摘要:维持正常的维生素C水平对人体免疫系统的正常运作至关重要。用于监测汗液维生素C的实时无创可穿戴式传感器的开发在指导个性化健康管理方面具有重要的应用前景。在此,这项工作提出了一种基于二维锌卟啉MOF纳米片/多壁碳纳米管(2D-TCPP(Zn)/MCNTs)的智能手机光驱动的无酶可穿戴式光电化学(PEC)传感器,用于监测汗液维生素C。对维生素C实现了3.61 μM的低检测限和10 ~ 1100 μM的宽检测范围。同时,所提出的电极具有优异的选择性和稳定性。此外,本工作还设计了一种新型的低成本柔性可穿戴PEC传感器贴片,用于有效收集和持续监测汗液中的维生素C。该智能手机光驱动的无酶可穿戴PEC传感器可以准确地检测真实汗液中的维生素C浓度,这将有助于确保人体适当的营养平衡。
方案详情

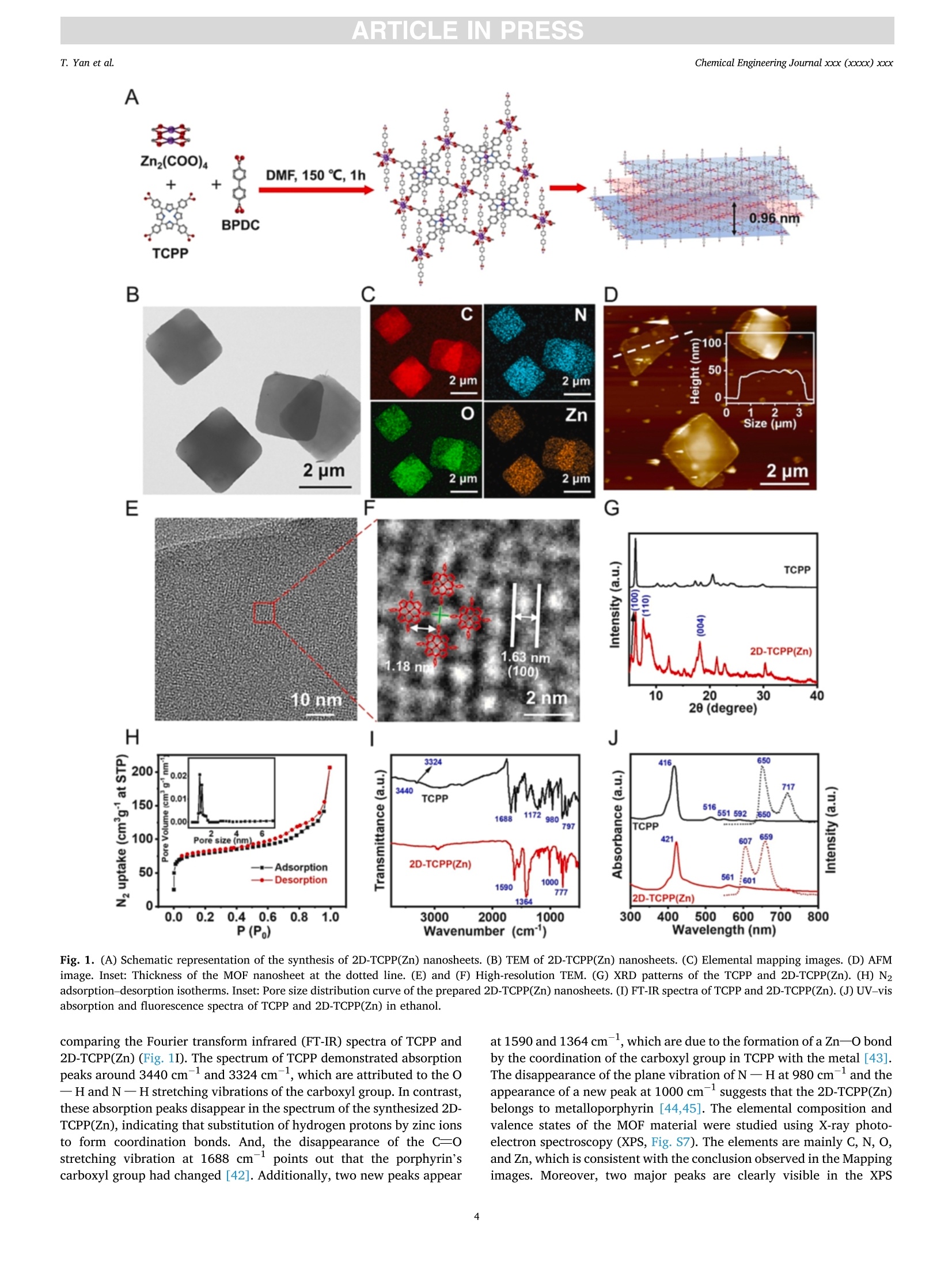
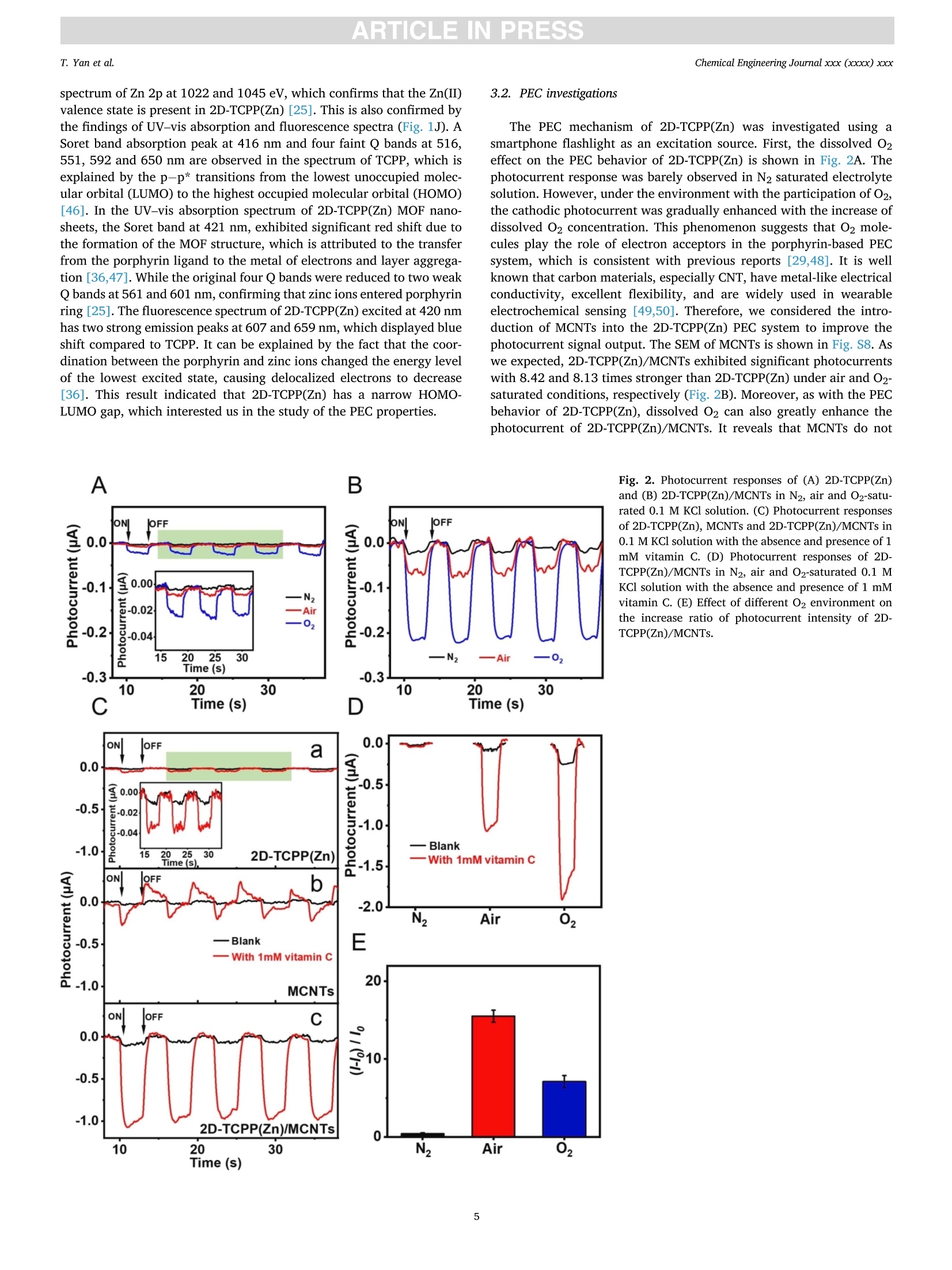
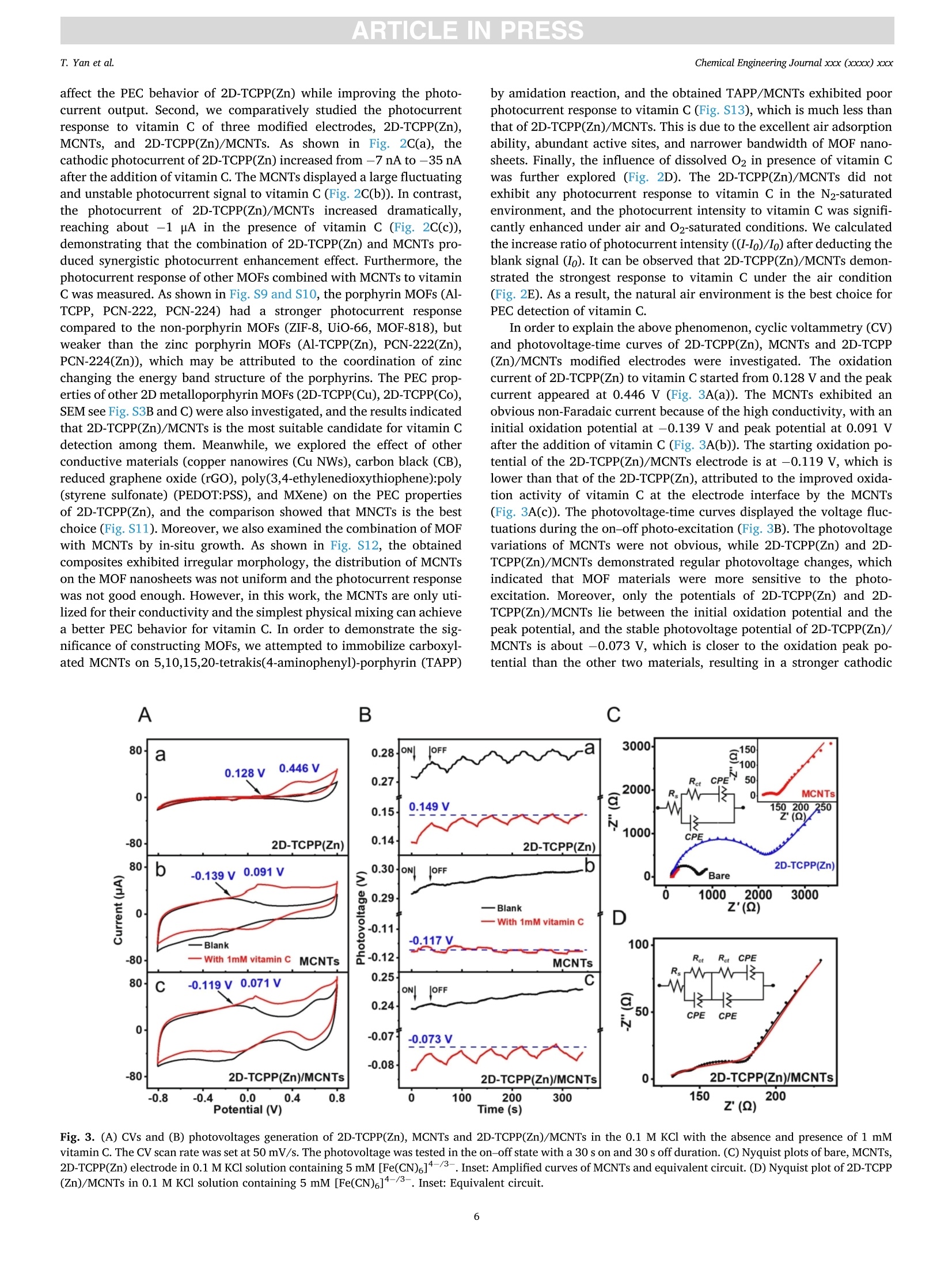
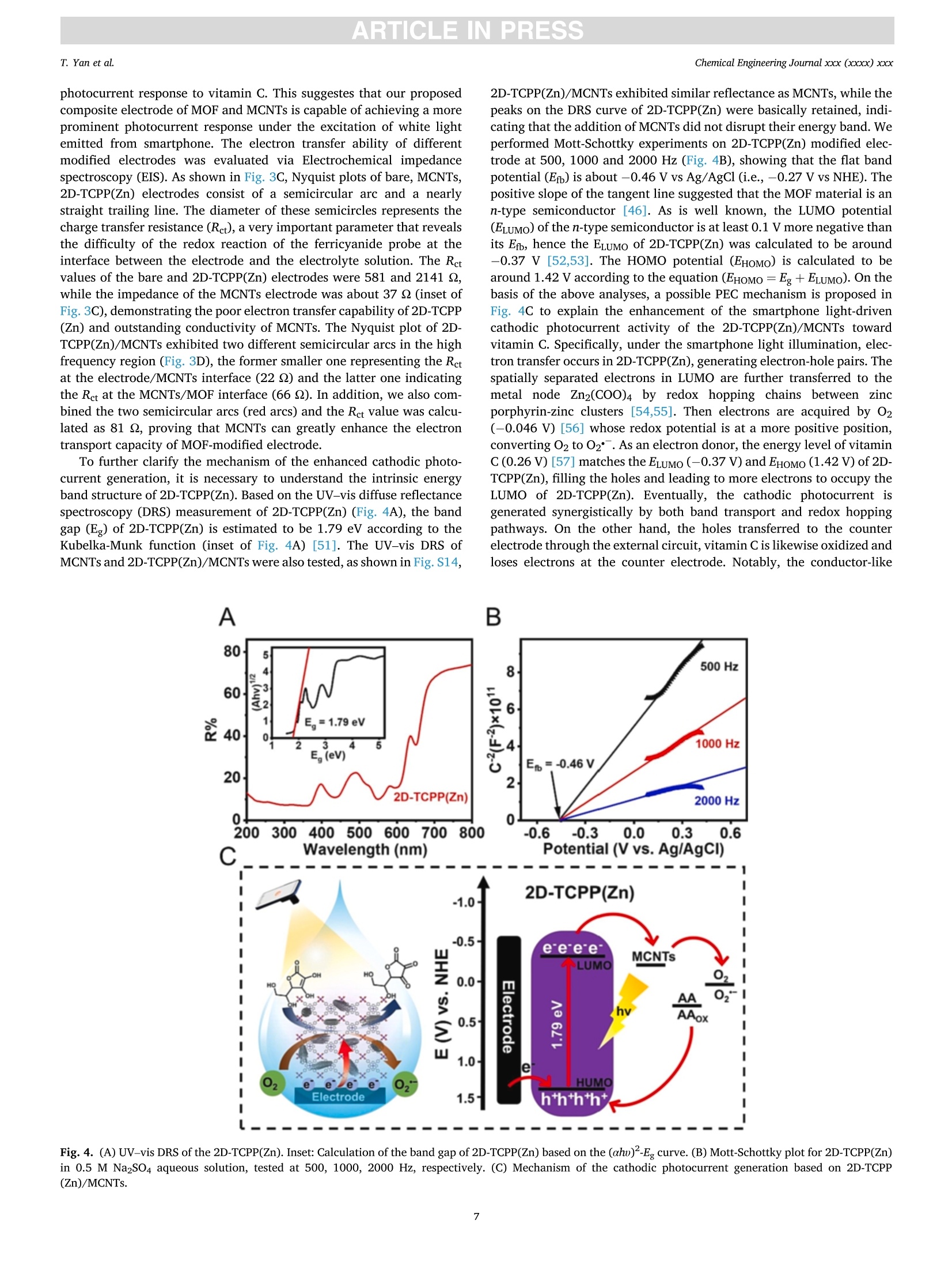
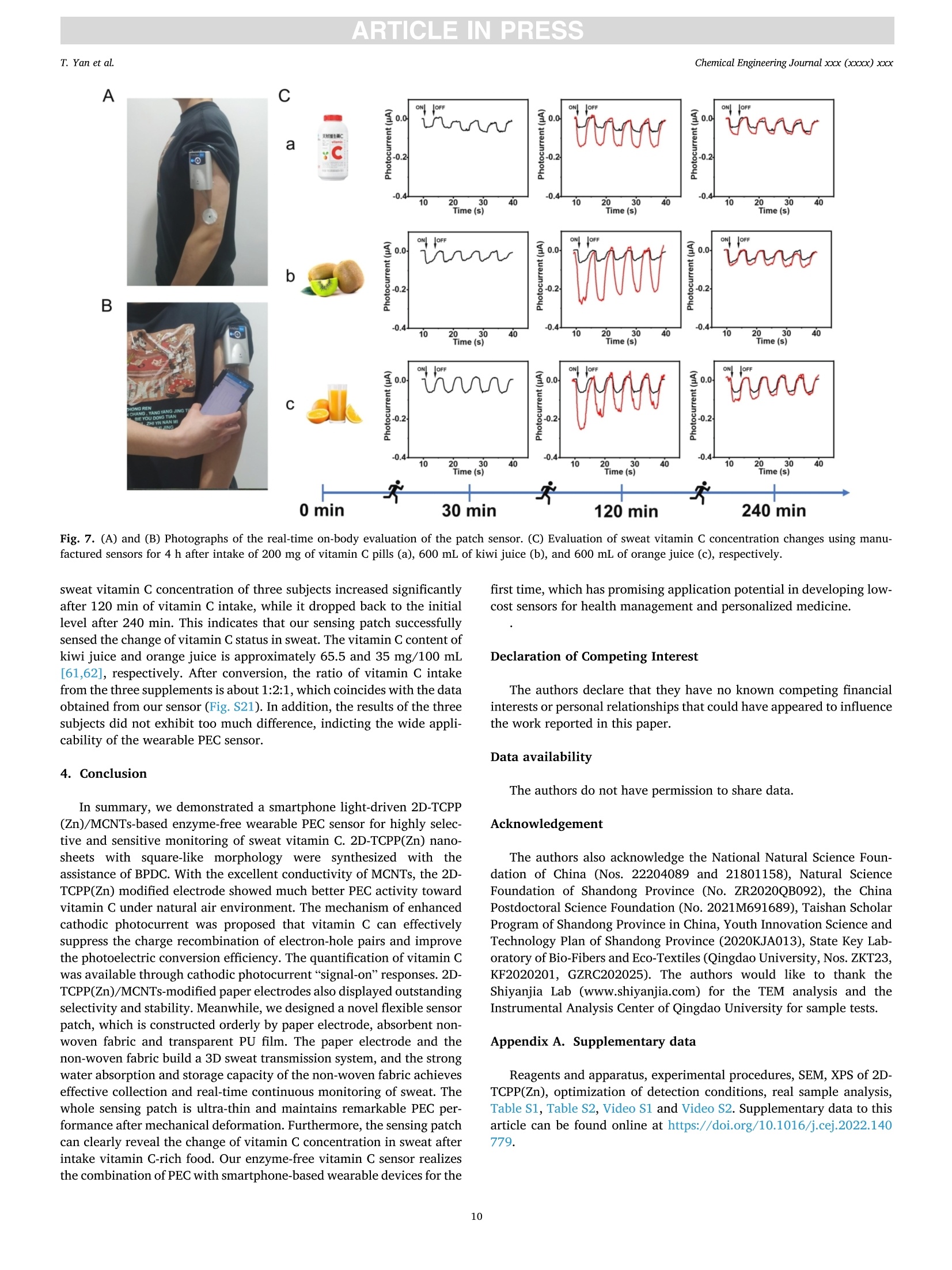
摘要:维持正常的维生素C水平对人体免疫系统的正常运作至关重要。用于监测汗液维生素C的实时无创可穿戴式传感器的开发在指导个性化健康管理方面具有重要的应用前景。在此,这项工作提出了一种基于二维锌卟啉MOF纳米片/多壁碳纳米管(2D-TCPP(Zn)/MCNTs)的智能手机光驱动的无酶可穿戴式光电化学(PEC)传感器,用于监测汗液维生素C。对维生素C实现了3.61 μM的低检测限和10 ~ 1100 μM的宽检测范围。同时,所提出的电极具有优异的选择性和稳定性。此外,本工作还设计了一种新型的低成本柔性可穿戴PEC传感器贴片,用于有效收集和持续监测汗液中的维生素C。该智能手机光驱动的无酶可穿戴PEC传感器可以准确地检测真实汗液中的维生素C浓度,这将有助于确保人体适当的营养平衡。原文链接:https://doi.org/10.1016/j.cej.2022.140779本文中进行电化学测试的仪器为荷兰PalmSens,型号:EmStat3Blue便携式电化学分析仪,由雷迪美特中国有限公司提供。需要了解更多电化学方面的知识,请关注我们或进入PalmSens中文网站:“http://www.palmsens.cn/”。ARTICLE IN PRESSChemical Engineering Journal xxx (xxxx) xxxChemical Engineering Journaljournal homepage: www.elsevier.com/locate/cej ARTICLE IN PRESSChemical Engineering Journal xxx (xxxx) xxxT. Yan et al. Contents lists available at ScienceDirect Smartphone light-driven zinc porphyrinic MOF nanosheets-basedenzyme-free wearable photoelectrochemical sensor for continuous sweatvitamin C detection Tingyi Yana, Guangyao Zhang a,l,, Kun Yu, Huining Chai, Mingwei Tian, Lijun Quar,Haifeng Dong,Xueji ZhangC aIntelligent Wearable Engineering Research Center of Qingdao, Research Center for Intelligent and Wearable Technology, College of Textiles and Clothing, QingdaoUniversity, Qingdao 266071, China DSchool of Environmental and Municipal Engineering, Qingdao University of Technology, Qingdao 266520, China CSchool of Biomedical Engineering, Health Science Center, Shenzhen University, Shenzhen 518060, China ARTICLEINFO ABSTRACT Keywords: Sweat vitamin C detection Zinc porphyrinic MOF nanosheets Wearable photoelectrochemical sensor Flexible patch Smartphone Maintaining normal vitamin C levels is essential for the proper functioning of the body’s immune system. Thedevelopment of real-time non-invasive wearable sensors for monitoring sweat vitamin C holds important promisein guiding personalized health management. Here, we present a smartphone light-driven enzyme-free wearablephotoelectrochemical (PEC) sensor based on two-dimensional zinc porphyrinic MOF nanosheets/multi-walledcarbon nanotubes (2D-TCPP(Zn)/MCNTs) for the precise monitoring of sweat vitamin C. 2D-TCPP(Zn) nano-sheets are prepared with the modulation of the pillared 4,4'-biphenyldicarboxylic acid (BPDC) molecules to formthe final square-like structure. After the introduction of MCNTs, the developed 2D-TCPP(Zn)/MCNTs modifiedscreen printing paper electrode exhibits an enhanced cathodic photocurrent response to vitamin C driven bysmartphone light due to the effective charge separation with electron injection from 2D-TCPP(Zn) to MCNTs. Alow limit of detection (LOD) of 3.61 uM and a wide detection range of 10-1100 uM for vitamin C are achieved.Also, the proposed electrode possesses high selectivity, satisfactory PEC stability and long-time storage stability.Moreover, a novel flexible PEC sensing patch was designed for the effective collection and continuous monitoringof sweat. The smartphone light-driven enzyme-free wearable PEC sensor detects the vitamin C concentration inreal sweat with good accuracy, which can help to ensure proper nutritional balance. 1. Introduction Vitamin C, also known as ascorbic acid (AA), contributes to healthygrowth of bones and blood vessels, promotes wound healing, and isindispensable for maintaining the proper functioning of the immunesystem [1,2]. Vitamin C deficiency results in scurvy, swollen joints,metabolic disorders, anemia and low immunity [3-6]. Unfortunately,the human body cannot synthesize vitamin C spontaneously and mustintake it via food and medications. Therefore, tracking vitamin C levelsin real time can help people supplement this important compoundtimely. Traditional assays are generally laboratory-based, directlyscreening biomarkers in blood samples, which is not only extremely timeconsuming, but invasive blood sampling needs to damage the skin, causing discomfort and potential cross-contamination risk [7]. As aresult, the desire to develop new technologies for in situ, continuous,noninvasive monitoring of vitamin C is growing. Recently, wearable biosensors are dedicated to extracting health-related information from analytes in body fluids such as blood, sweat,urine or tears [8-12]. In particular, sweat-based wearable biosensorshave received great attention for their real-time, non-invasive andcontinuous personalized health monitoring features. Moreover, sweatcontains the physiological information as rich as blood and is morereadily available than tears and urine, which has made wearable sweatsensors popular [13,14]. The reported wearable biosensors for vitamin Cmonitoring in sweat are mainly based on colorimetric and electro-chemical methods [15-17]. Rogers’ group has fabricated a skin- E-mail addresses: gyzhang@qdu.edu.cn (G. Zhang), lijunqu@qdu.edu.cn (L. Qu). These authors contribute equally to this work. https://doi.org/10.1016/j.cej.2022.140779 Received 8 August 2022; Received in revised form 28 November 2022; Accepted 1 December 2022Available online 5 December 2022 1385-8947/C 2022 Elsevier B.V. All rights reserved. interfaced microfluidic analysis and delivery patch for colorimetricdetection of sweat nutrients, including vitamin C [16]. This low-costwearable sensor has the advantage of simplicity of operation andrapid response, but its accuracy may be affected by subjective errorsowing to sweat impregnation. Sempionatto et al. achieved non-invasiveelectrochemical detection of sweat vitamin C by immobilizing ascorbateoxidase (AAOx) on a flexible printable tattoo electrode [18]. Suchwearable biosensors using electrochemical methods are favored due totheir high sensitivity and excellent accuracy. Nevertheless, electro-chemical sensors still have some problems, for example, high back-ground noise. Furthermore, enzyme-based electrochemical sensors aresensitive to environmental conditions, resulting in unstable signaloutput and high cost. As anextension0o1f theelectrochemical method1.,Pphoto-electrochemical (PEC) sensor is a promising analytical technique basedon a photoinduced electron transfer (PET) process between the analyte,the photoactive material, and the electrode [19]. This sensing modeseparates the excitation and detection signals, effectively reducing thebackground noise, thus making the PEC sensor ultrahigh sensitivity andexcellent repeatability. At present, PEC sensors have been widely used inthe detection of biological and environmental samples [20-22]. How-ever, limited by the introduction of excitation light sources, preparationof novel high-efficiency PEC activity materials and wearable conditionsrequirements, few studies have reported the construction of wearablePEC sensors for continuous monitoring of sweat. Metal-organic frameworks (MOFs), a class of porous crystallinematerials assembled from metals and organic ligands, have been widelyused in adsorption, separation, catalysis, sensing, diagnosis, etc.