
蜡菊属(永久花)生物勘探:化学性质、植物化学性质,以及抗灰孢菌的抑菌作用Bioprospecting of Helichrysum Species Chemical Profile, Phytochemical Properties, and Antifungal Efficacy against Botrytis cinerea
使用格哈特公司重载振荡器振荡72小时
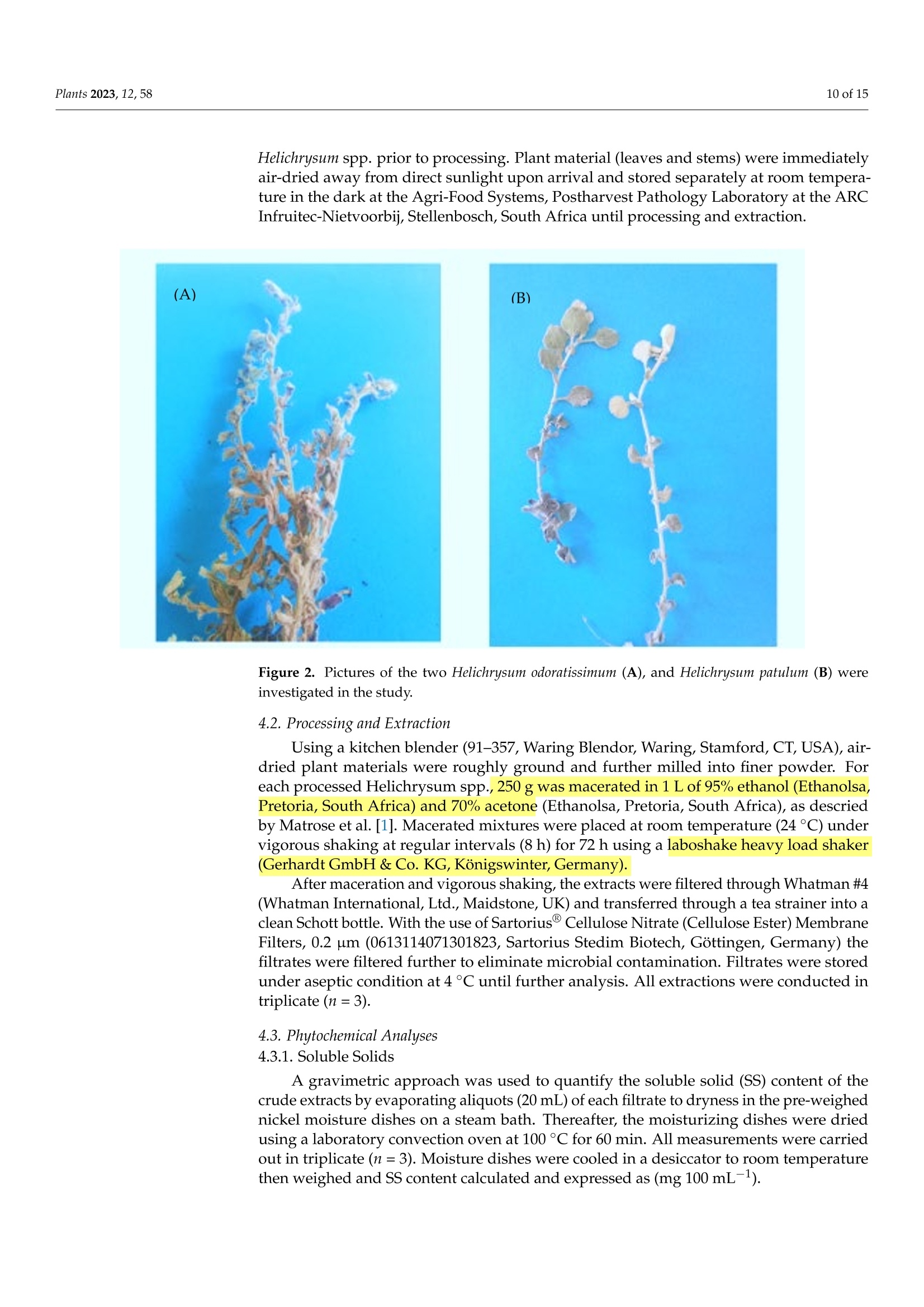
For each processed Helichrysum spp., 250 g was macerated in 1 L of 95% ethanol and 70% acetone. Macerated mixtures were placed at room temperature (24 °C) under vigorous shaking at regular intervals (8 h) for 72 h using a laboshake heavy load shaker (Gerhardt GmbH & Co. KG, Königswinter, Germany).
方案详情

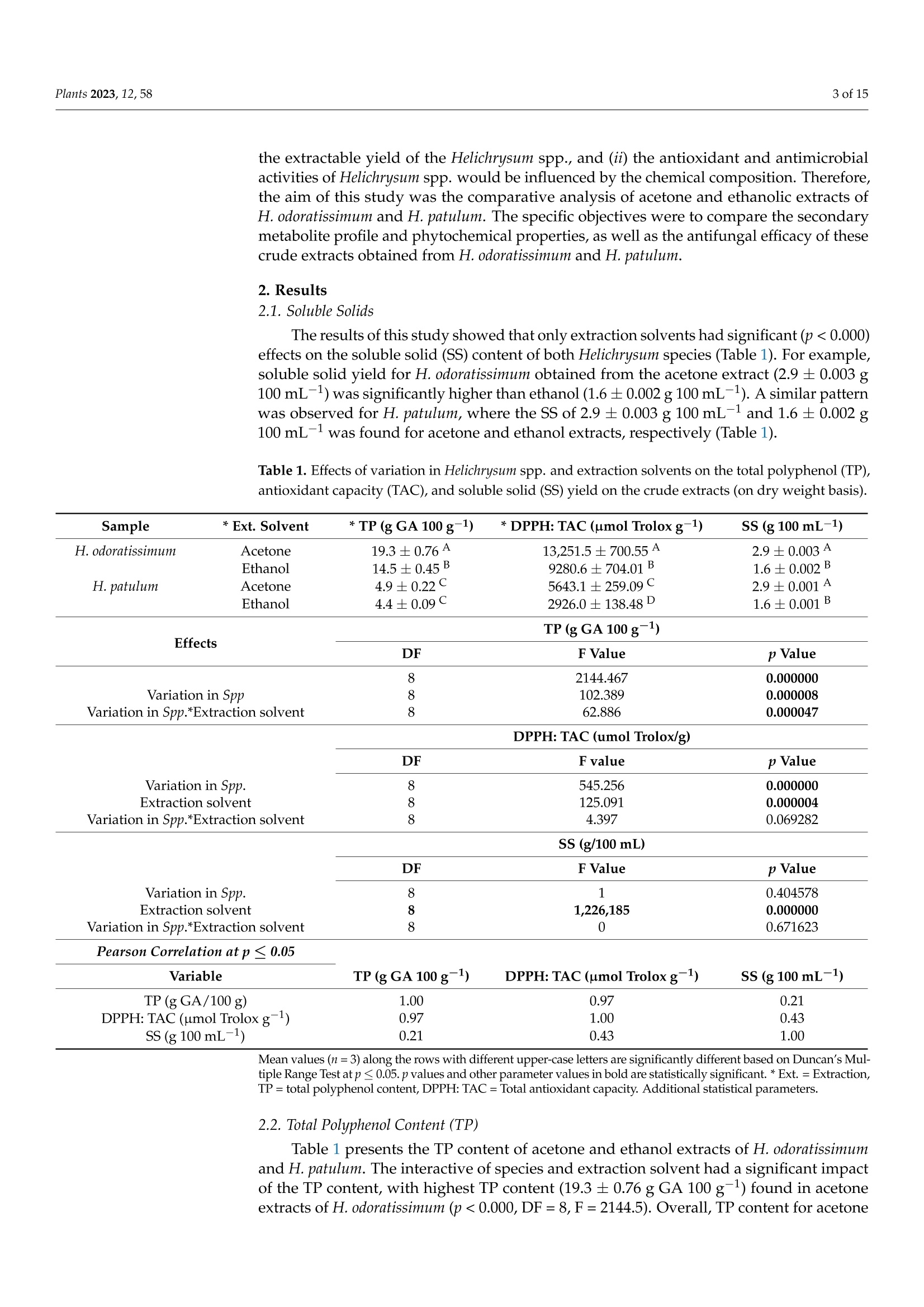
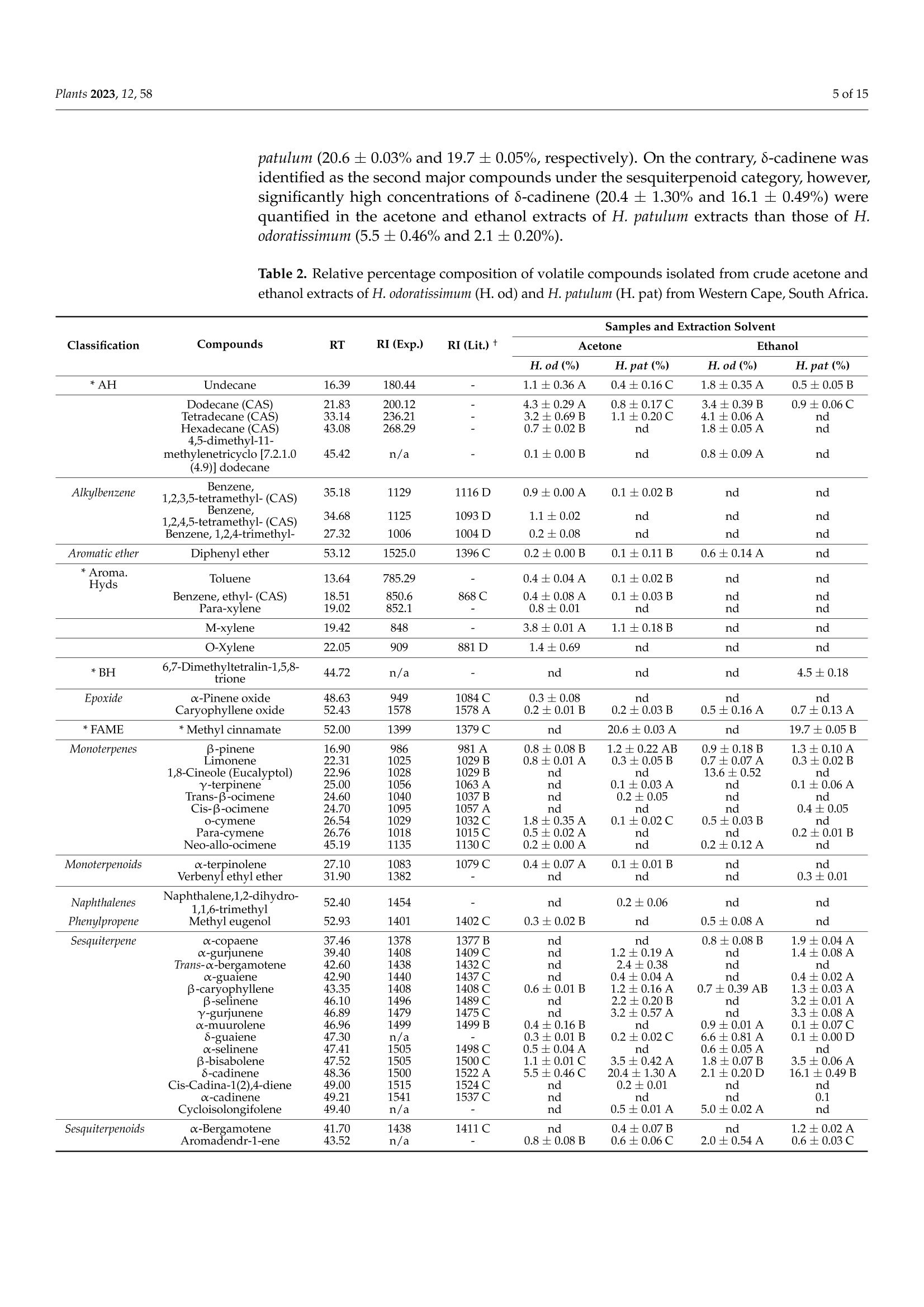
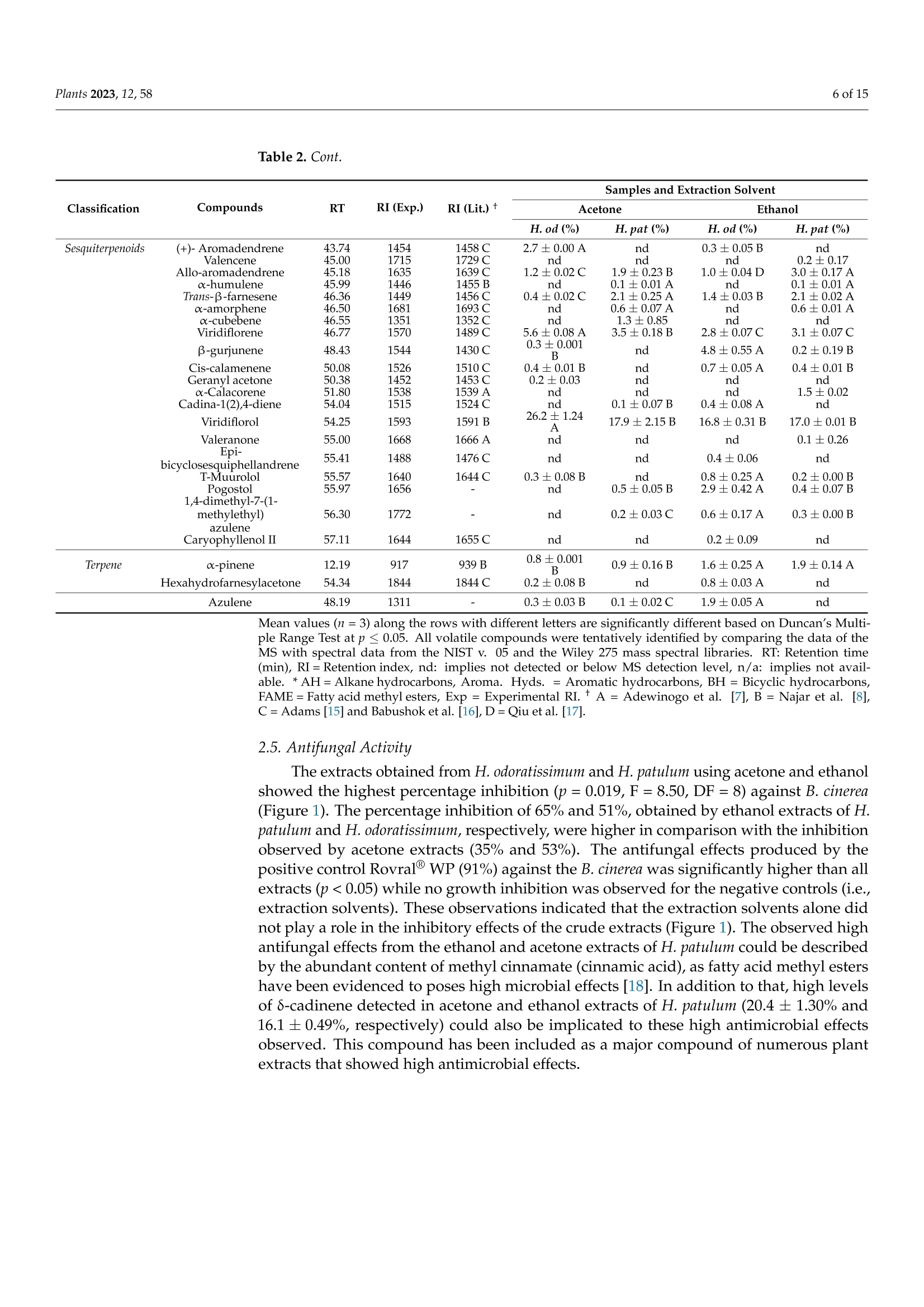
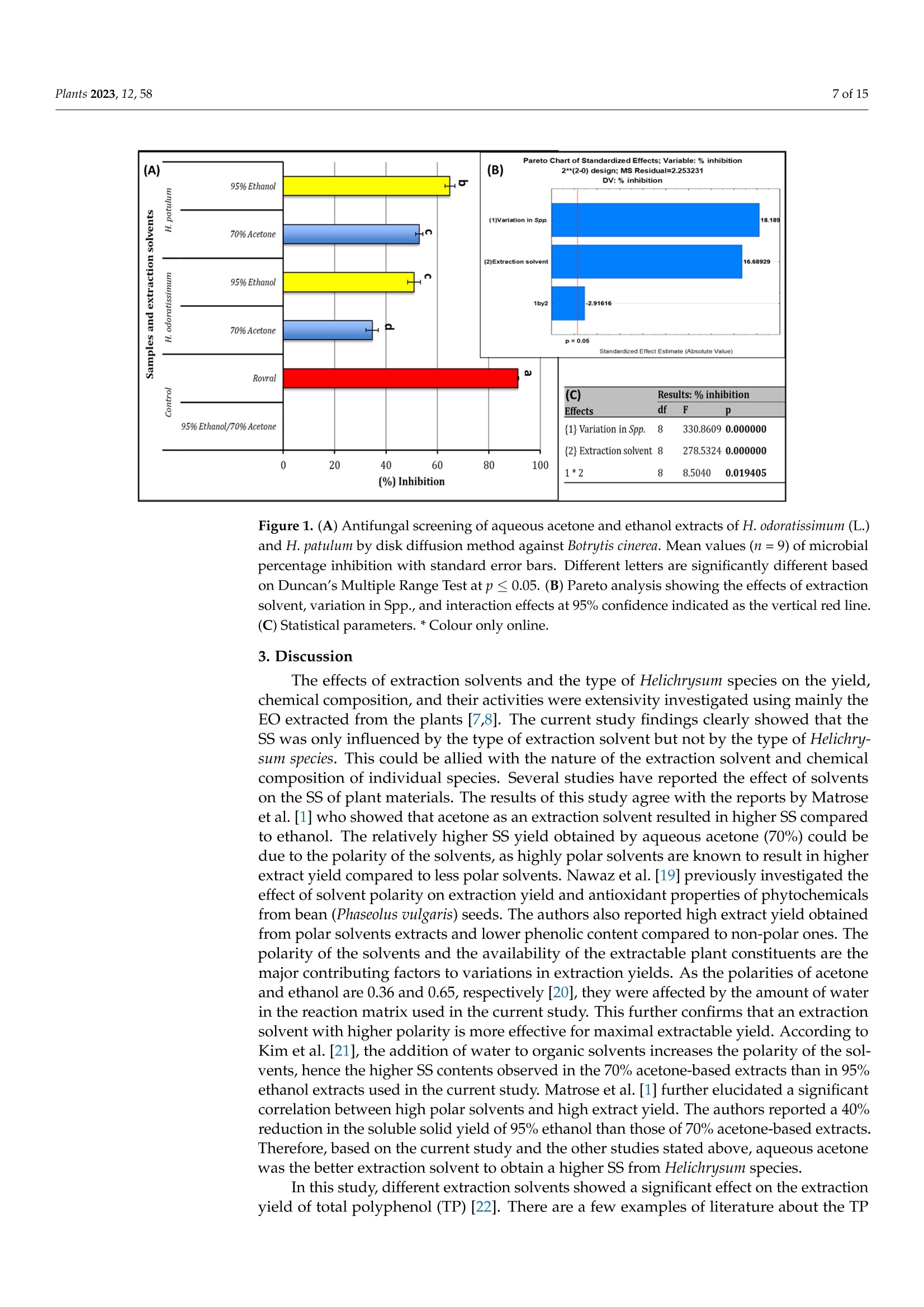
蜡菊属(永久花)生物勘探:化学性质、植物化学性质,以及抗灰孢菌的抑菌作用Bioprospecting of Helichrysum Species Chemical Profile, Phytochemical Properties, and Antifungal Efficacy against Botrytis cinereaplants Plants 2023, 12, 582 of 15 Citation: Matrose, N.A.; Belay,Z.A.;Obikeze, K.; Mokwena, L.; Caleb, O.J .Bioprospecting of Helichrysum Species: Chemical Profile,Phytochemical Properties, and Antifunga l Ef f icacy against Botrytis cinerea. Plants 2023,12,58. https://doi .org/10.3390/plants12010058 Academic Editors: Milan S. Stankov i c and Christophe Hano Received: 16 November 2022 Revised: 15 Decembe r 2022 Accepted: 16 December 2022 Published: 22 December 2022 Copyright: o 2022 by t he authors.Licensee MDPI, Basel, Switzerland.This article is an open access article distributed under the terms and conditions of the Creat i ve Commons A t t r i bution (CC BY ) l icense (https://creativecommons.org/licenses/by/4.0/) Post-Harvest and Agro-Processing Technologies (PHATs), Agricultural Research Council (ARC) Infruitec-Nietvoorbij, Stellenbosch 7599, South Africa 2 School of Pharmacy, Faculty of Science, University of the Western Cape, Bellville 7535, South Africa 3 Central Analy t ical Facil i ty, Stellenbosch Universi t y, Matieland 7602, South Africa 4 Department of Food Science, Faculty of AgriSciences, Stellenbosch University, Matieland 7602,South Africa 5 African Institute for Postharvest Technology, Faculty of AgriSciences, Stellenbosch University, Private Bag X1,Matieland 7602,South Africa Correspondence: calebo@arc .agric.za Abstract: Variation in plant species and extraction solvents play a crucial role in the recovery of thei r bioactive compounds and antifungal efficacy. Thus, in this study, a comparative i nvestigation was carried out using extraction solvents: 70% acetone and 95% ethanol to obtain crude aqueous extracts from Helichrysum odoratissimum and H. patulum. Crude aqueous extracts were screened using gas chromatography-mass spectrometry (GC -MS), to gain insight into their chemical com-position. Phytochemical properties (total polyphenols (TP) and radical scavenging capaci t y via 2,2-diphenyl-1-picrylhydrazyl (DPPH)), and antifungal activity against Bot r yt i s c i nerea of t he crude extracts were evaluated. Fungicide (Rovral@ WP) and extraction solvents were used as controls.Variation i n Helichrysum spp. and extraction solvent had influence on t he chemical composition,phytochemicals, and antifungal activ i ties. Metabolites such as y-terpinene (≈0.1%),x-amorphene (≈0.6%) α-gurjunene (≈1.4%), B-selinene (2.2-3.2%),Y-gurjunene (≈3.3%), and methyl cinnamate (≈20%) were detected only in extracts of H. patulum. Crude extract of H. odoratissimum using 70%acetone had the highest TP (19.3± 0.76 g GA 100 g-l), and DPPH capacity (13,251.5±700.55 umol Trolox g-1) compared to H. patulum (p≤0.05). Ethanolic extracts of H. patulum showed highest antifungal efficacy (≈65%) against B. cinerea (p≤0.05) compared to other crude extracts. T his study showed that Helichrysum spp. differ in their potential as a source for bioactive compounds and antifungal treatments/formulations. Keywords: antioxidant capacity; crude extracts; extraction solvents; imphepho'; secondary metabolites; sesquiterpenoids 1. Introduction Crude extracts of medicinal plants have been demonstrated as rich sources of bioactive molecules with curative capability. This curative potential i s attributed to the complex mixtures of t hese bioactive compounds of di f ferent chemical classes, with antioxidant and antimicrobial activity [1]. Allopathic medicine is emerging as a new f ield of research globally as potential source of novel active/functional compounds for new drugs [2]. In the agricultural sector, disease-causing fungal pathogens resul t in serious deterioration of fresh horticultural commodities after leaving the farm gate and during storage. Synthetic fungicides and bio-based fungicides have been successful in mitigating the impact of t hese pathogens [1,3]. According to t he Fungicides Global Market Report [4], it i s estimated that the global fungicides market will grow from 2021 value of approximately $19.32 billion to $20.64 billion in 2022, and to $26.09 billion in 2026. This is a huge cost for the management of agricultural produce. Therefore, bioprospecting for new natural bioactive compounds with anti-fungal properties as alternatives to existing fungicides is crucial f or the development biocontrol treatment of funga l pathogens. Helichrysum (Asteraceae), locally known i n South African t raditional/folk medicine by a collective name as 'Imphepho’, is one of the mostly used species to combat ailments,such as t he t reatment of wounds, burns, pimples, eczema, as well as coughs and colds [5,6].Traditional uses of the species also include the treatment of abdominal pains, catarrh,headache, fever, menstrual disorders, and urinary tract i nfections, suggesting that these plants parade anti-microbial and -inflammatory effects [7]. About 245-250 species, f rom the genus consisting of roughly 500-600 species, are indigenous to South Africa [1,7]. Several studies have reported on different pharmacological properties of the essential oils (EOs) extracted from Helichrysum spp. Adewingo et al . [8] explored t he biological activities of EO extracted from H. petiolare, H. odoratissimum, and H. cymosum and found out that the EOs possessed low antibacterial , anti-tyrosinase activity but promising antioxidant capacity. The