方案详情
文
本文报道了先进的纳米酶生物传感器,能够无创地同时监测糖尿病和缺氧。用核壳普鲁士蓝-六氰基高铁酸镍纳米酶浸渍涂层可产生稳定和灵敏的过氧化氢传感器。所得生物传感器的最佳性能特性是由直径为50 nm的纳米颗粒提供的,该纳米颗粒包含35–37 nm(ø)普鲁士蓝核。基于流通式多生物传感器,通过连续汗液分析操作的无创监测仪,用于同时检测葡萄糖和乳酸。安装在人体皮肤表面的特制葡萄糖乳酸盐监测仪,可直接测量未稀释人体汗液中葡萄糖和乳酸盐的真实浓度。结合已开发的生物传感器应用于可穿戴设备,显然将为缺氧和血糖的无创连续监测开辟新的视野。
方案详情

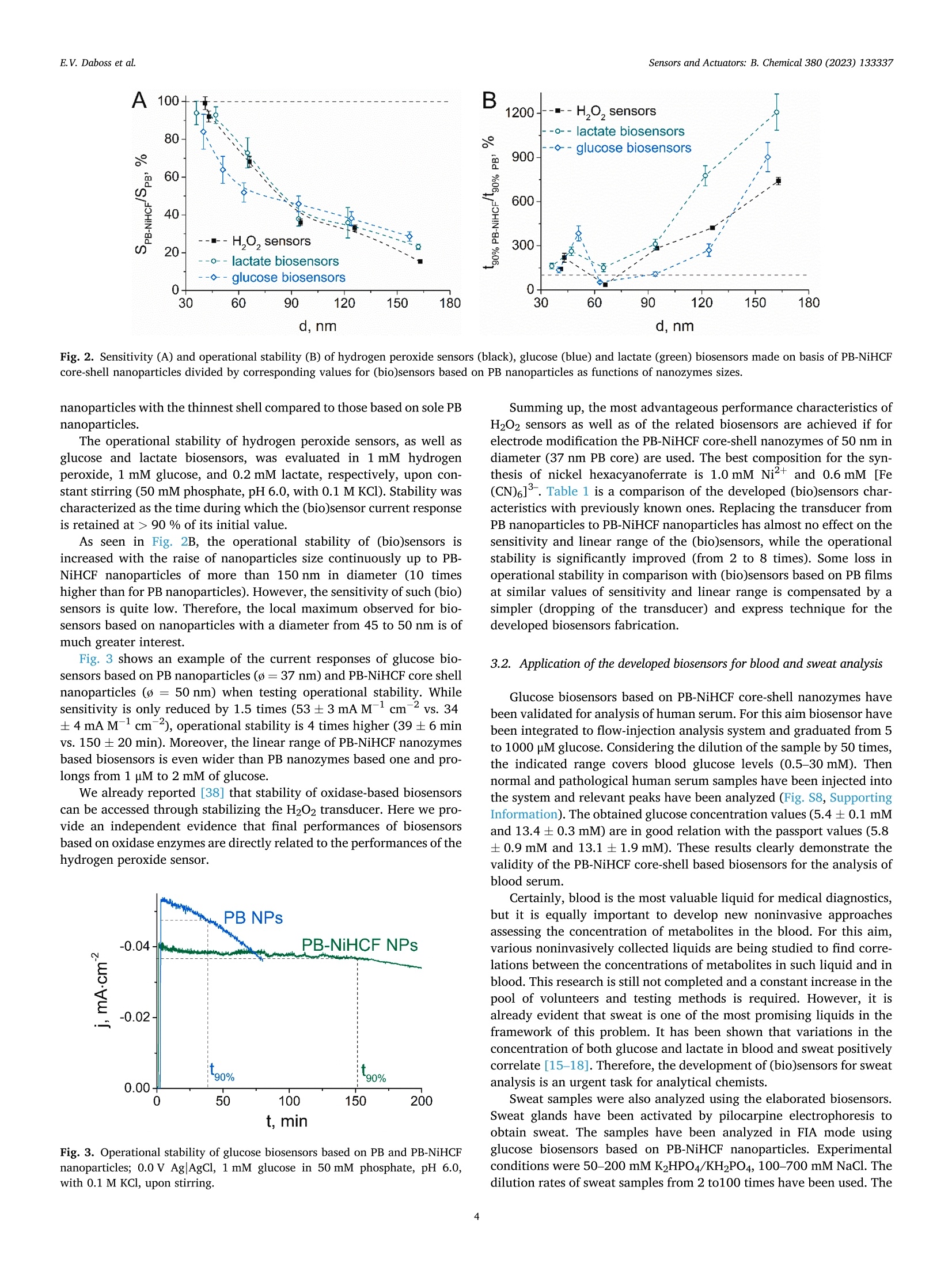
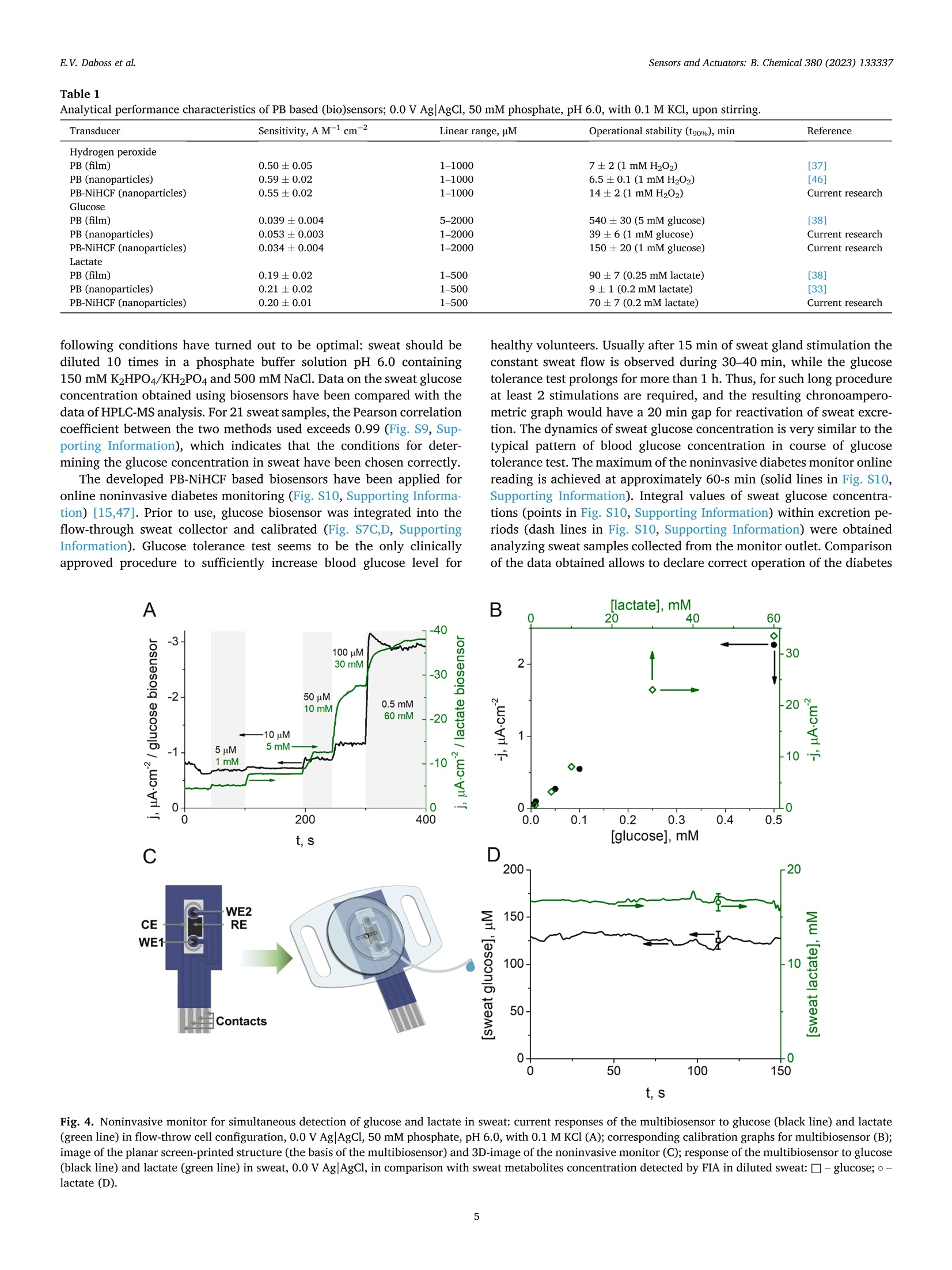
本文报道了先进的纳米酶生物传感器,能够无创地同时监测糖尿病和缺氧。用核壳普鲁士蓝-六氰基高铁酸镍纳米酶浸渍涂层可产生稳定和灵敏的过氧化氢传感器。所得生物传感器的最佳性能特性是由直径为50 nm的纳米颗粒提供的,该纳米颗粒包含35–37 nm(ø)普鲁士蓝核。基于流通式多生物传感器,通过连续汗液分析操作的无创监测仪,用于同时检测葡萄糖和乳酸。安装在人体皮肤表面的特制葡萄糖乳酸盐监测仪,可直接测量未稀释人体汗液中葡萄糖和乳酸盐的真实浓度。结合已开发的生物传感器应用于可穿戴设备,显然将为缺氧和血糖的无创连续监测开辟新的视野。--摘自ScienceDirect Sensors & Actuators: B. Chemical 380 (2023) 133337原文链接:https://doi.org/10.1016/j.snb.2023.133337Sensors &Actuators: B.Chemical 380 (2023) 133337 Contents lists available at ScienceDirect Sensors and Actuators: B. Chemical journal homepage: www.elsevier.com/locate/snb Simultaneous noninvasive monitoring of diabetes and hypoxia using core-shell nanozyme – oxidase enzyme biosensors Elena V. Daboss*, Elizaveta V. Shcherbacheva, Arkady A. Karyakin Chemistry Faculty of M.V. Lomonosov Moscow State University, 119991 Moscow, Russia Keywords: Biosensor Core-shell nanozyme Prussian Blue Nickel hexacyanoferrate Glucose Lactate A B S T R A C T We report on advanced nanozyme-enzyme biosensors enabling noninvasive simultaneous monitoring of diabetes and hypoxia. Dip-coating with core-shell Prussian Blue – nickel hexacyanoferrate nanozymes results in both stable and sensitive hydrogen peroxide transducers. The best performance characteristics of the resulting bio-sensors are provided by nanoparticles of 50 nm in diameter containing 35–37 nm (ø ) Prussian Blue core. The noninvasive monitor operated via continuous sweat analysis is based on a flow-through multibiosensor for the simultaneous detection of glucose and lactate. As shown, the elaborated glucose-lactate monitor being attached to the human skin surface returns the true concentration for both glucose and lactate in undiluted human sweat. The application of the wearable device incorporating developed biosensors would obviously open new horizons in noninvasive continuous monitoring of hypoxia and glycemia. 1. Introduction The leading positions among the key low molecular weight blood metabolites, the level of which must be monitored daily, obviously belong to glucose and lactate. Glucose is the main indicator of the metabolism of carbohydrates in the blood, the main energy substrate of the body. Blood glucose concentration is traditionally used as a biomarker of diabetes [1,2]. Lactate detection certainly attracts much less attention compared to glucose. Blood lactate concentration can indicate disease states such as sepsis, hypoxia, and meningitis [3–5]. In addition, tracking the blood lactate level is widely used in sports med-icine to assess the endurance and fatigue of athletes [5–7]. Some studies have shown that lactate can act as an independent factor that should be given the same importance as glucose for monitoring obesity and type 2diabetes [8]. Furthermore, the monitoring of both of these metabolites is of interest to sports medicine: during exhausting workouts, not only the lactate concentration changes [6], but also glucose [9]. Thus, the control of glucose and lactate concentration is an important task for medical diagnostics. Currently, there is a trend towards continuous monitoring of these metabolites, which is refelcted in the development of various wearable devices [10], as well as the emergence and development of noninvasive methods of analysis [11–13]. Such methods are preferred for diagnosis, since they exclude not only trauma to the blood vessels, but also damage to the surface of the skin, which avoids pain and potential infection to patients. The idea of metabolite detection in sweat appears to be rather obvious and unpretentious, considering the fact of an existing clinically approved method for sweat glands activation [14], and that sweat is spontaneously released during physical exercise. Positive correlations in variation rates of glucose [15,16] and lactate [17,18] concentrations between blood and collected sweat reported by us obviously confrim the possibility for noninvasive monitoring of diabetes and hypoxia. The most attractive devices for sweat analysis are based on electro-chemical (bio)sensors [11,19,20]. There are some studies devoted to the development of biosensors for the simultaneous glucose and lactate detection not only in blood [21–23], but also in artifciial sweat [24]. In most cases, oxidases are used as signal generation enzymes to create glucose and lactate biosensors [25]. About 50 years ago, it was shown that the detection of H2O2 is the most progressive way to ensure the operation of the corresponding biosensors [26]. In contrast to the commonly used platinum requiring high potential (0.6 vs. Ag/AgCl) for detection of hydrogen peroxide, it was shown in the mid 90 s of the last century that Prussian Blue (iron hexacyanoferrate) is a selective elec-trocatalyst for hydrogen peroxide reduction allowing its low-potential (0.0 V vs. Ag/AgCl) detection in the presence of oxygen (j(H2O2)/j (O2) =400–600) [27,28]. Besides, the catalytic activity of Prussian blue is three orders of magnitude higher than the activity of platinum [29]. Furthermore, low-potential detection with Prussian Blue based 0925-4005/© 2023 Elsevier B.V.All rights reserved. electrodes allows decreasing of reductants background signals. It has been shown that a 20-fold excess of ascorbate is required to mask