[23-25].Recently, they have also been reported in wearable biosensingresearch. Shu et al. developed a highly stretchable electrochemicalsensor based on Ni-Co MOF nanosheets decorated silver/reduced gra-phene oxide/urethane (Ag/rGO/PU) fiber for continuous monitoring ofglucose in sweat [26]. Moreover, MOFs displayed remarkable ability inPEC biosensing. Xu et al. proposed an anodic PEC sensing platform basedon meso-tetra(4-carboxyphenyl)porphyrin (TCPP) MOF for the deter-mination of ascorbic acid in serum [27], and our group constructed aTCPP MOF-based cathodic PEC biosensor for phosphate protein detec-tion and immunoassay [28,29]. As far as we know, the PEC behavior ofporphyrin MOFs is not invariant, Idan Hod’s group reported thatporphyrin MOFs may have both photoanodic and photocathodicbehavior, and the anodic-to-cathodic transition can be achieved bysimply changing the properties of the redox medium in the electrolyte[30]. While the development of a stable cathodic photocurrent sensingtechnique is necessary, owing to the fact that anodic photocurrents aresusceptible to interference from various reducing agents in real biolog-ical samples, which makes the signal response of the system unrealistic[31]. Herein, we present a smartphone light-driven zinc porphyrinicMOF nanosheets-based enzyme-free wearable photoelectrochemicalsensor for continuous sweat vitamin C detection, as shown in Scheme 1. Two-dimensional (2D) TCPP MOFs with Zn as the node (2D-TCPP(Zn))could generate electron-hole pairs stably under the stimulation of a lightsource. The significant photoelectric conversion ability was utilized topromote the oxidation reaction of vitamin C in sweat, enabling us toconstruct a vitamin C detection system. The electron transfer efficiencyin 2D-TCPP(Zn) was greatly improved with the introduction of multi-walled carbon nanotubes (MCNTs), and the developed 2D-TCPP(Zn)/MCNTs exhibited a satisfactory enhanced cathodic photocurrentresponse to vitamin C driven by smartphone light. Substrate materialsplay an important role in the design of wearable sensors (Table S1) [32],paper and fabric are considered as ideal substrates with the advantagesof low cost, flexibility, and hydrophilicity [33,34]. Accordingly, wefabricated a highly integrated paper electrode sensing patch (Fig. S1 andS2). Due to the strong water absorption ability of filter paper and non-woven fabric, sweat can spread rapidly along the axial direction,reducing the discomfort caused by the accumulation of sweat on thehuman-machine surface. Nowadays, people are increasingly insepa-rable from smartphones, and building a smartphone-based sensingplatform can streamline the testing process [35]. In our work, thesmartphone is the initiator of the excitation signal and the receiver of theresponse signal. As far as we know, this is the first time that asmartphone-based enzyme-free wearable sensor is combined with PECmethod. The experimental results suggest that our enzyme-free wear-able PEC sensor can accurately measure the concentration changes ofvitamin C in sweat, which is promising to be applied in personalizeddaily health management and guide people to make wise dietarychoices. 2. Experimental section. 2.1. Preparation of 2D-TCPP(Zn) nanosheets The 2D-TCPP(Zn) was synthesized based on the previous report withsome modifications [36]. Firstly, zinc nitrate (Zn(NO3)2,5 mg), TCPP (2mg), and 4,4'-biphenyldicarboxylic acid (BPDC, 2 mg) were ultrasoni-cally dissolved in 10 mL of N,N-dimethylformamide (DMF). Then, themixture was heated in an oil bath to 150°C for 1 h. After cooling to roomtemperature, a purple powdered sample was obtained via centrifuga-tion, washing, and drying. 2.2. Preparation of a paper electrode Screen printing templates were customized, and the electrodepattern was drawn using CorelDRAW software. Low resistance carboninks containing 7% diluent were printed on filter paper, used as workingelectrode and counter electrode, respectively.Ag/AgCl paste was usedas reference electrode. The filter paper was dried in a 50 °C oven for 10min after each printing. UV insulating oil was printed around the elec-trodes, front and back, then cured by irradiation with a mercury lamp for Scheme 1. Schematic diagram of smartphone light-driven enzyme-free wearable PEC sensor for sweat vitamin C detection. 2.3. Vitamin C detection 5 pL of 4 mg/mL 2D-TCPP(Zn) and 5 pL of 4 mg/mL MCNTs (ratio of1:1) were mixed and sonicated for 5 min, then spread on the surface ofworking electrode. The modified electrodes were dried in the oven at50°C for 1 h. Portable dark box that can be installed on mobile phonewas made from acrylic sheets. The opaque acrylic sheets (1 mm thick)were cut using laser scribe instrument with 60 % power and 20 % rate,repeat 4 times, the shape is drawn with CorelDRAW software. Acrylicsheets were glued together and dried at room temperature, and thesmartphone light intensity received on the electrode surface wasmeasured to be 2.5 W. The 0.1 M potassium chloride (KCl) solutioncontaining different concentrations of vitamin C was dropped onto thepaper electrode sensing area. The smartphone light source was turnedon 3 s and off 3 s for 40 s. Then calibrated the prepared vitamin C sensorthrough recording its photocurrent response. Selectivity was tested bycomparing the responses of 2D-TCPP(Zn)/MCNTs to 1 mM vitamin Cand interferents (uric acid (UA), lactic acid (LA), Nat, Ca, cysteine(Cys), glucose (Glc), dopamine (DA)) in the same concentration. Theanti-interference experiments of the prepared mixture were investigatedby sequential dropwise addition of vitamin C and interferents, andfinally adding vitamin C again. The stability of the photocurrentresponse was tested by continuously on-off light source with 3 s singleswitching duration for 170 s. The switching frequency and time werecontrolled via Flash bulb App. The prepared material was stored in arefrigerator at 4C when not in use, and the storage stability wasinvestigated by performing PEC measurements on the same concentra-tion of vitamin C every 3 days for 15 days. 2.4. Fabrication of a flexible patch sensor The flexible patch has five layers in total. The top and bottom layersare transparent PU film, which wraps the paper electrode layer and thesweat-absorbing non-woven fabric. Between the paper electrode layerand the sweat-absorbing layer, a ring-shaped PU film is added to preventsweat from wetting the wires. Small holes were printed on the top andbottom PU films using a laser scribe instrument for the transfer of sweatfrom the skin to the electrode surface with further flow and evaporation.All the pattern design was completed on CorelDRAW software. 2.5. Performance testing of flexible patch sensor The flexibility of developed sensor was evaluated by comparing thephotocurrent intensity changes after being subjected to compress, press,twist and stretch. To further explore the sensor’s ability to consistentlydetect vitamin C in sweat, the sweat flow process was simulated bycontinuously injecting 0.1 M KCl and 100 uM vitamin C alternately usinga syringe, and recorded the photocurrent responses after the potentialstabilized. 2.6. On-body measurements Three health volunteers (2 males, aged 24 and 28, 1 female, aged 24)were tested for vitamin C concentration in the sweat. The volunteersgave their full agreement to the presentation of the wearable PEC sensorand publishing test dates to the readers. After the subjects appeared tosweat enough for 30 min of running exercise, the patch sensor wasapplied to the upper extremity or chest to absorb sufficient sweat for 1min. Open the PStouch App, select the chronopotentiometry techniqueand wait for the open circuit potential to stabilize before starting themeasurement. Turn on the Flash bulb App to control the frequency andduration of the light. We selected vitamin C pills (200 mg), kiwi juice(600 mL) and orange juice (600 mL) to supplement the volunteers withvitamin C. The photocurrent responses of the sensor to sweat vitamin C were recorded at 30, 120 and 240 min after vitamin C intake. Allexperimental protocols were approved by the Scientific Ethics Com-mittee of Qingdao University. 