study conducted by Najar et al . [8] demonstrated a good antimicrobial activ-ity for EOs of H. pandurifolium and H. t rilineatum whereas, EOs f rom H. edwardsii and H.pandurifolium did not show any antimicrobial activity. A recent study by Serabele et al. [9]investigated the chemical profiling and antimicrobial activity of aqueous methanol of H.odoratissimum and H. petiolare. T he authors reported significantly higher antimicrobial effects of the methanolic extracts obtained from H. odorutissimum towards a larger range of bacteria in comparison to H. petiolare extrac t s with minimum inhibitory concentration (MIC)of 0.13 mg mL -1 alkanes (10.6%)> aromatic hydrocarbons (10.2%) > sesquiterpenes (8.7%)> monoterpenoids (4.3%)>monoterpenes (1.8%) > terpene (1.2%)> epoxide (0.8%)> phenylpropene and terpenoid (0.3%)> bromodiphenyl ethers (0.2%). On the other hand, the most abundant classes volatile compounds in ethanolic extracts of H. odoratissimum were in the following order:sesquiterpenoids (37.8%)> sesquiterpenes (18.6%)> oxanes (14%)> alkane hydrocarbons (12.4%) > terpene (3.3%)> monoterpenes (1.9%) > terpenoid (1.6%) > epoxide, phenyl-propene, and bromodipheny l ethers (0.7%)> monoterpenoids (0.6%)> aliphatic compounds (0.3%). A similar pattern was obtained for H. patulum for each given solvent with lesser quantities of mostly commonly identified compounds Sesquiterpenoids are modified class of terpenes with different functional groups and oxidized methyl groups moved or removed at various positions with three carbon isoprene units. These terpene derivatives are equipped with high pharmacologica l properties [14].Thus, their presence in plant extracts signals its potential therapeutic effec t s. Viridi f lorol , a sesquiterpenoid, was the major compound across all Helichrysum spp. and both extraction solvent tested. This study showed the relative percentage/concentration of viridiflorol (26.2±1.24% and 16.8±0.31%) for acetone and ethanol extracts of H. odoratissimum; the rel-ative percentage/concentration of viridiflorol (17.9±2.15%) for acetone and (17.0±0.01%)ethanol extracts of H. patulum extracts were significantly (p<0.05) l ower t han t he acetone extracts of H. odoratissimum. Relatively, higher concentrations of alkane hydrocarbons such as tetradecane and hexadecane were identified for H. odoratissimum extracts regardless of extraction solvents compared to H. patulum. Furthermore, compounds such as xylene, para-xylene, and x-pinene oxide were only identified under acetone extracts of H. odoratissimum, whereas a monoterpene: 1,8-Cineole (percentage: 13.6±0.52%) was only recorded in the ethanol extract of H. odorat i ssimum.Relative percentage of sesquiterpenes: α-gurjunene (1.2±0.19% and 1.4±0.08%),β-caryophyl l ene (1.2±0.16% and 1.3±0.03%), B-selinene (2.2±0.20% and 3.2±0.01%),and y-gurjunene (3.2±0.57% and 3.3±0.08%), were only identified in H. patulum for both acetone and ethanol solvents, respectively. Similarly, a significant l y higher (p<0.05)concentration of a fatty acid methyl ester methyl cinnamate (cinnamic acid, methy l ester),which is a sesquiterpene, was only identified in the acetone and ethanol extracts of H. patulum (20.6±0.03% and 19.7±0.05%, respectively). On the contrary, 8-cadinene was identified as the second major compounds under the sesquiterpenoid category, however,significantly high concentrations of 8-cadinene (20.4±1.30% and 16.1 ±0.49%) were quantified in the acetone and ethanol extracts of H. patulum extracts than those of H.odoratissimum (5.5±0.46% and 2.1±0.20%). Table 2. Relative percentage composition of volatile compounds i solated from crude acetone and ethanol extracts of H. odoratissimum (H. od) and H. patulum (H. pat) from Western Cape, South Africa. Classification *AH Compounds RT RI (Exp.) RI (Lit.) + Acetone Ethanol Samples and Extraction Solvent H.od(%) H. pat (%) H. od(%) H. pat (%) Undecane 16.39 180.44 一 1.1±0.36A 0.4±0.16C 1.8±0.35A 0.5±0.05B Dodecane (CAS) 21.83 200.12 一 4.3±0.29A 0.8±0.17S 3.4±0.39B 0.9±0.06C Tetradecane (CAS) 33.14 236.21 3.2±0.69B 1.1±0.20C 4.1±0.06A nd Hexadecane (CAS) 4,5-dimethyl-11- methylenetricyclo [7.2.1.0 43.08 268.29 一 0.7±0.02B nd 1.8±0.05A nd 45.42 n/a 一 0.1±0.00B nd 0.8±0.09A nd (4.9)] dodecane Alkylbenzene Benzene, 1,2,3,5-tetramethyl-(CAS) 35.18 1129 1116 D 0.9±0.00A 0.1±0.02B nd nd Benzene, 1,2,4,5-tetramethyl-(CAS) 34.68 1125 1093 D 1.1±0.02 nd nd nd BLenenzene, 1,2,4-trimethyl- 27.32 1006 1004D 0.2±0.08 nd nd nd Aromatic ether Diphenyl ether 53.12 1525.0 1396C 0.2±0.00B 0.1±0.11B 0.6±0.14A nd *Aroma.Hyds Toluene 13.64 785.29 一 0.4±0.04A 0.1±0.02B nd nd Benzene, ethyl-(CAS) 18.51 850.6 868 C 0.4±0.08A 0.1±0.03 B nd nd Para-xylene 19.02 852.1 一 0.8±0.01 nd nd nd M-xylene 19.42 848 一 3.8±0.01A 1.1±0.18B nd nd O-Xylene 22.05 909 881D 1.4±0.69 nd nd nd *BH 6,7-Dimethyltetralin-1,5,8- trione 44.72 n/a 一 nd nd nd 4.5±0.18 Epoxide xx-Pinene oxide 48.63 949 1084 C 0.3±0.08 nd nd nd Caryophyllene oxide 52.43 1578 1578 A 0.2±0.01B 0.2±0.03 B 0.5±0.16A 0.7±0.13A *FAME *Methyl cinnamate 52.00 1399 1379 C nd 20.6±0.03A nd 19.7±0.05B Monoterpenes B-pinene 16.90 986 981A 0.8±0.08B 1.2±0.22AB 0.9±0.18B 1.3±0.10A Limonene 22.31 1025 1029 B 0.8±0.01A 0.3±0.05B 0.7±0.07A 0.3±0.02B 1,8-Cineole (Eucalyptol) 22.96 1028 1029 B nd nd 13.6±0.52 nd Y-terpinene 25.00 1056 1063A nd 0.1±0.03A nd 0.1±0.06A Trans-B-ocimene 24.60 1040 1037 B nd 0.2±0.05 nd nd Cis-B-ocimene o-cymene 24.70 26.54 1095 1029 1032C nd 1.8±0.35A nd 0.1±0.02C nd 0.5±0.03 B 0.4±0.05 nd Para-cymene 26.76 1018 1015C 0.5±0.02A nd nd 0.2±0.01B Neo-allo-ocimene 45.19 1135 1130 C 0.2±0.00A nd 0.2±0.12A nd Monoterpenoids 心-terpinolene 27.10 1083 1079 C 0.4±0.07A 0.1±0.01B nd nd VerbenyI ethyl ether 31.90 1382 nd nd nd 0.3±0.01 Naphthalenes Naphthalene,1,2-dihydro- 1,1,6-trimethyl 52.40 1454 一 nd 0.2±0.06 nd nd Phenylpropene Methyl eugeriol 52.93 1401 1402 C 0.3±0.02B nd 0.5±0.08A nd Sesquiterpene x-copaene 37.46 1378 1377 B nd nd 0.8±0.08B 1.9±0.04A x-gurjunene 39.40 1408 1409 S nd 1.2±0.19A nd 1.4±0.08A Trans-c-bergamotene 42.60 1438 1432S nd 2.4±0.38 nd nd x-guaiene 42.90 1440 1437 S nd 0.4±0.04A nd 0.4±0.02A B-caryophyllene 