the response of Prussian Blue based sensors to hydrogen peroxide. The sensor response to paracetamol is even an order of magnitude lower [30]. Catalytic synthesis of Prussian Blue nanoparticles resulted in an artifciial substitute for the natural enzyme peroxidase [31]. (Bio)sensors based on such nanozymes possess a high sensitivity and a wide con-centration range, but their operational stability is not suffciient for long-term monitoring [32,33]. As known Prussian Blue can be solubi-lized by hydroxyl ions, a product of H2O2 reduction [29]. Covering the electrocatalyst with nickel hexacyanoferrate, which is more stable than Prussian Blue and isostructural to it but silent in electrocatalysis [34], is one of the best ways to improve the stability of Prussian Blue nano-particles [35] and prolong the lifetime of the (bio)sensor [36–38]. The concentration of glucose in sweat is considerably lower than in blood [39]. The linear range of glucose biosensors based on Prussian Blue, developed by us about 20 years ago [40], completely covers the physiological range of glucose concentrations in sweat. At the same time, the concentration of sweat lactate, on the contrary, is much higher than blood lactate [39,41]. Recently we ’ve reported on biosensor which linear range fully covers the range of feasible lactate concentrations in sweat [42]. Hence, it is possible to carry out continuous analysis of both glucose and lactate in sweat, thus achieving simultaneous monitoring of diabetes and hypoxia. We propose nanozyme-oxidase biosensors for detection of the most demanded metabolites, glucose and lactate. The combination of high s s e e n n s s i i t t i i v v i i t t y y a a n n d d s s i i g g n n i i f f ic ci a a n n t t l l y y i i m m p p r r o o v v e e d d o o p p e e r r a a t t i i o o n n a a l l s s t t a a b b i i l l i i t t y y o o f f t t h h e e developed biosensors allows them to be used for various tasks. In particular, we have demonstrated the possibility of their application for blood and sweat analysis. Furthermore, based on the four-electrode planar structure, a multibiosensor has been developed that can be applied to the simultaneous monitoring of glucose and lactate in undi-luted sweat. 2. Material and methods 2.1. Materials Experiments were carried out with Millipore Milli-Q water (re-sistivity 18.2 M Q.cm at room temperature). All inorganic salts, organic s s o o l l v v e e n n t t s s ,, a a n n d d h h y y d d r r o o g g e e n n p p e e r r o o x x i i d d e e ((3300 %% s s o o l l u u t t i i o o n n )) w w e e r r e e o o b b t t a a i i n n e e d d commercially from Reachim (Moscow, Russia) in the highest purity and used as received. D-glucose, potassium L-lactate, y -amino-propyltriethoxysilane (APTES), and glucose oxidase (EC 1.1.3.4, 200 IU mg - 1) from Aspergillus niger (type VII, lyophilized powder) were ob-tained from Sigma-Aldrich (Germany). Ionomer MF4 SK (per-fulorosulfonated ionomer, Nafoin analogue) was purchased from Plastpolymer (Saint Petersburg, Russia). Lactate oxidase (EC 1.1.3.2,109 U mg 1) from microorganism was obtained from Sorachim (Switzerland). Pilocarpine hydrochloride (1 %) was purchased from Renewal (Novosibirsk, Russia). Normal and pathological Spintrol “H ”human serum was obtained from Spinreact (Spain). Planar (bio)sensors were made on the basis of three-electrode or four-electrode screen printed structures with a carbon working electrodes (Ø =1.8 mm), produced by Rusens Ltd. (Moscow, Russia). To form the folw-through sweat collectors, clear photoactive resin, Formlabs Clear V4 (USA) was used. 2.2. Instrumentation Synthesis and ultrasonication of Prussian Blue (PB) and Prussian Blue – nickel hexacyanoferrate (PB-NiHCF) nanoparticles were carried out using high-speed centrifuge Microspin 12 (Biosan, Latvia) and ultra-sonic bath PSB-Gals (Russia). Nanoparticles sizing was performed with Malvern Zetasizer Nano ZS (Malvern Instruments Ltd, UK). UV/Vis measurements were carried out using Lambda 35 Spectrophotometer (Perkin Elmer, USA) operated in transmission mode. X-ray powder diffraction experiment was performed with the use of a HUBER G670Guinier camera (CoK f radiation, f r 1.78965 Å). The analysis of microstructure and elemental distribution in PB-NiHCF was carried out with transmission electron microscopy studies at Libra 200 (Carl Zeiss, Germany) chromatic aberration-corrected electron microscope equip-ped by Q -fliter and EDX Х M ах 80 T detectors. EDX linescans and maps were collected with 1.0 nm probe size and a typical point-to-point step of 0.7–1 nm. Energy flitered transmission electron microscopy images for nickel were acquired at Ni L-edge using 4 eV slit and monochromatic incident beam with energy dispersion of < 0.2 eV. Scanning electron microscopy (SEM) imaging was performed using a Helios NanoLab 600,(ThermoFisher Scientifci, Netherlands) and EDX mapping was per-formed with EDAX, SDD Apollo XV (EDAX, USA). Drying of the catalytic layer of nanoparticles was carried out using a hot air oven Vityaz GP-40–3(Vityaz, Belarus). Voltammetry, amperometry, and electro-chemical impedance spectroscopy (EIS) were carried out using a Palm-sens 4 potentiostat with BiPot module (PalmSens BV, The Netherlands). To record the current response in power generation mode, a digital multimeter Tektronix DMM4020 (Tektronix Inc., USA) connected to a PC was used. The home-made folw-injection analyzer setup consisted of a syringe pump Perfusor Compact S (Braun, Germany), a home-made folw-through wall-jet cell with a 0.5 mm nozzle, and an injector (IDEX Health & Science LLC, USA). The folw rate of 0.67 mL min - 1 was used. Potok-1 (EMA Plant, Russia) was used for electrophoresis. HPLC-MS was performed using HPLC Dionex Ultimate 3000 (USA), a system with a binary analytical pump, an automatic sample injector, a DAD detector coupled on-line with AB Sciex Qtrap 3200 (Canada) mass spectrometer with an ESI source. A Form 3 SLA 3D printer (Formlabs, USA) was used for folw-through sweat collectors fabrication. 