3. Results and discussion 3.1. Characterization of the 2D-TCPP(Zn) 2D-TCPP(Zn) was obtained from the solvothermal reaction of TCPPwith Zn(NO3)2 and BPDC. As shown in Fig. 1A, zinc ions enter theporphyrin ring to make TCPP metallized. Meanwhile, the porphyrinligand is coordinated with the Zn(II) paddlewheel metal node(Zn2(COO)4, secondary structure unit) to form the 2D “checkerboard-style"nanolayer structure. According to our previous study [37], it isknown that the carboxyl functional group in BPDC can bind to zinc ionsselectively through coordination bonding. Therefore, we added BPDC asa regulator to control the growth of 2D MOF nanosheets to form a moreordered square sheet-like structure. The tendency of anisotropic growthof MOF was suppressed, and further determined as the space structure ofAB stacking mode by BPDC [36,38,39]. The morphology of the syn-thesized 2D-TCPP(Zn) was described via transmission electron micro-scopy (TEM, Fig. 1B) and scanning electron microscopy (SEM, Fig. S3A).It can be clearly observed that 2D-TCPP(Zn) is in the form of regularsquare sheets with length of 2.1 ±0.3 um. The corresponding elementalmapping exhibits that C, N, O, and Zn elements are uniformly distrib-uted in the 2D-TCPP(Zn) MOF nanosheets (Fig. 1C). The atomic forcemicroscopy (AFM) measurement indicated that the thickness of 2D-TCPP(Zn) MOF nanosheets is about 50 nm (Fig. 1D). High-resolutionTEM (HRTEM) images displayed inter-lattice distance of 1.63 nm andpore diameter of 1.18 nm on the surface of 2D-TCPP(Zn) MOF nano-sheets (Fig. 1E and F). The lattice distance is in exactly accordance withthe theoretical value calculated by the Bragg equation according to the(100) plane of the 2D-TCPP(Zn) crystal in the X-ray diffraction (XRD)spectrum (Fig. 1G) [36]. Also, easily noticed the broad diffraction peakon 18.13° corresponding to the (004) plane. The layer spacing of 0.48nm (interlayer distance of 0.96 nm), calculated using the Bragg equa-tion, approximately equal to the theoretical layer spacing of 0.93 nm forthe AB stacking mode [40]. The water stability of 2D-TCPP(Zn) MOFnanosheets was also explored, and comparing the XRD spectra beforeand after dispersion in deionized water and storage for one week(Fig. S4), the characteristic peaks did not change much, indicating thatour synthesized MOF nanosheets have excellent water stability, which ismainly attributed to the crystal stability of the prepared Zn node-basedMOF materials [36]. The surface area of 2D-TCPP(Zn) is shown to be243 m²/g as measured by N2 adsorption-desorption isotherms (BET)experiments (Fig. 1H). From the pore size distribution curves in inset ofFig. 1H, the pore diameter is about 1.2 nm, which correspond with thedata obtained in HRTEM. The other micropores may be slit-like poresdue to the low-temperature storage process of the MOF nanosheets [25].Moreover, as an electron acceptor, the participation of 02 is veryimportant for the PEC detection of vitamin C. As shown in Fig. S5, the O2adsorption quantity of 2D-TCPP(Zn)MOF nanosheets increasedcontinuously and linearly with pressure,reaching 0.135 mmol/g at 750mmHg, indicating that this regular porous structure provides a high gasstorage capacity [41], which is beneficial for the adsorption of 02molecules. Notably, it is difficult to observe a stable lattice structure inmost MOF materials due to the susceptibility to electron beam damageduring testing. However, this problem did not exist in our synthesizedMOFs, reflecting the superior stability of the 2D-TCPP(Zn) MOF nano-sheets. Additionally, thermogravimetric property (TGA) showed that the2D-TCPP(Zn) MOF nanosheets started to disintegrated at 400 °C(Fig. S6), indicating a good heat resistance, while the weight slowlydecreased before 400°C, probably due to the partial collapse of thepaddlewheel structure caused by water binding and thermally inducedamorphization in the MOF material [37]. We further investigated the binding mode of porphyrins to Zn(II) by A 名 Fig. 1. (A) Schematic representation of the synthesis of 2D-TCPP(Zn) nanosheets. (B) TEM of 2D-TCPP(Zn) nanosheets. (C) Elemental mapping images. (D) AFMimage. Inset: Thickness of the MOF nanosheet at the dotted line. (E) and (F) High-resolution TEM. (G) XRD patterns of the TCPP and 2D-TCPP(Zn). (H) N2adsorption-desorption isotherms. Inset: Pore size distribution curve of the prepared 2D-TCPP(Zn) nanosheets. (I) FT-IR spectra of TCPP and 2D-TCPP(Zn). (J)UV-visabsorption and fluorescence spectra of TCPP and 2D-TCPP(Zn) in ethanol. comparing the Fourier transform infrared (FT-IR) spectra of TCPP and2D-TCPP(Zn) (Fig. 1I). The spectrum of TCPP demonstrated absorptionpeaks around 3440 cm- and 3324 cm-, which are attributed to the O-H and N -H stretching vibrations of the carboxyl group. In contrast,these absorption peaks disappear in the spectrum of the synthesized 2D-TCPP(Zn), indicating that substitution of hydrogen protons by zinc ionsto form coordination bonds. And, the disappearance of the C=0stretching vibration at 1688 cmpoints out that the porphyrin’scarboxyl group had changed [42]. Additionally, two new peaks appear at 1590 and 1364 cm, which are due to the formation of a Zn-o bondby the coordination of the carboxyl group in TCPP with the metal [43].The disappearance of the plane vibration of N - H at 980 cm-1 and theappearance of a new peak at 1000 cm- suggests that the 2D-TCPP(Zn)belongs to metalloporphyrin [44,45]. The elemental composition andvalence states of the MOF material were studied using X-ray photo-electron spectroscopy (XPS, Fig. S7). The elements are mainly C, N, O,and Zn, which is consistent with the conclusion observed in the Mappingimages. Moreover, two major peaks are clearly visible in the XPS spectrum of Zn 2p at 1022 and 1045 eV, which confirms that the Zn(II)valence state is present in 2D-TCPP(Zn) [25]. This is also confirmed bythe findings of UV-vis absorption and fluorescence spectra (Fig. 1J). ASoret band absorption peak at 416 nm and four faint Q bands at 516,551,592 and 650 nm are observed in the spectrum of TCPP, which isexplained by the p-p* transitions from the lowest unoccupied molec-ular orbital (LUMO) to the highest occupied molecular orbital (HOMO)[46]. In the UV-vis absorption spectrum of 2D-TCPP(Zn) MOF nano-sheets, the Soret band at 421 nm, exhibited significant red shift due tothe formation of the MOF structure, which is attributed to the transferfrom the porphyrin ligand to the metal of electrons and layer aggrega-tion [36,47]. While the original four Q bands were reduced to two weakQ bands at 561 and 601 nm, confirming that zinc ions entered porphyrinring [25]. The fluorescence spectrum of 2D-TCPP(Zn) excited at 420 nmhas two strong emission peaks at 607 and 659 nm, which displayed blueshift compared to TCPP. It can be explained by the fact that the coor-dination between the porphyrin and zinc ions changed the energy levelof the lowest excited state, causing delocalized electrons to decrease[36]. This result indicated that 2D-TCPP(Zn) has a narrow HOMO-LUMO gap, which interested us in the study of the PEC properties. 3.2. PEC investigations The PEC mechanism of 2D-TCPP(Zn) was investigated using asmartphone flashlight as an excitation source. First, the dissolved 02effect on the PEC behavior of 2D-TCPP(Zn) is shown in Fig. 2A. Thephotocurrent response was barely observed in N2 saturated electrolytesolution. However, under the environment with the participation of O2,the cathodic photocurrent was gradually enhanced with the increase ofdissolved O2 concentration. This phenomenon suggests that O2 mole-cules play the role of electron acceptors in the porphyrin-based PECsystem, which is consistent with previous reports [29,48]. It is wellknown that carbon materials, especially CNT, have metal-like electricalconductivity, excellent flexibility, and are widely used in wearableelectrochemical sensing [49,50]. Therefore, we considered the intro-duction of MCNTs into the 2D-TCPP(Zn) PEC system to improve thephotocurrent signal output. The SEM of MCNTs is shown in Fig. S8. Aswe expected, 2D-TCPP(Zn)/MCNTs exhibited significant photocurrentswith 8.42 and 8.13 times stronger than 2D-TCPP(Zn) under air and O2-saturated conditions, respectively (Fig.2B). Moreover, as with the PECbehavior of 2D-TCPP(Zn), dissolved O2 can also greatly enhance thephotocurrent of 2D-TCPP(Zn)/MCNTs. It reveals that MCNTs do not Fig. 2. Photocurrent responses of (A) 2D-TCPP(Zn)and (B) 2D-TCPP(Zn)/MCNTs in N2, air