43.35 1408 1408 C 0.6±0.01B 1.2±0.16A人 0.7±0.39 AB 1.3±0.03A B-selinene 46.10 1496 1489 S nd 2.2±0.20 B nd 3.2±0.01A Y-gurjunene 46.89 1479 1475 C nd 3.2±0.57A nd 3.3±0.08A x-muurolene 46.96 1499 1499 B 0.4±0.16B nd 0.9±0.01A 0.1±0.07S 8-guaiene 47.30 n/a 0.3±0.01B 0.2±0.02C 6.6±0.81A 0.1±0.00D c-selinene 47.41 1505 1498 C 0.5±0.04A nd 0.6±0.05A nd B-bisabolene 47.52 1505 1500 C 1.1±0.01S 3.5±0.42A 1.8±0.07B 3.5±0.06A S-cadinene 48.36 1500 1522A 5.5±0.46C 20.4±1.30A 2.1±0.20D 16.1±0.49B Cis-Cadina-1(2),4-diene 49.00 1515 1524S nd 0.2±0.01 nd nd x-cadinene, 49.21 1541 1537C nd nd nd 0.1 Cycloisolongifolene 49.40 n/a 一 nd 0.5±0.01A 5.0±0.02A nd Sesquiterpenoids x-Bergamotene 41.70 1438 1411 C nd 0.4±0.07B nd 1.2±0.02A Aromadendr-1-ene 43.52 n/a 一 0.8±0.08B 0.6±0.06C 2.0±0.54A 0.6±0.03C Table 2. Cont. Classification Compounds RT RI (Exp.) Samples and Extraction Solvent RI (Lit.) + Acetone Ethanol H. od(%) H. pat (%) H. od(%) H. pat (%) Sesquiterpenoids (+)-Aromadendrene 43.74 1454 1458 C 2.7±0.00A nd 0.3±0.05B nd Valencene 45.00 1715 1729 C nd nd nd 0.2±0.17 Allo-aromadendrene 45.18 1635 1639S 1.2±0.02 C 1.9±0.23B 1.0±0.04D 3.0±0.17A c-humulene 45.99 1446 1455 B nd 0.1±0.01A nd 0.1±0.01A Trans-B-farnesene 46.36 1449 1456C 0.4±0.02C 2.1±0.25A 1.4±0.03 B 2.1±0.02A x-amorphene 46.50 1681 1693 C nd 0.6±0.07A nd 0.6±0.01A x-cubebene 46.55 1351 1352 C nd 1.3±0.85 nd nd Viridiflorene 46.77 1570 1489 C 5.6±0.08A 3.5±0.18B 2.8±0.07C 3.1±0.07C B-gurjunene 48.43 1544 1430 C 0.3±0.001B nd 4.8±0.55A 0.2±0.19B Cis-calamenene 50.08 1526 1510 C 0.4±0.01B nd 0.7±0.05A 0.4±0.01B Geranyl acetone 50.38 1452 1453C 0.2±0.03 nd nd nd xx-Calacorene 51.80 1538 1539A nd nd nd 1.5±0.02 Cadina-1(2),4-diene 54.04 1515 1524C nd 0.1±0.07B 0.4±0.08A nd Viridiflorol 54.25 1593 1591 B 26.2±1.24A 17.9±2.15B 16.8±0.31B ¥17.0±0.01B Valeranone 55.00 1668 1666A nd nd nd 0.1±0.26 Epi- bicyclosesquiphellandrene 55.41 1488 1476 C nd nd 0.4±0.06 nd T-Muurolol 55.57 1640 1644 C 0.3±0.08B nd 0.8±0.25A 0.2±0.00B Pogostol7 55.97 1656 nd 0.5±0.05B 2.9±0.42A 0.4±0.07B 1,4-dimethyl-7-(1- methylethyl) azulene Caryophyllenol II 56.30 1772 nd 0.2±0.03C 0.6±0.17A 0.3±0.00B 57.11 1644 1655C nd nd 0.2±0.09 nd Terpene 仑-pinene 12.19 917 939 B 0.8±0.001 B 0.9±0.16B 1.6±0.25A 1.9±0.14A Hexahydrofarnesylacetone 54.34 1844 1844C 0.2±0.08B nd 0.8±0.03A nd Azulene 48.19 1311 一 0.3±0.03B 0.1±0.02C 1.9±0.05A nd Mean values (n=3) along the rows with different l etters are significantly different based on Duncan's Mul t i-ple Range Test at p<0.05. All volat i le compounds were tentatively identi f ied by comparing the data of the MS with spectral data from the NIST v . 05 and the Wiley 275 mass spectral libraries. RT: Retention time (min), RI=Retention index, nd: i mplies not detected or below MS detection l evel, n/a: i mplies not avail-able. * AH=Alkane hydrocarbons, Aroma. Hyds. = Aromatic hydrocarbons, BH= Bicyclic hydrocarbons,FAME= Fatty acid methyl esters, Exp = Experimental RI . t A =Adewinogo et al. al.[8]C=Adams [15] and Babushok et al. [16],D=Qiu et al . [17]. 2.5. Antifungal Activity The extracts obtained from H. odoratissimum and H. patulum using acetone and ethanol showed the highest percentage inhibition (p=0.019, F=8.50, DF=8) against B. cinerea (Figure 1). The percentage inhibition of 65% and 51%, obtained by ethanol extracts of H.patulum and H. odoratissimum, respectively, were higher in comparison with t he inhibition observed by acetone extracts (35% and 53%). The antifungal effects produced by the positive control Rovral@ WP (91%) agains t t he B. cinerea was significantly higher t han all extracts (p<0.05) while no growth i nhibition was observed for the negative controls (i.e.,extraction solvents). These observations indicated that t he extraction solvents alone did not play a role in the inhibitory effects of the crude extracts (Figure 1). The observed high antifungal effects from the ethanol and acetone extracts of H. patulum could be described by the abundant content of methyl cinnamate (cinnamic acid), as fatty acid methyl esters have been evidenced to poses high microbial effects [18]. In addition to that, high levels of 8-cadinene detected in acetone and ethanol extracts of H. patulum (20.4±1.30%and 16.1±0.49%, respectively) could also be implicated to these high antimicrobial effects observed. This compound has been i nc l uded as a major compound of numerous plant extracts that showed high antimicrobial effects. Figure 1. (A) Antifungal screening of aqueous acetone and ethanol extracts of H. odoratissimum (L.)and H. patulum by disk diffusion method against Botrytis c i nerea. Mean values (n=9) of microbial percentage inhibition with standard error bars. Different l etters are signi f icantly different based on Duncan’s Multiple Range Test at p≤0.05. (B) Pareto analysis showing the ef f ects of extraction solvent, variation in Spp., and interaction effects at 95% confidence indicated as the vertical red l ine.(C) Statistica l parameters. * Colour only online 3. Discussion The effects of extraction solvents and the type of Helichrysum species on the yield,chemical composition, and their activities were extensivity investigated using mainly the EO extracted from the plants [7,8]. The current study findings clearly showed that the SS was only influenced by the type of extraction solvent but not by the type of Helichry-sum species. This could be allied with the nature of the extraction solvent and chemical composi t ion of individual species. Several studies have reported the effect of solvents on the SS of plant materials. The results of this study agree with t he reports by Matrose et al. [1] who showed that acetone as an extraction solvent resulted i n higher SS compared to ethanol. The relatively higher SS yield obtained by aqueous acetone (70%) could be due to the polarity of the solvents, as highly polar solvents are known to result in higher extract yield compared to l ess polar solvents. Nawaz et al. [19] previously i nvestigated t he effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. The authors also reported high extract yield obtained from polar solvents extracts and lower