2.3. Methods PB nanoparticles were synthesized in 1:1 mixture of FeCl3 and K3[Fe (CN)6] (75 mM) dissolved in 0.1 M KCl with 0.1 M HCl under continuous stirring and ultrasonication. The precipitation process was initiated by adding 50 mM H2O2. The resulting colloidal solution was centrifuged at 13400 rpm for 30 s and the obtained precipitate was re-dispersed into 0.1 M KCl and 0.1 M HCl solution under ultrasonication (5–7 times). The concentration of PB in colloidal solution was determined spectropho-tometrically (e 700 nm (per PB unit cell) = 4.85-104 M - 1 cm - 1), and the nanoparticle diameter was estimated using dynamic light scattering (DLS). For stabilization of PB nanoparticles, the resulting suspension was added to 0.3–2.1 mM K3[Fe(CN)6] and 0.5–3.5 mM NiCl2)6 H2O dis-solved in a solution containing 0.5 M KCl and 0.1 M HCl and was ultrasonicated for 20 s. To avoid aggregation of nanoparticles, after one of the resulting suspension centrifugations, the obtained nanoparticles were added to 7 mM sodium hexametaphosphate with 0.5 M KCl and 0.1 M HCl. The suspension containing PB or stabilized PB nanoparticles were stored at pH 1.1 and were ultrasonicated prior to use. For hydrogen peroxide sensors manufacture, a 2 µ L nanoparticles suspension was cast onto the working electrode surface of planar sensor structures and was dried at room temperature for 24 h and at 100 C for 1h in a hot air oven. Impedance spectra were recorded at 5 mV amplitude at the redox potential of PB found from its cyclic voltammograms (≈0.12 V) [43]. Biosensors were made by casting a mixture containing the glucose or lactate oxidase onto the catalytic nanoparticles layer modifeid working e e l l e e c c t t r r o o d d e e .. G G l l u u c c o o s s e e a a n n d d l l a a c c t t a a t t e e o o x x i i d d a a s s e e m m i i x x t t u u r r e e s s w w e e r r e e p p r r e e p p a a r r e e d d b b y y blending an aqueous enzyme solution (10 mg/mL) with 0.3 % per-fulorosulfonated ionomer in 85 % isopropanol or 2 %y -amino-propyltriethoxysilane in 90 % isopropanol, respectively. After solvent was evaporated at room temperature, the membranes were kept in the refrigerator (+4 °C) for 24 h. The HPLC separation of sweat samples was conducted on a polymer-based column for HILIC (Shodex (Japan); Asahipak NH2P-40 2D; 4.0µ m, 2.0 × 150 mm) at 40 °C. Two solvents were used (A) deionized water, and (B) MeCN. The column effulent was analyzed by ESI-MS/MS in a negative-ion mode. The glucose tolerance test was carried out with the participation of a healthy human volunteers who gave informed consent prior to an experiment. All work was performed in accordance with GCP regula-tions. Sweat gland activation was carried out according to the accepted worldwide clinically relevant procedure based on stimulation of the sweat gland with 1 % pilocarpine through electrophoresis for 15 min 3. Results and discussion 3.1.(Bio)sensors based on Prussian Blue – nickel hexacyanoferrate core-shell nanozymes First, Prussian Blue (PB) nanoparticles with an average diameter of 31–37 nm have been obtained by catalytic synthesis as described in [31]. Next, nickel hexacyanoferrate (NiHCF) shells have been synthe-sized around the PB cores according to [35]. Higher concentrations of precursors lead to larger diameters (up to 160 nm) of the resulting nanozymes (Fig. S1, Supporting Information). To prove the crystalline nature of the nanoparticles, XRD proflies have been plotted (Fig. S2, Supporting Information). All scattering peaks can be well indexed based on a face-centered cubic structure with space group of Fm-3m , which is the typical character of metal ferricyanides. According to the trans-mission electron microscopy (TEM) images (Fig. S3, Supporting Infor-mation), the obtained PB-NiHCF nanoparticles consist of small crystalline grains with an average size of 7 - 20 nm, which aggregate to larger particles (40–160 nm). The presence of Ni atoms on the surface (Fig. S3B, Supporting Information) confrims the existence of a NiHCF shell. Both EFTEM and EDX mappings (Fig. S3C, D, Supporting Infor-mation) illustrate rather homogeneous distribution of nickel over the specimens. Since the thickness of the NiHCF shell affects both activity and sta-bility of the PB-NiHCF nanozymes [35], the sensitivity and operational stability of biosensors based on such nanozymes have been studied. A 2µ L drop of suspension containing PB or PB-NiHCF nanoparticles has been casted onto the working electrode surface of planar sensor struc-tures to produce hydrogen peroxide sensors [31,35]. According to the SEM images, the PB-NiHCF nanozymes completely cover the carbon electrode surface (Fig. 1A) with small particles (Fig. 1C), while the PB-NiHCF flim [36,37] is composed of much larger particles (Fig. 1B). To confrim the presence of both iron and nickel on the PB-NiHCF nanoparticles modifeid-electrode surface and the absence of nickel in case of using PB nanoparticles, EDX spectra (Fig. S4, Supporting Infor-mation) have been obtained. The nickel-to-iron atomic ratio, estimated from the EDX spectra, follows a hyperbolic