and O2-satu-rated 0.1 M KCl solution. (C) Photocurrent responsesof 2D-TCPP(Zn), MCNTs and 2D-TCPP(Zn)/MCNTs in0.1 M KCl solution with the absence and presence of 1mM vitamin C. (D) Photocurrent responses of 2D-TCPP(Zn)/MCNTs in N2, air and O2-saturated 0.1 MKCl solution with the absence and presence of 1 mMvitamin C. (E) Effect of different O2 environment onthe increase ratio of photocurrent intensity of 2D-TCPP(Zn)/MCNTs. affect the PEC behavior of 2D-TCPP(Zn) while improving the photo-current output. Second, we comparatively studied the photocurrentresponse to vitamin C of three modified electrodes, 2D-TCPP(Zn),MCNTs, and 2D-TCPP(Zn)/MCNTs. As shown in Fig. 2C(a), thecathodic photocurrent of 2D-TCPP(Zn) increased from -7 nA to-35 nAafter the addition of vitamin C. The MCNTs displayed a large fluctuatingand unstable photocurrent signal to vitamin C (Fig. 2C(b)). In contrast,the photocurrent of 2D-TCPP(Zn)/MCNTs increased dramatically,reaching about -1 uA in the presence of vitamin C (Fig. 2C(c)),demonstrating that the combination of 2D-TCPP(Zn) and MCNTs pro-duced synergistic photocurrent enhancement effect. Furthermore, thephotocurrent response of other MOFs combined with MCNTs to vitaminC was measured. As shown in Fig. S9 and S10, the porphyrin MOFs (Al-TCPP, PCN-222, PCN-224) had a stronger photocurrent responsecompared to the non-porphyrin MOFs (ZIF-8, UiO-66, MOF-818), butweaker than the zinc porphyrin MOFs (Al-TCPP(Zn), PCN-222(Zn),PCN-224(Zn)), which may be attributed to the coordination of zincchanging the energy band structure of the porphyrins. The PEC prop-erties of other 2D metalloporphyrin MOFs (2D-TCPP(Cu), 2D-TCPP(Co),SEM see Fig. S3B and C) were also investigated, and the results indicatedthat 2D-TCPP(Zn)/MCNTs is the most suitable candidate for vitamin Cdetection among them. Meanwhile, we explored the effect of otherconductive materials (copper nanowires (Cu NWs), carbon black (CB),reduced graphene oxide (rGO), poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS), and MXene) on the PEC propertiesof 2D-TCPP(Zn), and the comparison showed that MNCTs is the bestchoice (Fig. S11). Moreover, we also examined the combination of MOFwith MCNTs by in-situ growth. As shown in Fig. S12, the obtainedcomposites exhibited irregular morphology, the distribution of MCNTson the MOF nanosheets was not uniform and the photocurrent responsewas not good enough. However, in this work, the MCNTs are only uti-lized for their conductivity and the simplest physical mixing can achievea better PEC behavior for vitamin C. In order to demonstrate the sig-nificance of constructing MOFs, we attempted to immobilize carboxyl-ated MCNTs on 5,10,15,20-tetrakis(4-aminophenyl)-porphyrin (TAPP) by amidation reaction, and the obtained TAPP/MCNTs exhibited poorphotocurrent response to vitamin C (Fig. S13), which is much less thanthat of 2D-TCPP(Zn)/MCNTs. This is due to the excellent air adsorptionability, abundant active sites, and narrower bandwidth of MOF nano-sheets. Finally, the influence of dissolved O2 in presence of vitamin Cwas further explored (Fig. 2D). The 2D-TCPP(Zn)/MCNTs did notexhibit any photocurrent response to vitamin C in the N2-saturatedenvironment, and the photocurrent intensity to vitamin C was signifi-cantly enhanced under air and O2-saturated conditions. We calculatedthe increase ratio of photocurrent intensity ((I-Io)/Io) after deducting theblank signal (Io). It can be observed that 2D-TCPP(Zn)/MCNTs demon-strated the strongest response to vitamin C under the air condition(Fig. 2E). As a result, the natural air environment is the best choice forPEC detection of vitamin C. In order to explain the above phenomenon, cyclic voltammetry (CV)and photovoltage-time curves of 2D-TCPP(Zn), MCNTs and 2D-TCPP(Zn)/MCNTs modified electrodes were investigated. The oxidationcurrent of 2D-TCPP(Zn) to vitamin C started from 0.128 V and the peakcurrent appeared at 0.446 V (Fig. 3A(a)). The MCNTs exhibited anobvious non-Faradaic current because of the high conductivity, with aninitial oxidation potential at -0.139 V and peak potential at 0.091 Vafter the addition of vitamin C (Fig. 3A(b)). The starting oxidation po-tential of the 2D-TCPP(Zn)/MCNTs electrode is at -0.119 V, which islower than that of the 2D-TCPP(Zn), attributed to the improved oxida-tion activity of vitamin C at the electrode interface by the MCNTs(Fig. 3A(c)). The photovoltage-time curves displayed the voltage fluc-tuations during the on-off photo-excitation (Fig.3B). The photovoltagevariations of MCNTs were not obvious, while 2D-TCPP(Zn) and 2D-TCPP(Zn)/MCNTs demonstrated regular photovoltage changes, whichindicated that MOF materials were more sensitive to the photo-excitation. Moreover, only the potentials of 2D-TCPP(Zn) and 2D-TCPP(Zn)/MCNTs lie between the initial oxidation potential and thepeak potential, and the stable photovoltage potential of 2D-TCPP(Zn)/MCNTs is about -0.073 V, which is closer to the oxidation peak po-tential than the other two materials, resulting in a stronger cathodic A B C Fig. 3. (A) CVs and (B) photovoltages generation of 2D-TCPP(Zn), MCNTs and 2D-TCPP(Zn)/MCNTs in the 0.1 M KCl with the absence and presence of 1 mMvitamin C. The CV scan rate was set at 50 mV/s. The photovoltage was tested in the on-off state with a 30 s on and 30 s off duration. (C) Nyquist plots of bare, MCNTs,2D-TCPP(Zn) electrode in 0.1 M KCl solution containing 5 mM [Fe(CN) ]4-/3-. Inset: Amplified curves of MCNTs and equivalent circuit. (D) Nyquist plot of 2D-TCPP(Zn)/MCNTs in 0.1 M KCl solution containing 5 mM [Fe(CN)。]4-/3-. Inset: Equivalent circuit. photocurrent response to vitamin C. This suggestes that our proposedcomposite electrode of MOF and MCNTs is capable of achieving a moreprominent photocurrent response under the excitation of white lightemitted from smartphone. The electron transfer ability of differentmodified electrodes was evaluated via Electrochemical impedancespectroscopy (EIS). As shown in Fig. 3C, Nyquist plots of bare, MCNTs,2D-TCPP(Zn) electrodes consist of a semicircular arc and a nearlystraight trailing line. The diameter of these semicircles represents thecharge transfer resistance (Rct), a very important parameter that revealsthe difficulty of the redox reaction of the ferricyanide probe at theinterface between the electrode and the electrolyte solution. The Retvalues of the bare and 2D-TCPP(Zn) electrodes were 581 and 2141 Q,while the impedance of the MCNTs electrode was about 37 Q (inset ofFig. 3C), demonstrating the poor electron transfer capability of 2D-TCPP(Zn) and outstanding conductivity of MCNTs. The Nyquist plot of 2D-TCPP(Zn)/MCNTs exhibited two different semicircular arcs in the highfrequency region (Fig. 3D), the former smaller one representing the Retat the electrode/MCNTs interface (22 Q) and the latter one indicatingthe Ret at the MCNTs/MOF interface (66 Q). In addition, we also com-bined the two semicircular arcs (red arcs) and the Ret value was calcu-lated as 81 Q, proving that MCNTs can greatly enhance the electrontransport capacity of MOF-modified electrode. To further clarify the mechanism of the enhanced cathodic photo-current generation, it is necessary to understand the intrinsic energyband structure of 2D-TCPP(Zn). Based on the UV-vis diffuse reflectancespectroscopy (DRS) measurement of 2D-TCPP(Zn) (Fig. 4A), the bandgap (Eg) of 2D-TCPP(Zn) is estimated to be 1.79 eV according to theKubelka-Munk function (inset of Fig. 4A) [51]. The UV-vis DRS ofMCNTs and 2D-TCPP(Zn)/MCNTs were also tested, as shown in Fig. S14, 2D-TCPP(Zn)/MCNTs exhibited similar reflectance as MCNTs, while thepeaks on the DRS curve of 2D-TCPP(Zn) were basically retained, indi-cating that the addition of MCNTs did not disrupt their energy band. Weperformed Mott-Schottky experiments on 2D-TCPP(Zn) modified elec-trode at 500, 1000 and 2000 Hz (Fig. 4B), showing that the flat bandpotential (Efb) is about -0.46 V vs Ag/AgCl (i.e., -0.27 V vs NHE). Thepositive slope of the tangent line suggested that the MOF material is ann-type semiconductor [46]. As is well known, the LUMO potential(ELUMO) of the n-type semiconductor is at least 0.1 V more negative thanits Efb, hence the ELuMo of 2D-TCPP(Zn) was calculated to be around-0.37 V [52,53]. The HOMO potential (EHoMo) is calculated to bearound 1.42 V according to the equation (EHOMO=Eg+ELUMO). On thebasis of the above analyses, a possible PEC mechanism is proposed inFig. 