phenolic content compared to non-polar ones. The polarity of the solvents and the availability of the extractable plant constituents are the major contributing factors to variations in extrac t ion yields. As the polarities of acetone and ethanol are 0.36 and 0.65, respectively [20], they were affected by the amount of water in the reaction matrix used in the current study. This f urther confirms that an extraction solvent with higher polarity is more effective for maximal extractable yield. According to Kim et al. [21], the addition of water to organic solvents increases the polarity of the sol-vents, hence the higher SS contents observed i n t he 70% acetone-based extract s t han in 95%ethanol extracts used in the current study. Matrose et al. [1] further elucidated a significant correlation between high polar solvents and high extract yield. The authors reported a 40%reduction in the soluble solid yield of 95% ethanol t han t hose of 70% acetone-based extracts.Therefore, based on the current study and the other studies stated above, aqueous acetone was the better extraction solvent to obtain a higher SS from Helichrysum species. In this study, different extraction solvents showed a significant effect on the extraction yield of total polyphenol (TP)[22]. There are a few examples of l iterature about the TP The antioxidant capacity of H. odoratissimum extracts was significantly more affected than that of H. patulum by the extraction solvents. Results in the current study were supported by various studies who reported the influence of extraction solvents and dis-crepancy on the chemical profiles among plant species on the antioxidant capacity of different plants of the same genus. Even though the two species were obtained f rom the same agro-climatic zones, the differences observed in t heir antioxidant capacity could be influenced by a couple of factors (i.e., variability in t he chemical composition of each plant species, selectivity of solvents, and the solubility of plant chemical compounds), which affects extraction yield [1,23,27]. A direct correlation was again confirmed in a study by Lim et al. [28]. The authors investigated the correlation between the extraction yield of mangiferin to the antioxidant activ i ty, total phenolic, and total f lavonoid content of Phaleria macrocarpa fruits, and a significant (p≤ 0.05) correlation between the extraction solvent (methanol), method, extraction yield of mangiferin, and DPPH scavenging capacity was observed . In addition, Swarts [29] investigated the chemical profile of EOs obtained f rom H. patulum; t he author reported on high antioxidant activity of the oils, which i s allied to the presence of the phenolic constituent, arbutin. An earlier study by Takebayashi et al. [30] also reported weak antioxidant activity of arbutin. The authors, however, f urther discussed that the potency of the antioxidant of t he compound to be directly dependent on the type of assay employed. Furthermore, there are several studies that showed the strong correlation between DPPH and TP content, which could explain the higher DPPH scavenging ability of H. odoratissimum than H. patulum. For instance, t he results of t his study indicated a significant (p ≤0.05) correlation between the total polyphenol content and the DPPH scavenging capacity of the extracts (Table 1). The GC-MS analysis of this study identified higher concentrations of alkane hydro-carbons (tetradecane and hexadecane) i n the H. odoratissimum extracts. These could be responsible for the higher antioxidant activity t hat was obtained in the H. odoratissimum ex-tracts than H. patulum extracts. This could be supported by the findings of Ashraf et al . [31].The authors compared chemical composi t ion, antioxidant, and antimicrobial activities of EOs obtained from different parts (leaves and stems) of Daphne mucronata Royle. Their study reported a significantly high antioxidant activity in the EOs obtained from the l eaves than that of the stems and linked this to alkane hydrocarbons. Pentadecane (12.75%),2-methyl hexadecane (8.90%), 7,9-dimethyl hexadecane (8.90%), tetradecane (7.32%), 5-Propyl decane (6.16%), 2,3,5,8 tetramethyl hexadecane (5.81%), 2-methyl6-propyl dodecane (5.11%), and 5-methyl tetradecane (5.10%) were identified as the major constituents of the potent plant part. Generally, most of these compounds are reported to exhibit high antioxidant and high microbial effects [32]. In this study, t he concertation of alkylbenzene and aromatic hydrocarbons were below the detected l imi t for ethanol extracts of both H. patulum and H. odoratissimum, suggesting the type of solvent could affect the volatile emission from the extracts. This could be due to the high concentration of ethanol (95%)in the extraction solvent; as stated by Feng et al. [25], high ethanol concentration leads to protein denaturation, preventing the dissolution of polyphenols leading to lowered extract i on rate. In addition, acetone can dissolve both polar and nonpolar substances while ethanol amongst other solvents can only dissolve one or the other. T he f indings from this study are consistent with the results of De Canha et al . [33]. The authors reported a relatively high abundance of sesquiterpenoids and sesquiterpenes for the crude methanolic extracts of H. odoratissimum obtained from Kwazulu Natal . An earlier report by Gundidza and Zwaving [18] also reported similar results i n a Zimbabwean H. odoratissimum wi t h sesquiterpenoids (>22.6%) as major components. Similarly, Bougatsos et al. [34] reported the sesquiterpenoids (B-caryophyllene, 30.7% and 12.6%) as a major component of both H. kraussii and H. rugulosum, respectively. It is noteworthy that the phytochemistry re-ported for crude plant extract of H. odorutissimum and H. patulum in t his study differs in the relative abundance of major and minor chemical classes from those reported i n the literature for EOs. For instance, Najar et al . [35], identified a- and β-pinene (27.6% and 44.9%, respectively) as the major compounds i n the EOs of H. patulum. It is, however,noteworthy that factors such as extrac t ion solvent, plant