dependence on the size of the composite nanoparticles (Fig. S4B, Supporting Information). Zero nickel content corresponds to PB nanozymes (ø = 37nm) modifeid electrode. For large nanoparticles with a thick shell over the same core, the ratio clearly matches the case of nickel hexacyanoferrate. On square-wave voltammograms of electrodes modifeid with PB-NiHCF core-shell nanoparticles (Fig. S1, Supporting Information) we can see a peak at a potential of 0.15V corresponding to the redox transition Prussian White – Prussian Blue. Peaks at potentials of 0.4 V and 0.55V are characteristic of the process of iron oxidation-reduction of K *-depleted and K *-rich nickel hexacyanoferrate forms [44]. Applicability of impedance spectroscopy as microscopy-free tool for estimation of flim continuity was demonstrated by our group [43]. The continuity of the electrocatalyst (PB) on the electrode surface according to [43] can be evaluated from the dependence of charge transfer resis-tance (found from the impedance spectra of PB or PB-NiHCF nanozymes modifeid electrodes) on the amount of Prussian Blue (Fig. S5, Supporting Information). The charge transfer resistance has been estimated from impedance spectra ftited to the Randles-Sevcik circuit complicated by the diffusion impedance with refelctive boundary conditions (shown in the inset of Fig. S5). As expected, the dependence reaches a plateau when the amount of PB is about 1–2 nmol cm - 2, which considering the chosen deposition procedure (see Experimental) relates to PB-NiHCF nanoparticles with diameter 65 nm and less. Thus, for nanozymes of this size the electrocatalyst is distributed over the entire surface of the working electrode. Glucose and lactate biosensors based on PB and PB-NiHCF nano-particles have been elaborated. In the presence of the corresponding enzyme, the analyte is oxidized by atmospheric oxygen, with hydrogen peroxide acting as one of the reaction products. Then, at 0.0 V vs. Ag/AgCl, H2O2 is reduced to hydroxyl ions by Prussian White (PW), thereby oxidizing it to PB, which is immediately reduced back to PW (Fig. S6, Supporting Information). Thus, the hydrogen peroxide reduction cur-rent is recorded. The enzyme immobilization protocols frist proposed by our group more than 10 years ago [40,45] have been used. All (bio) sensors have been tested in chronoamperometry mode upon stirring in neutral media (50 mM phosphate, pH 6.0, with 0.1 M KCl) at 0.0 V vs. AgAgCl. The analyte (H2O2, glucose or lactate) concentration in the solution has been increased stepwise. Response time after addition of a new analyte portion, is within 10 s (Fig. S7A, Supporting Information). The sensitivity, as usual, has been calculated as the slope of the current density vs. the analyte concentration curve (Fig. S7B, Supporting information). As seen in Fig. 2A, the (bio)sensor sensitivity is decreased as the size of nanoparticles is increased. This can be explained in terms of the increased thickness of the NiHCF shell causing a decrease in the amount of electrocatalyst on the electrode surface. We note there is no signif-i cant change in sensitivity for (bio)sensors based on stabilized Fig. 1. SEM images of a bare carbon SPE (A), a H2O2 sensor based on PB-NiHCF bilayer (B), a H2O2 sensor based on PB-NiHCF core-shell nanoparticles (C). The scale bar is 200nm. Fig. 2. Sensitivity (A) and operational stability (B) of hydrogen peroxide sensors (black), glucose (blue) and lactate (green) biosensors made on basis of PB-NiHCF core-shell nanoparticles divided by corresponding values for (bio)sensors based on PB nanoparticles as functions of nanozymes sizes. nanoparticles with the thinnest shell compared to those based on sole PB nanoparticles. The operational stability of hydrogen peroxide sensors, as well as g g l l u u c c o o s s e e a a n n d d l l a a c c t t a a t t e e b b i i o o s s e e n n s s o o r r s s ,, w w a a s s e e v v a a l l u u a a t t e e d d i i n n 11 m m M M h h y y d d r r o o g g e e n n peroxide, 1 mM glucose, and 0.2 mM lactate, respectively, upon con-stant stirring (50 mM phosphate, pH 6.0, with 0.1 M KCl). Stability was characterized as the time during which the (bio)sensor current response is retained at 90 % of its initial value. As seen in Fig. 2B, the operational stability of (bio)sensors is increased with the raise of nanoparticles size continuously up to PB-NiHCF nanoparticles of more than 150nm in diameter (10 times higher than for PB nanoparticles). However, the sensitivity of such (bio) sensors is quite low. Therefore, the local maximum observed for bio-sensors based on nanoparticles with a diameter from 45 to 50nm is of much greater interest. Fig. 3 shows an example of the current responses of glucose bio-sensors based on PB nanoparticles (ø =37nm) and PB-NiHCF core shell nanoparticles (ø 0 50nm) when testing operational stability. While sensitivity is only reduced by 1.5 times (53±3mAM M 1 cm - 2 vs. 34±4mAM M 1 cm - 2), operational stability is 4 times higher (39±6min vs. 150±20min). Moreover, the linear range of PB-NiHCF nanozymes based biosensors is even wider than PB nanozymes based one and pro-longs from 1 u M to 2 mM of glucose. We already reported [38] that stability of oxidase-based biosensors can be accessed through stabilizing the H2O2 transducer. Here we pro-vide an independent evidence that fnial performances of biosensors based on oxidase enzymes are directly related to the performances of the hydrogen peroxide sensor. Fig. 3. Operational stability of glucose biosensors based on PB and PB-NiHCF nanoparticles; 