4C to explain the enhancement of the smartphone light-drivencathodic photocurrent activity of the 2D-TCPP(Zn)/MCNTs towardvitamin C. Specifically, under the smartphone light illumination, elec-tron transfer occurs in 2D-TCPP(Zn), generating electron-hole pairs. Thespatially separated electrons in LUMO are further transferred to themetal node Zn2(COO)4 by redox hopping chains between zincporphyrin-zinc clusters [54,55]. Then electrons are acquired by O2(-0.046 V) [56] whose redox potential is at a more positive position,converting 02 to 02. As an electron donor, the energy level of vitaminC (0.26V) [57] matches the ELUMO(-0.37 V) and EHoMo (1.42 V) of 2D-TCPP(Zn), filling the holes and leading to more electrons to occupy theLUMO of 2D-TCPP(Zn). Eventually, the cathodic photocurrent isgenerated synergistically by both band transport and redox hoppingpathways. On the other hand, the holes transferred to the counterelectrode through the external circuit, vitamin C is likewise oxidized andloses electrons at the counter electrode. Notably, the conductor-like Fig. 4. (A) UV-vis DRS of the 2D-TCPP(Zn). Inset: Calculation of the band gap of 2D-TCPP(Zn) based on the (ahw)-Eg curve. (B) Mott-Schottky plot for 2D-TCPP(Zn)in 0.5 M Na2SO4 aqueous solution, tested at 500, 1000, 2000 Hz, respectively. (C) Mechanism of the cathodic photocurrent generation based on 2D-TCPP(Zn)/MCNTs. MCNTs greatly promote electron transfer and enables easier oxidation ofvitamin C. The electrons lost by vitamin C are efficiently transported tothe dissolved o2 molecules, resulting in an enhanced cathodicphotocurrent. 3.3. Analytical performance of 2D-TCPP(Zn)/MCNTs The concentration ratio of MOF to MCNTs has a great influence onthe PEC sensing system of vitamin C. Both the background and theresponse photocurrents to vitamin C were enhanced as the ratioincreased, until reaching saturation at a ratio of 1:1 (Fig. S15A and B).This was attributed to the conductivity of electrodes increasing with theMCNTs ratio, as shown in Fig. S15C and D, the conductivity andphotocurrent response of the bare electrodes were poor but improvedwith the introduction of MCNTs. Thus, the concentration ratio of MOF/MCNTs of 1:1 was used in the subsequent PEC detection experiments ofvitamin C. Then, Fig. 5A displayed the increased photocurrent intensitywith increasing vitamin C concentration at the 2D-TCPP(Zn)/MCNTselectrode at open circuit potential. Two linear relationships exist between photocurrent intensity and vitamin C concentration withregression equations of y=0.009x+7.026(10-100pM) andy=0.077x- 0.787 (100-1100 uM), respectively (Fig. 5B). The limit of detection(LOD) was estimated to be 3.61 uM (signal-to-noise ratio of 3), whichcan be compared with the previously reported vitamin C sensor(Table S2). The excellent analytical performance of the paper electrodewas attributed to the outstanding PEC activity of 2D-TCPP(Zn)/MCNTstoward vitamin C. The complex composition of sweat may affect theaccuracy of the sensor in detecting vitamin C. Therefore, some possibleinterfering components (UA, LA, NaCl, CaCl2, Cys, Glc, DA) were tested.The results revealed that the sensor did not respond significantly to theseinterferents (Fig. 5C). Moreover, the anti-interference performance ofthe sensor was evaluated through successive drops of vitamin C andIVIthese interferents. As shown in Fig. 5D, the photocurrent response wasnot affected by the interferents, and significantly enhanced only afterthe addition of vitamin C. These results demonstrate that the proposedpaper-based PEC sensor possesses high selectivity and anti-interferencecapability that can be applied to the detection of vitamin C in real sweatsamples. No significant changes in photocurrent response of the sensor Fig. 5. (A) Photocurrent responses and (B) cali-bration plots for the detection of vitamin C by thePEC sensor. The concentrations of vitamin C are0,10,20,30,40,50,60,100,300,500,700,900,1100,1300 uM (from a to n). (C) Selectivity test:Including vitamin C, UA, LA, NaCl, CaCl2, Cys,Glc, DA. (D) anti-interference test: Continuousaddition of vitamin C, UA, LA, NaCl, CaCl2, Cys,Glc, DA and vitamin C. (E) Photocurrent responsestability test: The light source turns on for 3 s,turns off for 3 s, and lasts for 170 s. (F) Storagestability test: The same concentration of vitaminC was tested every 3 days for 16 days. were observed when tested in a 0.1 M KCl solution containing 100 uMvitamin C for 27 cycles using the smartphone light source switch(Fig. 5E), suggesting the satisfactory PEC stability for vitamin C detec-tion. Furthermore, the proposed PEC sensor was stored at 4°C and 100uM of vitamin C was measured every 3 days, and the results werenormalized with the data of the first day to describe its long-term sta-bility. The photocurrent remained at 95.8 % of the original responseafter 16 days (Fig. 5F), indicating that the constructed sensor has goodstorage stability and satisfactory reusability. 3.4. Characterization of flexible wearable PEC sensing patch To achieve wearable sweat sensing, we developed a wearable patchbased on the above paper electrode (Fig.6A). The patch consists of thepaper electrode, absorbent non-woven fabric and flexible transparentPU film. It is very small, with a diameter of 5 cm and thickness no morethan 0.5 mm, and is composed entirely of flexible materials, eliminatingthe discomfort of the human-machine contact interface. The structuralanatomy of the sensing patch is shown in Fig. 6B. The non-woven fabrichas no warp and weft threads, is very convenient to cut, and easy toshape, well suitable for the manufacture of wearable devices. Besides,non-woven fabric has stronger water absorption and storage capacitythan filter paper. Therefore, combining filter paper with non-wovenfabric can effectively allow sweat to flow inside the sensing patchwithout excessive structural design, making the fabrication processmore simplified. In order to evaluate the sweat collection ability of thepatch, we simulated a sweat flow experiment (Fig. S16 and Video S1).After dropping blue ink, the ink was observed to diffuse into the sweatabsorption layer within 5 s with the capillary effect of the filter paper.Then, red ink drops were added and, similarly, diffused into the sweatabsorption layer within 5 s and pushed the blue ink to spread around.This result revealed that the printed ultraviolet (UV) insulating oilsuccessfully generated a hydrophobic barrier that allowed for thedirectional transport of sweat in the hydrophilic channel (Fig. S17).Moreover, epidermal deformation is a necessary consideration forwearable sensors, which may affect the accuracy and stability of thesignal. Fig. 6C demonstrated the photographs of the patch attached tothe forearm under compression, compression, twisting and stretchingdeformation. Compared with the normal condition, the patch sensor that underwent the above deformations did not change much in photocur-rent response and resistance (Fig. 6D and Fig. S18), indicating that ourPEC sensing patch can perform reliably for vitamin C detection evenunder epidermal deformations. To examine the ability of the sensingpatch for continuous real-time detection of vitamin C, we simulatedsweat flow using sequential injections of artificial sweat in the presenceand absence of vitamin C. As shown in Fig. 6E, the resulting photocur-rent response signal presents periodic variations, which suggests that thesensing patch we developed eliminates any residual effects and provesits value in real-time sweat monitoring applications. To further explorethe ability of our sensor to cope with the complex and realistic sweatenvironment, we tested the effect of different temperature and pH on thePEC performance of the patch sensor, as shown in Fig. S19. In the normalhuman skin temperature range [58,59], these data changed insignifi-cantly with increasing temperature. The signal intensity of MOF nano-sheets decreased in alkaline environments, but the pH of sweat is usuallymoderately acidic to neutral, between 4.5 and 7.0 [59,60], thus thiseffect can be completely eliminated in practice by calibrating the data atdifferent pH values. 