parts, extraction method, sample type (crude extracts or EOs), geographical location, and species used play a crucial role on t S he extrac t yield and chemical constituents [1]. The antimicrobial i nhibitory effects of Helichrysum spp. have been reported by previous studies [36]. The current study also confirmed marked growth i nhibitory effects of both H. odoratissimum and H. patulum. Generally, H. patulum poses higher antifungal effects in comparison with H. odoratissimum. The findings of the current study were in support of the previous study by Louranse et al . [5]. The authors showed t hat H. patulum displayed antimicrobial activity against Staphylococcus aureus in the disc diffusion assay that was comparable to that of the posi t ive control: ciprofloxacin. Phytochemical studies conducted in H. patulum extracts or Eos evidenced the presence of different compounds with high microbial activities. According to Najar et al . [35], sesquiterpenes are evident antimicrobials.The authors further noted t hat compounds such as the epoxide: caryophyllene oxide,sesquiterpenoid: viridiflorol for high antimicrobial activity thus could lead to the observation of the current study, as a high presence of the latter compounds were detected i n all extracts tested. Khundu et at. [36] evaluated f ive cadinene derivatives (cadinan-3-ene-2,7-dione,7-hydroxycadinan-3-ene-2-one, 5,6-dihydroxycadinan-3-ene-2,7-dione, cadinan-3,6-diene-2,7-dione, and 2-acetyl-cadinan-3,6-diene-7-one) fractionated from ethyl acetate extract of the l eaves of Eupatorium adenophorum. The authors revealed that all compounds exhibi t a high but selective antifungal activity. A previous study by Gonzalez et al. [37] identified 8-cadinene as a major compound in the methanol extracts of Xenophyllum poposum. The authors associated the high antifungal activity of the extracts with the presence of the l ater compound. As aforementioned, even though not in high concentration, B-selinene and y-gurjunene were only identified i n H. patulum extracts. High antimicrobial activity of t hese sesqui t erpenes has been reported by Salleh et al. [38]. The authors further noted a direct correlation between the total polyphenol content of t he extracts and antimicrobial activity. 4. Materials and Methods 4.1. Plant Material Fresh plant material (areal parts: leaves and stems) of H. odoratissimum L. [Sweet](Voucher No: HO370) were collected from t he Botrivier area in the Overberg region (Lati-tude: -34°13'0.02" Longitude: 19°12’0"), Hermanus, South Africa and supplied by AfriNat-ural (Cape Town, South Africa). H. patulum was collected and identified (Voucher No:A9006) by the Tygerberg Nature Reserve on the Tygerberg hil l s i n the northern suburbs (-33.8793° S: 18.5966° E) of Cape Town, South Africa. Figure 2 presents images of t he Helichrysum spp. prior to processing. Plant material (leaves and stems) were immediately air-dried away from direct sunlight upon arrival and stored separately at room tempera-ture in the dark at the Agri-Food Systems, Postharvest Pathology Laboratory at t he ARC Infruitec-Nietvoorbi j, Stel l enbosch, South Africa until processing and extraction. (A) (B) Figure 2. Pictures of the two Helichrysum odoratissimum (A), and Helichrysum patulum (B) were investigated in t he study. 4.2. Processing and Extraction Using a kitchen blender (91-357, Waring Blendor, Waring, Stamford, CT, USA), air-dried plant materials were roughly ground and further milled i nto f iner powder. For each processed Helichrysum spp., 250 g was macerated in 1 L of 95% ethanol (Ethanolsa,Pretoria, South Africa) and 70% acetone (Ethanolsa, Pretoria, South Africa), as descried by Matrose et al. [1]. Macerated mixtures were placed at room temperature (24°C) under vigorous shaking at regular intervals (8 h) f or 72 h using ala boshake heavy load shaker (Gerhardt GmbH & Co. KG, Konigswinter, Germany). After maceration and vigorous shaking, the extracts were filtered through Whatman #4(Whatman International , Ltd., Maidstone, UK) and transferred through a tea strainer into a clean Schott bottle. With the use of Sartorius@ Cellulose Nitrate (Cellulose Ester) Membrane Filters, 0.2 um (0613114071301823, Sartorius Stedim Biotech, Gottingen, Germany) the filtrates were filtered further to eliminate microbial contamination. F i ltrates were stored under aseptic condition at 4 °C until further analysis. Al l extractions were conducted in triplicate (n=3). 4.3. Phytochemical Analyses 4.3.1. Soluble Solids A gravimetric approach was used to quantify the soluble solid (SS) content of the crude extracts by evaporating aliquots (20 mL) of each f iltrate to dryness in the pre-weighed nickel moisture dishes on a steam bath. Thereafter, the moisturizing dishes were dried using a laboratory convection oven at 100 °C for 60 min. All measurements were carried out in triplicate (n=3). Moisture dishes were cooled in a desiccator to room temperature then weighed and SS content calculated and expressed as (mg 100 mL-1). 4.3.2. Total Polyphenol Analysis BioTek Synergy HT multi-plate reader (BioTek Instruments, Winooski, VT, USA) was used to quantify t he total polyphenol content of all the crude extracts. The Folin-Ciocalteu method by Singleton and Ross [39], which was further adapted for micro-palate reader format by Arthur et al. [40], was used in this study. Gallic acid (Sigma-Aldrich, Burlington,MA, USA) was used as the standard for t he calibration curve ranging in concentration from 1 mg L-1 to 10 mg L-1. T he samples stock solutions (1 mg mL-1) were diluted (300 uL sample di l uted to a final volume of 1000 uL with deionized water) to obtain absorbance values within the range of the calibration curve. Gallic acid standards (20 uL) with the samples and the assay control (deionized water) were transferred in triplicate into a 96-well polystyrene flat-bottomed micro-plate (Greiner Bio-One, Frickenhausen, Germany). Folin-Ciocalteu's reagent (10× diluted; 100 uL) and sodium carbonate (7.5%w/o;80 uL, Merck,Darmstadt, Germany) solution were added into the reaction mixture, followed by mixing of the well contents using an Eppendorf Mix Mate micro-plate shaker (Merck, Darmstadt,Germany). The micro-plates were incubated for 2 h at 30°C to allow for development of a blue-color complex resulting f rom oxidation of the phenolic compounds. The absorbance was measured at 765 nm and total polyphenol content (TP) in each extract was expressed as g Gallic Acid Equivalents (GAE) 100 g-1 dry weight of the leaves. 