0.0 V AgAgCl, 1 mM glucose in 50mM phosphate, pH 6.0, with 0.1 M KCl, upon stirring. Summing up, the most advantageous performance characteristics of H2O2 sensors as well as of the related biosensors are achieved if for electrode modifciation the PB-NiHCF core-shell nanozymes of 50 nm in diameter (37 nm PB core) are used. The best composition for the syn-thesis of nickel hexacyanoferrate is 1.0 mM Ni2+ and 0.6 mM [Fe –(CN)6]3. Table 1 is a comparison of the developed (bio)sensors char-acteristics with previously known ones. Replacing the transducer from PB nanoparticles to PB-NiHCF nanoparticles has almost no effect on the sensitivity and linear range of the (bio)sensors, while the operational stability is signifciantly improved (from 2 to 8 times). Some loss in operational stability in comparison with (bio)sensors based on PB flims at similar values of sensitivity and linear range is compensated by a simpler (dropping of the transducer) and express technique for the developed biosensors fabrication. 3.2. Application of the developed biosensors for blood and sweat analysis Glucose biosensors based on PB-NiHCF core-shell nanozymes have been validated for analysis of human serum. For this aim biosensor have been integrated to folw-injection analysis system and graduated from 5to 1000 u M glucose. Considering the dilution of the sample by 50 times, the indicated range covers blood glucose levels (0.5–30mM). Then normal and pathological human serum samples have been injected into the system and relevant peaks have been analyzed (Fig. S8, Supporting Information). The obtained glucose concentration values (5.4±0.1 mM and 13.4±0.3 mM) are in good relation with the passport values (5.8±0.9 mM and 13.1±1.9 mM). These results clearly demonstrate the validity of the PB-NiHCF core-shell based biosensors for the analysis of blood serum. Certainly, blood is the most valuable liquid for medical diagnostics, but it is equally important to develop new noninvasive approaches assessing the concentration of metabolites in the blood. For this aim, various noninvasively collected liquids are being studied to fnid corre-lations between the concentrations of metabolites in such liquid and in blood. This research is still not completed and a constant increase in the pool of volunteers and testing methods is required. However, it is already evident that sweat is one of the most promising liquids in the framework of this problem. It has been shown that variations in the concentration of both glucose and lactate in blood and sweat positively correlate [15–18]. Therefore, the development of (bio)sensors for sweat analysis is an urgent task for analytical chemists. Sweat samples were also analyzed using the elaborated biosensors. Sweat glands have been activated by pilocarpine electrophoresis to obtain sweat. The samples have been analyzed in FIA mode using glucose biosensors based on PB-NiHCF nanoparticles. Experimental conditions were 50–200mMK2HPO4/KH2PO4, 100–700mM NaCl. The dilution rates of sweat samples from 2 to100 times have been used. The Table 1 Analytical performance characteristics of PB based (bio)sensors; 0.0 V AgAgCl, 50 mM phosphate, pH 6.0, with 0.1 M KCl, upon stirring. Transducer Sensitivity, A M- 1 cm- 2 Linear range, uM Operational stability (t90%), min Reference Hydrogen peroxide [37] PB (flim) 0.50±0.05 1–1000 7±2 (1 mM H2O2) PB (nanoparticles) 0.59±0.02 1–1000 6.5±0.1 (1 mMH2O2) [46] PB-NiHCF (nanoparticles) 0.55±0.02 1–1000 14±2 (1 mMH2O2) Current research PB (flim) 0.039±0.004 5–2000 540±30 (5 mM glucose) [38] PB (nanoparticles) 0.053±0.003 1–2000 39±6 (1 mM glucose) Current research PB-NiHCF (nanoparticles) 0.034±0.004 1–2000 150±20 (1 mM glucose) Current research Lactate PB (flim) 0.19±0.02 1–500 90±7 (0.25 mM lactate) [38] PB (nanoparticles) 0.21±0.02 1–500 9±1 (0.2 mM lactate) [33] PB-NiHCF (nanoparticles) 0.20±0.01 1–500 70±7 (0.2 mM lactate) Current research following conditions have turned out to be optimal: sweat should be diluted 10 times in a phosphate buffer solution pH 6.0 containing 150mMK2HPO4/KH2PO4 and 500mM NaCl. Data on the sweat glucose concentration obtained using biosensors have been compared with the data of HPLC-MS analysis. For 21 sweat samples, the Pearson correlation coeffciient between the two methods used exceeds 0.99 (Fig. S9, Sup-porting Information), which indicates that the conditions for deter-mining the glucose concentration in sweat have been chosen correctly. The developed PB-NiHCF based biosensors have been applied for online noninvasive diabetes monitoring (Fig. S10, Supporting Informa-tion) [15,47]. Prior to use, glucose biosensor was integrated into the folw-through sweat collector and calibrated (Fig. S7C,D, Supporting Information). Glucose tolerance test seems to be the only clinically approved procedure to suffciiently increase blood glucose level for healthy volunteers. Usually after 15 min of sweat gland stimulation the constant sweat folw is observed during 30–40min, while the glucose tolerance test prolongs for more than 1h. Thus, for such long procedure at least 2 stimulations are required, and the resulting chronoampero-metric graph would have a 20min gap for reactivation of sweat excre-tion. The dynamics of sweat glucose concentration is very similar to the typical pattern of blood glucose concentration in course of glucose tolerance test. The maximum of the noninvasive diabetes monitor online reading is achieved at approximately 60-s min (solid lines in Fig. S10, Supporting Information). Integral values of sweat glucose concentra-tions (points in Fig. S10, Supporting Information) within excretion pe-riods (dash lines in Fig. S10, Supporting Information) were obtained analyzing sweat samples collected from the monitor outlet. Comparison of the data obtained allows to declare correct operation of the diabetes Fig. 4. Noninvasive monitor for simultaneous detection of glucose and lactate in sweat: current responses of the multibiosensor to glucose (black line) and lactate (green line) in flow-throw cell configuration, 0.0 V AgAgCl, 50 mM phosphate, pH 6.0, with 0.1 M KCl (A); corresponding calibration graphs for multibiosensor (B); image of the planar screen-printed structure (the basis of the multibiosensor) and 3D-image of the noninvasive monitor (C); response of the multibiosensor to glucose (black line) and lactate (green line) in sweat, 0.0 V AgAgCl, in comparison with sweat metabolites concentration detected by FIA in diluted sweat: – glucose; -– lactate (D). monitor in undiluted sweat. Accordingly, the developed glucose oxidase – nanozyme based biosensor integrated to the folw-through cell can be used for noninvasive assessment of blood glucose concentration. As mentioned, the concentration of both lactate and glucose is of great interest for sports medicine as well as for diabetic patients with an active lifestyle. For simultaneous metabolites monitoring, we have used the design of a printed structure with two working electrodes (Fig. 4C)[21]. Both electrodes have been modifeid with PB-NiHCF nanoparticles, and then glucose oxidase have been immobilized on one of the elec-trodes [40], and lactate oxidase have been immobilized on the other [42]. Such a multibiosensor have been integrated into folw-through cell (Fig. 4 C, Fig. S11, Supporting Information) which operates as follows. Sweat enters the cell through a hole in the center, folws to the reference electrode (RE), then folws through the auxiliary electrode (CE). Next, the sweat stream is divided into two parts, each gets on the one of two working electrodes (WE1 and WE2). Then the streams are again com-bined into one and folw out through the outlet capillary. This design makes it possible to exclude the mutual infulence of reactions occurring on the working electrodes: hydrogen peroxide released during catalysis, both on glucose and lactate biosensors, gets into the waste. Prior to use, current responses to mixtures of glucose (from 5 to 500 u M) and lactate (from 1 to 60 mM) have been recorded at 0.0 V using a bipotentiostat (Fig. 4 A) and corresponding calibration graphs have been plotted (Fig. 4B). Further, after 15 min of sweat gland stim-ulation noninvasive monitor has been placed on the surface of the skin of the volunteer ’s forearm. Fig. 4D displays the corresponding online readings of the glucose-lactate monitor. As seen the readings are stable with average values of 120 10 µ M for glucose and of 16.7±0.8 mM for lactate. Validation of the glucose-lactate monitor has been carried out col-lecting sweat samples from the monitor outlet (Fig. 4 C) with their subsequent analysis by means home-made folw-injection system. As seen (Fig. 4D), the corresponding points (125±9 µ M glucose and 16.6±0.9 mM lactate) belong to the same concentration-time dependence as the readings of the monitor. Hence the elaborated glucose-lactate monitor being attached to the human skin surface returns true concen-tration for both glucose and lactate in undiluted human sweat. Since the developed multibiosensor is reusable, and it is also planned to be used for continuous metabolites monitoring, the repeatability and operational stability have been studied (Fig. S12, Supporting Informa-tion). Repeatability was tested in folw-injection-analysis mode. The deviation of the results of 60 injections of 0.1 mM glucose and 20mM lactate does not exceed 10 %. It has been demonstrated that both glucose and lactate biosensors display stable response ( 100 %) for at least 4 h of continuous operation in 0.1 mM glucose and 20mM lactate folw (folw rate 0.2 mLh 1), which is suffciient for long-term sweat analysis. Thus, it has been shown one device provides two important in-dicators (glucose and lactate levels) simultaneously and it requires only a small amount of freshly excreted sweat. Tracked live data changes by such a device can be converted to corresponding changes in blood and interpreted according to medical diagnostics knowledge accumulated over many years. Using the developed core-shell nanozyme – oxidase enzyme biosensor, it is possible to noninvasively monitor hypoxia and glycemia, in particularly, during exercises. This fact opens great pros-pects for testing athletes. 4. Conclusions Prussian Blue nanoparticles are increasingly being used for (bio) sensing applications. Here we present glucose and lactate biosensors based on oxidases and core-shell nanozymes Prussian Blue – nickel hexacyanoferrate. A simple highly reproducible one-step procedure of casting a stabilized transducer, combined with high sensitivity and wide linear and dynamic ranges, being not inferior to previous sensors based on Prussian Blue, and at least 3 times improved operational stability, makes these biosensors unique. Our earlier hypothesis of the stability of the transducer making a substantial contribution to the stability of the biosensor was fully confrimed. The developed biosensors are applicable in various feilds, primarily for the analysis of biological fulids. As part of the development of the noninvasive diagnostics, which we have been actively cultivating over the past 10 years, a new design of