3.5. On-Body in situ sweat sensing results and analysis We further displayed in situ human sweat sensing system constructedbased on 2D-TCPP(Zn)/MCNTs. Fig. 7A presented that the Palmsensportable electrochemical analyzer and patch were fixed to the upper armof a volunteer with PU tape. The analyzer is paired and connected to asmartphone via Bluetooth communication function and the light fre-quency is controlled using a customized Flash bulb App. It is worthnoting that the split-screen function of the smartphone can operate twoApps on one screen, which is greatly convenient for users. We placed thesmartphone flashlight equipped with a portable dark box (Fig. S20) infront of the working area of the paper electrode and tested the photo-current at open-circuit voltage (Fig. 7B and Video S2). Three vitamin C-rich foods were selected for testing, including vitamin C pills, kiwi juice,and orange juice. After taking these foods, three subjects jogged at thesame speed to ensure that the exercise intensity was at the same level.30 min later, enough sweat samples were collected at the electrodes tostart the test and recorded by a smartphone. Then, another recordingwas made after 120 and 240 min, respectively. As shown in Fig.7C, the UV Insulating oil Sweat Fig. 6. (A) Photographs of the paper electrode patch. (B) Structural anatomy of paper electrode patch. (C) Photographs of the paper electrode patch attached to theskin under various mechanical deformations. (D) The photocurrent changes of the paper electrode patch in detecting 100 pM vitamin C after experiencing the abovedeformations 20 times respectively. (E) Continuous testing performance of the paper electrode patch: Photocurrent responses after continuous addition of 100 uMvitamin C and artificial sweat. Fig. 7. (A) and (B) Photographs of the real-time on-body evaluation of the patch sensor. (C) Evaluation of sweat vitamin C concentration changes using manu-factured sensors for 4 h after intake of 200 mg of vitamin C pills (a), 600 mL of kiwi juice (b), and 600 mL of orange juice (c), respectively. sweat vitamin C concentration of three subjects increased significantlyafter 120 min of vitamin C intake, while it dropped back to the initiallevel after 240 min. This indicates that our sensing patch successfullysensed the change of vitamin C status in sweat. The vitamin C content ofkiwi juice and orange juice is approximately 65.5 and 35 mg/100 mL[61,62], respectively. After conversion, the ratio of vitamin C intakefrom the three supplements is about 1:2:1, which coincides with the dataobtained from our sensor (Fig. S21). In addition, the results of the threesubjects did not exhibit too much difference, indicting the wide appli-cability of the wearable PEC sensor. 4. Conclusion In summary, we demonstrated a smartphone light-driven 2D-TCPP(Zn)/MCNTs-based enzyme-free wearable PEC sensor for highly selec-tive and sensitive monitoring of sweat vitamin C. 2D-TCPP(Zn) nano-sheets with square-like morphology were synthesized with theassistance of BPDC. With the excellent conductivity of MCNTs, the 2D-TCPP(Zn) modified electrode showed much better PEC activity towardvitamin C under natural air environment. The mechanism of enhancedcathodic photocurrent was proposed that vitamin C can effectivelysuppress the charge recombination of electron-hole pairs and improvethe photoelectric conversion efficiency. The quantification of vitamin Cwas available through cathodic photocurrent“signal-on”responses. 2D-TCPP(Zn)/MCNTs-modified paper electrodes also displayed outstandingselectivity and stability. Meanwhile, we designed a novel flexible sensorpatch, which is constructed orderly by paper electrode, absorbent non-woven fabric and transparent PU film. The paper electrode and thenon-woven fabric build a 3D sweat transmission system, and the strongwater absorption and storage capacity of the non-woven fabric achieveseffective collection and real-time continuous monitoring of sweat. Thewhole sensing patch is ultra-thin and maintains remarkable PEC per-formance after mechanical deformation. Furthermore, the sensing patchcan clearly reveal the change of vitamin C concentration in sweat afterintake vitamin C-rich food. Our enzyme-free vitamin C sensor realizesthe combination of PEC with smartphone-based wearable devices for the first time, which has promising application potential in developing low-cost sensors for health management and personalized medicine. Declaration of Competing Interest The authors declare that they have no known competing financialinterests or personal relationships that could have appeared to influencethe work reported in this paper. Data availability The authors do not have permission to share data. Acknowledgement The authors also acknowledge the National Natural Science Foun-dation of China (Nos. 22204089 and 21801158), Natural ScienceFoundation of Shandong Province (No. ZR2020QB092), the ChinaPostdoctoral Science Foundation (No. 2021M691689), Taishan ScholarProgram of Shandong Province in China, Youth Innovation Science andTechnology Plan of Shandong Province (2020KJA013), State Key Lab-oratory of Bio-Fibers and Eco-Textiles (Qingdao University,Nos.ZKT23,KF2020201, GZRC202025). The authors would like to thank theShiyanjia Lab (www.shiyanjia.com) for the TEM analysis and theInstrumental Analysis Center of Qingdao University for sample tests. Appendix A. Supplementary data Reagents and apparatus, experimental procedures, SEM, XPS of 2D-TCPP(Zn), optimization of detection conditions, real sample analysis,Table S1, Table S2, Video S1 and Video S2. Supplementary data to thisarticle can be found online at https://doi.org/10.1016/j.cej.2022.140779. ( [1 ] A. Magr i , G. Germano, A. Lorenzato, S. Lamba, R. Chila, M. Montone, V. Amodio,T. Ceruti, F. Sassi, S. Arena, S. Abrignani, M. D’Incalci, M. Zucchetti , F. DiNicolantonio, A. Bardelli, High-dose vitamin C enhances cancer immunotherapy, Sci. Transl . Med. 12 (532) (2020) eaay8707, h tt p s :// d o i .o rg / 1 0 . 1 126 / s c i tra n slme d .aay8707 . ) ( [2] K. Dhara, R.M. Debiprosad, Review on nanomaterials-enabled electrochemicalsensors for ascorbic acid detection, Anal. Biochem. 586 (2019), 113415, https : // doi . org / 10.10 1 6 / j. ab.201 9 .1 1 3415 . ) ( [3] C.J. Schorah, A. Newill, D.L. Scott, D.B. Morgan, Clinical effects of vitamin C inelderly inpatients with low blood-vitamin-C levels, Lancet 1 (8113) (1979) 403-405, https: // d o i.or g / 1 0.10 1 6 / s0140-67 3 6(79)90882- 1 . ) ( [4] P.C. Calder, A.C. Carr, A.F. Gombart, M. Eggersdorfer, Opt i mal nutritional statusfor a well-functioning immune system is an important factor to protect against viralinfections, Nutrients 12 (4) (2020) 1181 , h ttps: / / doi . o rg / 1 0.3390 /n u 1 2041 1 81 . ) ( [5] M. Gleeson, D.C. Nieman, B.K. Pedersen, Exercise, nutrition and immune function, J. Sport . Sci. 22 (1) (2004) 115-125, https :/ / doi . org / 10.1080 / 02640410 3 1000140590 . ) ( [6] K. Norman, C. Pichard, H. Lochs, M. Pirlich, Prognostic impact of disease-related malnutrition, Clin. Nutr . 27 (1) (2008) 5-15, ht t p s:// doi .o rg / 1 0.10 1 6/ j . c l n u. 2007 . 10 . 007 . ) ( [7] U. Holler, S.J.L. Bakker, A. Diisterloh, B. Frei, J. Kohrle, T. Konz, G. Lietz,A. McCann, A.J. Michels, A.M. Molloy, H. Murakami, D. Rein, W.H.M. Saris,K. Schmidt, K. Shimbo, S. Schumacher, C. Vermeer, J. Kaput, P. Weber,M. Eggersdorfer, S. Rezzi, Micronutrient status assessment in humans: current methods of analysis and future trends, TrAC-Trends Anal . Chem. 102 (2018)110-122, ht t ps :// doi . or g/ 1 0.10 1 6/ j. tr a c . 