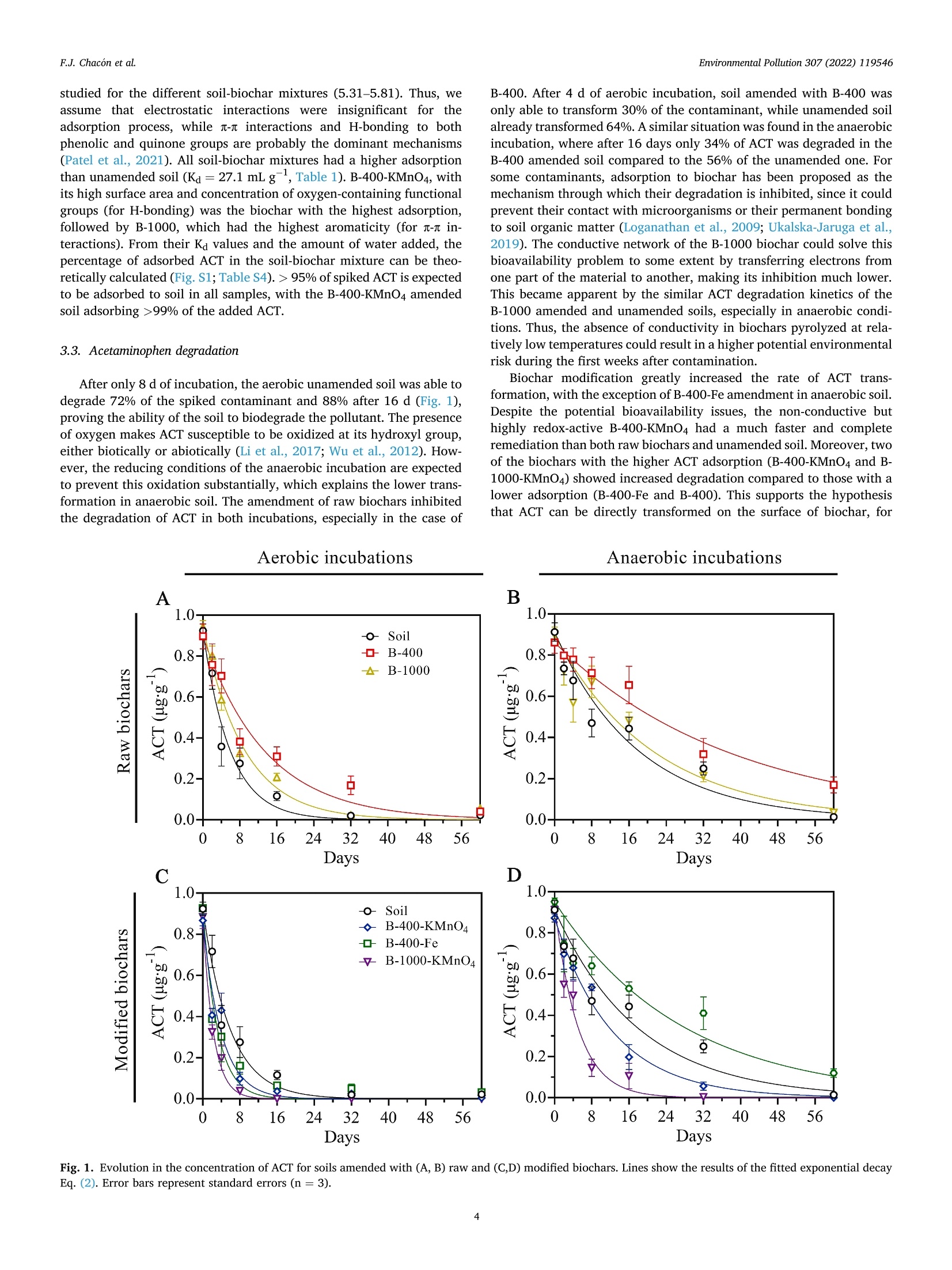
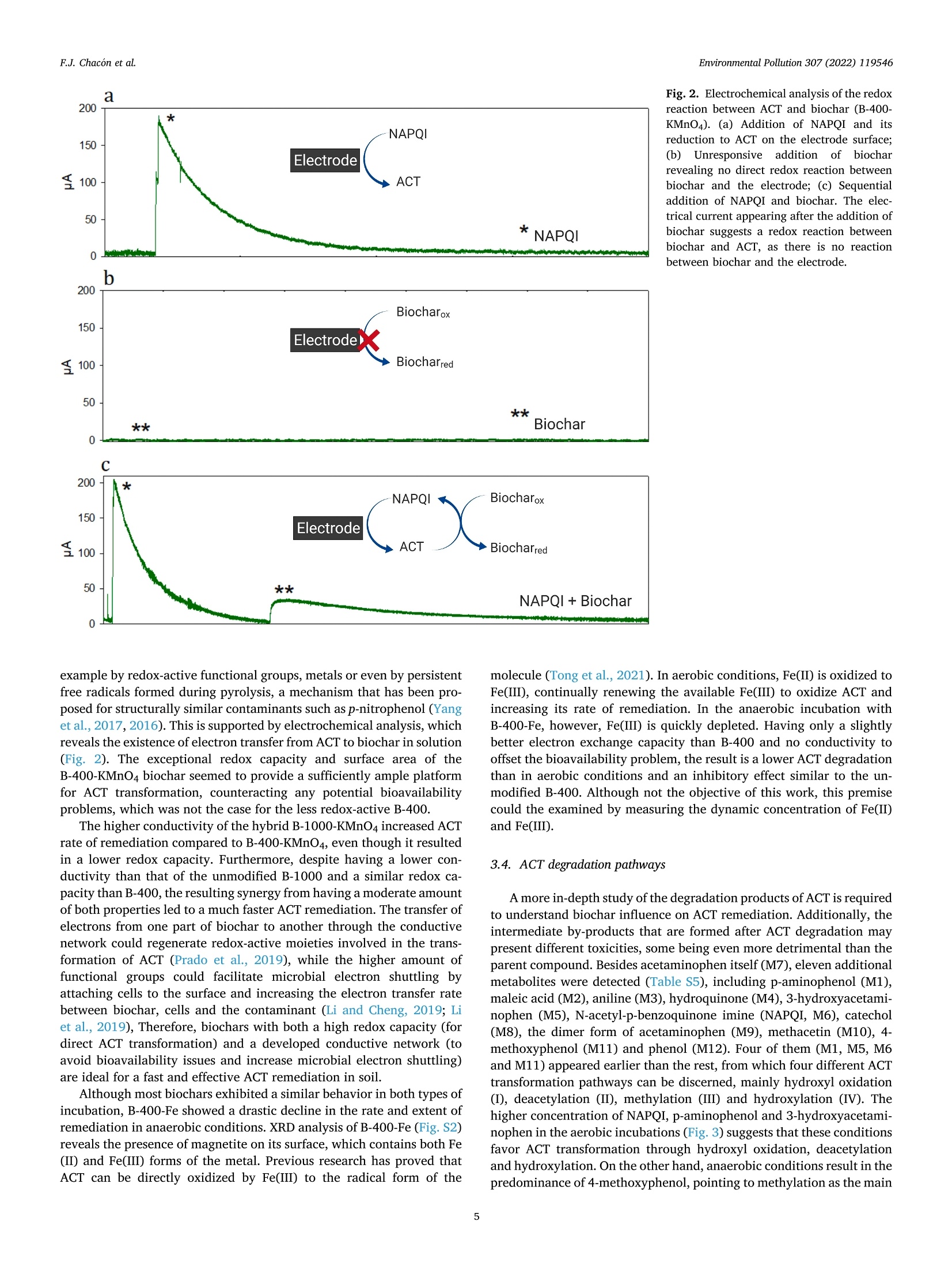