the folw-through sweat collector was created, compatible with a four-electrode planar printed structure, which made it possible to simultaneously detect lactate and glucose in sweat. CRediT authorship contribution statement Elena V. Daboss: Investigation, Visualization, Conceptualization, Methodology, Formal analysis, Writing – original draft. Elizaveta V. Shcherbacheva: Investigation, Visualization. Arkady A. Karyakin :Supervision, Conceptualization, Writing –original draft, Funding acquisition. Declaration of Competing Interest The authors declare that they have no known competing fniancial interests or personal relationships that could have appeared to infulence the work reported in this paper. Data availability No data was used for the research described in the article. Acknowledgements Financial support of the Russian Science Foundation (RSF) through Grant No. 19-13-00131 (https://rscf.ru/en/project/19-13-00131/) is greatly acknowledged. Appendix A. Supporting information Supplementary data associated with this article can be found in the online version at doi:10.1016/j.snb.2023.133337. References [1] D. Olczuk, R. Priefer, A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus, Diabetes Metab. Syndr. Clin. Res. Rev. 12 (2018)181–187, https://doi.org/10.1016/J.DSX.2017.09.005. [2] B.C. Mulukutla, A. Yongky, T. Le, D.G. Mashek, W.S. Hu, Regulation of glucose metabolism – a perspective from cell bioprocessing, Trends Biotechnol. 34 (2016)638–651, https://doi.org/10.1016/J.TIBTECH.2016.04.012. [3] R. Dringen, R. Gebhardt, B. Hamprecht, Glycogen in astrocytes: possible function as lactate supply for neighboring cells, Brain Res. 623 (1993) 208–214, https://doi. org/10.1016/0006-8993(93)91429-V. [4] K. Rathee, V. Dhull, R. Dhull, S. Singh, Biosensors based on electrochemical lactate detection: a comprehensive review, Biochem. Biophys. Rep. 5 (2016) 35–54, https://doi.org/10.1016/J.BBREP.2015.11.010. [5] A. Moioli, B. Maresca, A. Manzione, · Antonello, M. Napoletano, D. Coclite, N. Pirozzi, G. Punzo, P. Men ,e, Metformin Associated Lactic Acidosis (MALA): Clinical Profliing and Management, (n.d.). (https://doi.org/10.1007/s40620–016-0267–8). [6]I. Jacobs, Blood lactate, Sport. Med. 3 (1986) 10–25, https://doi.org/10.2165/ 00007256-198603010-00003. [7] G.A. Brooks, The science and translation of lactate shuttle theory, Cell Metab. 27(2018) 757–785, https://doi.org/10.1016/J.CMET.2018.03.008. [8] F. Guerif, P. Mckeegan, H.J. Leese, R.G. Sturmey, A simple approach for consumption and release (CORE) analysis of metabolic activity in single mammalian embryos, PLoS One 8 (2013) 67834, https://doi.org/10.1371/journal. pone.0067834. [9] A. Wiorek, M. Parrilla, M. Cuartero, G.A. Crespo, Epidermal patch with glucose biosensor: PH and temperature correction toward more accurate sweat analysis during sport practice, Anal. Chem. 92 (2020) 10153–10161, https://doi.org/10.1021/acs.analchem.0c02211. [10] Biosensors Market with COVID-19 Impact by Type, Product (Wearable, Non-wearable), Technology, Application (POC, Home Diagnostics, Research Lab, Environmental Monitoring, Food & Beverages, Biodefense) and Region - Global Forecast to 2026, Markets and Markets, (2021) 1–223. (https://www.marketresear ch.com/MarketsandMarkets-v3719/Biosensors-COVID-Impact-Type-Product-14457655/). [11] J. Heikenfeld, J. Rogers, T. Pan, M. Khine, J. Wang, J. Heikenfeld, A. Jajack, bg P. Gutruf, L. Tian, R. Li, J. Kim, Wearable sensors: modalities, challenges, and prospects, Lab Chip 18 (2018) 217–248, https://doi.org/10.1039/c7lc00914c. [12] H. Teymourian, A. Barfdiokht, J. Wang, Electrochemical glucose sensors in diabetes management: an updated review (2010-2020), Chem. Soc. Rev. 49 (2020)7671, https://doi.org/10.1039/d0cs00304b. [13] R.D. Crapnell, A. Tridente, C.E. Banks, N.C. Dempsey-Hibbert, Evaluating the possibility of translating technological advances in non-invasive continuous lactate monitoring into critical care, Sensors 21 (2021) 879, https://doi.org/10.3390/s21030879. [14] J.H. Burn, The secretion of sweat and vaso-dilatation produced by pilocarpine, J. Physiol. 60 (1925) 365–378, https://doi.org/10.1113/jphysiol.1925.sp002255. [15] E.V. Karpova, E.V. Shcherbacheva, A.A. Galushin, D.V. Vokhmyanina, E. E. Karyakina, A.A. Karyakin, Noninvasive diabetes monitoring through continuous analysis of sweat using folw-through glucose biosensor, Anal. Chem. 91 (2019)3778–3783, https://doi.org/10.1021/acs.analchem.8b05928. [16] J. Moyer, D. Wilson, I. Finkelshtein, B. Wong, R. Potts, Correlation between sweat glucose and blood glucose in subjects with diabetes, Diabetes Technol. Ther. 14(2012) 398–402, https://doi.org/10.1089/dia.2011.0262. [17] E.V. Karpova, A.I. Laptev, E.A. Andreev, E.E. Karyakina, A.A. Karyakin, Relationship between sweat and blood lactate levels during exhaustive physical exercise, ChemElectroChem 7 (2020), https://doi.org/10.1002/celc.201901703. [18] D.A. Sakharov, M.U. Shkurnikov, M.Y. Vagin, E.I. Yashina, A.A. Karyakin, A. G. Tonevitsky, Sports medicine relationship between lactate concentrations in active muscle sweat and whole blood, Transl. Byulleten ’ Eksp. Biol. i Meditsiny.150 (2010) 94–96. [19] J. Xu, Y. Fang, J. Chen, Wearable biosensors for non-invasive sweat diagnostics, Biosensors 11 (2021), https://doi.org/10.3390/bios11080245. [20] M. Falk, C. Psotta, S. Cirovic, S. Shleev, Non-Invasive Electrochemical Biosensors Operating in Human Physiological Fluids, (n.d.). (https://doi.org/10.3390/s20216352). [21] D.V. Vokhmyanina, E.E. Karyakina, E.A. Andreev, A.A. Karyakin, Prussian blue-based