201 8 .02.0 0 1 . ) ( [8] Y. Jiang, T. Xia, L. Shen, J. Ma, H. Ma, T. Sun, F. Lv, N. Zhu, Facet-dependent Cu2O electrocatalysis for wearable enzyme-free smart sensing, ACS Catal. 11 (5) (2021)2949-2955, https: // d o i . or g/ 10.1021 / ac s cat a l . 0c04797 . ) ( [9] J. Kim, A.S. Campbell , B.E. de Avila, J. Wang, Wearable biosensors for healthcaremonitoring, Nat. Biotechnol. 37 (4) (2019) 389-406, http s:// doi . org / 10.10 3 8 / s 4158 7 -01 9 - 0 045 - y . ) ( [10] P.Q. Nguyen, L.R. Soenksen, N.M. Donghia, N.M. Angenent-Mari, H. de Puig,A. Huang, R. Lee, S. Slomovic, T. Galbersanini, G. Lansberry, H.M. Sallum, E.M. Zhao, J.B. Niemi, J.J. Col l ins, Wearable materials with embedded syntheticbiology sensors for biomolecule detection, Nat. Biotechnol. 39 (11 ) (2021) 1366-1374, https : / / do i . org / 10.1038/s41587 - 021 - 00950-3 . ) ( [11] F . Te h r an i , H . T ey mour i a n, B. Wue rs tle , J . K a vner , R . Pat e l , A . F urmi d g e, R. Agha va li , H . H osse in i- Toudeshki , C . Brow n , F . Z ha n g, K . Mahato , Z . L i , A. B a rfi d okht , L . u . Yin, P . Warren, N . Huang, Z . P a tel, P . P . M erc i er , J. Wang , A n i n te gr a t ed w ea r a ble mi croneedle a r r a y f o r the c ont i nu o us m onit o ri ng o f m ultip l e bioma r kers in interst i t i al fl u id, N a t . Biomed . E ng . 6 (11) (2022) 1 214 -1 22 4. ) ( [12] X. Jin, G. Li, T. Xu, L. Su, D. Yan, X. Zhang, Fully integrated flexible biosensor forwearable cont i nuous glucose monitoring, Biosens. Bioelectron. 196 (2022), 113760, http s : // doi . or g/ 10.1016 /j .bios . 2021 . 1 1 3 760 . ) ( [13] M. Bariya, H.Y.Y. Nyein, A. Javey, Wearable sweat sensors, Nat. Electron. 1 (3) (2018) 160-171 , ht tp s : / / doi . o r g/ 10 . 1038 /s 41928 - 018-0043-y . ) ( [14 ] W. Gao, S. Emaminejad, H.Y.Y. Nyein, S. Challa, K. Chen, A. Peck, H.M. Fahad,H. Ota, H. Shiraki, D. Kiriya, D.H. Lien, G.A. 1 Brooks, R.W. Davis, A. Javey, Fullyintegrated wearable sensor arrays for multiplexed in situ perspiration analysis, Nature 529 (7587)(2016)50-514, h t t ps :// doi .o r g/ 1 0. 1 0 3 8 / n a ture1 6 521 . ) ( [15] M. Bariya, L. Li, R. Ghattamaneni, C.H. Ahn, H.Y.Y. Nyein, L .C. Tai, A. Javey,Glove-based sensors for multimodal monitoring of natural sweat, Sci. Adv. 6 (35) (2020) eabb8308, h ttp s :// do i .or g/ 10. 1 1 26 /s ci a dv . a bb8 3 08 . ) ( [16] J . Kim, Y . Wu , H . Lu a n, D. S. Ya n g, D . C ho, S.S . Kwa k , S . Liu, H. R yu , R . Ghaffari , J . A . Rogers , A s kin- i n terf a ced, mi n i a tur i z ed mic r ofluidic a na l ysi s a nd deliv e ry 1 - 1 1 1 s y s tem f o r color i m etr i c m e a surement s o f nut r i e nt s in s w e a t an d s up p l y o f v ita mi n s thr o ug h the s k i n , Adv . S ci . 9 ( 2 ) ( 2 022) e2 103331 . ) ( [17] J.Zha o, H .Y . Y . Ny e i n , L . H ou , Y . Lin, M . Bariya , C . H. A hn, W. J i, Z . F a n, A . Javey , A w ea r a ble n utrit io n tracker , Adv . M a ter . 33 (1 ) ( 2 02 1 ) e 2 0 0 6444 . ) ( [18] ]J.R. Sempionatto, A.A. Khorshed, A. Ahmed, E.S.A.N. De Loyola, A. Barfidokht,L. Yin, K.Y. Goud, M.A. Mohamed, E. Bailey, J. May, C. Aebischer, C. Chatelle, J. Wang, Epidermal enzymatic biosensors for sweat vitamin c: toward personalized nutrition, ACS Sens. 5 (6) (2020) 1804-1813, http s : / / d o i . or g/1 0.1021 / acssensor s. Oc00604 . ) ( [19] W.-W. Zhao,J.-J. Xu, H.-Y. Chen, Photoelectrochemical bioanalysis: the state of the art , Chem. Soc. Rev . 44 (3) (2015) 729-741 , ht tp s : / / d oi . org / 10.1 0 3 9/ C4CS0 0 228 H . ) ( [20] A. Victorious, S. Saha, R. Pandey, L. Soleymani, Enhancing the sensitivity ofphotoelectrochemical DNA biosensing using plasmonic DNA barcodes anddifferential signal readout, Angew. Chem. Int. Ed. 60 (13) (2021)7316-7322, http s:// d o i . o r g / 10.1002 / a nie . 202014329 . ) ( [21] C . Wang, Y . W a ng, K . O . Kir l i k o v a l i, K. M a , Y . Z hou, P. Li, O . K . F a r ha , Ultrafi n e s ilver nanopar t ic l e enc a psu l a ted porous molec u l a r tr a ps for discrimi n ative photoe l ect r o c h emic a l detect i on o f must a rd ga s s imul a nt s by s y n ergi s t ic size - e x c l u s i o n a n d s i t e - s p ec i f i c r eco gn i t i o n, A d v . Mat e r . 3 4 ( 35 ) ( 2 02 2 ) e 2 202287 . ) ( [22] J . Shu, D. Tang, Recent advances in photoelectrochemical sensing: from engineeredphotoactive materials to sensing devices and detection modes, Anal . Chem. 92 (1) (2020) 363 - 377, htt p s :// d o i .o rg / 10.1021 / a c s. a nalchem.9 b 041 9 9 . ) ( [23] Z. Zhou, S. Mukherjee, S. Hou, W. Li, M. Elsner, R.A. Fischer, Porphyrinic MOF filmfor multifaceted electrochemical sensing, Angew. Chem. Int. Ed. 60 (37) (2021) 20551-20557, ht t ps: // doi .o r g/ 10.1002 / a nie.202107860 . ) ( [24 ] Y. Zhong, B. Cheng, C. Park, A. Ray, S. Brown, F. Mujid, J.U. Lee, H. Zhou, J. Suh,K.H. Lee, A.J. Mannix, K. Kang, S.J. Sibener, D.A. Muller, J. Park, Wafer-scale ) ( synthesis of monolayer two-dimensional porphyrin polymers for hybridsuperlattices, Science 366 (6471) (2019) 1379-1384, h ttps :// d o i .o rg / 1 0.1126 / sc i enc e. aax9385 . ) ( [25] Y. Zhao, J. Wang, R. Pei, Micron-sized ultrathin metal-organic framework sheet, J . Am. Chem. Soc. 142 (23) (2020) 10331-10336, h t t p s: / / d o i . org /1 0 . 1 021 / ia c s . Oc04442 . ) ( [26] Y. Shu, T. Su, Q. Lu, Z. Shang, Q. Xu, X. Hu, Highly stretchable wearableelectrochemical sensor based on Ni-Co MOF nanosheet-decorated Ag/rGO/PU fiberfor continuous sweat glucose detection, Anal. Chem. 93 (48) (2021 ) 16222-16230, h ttps ://d oi . org / 10. 1 02 1/a cs .a nalc h em. 1 c0 4 106 . ) ( [27] X. Xu, C.-H. Li, H. Zhang, X.-M. Guo, Construction of electrochemical andphotoelectrochemical sensing platform based on porphyrinic metal-organicframeworks for determination of ascorbic acid, Nanomaterials 12 (3)(2022), h ttps : //d oi . org / 10.3390 / n a n o 12030 4 8 2 . ) ( [28] G. Zhang, D. Shan, H. Dong, S. Cosnier, K.A. Al-Ghanim, Z. Ahmad, S. Mahboob,X. Zhang, DNA-mediated nanoscale metal-organic frameworks for ultrasensi t ivephotoelectrochemical enzyme-free immunoassay, Anal. Chem. 90(20)(2018) 12284-12291, https : // d o i . o rg/ 1 0 . 1021 / acs . an a lch e m.8b03 7 6 2 . ) [29] G. Zhang, Y. Zhuang, D. Shan, G. Su, S. Cosnier, X. Zhang, Zirconium-basedporphyrinic metal-organic framework (PCN-222): enhanced photoelectrochemicalresponse and its application for label-free phosphoprotein detection, Anal. Chem.88 (22) (2016) 11207-11212, https://doi.org/10.1021/acs.analchem.6b03484. ( [30] R. Ifraemov, R. Shimoni, W. He, G. Peng, I . Hod, A metal-organic framework filmwith a switchable anodic and cathodic behaviour in a photo-electrochemical cell,J. Mater. Chem. A 7 (7) (2019) 3046-3053, h t tps :// doi .o rg / 10.10 3 9 / c8ta10 4 8 3 b. ) ( [31] Y.-T. Xu, S.-Y. Yu, Y.-C. Zhu, G.-C. Fan, D.-M. Han, P. Qu, W.-W. Zhao, Cathodic photoelectrochemical bioanalysis, TrAC Trends Anal. Chem. 114 (2019) 81-88, https: // doi . org / 10. 1 016 /j. trac.20 1 9.03 . 002 . ) ( [32] T. Yan, G. Zhang, H. Chai, L. Qu, X. Zhang, Flexible biosensors based oncolorimetry, f luorescence, and electrochemistry for point-of-care testing, Front.Bioeng. Biotechnol. 9 (2021), 753692, h ttps :/ / do i .org / 1 0 . 33 89 / fbioe . 2021 . 75 3692 . ) ( [33]I H. Yu, X. Tan, S. Sun, L. Zhang, C. Gao, S. Ge, Engineering paper-based visiblelight-responsive Sn-self doped domed SnO2 nanotubes for ultrasensitivephotoelectrochemical sensor, Biosens. Bioelectron. 185 (2021),113250, https: // doi . org /10 . 1 0 16 /j. b i os .2 021 .1 1 3 250 . ) ( [34] X. Liu, P.B. Lillehoj, Embroidered electrochemical sensors for biomoleculardetection, Lab Chip 16 (11 ) (2016) 2093-2098, ht t ps :// doi . or g / 10 . 