4.3.3. Determination of Antioxidant Activity The antioxidant activity of the plant extracts against 2,2-Diphenyl-2-picrylhydrazyl (DPPH) was determined using the method described by Arthur et al. [40]. The methanolic (Merck, Darmstadt, Germany) solut i on (0.05 mg mL-1) of DPPH (Sigma-Aldrich, Burling-ton, MA, USA) was prepared, covered with foil, and sonicated for 5 min. Using the BioTek Synergy HT multiplate reader (BioTek Instruments, Winooski, VT, USA), the DPPH-methanol solution absorbance value was adjusted to the range of 0.68-0.71 for assay standardization purposes. Trolox (Sigma-Aldrich, Burlington, MA, USA) was used as a calibration standard. A dilution range (1 ug mL-1 to 10 ug mL-1) of T r olox s tandard f rom stock solution (1 mM) was used to prepare a calibration curve in t he reaction volume. Thirty microliters each of the Trolox standards, samples, and assay control (deionized water) were transferred in t riplicate into corresponding wells of a 96 deep-well plate (Axygen Scientific Inc., Union City, CA, USA) and 270 uL DPPH solution (ca 0.7 absorbance value) was added. To prevent evaporation of methanol (Merck, Darmstadt Germany), t he sample-loaded plate was sealed with silicon mat. The contents in the 96 deep-well plate were then mixed for 30 s at 1650 rpm using an Eppendorf MixMate@ micro-plate shaker (Eppendorf AG, Hamburg, Germany). The micro-plates were incubated in a dark cupboard at room temperature for 2 h (to allow scavenging of DPPH by t he antioxidants, leading to a decrease in absorbance of the free radical solution). Thereafter, 200 uL of the reaction mixture were transferred into corresponding wel l s of a polystyrene flat-bottom 96-well micro-plate (Greiner bio-one, Frickenhausen, Germany) and t he absorbance measured at 515 nm using the BioTek Synergy HT multiplate reader The radical scavenging ability was expressed as umol Trolox Equivalents (TE) g -1 extract. 4.4. Secondary Metabolites Relative abundance of secondary metabolites of the Helichrysum spp. extracts were quantified by pipetting ~5 mL of each extract into 20 mL solid-phase micro-extraction (SPME)vials. Each vial was allowed to equilibrate for 5 min at 50°C i n the CTC auto-sampler incubator at 250 rpm. Volatile compounds trapped i n the headspace of the vails were extracted using the static headspace (SPME) method [41]. Subsequently, SPME fiber (50/30 um) coated with divinylbenzene/-carboxen/-polydimethylsiloxane (DVB/CAR/PDMS) was exposed to the sample headspace for 10 min at 50 °C. After volatile extraction from SHS of t he vials, desorption of the adsorbed compounds from the fiber coating was carried out in t he injection port of the gas chromatography-mass spectrometry (GC-MS) for 10 min. The injector temperature was maintained at 250°C. Separation and quantification of the volatile compounds were performed on a gas chromatograph using Agilent 6890 N (Agilent, Palo Alto, CA, USA), coupled with an Agilent mass spectrometer detector Agilent 5975 MS (Agilent, Palo Alto,CA, USA). The system was equipped with a polar ZB-FFAP column (Model No.: ZB 7KM-G009-17),with a nominal length of 60 m, 0.25 um internal diameter, and 0.5 um f ilm thickness.Helium gas was used as the carrier gas for these analyses at a flow of 2 mL min -1 with a nominal i nitial pressure of 196.0 kPa and an average veloc i ty of 36 cm s-1. The oven temperature program was as follows: 35 °C for 5 min; and then ramped up to 50°℃at 3 °C min -1 and held for 3 min, and again ramped up to 120 ℃ at 3°C min-1 with a 5 min holding time. Finally, the temperature was ramped up to 240 at 8°℃ min-1and held for 5 min. The MSD was operated in full scan mode and the ion source and quadrupole temperatures were maintained at 230 °C and 150 °C, respectively. The transfer line temperature was maintained at 250 °C. Compounds were tentatively identified by comparison of retention t imes (RT) and retention i ndex (RI) with those r egistered i n the National Institute of Standards and Technology (NIST v. 05, Gaithersbug, MD, USA) and the WHILEY 275 mass spectral l ibraries. Only compounds that occur across t he triplicates and with correlation coefficient ≥ 80% from the NIST MS l ibrary were considered for further analysis. For quantification, the calculated relative abundances were used. This experiment was i ndependently replicated in triplicate (n=3). 4.5. Antimicrobial Activity Analysis 4.5.1. Preparation of Test Fungal Pathogen Fungal pathogen B. Cinerea isolated from infected plums was obtained from Agri-cultural Research Council (ARC)—Plant Protec t ion I nstitute, Pretoria (Accession number:PPRI 7338). South Africa. B. cinerea was cultured on potato dextrose agar (PDA, Merck,Johannesburg, South Africa) at 25 °C for 3 days for mycelial plugs, and for 7 days to produce spores. The cultures of B. cinerea, were maintained on PDA slants at 4°C. Coni-dia were harvested from the medium surface with sterile distilled water with Tween 80(0.05% W/V) (Batch No.: 1006022, Merck, Darmstadt, Germany) and gentle agitating the plates to dislodge the spores. The f inal inoculum concentration was adjusted to 1 ×105conidia mL-1 using a haemocytometer (Marienfeld, Germany). 4.5.2. Ant i fungal Activi t y To determine the antifungal activi t y of the crude extracts against B. c inerea, a disc diffusion assay described by Matrose et al . was used [1]. Sterile Whatman #4 filter paper disks (4mm,o)were soaked in the perspective samples/standards/controls, i .e., (a) respective crude extracts concentration (250 mg mL -1) was selected for this investigation based on multiple preliminary study; (b) in 0.005 mg mL-1 of Rovral @ WP (Bayer Crop Science, Leverkusen, Germany) as negative control (standard); and (c) in the extraction solvents as positive controls. The PDA plates were spread -plated with 200 uL suspension of B. cinerea spores. Inoculated plates were allowed to dry before the treated discs were aseptically placed at the centre. Each plate was sealed with parafilm (DEMIS flexible packaging, Demis,USA), and incubated at 25 °C for 5 days. The zone of inhibition on day 5 was measured using a digital calliper (MAC-AFRIC-Adendorff Machinery Mart, Johannesburg, South Africa), the percentage inhibition was calculated, and antifungal activity expressed as %Inhibition [1]. This experiment was independently replicated in t riplicate and three t imes (n=9). 