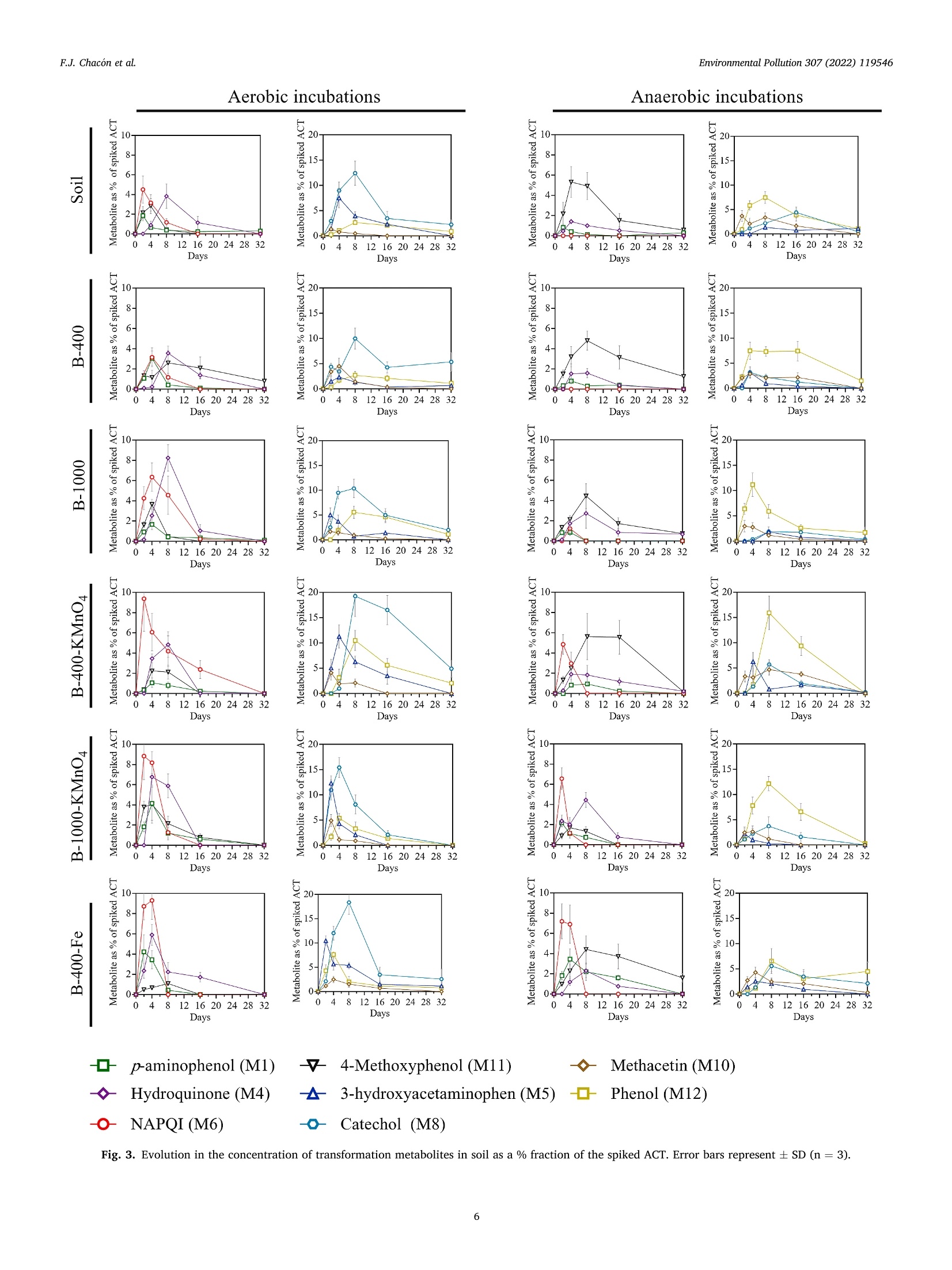
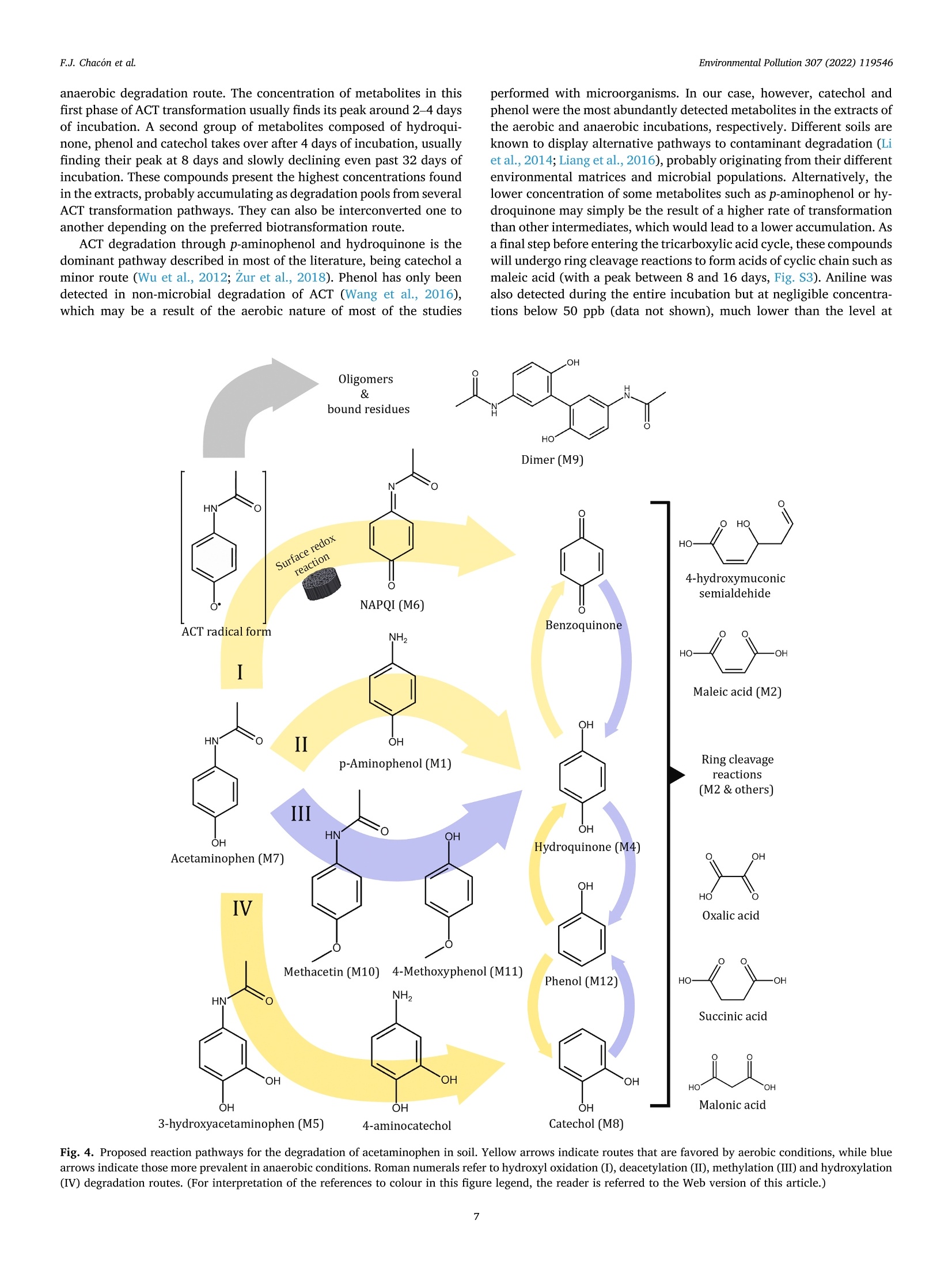




还剩9页未读,是否继续阅读?
中国格哈特为您提供《土壤中扑热息痛(对乙酰氨基酚)含量的检测》,该方案主要用于土壤中有机污染物检测,参考标准《HJ 703-2014土壤和沉积物 酚类化合物的测定 气相色谱法》,《土壤中扑热息痛(对乙酰氨基酚)含量的检测》用到的仪器有格哈特全自动快速溶剂萃取仪Sox416、格哈特快速干燥仪STL56、格哈特强力高重现振荡器LS500/RO500、德国加液器MM、棕色避光防紫外线萃取杯
相关方案
更多
该厂商其他方案
更多