10 3 9 / c6lc 0 0307 a . ) ( [35]F K. Yu, M. Li , H. Chai, Q. Liu, X. Hai, M. Tian, L. Qu, T. Xu, G. Zhang, X. Zhang,MOF-818 nanozyme-based color i metric and electrochemical dual-mode smartphone sensing platform for in situ detection of H202 and H2S released from living cells, Chem. Eng. J. 451 (2023), 138321 , h ttp s:// d o i . org / 1 0 . 1 0 1 6/j . cej . 2022 . 138321 . ) ( [36] Y. Zhao, L. Jiang, L. Shangguan, L. Mi, A. Liu, S. Liu, Synthesis of porphyrin-basedtwo-dimensional metal-organic framework nanodisk with small size and fewlayers, J. Mater. Chem. A 6 (6) (2018) 2828-2833, htt p s: // doi .o rg / 10.103 9/ c7ta07911 g. ) ( [37] K. Yu, G. Zhang, H. Chai , L. Qu, D. Shan, X. Zhang, Two-stage ligand exchange i nMn(III)-based porphyrinic metal-organic frameworks for fluorescence watersensing, Sens. Actuators. B-Chem. 362 (2022), 131808, h t tps : // do i . o r g / 10.1016 / j . sn b.2022 . 131808 . ) ( [38] E.Y. Choi, P.M. Barron, R.W. Novotny, H.T. Son , C. Hu, W. Choe, Pillaredporphyrin homologous series: intergrowth i n metal-organic frameworks, Inorg Chem 48 (2) (2009) 426-428, h t t p s :/ /d oi . org / 10. 1 0 2 1/ i c801677y . ) ( [39] F. Cao, M. Zhao, Y. Yu, B. Chen, Y. Huang, J. Yang, X. Cao, Q. Lu, X. Zhang,Z. Zhang, C. Tan, H. Zhang, Synthesis of two-dimensional CoS1.097/nitrogen-dopedcarbon nanocomposites using metal-organic framework nanosheets as precursorsfor supercapacitor application, J . Am. Chem. Soc. 138 (22) (2016) 6924-6927, h ttps: //d o i . o rg / 10. 1 021 / ja cs.6b02540 . ) ( [40] E.-Y. Choi, C.A. Wray, C. Hu, W. Choe, Highly tunable metal-organic frameworkswith open metal centers, CrystEngComm 11 (4) (2009) 553-555, ht t ps :/ /do i.org / 10 . 1039 / B819707 P . ) ( [41 ] H. Liu, L. Yang, D. Yuan, G. Liu, J. Xing, Y. Xu, Z. Liu, Mixed-ligand metal-organicframeworks with coordinatively un-saturated Co(II) and Ni(II) sites for regenerableO2-selective ad-sorption over N2, Chem. Eng. J. 450 (2022),138214, https :// doi . org / 10.1016 /j .c e j . 2022 . 1 3821 4 . ) ( [42] J. Ma, W. Bai, X. Liu, J. Zheng, Electrochemical dopamine sensor based on bi-metallic Co/Zn porphyrin metal-organic framework, Microchim. Acta 189 (1) (2021) 20, h ttps: / / doi . org / 10.1007 / s00604 - 021 - 05 1 2 2-3 . ) ( [43] J. Ma, W. Bai, J. Zheng, Non-enzymatic electrochemical hydrogen peroxide sensingusing a nanocomposite prepared from silver nanoparticles and copper (II)- porphyrin derived metal-organic framework nanosheets, Microchim. Acta 186 (7) (2019) 482, htt ps:/ / d o i . org / 1 0 .1 0 07 /s 00 6 04 - 01 9- 3 551-1 . ) ( [44]I R. Makiura, S. Motoyama, Y. Umemura, H. Yamanaka, O. Sakata, H. Kitagawa,Surface nano-architecture of a metal - organic framework, Nat. Mater. 9 (7) (2010) 565-571 , https: / / d o i .o rg / 10.10 3 8/ n mat 27 69 . ) ( [45] M. Jian, H. Liu, T . Williams, J. Ma, H. Wang, X. Zhang, Temperature-inducedoriented growth of large area, few-layer 2D metal-organic framework nanosheets, Chem. Commun. 53 (98) (2017)13161-13164, https: // do i . org / 10.1039 / C7CC06988 J. ) ( [46] H.-Q. Xu, J. Hu, D. Wang, Z. Li, Q. Zhang, Y. Luo, S.-H. Yu, H.-L. Jiang, Visible-lightphotoreduction of CO2 in a metal-organic framework: boosting electron-holeseparation via electron trap states, J. Am. Chem. Soc. 137 (42)(2015) 13440-13443, htt p s: // doi . or g/ 10.1021 /jac s.5 b 08773 . ) ( [47] N.C. Maiti , S. Mazumdar, N. Periasamy, J-and h-aggregates of porphyrin-surfactant complexes: time-resolved f luorescence and other spectroscopic studies,J. Phys. Chem. B 102 (9) (1998) 1528-1538, htt p s : // d o i . org / 1 0.1021 / jp9723 3 72 . ) ( [48] Z.W. Jiang, Y.C. Zou , T.T. Zhao, S.J. Zhen, Y.F. Li, C.z. Huang, Controllablesynthesis of porphyrin-based 2D lanthanide metal-organic f rameworks withthickness- and metal-node-dependent photocatalytic performance, Angew. Chem. Int. Ed. 59 (8) (2020)3300 - 3306, https : // doi . org / 10.100 2/ anie . 2019 1 3748 . ) ( [49] Z. Yin, X. Liang, K. Zhou, S. Li, H. Lu, M. Zhang, H. Wang, Z. Xu, Y. Zhang,Biomimetic mechanically enhanced carbon nanotube f ibers by silk fibroin infiltration, Small 17 (19) (2021 ) 2100066, https : / / d oi .o rg / 1 0.1002 /s ml l.2 02 1 0 0 066 . ) ( [50] ]J . Lv, L. Yin, X. Chen, I. Jeerapan, C.A. Silva, Y. Li , M. Le, Z. Lin, L. Wang,A. Trifonov, S. Xu, S. Cosnier, J. Wang, Wearable biosupercapacitor: harvestingand storing energy from sweat, Adv. Func t . Mater. 31 (38) (2021)2102915, ht t ps : / / d o i . o r g / 1 0.1002 /a d fm.202102915 . ) ( [51] X. Li, Y. Pi, Q. Xia, Z. Li, J. Xiao, TiO2 encapsulated in Salicylaldehyde-NH2-MIL-101(Cr ) for enhanced visible light-driven photodegradation of MB, Appl. Catal., B- Environ. 191 (2016) 192-201, h t tps :/ / d oi .o r g / 10 . 1 0 16 / j. ap c a tb.2016.0 3 . 0 3 4 . ) ( [52] Y. Zhao, Y . Dong, F. Lu, C. Ju, L. Liu, J. Zhang, B. Zhang, Y. Feng, Coordinative integration of a metal-porphyrinic framework and TiO2 nanoparticles for theformation of composite photocatalysts with enhanced visible- l ight-driven photocatalytic activities, J. Mater. Chem. A 5 (29)(2017) 15380-15389, h ttp s : // doi . org / 10.1039 / c7t a 03840b . ) ( [53] J. Du, S. Ma, H. Liu, H. Fu, L. Li, Z. Li, Y. Li, J. Zhou, Uncovering the mechanism ofnovel AgInS2 nanosheets/TiO2 nanobelts composites for photocatalyticremediation of combined pollution, Appl. Catal. B-Environ. 259 (2019), 118062, https: //d oi . o rg / 10.1016 /j . a pcatb.20 1 9.118062 . ) ( [54] Z. Zhao, J. Bian, L. Zhao, H. Wu, S. Xu, L. Sun, Z. Li, Z. Zhang, L. Jing, Constructionof 2D Zn-MOF/BiVO4 S-scheme heterojunction for efficient photocatalytic CO2 conversion under visible light irradiation, Chin. J . Catal. 43 (5) (2022)1331-1340, https :// d o i . o r g / 10 . 1016 /s 1872 - 2067( 2 1 )64005 - 6 . ) ( [55] S.R. Ahrenholtz, C.C. Epley, A.J. Morris, Solvothermal preparation of anelectrocatalytic metalloporphyrin MOF thin f ilm and its redox hopping charge- U IPH transfer mechanism, J. Am. Chem. Soc. 136 (6)(2014)2464-2472, https :// doi . o rg / 10.102 1/ j a410684 q . ) ( [56]N M. Xu, L. Han, S. Dong, Facile fabrication of highly efficient g-C3N4/Ag2Oheterostructured photocatalysts with enhanced visible-light photocatalytic activity, ACS Appl. Mater. Inter.5 (23) (2013) 12533-12540, https : // doi . org / 10 . 1 0 2 1 /a m40 3 8307 . ) ( [57] S. Bilal, A. Akbar , A.A. Shah, Highly selective and reproducible electrochemicalsensing of ascorbic acid through a conductive polymer coated electrode, Polymers (Basel ) 11 (8) (2019) 1346, htt p s :// d o i . or g/ 10. 339 0 / polym1108 1 3 46 . ) ( [58] F.G. Benedict , W.R. Miles, A. Johnson, The temperature of the human skin, Proc.Natl. Acad. Sc i . U.S.A. 5 (6) (1919) 218 - 222, h t tps : // d o i .o rg /10 . 1 07 3/ p na s . 5 .6.218 . ) [59]A. Wiorek, M. Parrilla, M. Cuartero, G.A. Crespo, Epidermal patch with glucosebiosensor: pH and temperature correction toward more accurate sweat analysisduring sport practice, Anal. Chem. 92 (14) (2020) 10153-10161,https://doi.org/10.1021/acs.analchem.0c02211. [60] A.J. Bandodkar, V.W.S. Hung, W. Jia, G. Valdes-Ramirez, J.R. Windmiller, A.G. Martinez, J. Ramirez, G. Chan, K. Kerman, J. Wang, Tattoo-based potentiometricion-selective sensors for epidermal pH monitoring, Analyst 138 (1) (2013)123-128, https://doi.org/10.1039/C2AN36422K. [61] K. Stos, B. Przygoda, M. Jarosz, E. Matczuk, B. Groele, S. Skapska, J. Markowski,Oranges as fruit and as juice, Proc. Nutr. Soc. 79 (OCE2) (2020) E711, https://doi.org/10.1017/s0029665120006606. ( [62]I I. Nishiyama, Y. Yamashita, M. Yamanaka, A. Shimohashi, T. Fukuda, T. Oota, Varietal difference in vitamin C content in the fruit of kiwifruit and other actinidia species, J. Agric. Food Chem. 52 (17)(2004)5472-5475, h ttp s : // doi . org / 1 0 .1021 / j f049398z . ) Please cite this article as: Tingyi Yan, Chemical Engineering Journal, https://doi.org/j.cej.
确定







还剩10页未读,是否继续阅读?
雷迪美特中国有限公司为您提供《【EmStat3Blue电化学应用】基于锌卟啉MOF纳米片的智能手机光驱动的无酶可穿戴光电化学传感器,用于汗液维生素C检测》,该方案主要用于其他中生化检验检测,参考标准--,《【EmStat3Blue电化学应用】基于锌卟啉MOF纳米片的智能手机光驱动的无酶可穿戴光电化学传感器,用于汗液维生素C检测》用到的仪器有EmStat4X便携式电化学分析仪
推荐专场
该厂商其他方案
更多
