4.6. Statistical Analysis Data obtained were subjected to factorial analysis of variance (ANOVA) using Statis-tical software (vr. 13, StatSoft Inc. TIBCO Software Inc., Arlington, VA, USA). Effects of experimental factors: species (Spp.), extraction solvents (S), and t heir interactions (Spp. *S)on soluble solids (SS), total polyphenol (TP), antioxidant activity, and antifungal activities were analyzed. To test statist i ca l di f ferences between mean values, Duncan’s Mul t iple Range Test was at p≤0.05. To describe the relationship between TP, antioxidant activity and SS, Pearson correlation matrix was used . Besides the antifungal activity, all experimental data obtained were i n triplicate and the result presented as mean values (n=3) standard deviation (SD). 5. Conclusions This study showed that that extraction solvent plays a crucial role i n t he availability,bioactivity, and antifungal efficacy of bioactive compounds of Helichrysum Spp. Comparing between the antioxidant activities of Helichrysum Spp., H. odoratissimum had the highest f ree radical scavenging capacity for the extracts obtained with 70% acetone and 95% ethanol,while extracts from H. patulum showed lower scavenging activity. High antioxidant ac ti vity was consistent with the total polypheno l content. The GC-MS profiling of Helichrysum odoratissimum and H. patulum showed distinction variation in the secondary metabolites.Viridiflorol , a sesquiterpenoid with known antimicrobial activity, W wa a s S most abundant i n t he 70% acetone crude extract of H. odoratissimum. Similarly, 8-cadinene was abundant in the 70% acetone crude extract of H. patulum. Fatty acid methyl ester (methyl c i nnamate) was only detected in ethanolic extract of H. patulum. Thus, t his study emphasises the importance of species variation in bioprospecting for new plant-based bioactive compounds. Author Contributions: N.A.M.: conceptualization, data curation, writ i ng original draft , editing.Z.A.B.: conceptualization, superv i sion, visualization, writing-rev i ew & editing. K.O.: conceptu-alization, supervision, writing-review & editing. L.M.: methodology, visualization, data. O.J .C.:conceptualization, project administration, validation, writing-review & editing, funding acquisition.All authors have read and agreed to t he published version of the manuscript. Funding: This research was funded by t he National Research Foundation (NRF) of South Africa (Grant Nos.: 137990 and 146360) and (Grant No.: 138128) awarded to Caleb and Belay. The FoodBev SETA scholarship awarded to Neliswa Matrose is gratefully appreciated. Institutional Review Board Statement: Not applicable Informed Consent Statement: Not applicable. Data Availability Statement: The data that support t he finding of t his study are available f rom the corresponding author upon request. Acknowledgments: The research support provided by the Plant Bioactives Group, ARC I nfruitec-Nietvoorbij, Stellenbosch, South Af r ica is acknowledged. Conflicts of Interest: The authors declare no conflict of interest. References 1. Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Impact of spatia l variation and extraction solvents on bioactive compounds,secondary metabol i tes and antifunga l efficacy of South African Impepho [Helichrysum odoratissimum (L.) Sweet]. Food Biosci. 2021,42,101139. [CrossRef ] 3. Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41-52.[C r ossRef ] 4. Fungicides Global Market Report 2022. Available online: https://www.thebusinessresearchcompany.com/report/fungi ci des-global-market-report (accessed on 16 September 2022). 5. Lourens, A.C.U.; Viljoen, A.M.; Van Heerden, F.R. South African Helichrysum species: A review of the t raditional uses, biological ac t ivity and phytochemistry. J. Ethnopharmacol. 2008,119,630-652. [CrossRef] [PubMed] 6. Maroyi, A. A synthesis and review of medicinal uses, phytochemistry and biological activities of Helichrysum odoratissimum (L .)sweet. Asian J. Pharm. Clin. Res. 2019, 12, 15-23.[CrossRef ] 7. Najar, B.; Nardi, V.;Cervelli, C.; Mecacci, G.; Mancianti , F; Ebani, V.V.; Nardoni, S.; Pistelli, L. Volatilome analyses and in vitro antimicrobia l ac t ivity of the essential oils from f ive South African Helichrysum species. Molecules 2020,25,3196. [CrossRef ] 8. Adewinogo, S.O.; Sharma, R.; Africa, C.W.; Marnewick, J.L.; Hussein, A.A. Chemical Study and Comparison of t he Biological Activities of the Essentia l Oils of Helichrysum petiolare, H. cymosum, and H. odoratissimum. Plants 2022, 11, 2606. [CrossRef] 9. Serabele, K.; Chen, W.; Tankeu, S.; Combirinck, S.; Veale, C.G.L.; van Vuuren, S.; Chaudhary, S.K.; Viljoen, A. Comparative chemical profiling and antimicrobial activity of two i nterchangeable used Impepho' species (Helichrysum odorat i ssimum and Helichrysum pet i olate). S. Afr. J. Bot . 2021, 137, 117-132. [CrossRef ] 10. Zantanta, N.;Kambizi , L.; Etsassala, N.G.; Nchu, F. Comparing crop yield, secondary metabolite contents, and antifungal activity of extracts of Helichrysum odoratissimum cultivated in aquaponic, hydroponic, and field systems. Plants 2022, 11, 2696.[CrossRef ] 11. Acimovic, M.; Ljujic, J.; Vulic, J .; Zheljazkov, V.D.; Pezo, L.; Varga, A.; Saponjac, V.T. Helichrysum i talicum (Roth) G. Don essential oi l from Serbia: Chemical composition, classif i cation and biological activity-May i t be a suitable new crop for Serbia? Agronomy 2021,11, 1282.[CrossRef ] 12. Djihane, B.; Wafa, N.; Elkhamssa, S.; Pedro, H.J .;Maria, A.E.; Mihoub, Z.M. Chemical constituents of Helichrysum italicum (Roth)G. Don essential oil and their antimicrobial activity against gram-posi t ive and gram-negative bacteria, f ilamentous f ungi,and Candida albicans. Saudi Pharm. J. 2017,25,780-787.[CrossRef ] 13. Lawal, O.A.; Ogunwade, I .A.; Kasal i , A.A.; Opoku, A.R.; Oyedeji, A.O. Chemica l composition, antibacterial and cytotoxic activities of essential oils from the leave of Helichrysum odoratissimum growing in South Africa. J. Essent. Oil-Bear. Plants 2015,18,236-241. [CrossRef ] 14. Khor, G.K.; Uzir, M.H. Saccharomyces cerevisiae: A potential stereospeci f ic reduction t ool for biotransformation of mono-and sesquiterpenoids. Yeast 2011, 28, 93-107. [CrossRef] [P ubMed ] 15. Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 3rd ed.; Allured Publishing: Carol Stream, IL, USA, 2003. 16. Babushok, V.I.; Linstrom, P .J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys.Chem. Ref. Data 2011, 40,043101.[CrossRef ] 17. Qiu, X.; Cao, L .; Han, R. analysis of volatile components in dif f erent Ophiocordyceps sinensis and insect host products. Molecules 2020, 25,1603.[C r ossRef ] 18. Gundidza, M.G.; Zwaving, J.H. The chemical composition of the essential leaf oil of Helichrysum odoratissimum sweet from Zimbabwe. J. Essent. Oil Res. 1993,5,341-343.[CrossRef ] 19. Nawaz, H.; Shad,M.A.; Rehman, N.; Andaleeb,H.; Ullah, N. Effects of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J . Pharm. Sci. 2020, 56,1-9. [CrossRef ] 20. Abubakar, A.R.; Hague, M. Preparation of medicinal plants: Basic extraction and fractionation procedure f or experimental purposes. J. Pharm. Bioallied Sci. 2020,12,1-10. [CrossRef ] 21. Kim, H.; Lee, S.; Kim, B.; Lee, E.; Ryu, J. Effect of modifiers on the supercritical CO2 extraction of glycyrrhizin f rom licorice and the morphology of licorice t i ssue after extraction. Biotechnol. Bioprocess Eng. 2004, 9, 447-453.[CrossRef ] 22. Nguyen, N.Q.; Nguyen, M.T .; Nguyen, V.T .; Le, V.M.; T r ieu, L.H.; Le, X.T .; Khang, T.V.;Giang, N.T .L .; T hach, N.Q.; Hung, T.T. Th e effect of di f ferent extraction conditions on the polyphenol, flavonoids components and antioxidant activity of Polyscias f ruticose roots. Mater. Sci. Eng. 2020, 736,022067. 23. Iloki-Assanga, S.B.; Lewis-Lujah, L.M.; Lara-Espinoza, C.; Gil-Salido, A.A.; Fernandez-Angulo, D.;Rubio-Pino, J.L.; Haines,D.Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulat i ons for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res. Notes 2015,8,396. [CrossRef ] 24. Borges, J .G.; Garcia, V.A.D.; Osiro, D.; de Carvalho, R.A. Printing ethanol pomegranate extract i n films by ikjet technology. Ind.Crops Prod. 2019,140,111643. [CrossRe f ] 25. Feng, Y.; Yuan, D.; Kong, B.; Sun, F; Wang, M.; Wang, H.; Liu, Q. Structural changes and exposed amino acids of ethanol-modified whey proteins i solates promote i ts antioxidant potential. Curr. Res. Food Sci . 2022, 5, 1386-1394.[CrossRef ] 26. Ngo, T.V.; Scarlett, C.J .; Bowyer, M.C.; Ngo, P.D.; Vuong, Q.V. Impact of di f ferent extraction solvents on bioactive compounds and antioxidant capacity from the root of Salac i a chinensis L. J. Food Qual. 2017,2017,9305047. [CrossRef ] 27. Azabou, S.; Sebii, H.; Taheur, F.B.; Abid, Y.; Jridi, M.; Nashri , M. Phytochemical profile and antioxidant properties of tomato by-produc t s as af f ec t ed by extract i on solvents and potential applicat i on in refined ol i ve oils. Food Biosci. 2020, 36, 100664.[CrossRef ] 28. Lim, Y.P.; Pang, S.F.; Yusoff, M.M.; Mudalip, S.A.; Gimbun, J. Correlation between t he extraction yield of mangifer i n t o t he antioxidant activity, total phenolic and total flavonoid content of Pheleria macrocarpa f ruits. J. Appl. Res. Med. Aromat. Plants 2019,14,100224. 29. Swarts, V.G. Phytochemicalstudies of Helichrysum patulum; Department of Chemistry, University of t he Western Cape: Cape Town,South Africa, 2006. 30. Takebayashi, J.; Chen, R.I.; Matsumoto, T .; Ishimi, Y.; Tai, A. Reassessment of antioxidant activity of arbutin: Multifaceted evaluation using five antioxidant assay system. Free Radic. Res. 2010, 44,473-478. [CrossRef ] 32. Subramaniam, Y.; Ramalakshmi, S.; Neelavathy, R.; Jhnpaul, M. Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GC-MS. Glob. J . Pharmacol. 2012, 6,65-71. 33. De Canha, M.N.; Komarnytsky, S.; Langhansova, L.; Lall, N. Exploring t he anti-acne potential of I mpepho [Helichrysum odorat i ssimum (L.) Sweet] to Combat Cutibacterium acnes virulence. Front . Pharmacol. 2020, 10, 1559.[CrossRef] 34. Bougatsos, C.; Meyer, J .J .M.; Magiatis, P.; Vagias, C.; Chinou, I .B. Composition, and antimicrobial activity of t he essential oils of Helichrysum kraussi i Sch. Bip. and H. rugulosum Less. from South Af r ica. Flavour Fragr. J . 2003,18,48-51. [CrossRef ] 35. Najar, B.; Cervelli, C.; Ferri, B.; Cioni, P.L.; Pistell i , L. Essential oils and volati l e emission of eight South African species of Helichrysum grown in uniform environmental conditions. S. Afr . J. Bot. 2019,124,178-187. [CrossRef ] 36. Khundu, A.; Saha, S.; Walia, S.; Shakil , N.A.; Kumar, J.; Annapurna, K. Cadinene sesquiterpenes f rom Eupatorium adenophorum and their antifungal activity. J . Environ. Sci. Health 2013, 48 Pt B, 516-522. [CrossRe f ] 38. Salleh, W.M.N.H.W.; Ahmad, F; Yen, K.H.; Zulkifli, R.M. Chemical composition, and biological activi t ies of essential oils of Bei l schmiedia glabra. Nat . Prod. Commun. 2015,10,1297-1300.[CrossRef ] [PubMed] 39. Singleton, V.L .; Rossi, J .A. Colorimetry of total phenolics wi t h phosphomolybdic-phosphotungst i c acid reagents. Am. J. Enol.Vitic. 1965,16, 144-158. Disclaimer/Publisher's Note: The statements, opinions and data contained in all publications are solely those of t he i ndividual author(s) and contributor(s) and not of MDPI and/or t he editor(s). MDPI and/or t he editor(s) disclaim responsibility for any injury to people or property resulting from any ideas , methods, instructions or products referred to in the content.
确定










还剩13页未读,是否继续阅读?
中国格哈特为您提供《蜡菊属(永久花)植株样品的振荡提取》,该方案主要用于中药材和饮片中前处理检测,参考标准--,《蜡菊属(永久花)植株样品的振荡提取》用到的仪器有格哈特强力高重现振荡器LS500/RO500、格哈特快速干燥仪STL56、德国移液器MM
推荐专场
快速干燥仪
更多
相关方案
更多
该厂商其他方案
更多
