thin-layer folw-injection multibiosensor for simultaneous determination of glucose and lactate, Mosc. Univ. Chem. Bull. 73 (2018) 216–222, https://doi.org/10.3103/S0027131418050127. [22] M. Thapa, R. Sung, Y.S. Heo, A Dual Electrode Biosensor for Glucose and Lactate Measurement in Normal and Prolonged Obese Mice Using Single Drop of Whole Blood, (2021). (https://doi.org/10.3390/bios11120507). [23] X. Li, J. Zang, Y. Liu, Z. Lu, Q. Li, C.M. Li, Simultaneous detection of lactate and glucose by integrated printed circuit board based array sensing chip, Anal. Chim. Acta 771 (2013) 102–107, https://doi.org/10.1016/J.ACA.2013.02.011. [24] F. Poletti, B. Zanfrognini, L. Favaretto, V. Quintano, J. Sun, E. Treossi, M. Melucci, V. Palermo, C. Zanardi, Continuous capillary-folw sensing of glucose and lactate in sweat with an electrochemical sensor based on functionalized graphene oxide, Sens. Actuators B Chem. 344 (2021), 130253, https://doi.org/10.1016/J. SNB.2021.130253. [25] A.P.F. Turner, Biosensors: sense and sensibility, Chem. Soc. Rev. 42 (2013)3184–3196, https://doi.org/10.1039/C3CS35528D. [26] G.G. Guilbault, G.J. Lubrano, D.N. Gray, Glass-metal composite electrodes, Anal. Chem. 45 (1973) 2255–2258, https://doi.org/10.1021/ac60335a025. [27] A.A. Karyakin, O.V. Gitelmacher, E.E. Karyakina, A high-sensitive glucose amperometric biosensor based on Prussian Blue modifeid electrodes, Anal. Lett. 27(1994) 2861–2869, https://doi.org/10.1080/00032719408000297. [28] A.A. Karyakin, O.V. Gitelmacher, E.E. Karyakina, Prussian blue-based frist-generation biosensor. a sensitive amperometric electrode for glucose, Anal. Chem. 67 (1995) 2419–2423, https://doi.org/10.1021/ac00110a016. [29] A.A. Karyakin, E.E. Karyakina, L. Gorton, On the mechanism of H2O2 reduction at Prussian Blue modifeid electrodes, Electrochem. Commun. 1 (1999) 78–82, https://doi.org/10.1016/S1388-2481(99)00010-7. [30] M.A. Komkova, E.E. Karyakina, A.A. Karyakin, Noiseless performance of Prussian Blue based (bio)sensors through power generation, Anal. Chem. 89 (2017)6290–6294, https://doi.org/10.1021/acs.analchem.7b01142. [31] M.A. Komkova, E.E. Karyakina, A.A. Karyakin, Catalytically Synthesized Prussian Blue Nanoparticles Defeating Natural Enzyme Peroxidase, (2018). (https://doi. org/10.1021/jacs.8b05223). [32] M.A. Komkova, A.A. Zarochintsev, E.E. Karyakina, A.A. Karyakin, Electrochemical and sensing properties of Prussian Blue based nanozymes “artifciial peroxidase ”, J. Electroanal. Chem. 872 (2020), 114048 https://doi.org/10.1016/J. JELECHEM.2020.114048. [33] D.V. Vokhmyanina, K.D. Andreeva, M.A. Komkova, E.E. Karyakina, A.A. Karyakin,‘Artifciial peroxidase ’ nanozyme – enzyme based lactate biosensor, Talanta 208(2020), 120393, https://doi.org/10.1016/J.TALANTA.2019.120393. [34] N.A. Sitnikova, M.A. Komkova, I.V. Khomyakova, E.E. Karyakina, A.A. Karyakin, Transition metal hexacyanoferrates in electrocatalysis of H2O2 reduction: an exclusive property of Prussian Blue, Anal. Chem. 86 (2014) 4131–4134, https://doi.org/10.1021/ac500595v. [35] E.V. Karpova, E.V. Shcherbacheva, M.A. Komkova, A.A. Eliseev, A.A. Karyakin, Core-shell nanozymes “artifciial Peroxidase ”: stability with superior catalytic properties, J. Phys. Chem. Lett. 12 (2021), https://doi.org/10.1021/acs. jpclett.1c01200. [36] N.A. Sitnikova, A.V. Borisova, M.A. Komkova, A.A. Karyakin, Superstable advanced hydrogen peroxide transducer based on transition metal hexacyanoferrates, Anal. Chem. 83 (2011) 2359–2363, https://doi.org/10.1021/AC1033352. [37] E.V. Karpova, E.E. Karyakina, A.A. Karyakin, Iron-nickel hexacyanoferrate bilayer as an advanced electrocatalyst for H2O2 reduction, RSC Adv. 6 (2016)103328–103331, https://doi.org/10.1039/c6ra24128j. [38] E.V. Karpova, E.E. Karyakina, A.A. Karyakin, Communication - accessing stability of oxidase-based biosensors via stabilizing the advanced H2O2, Transducer, J. Electrochem. Soc. 164 (2017), https://doi.org/10.1149/2.0091705jes. [39] C.J. Harvey, R.F. LeBouf, A.B. Stefaniak, Formulation and stability of a novel artifciial human sweat under conditions of storage and use, Toxicol. Vitr. 24 (2010)1790–1796, https://doi.org/10.1016/j.tiv.2010.06.016. [40] A.A. Karyakin, E.A. Kotel ’nikova, L.V. Lukachova, E.E. Karyakina, J. Wang, Optimal Environment for Glucose Oxidase in Perfulorosulfonated Ionomer Membranes: Improvement of First-Generation Biosensors, (2002). (https://doi. org/10.1021/ac0155409). [41] L.S. Lamont, Sweat lactate secretion during exercise in relation to women ’s aerobic capacity, J. Appl. Physiol. 62 (1987) 194–198, https://doi.org/10.1152/jappl.1987.62.1.194. [42] E.V. Daboss, D.V. Tikhonov, E.V. Shcherbacheva, A.A. Karyakin, Ultrastable lactate biosensor linearly responding in whole sweat for noninvasive monitoring of hypoxia, Anal. Chem. (2022), https://doi.org/10.1021/acs.analchem.2c02208. [43] M.A. Komkova, E.V. Karpova, G.A. Sukhorukov, A.A. Sadovnikov, A.A. Karyakin, Estimation of continuity of electroactive inorganic flims based on apparent anti-Ohmic trend in their charge transfer resistance, Electrochim. Acta 219 (2016), https://doi.org/10.1016/j.electacta.2016.09.145. [44] S. Zamponi, M. Berrettoni, P.J. Kulesza, K. Miecznikowski, M.A. Malik, O. Makowski, R. Marassi, Infulence of experimental conditions on electrochemical behavior of Prussian blue type nickel hexacyanoferrate flim, Electrochim. Acta 48(2003) 4261–4269, https://doi.org/10.1016/J.ELECTACTA.2003.08.001. [45] E.I. Yashina, A.V. Borisova, E.E. Karyakina, O.I. Shchegolikhina, M.Y. Vagin, D. A. Sakharov, A.G. Tonevitsky, A.A. Karyakin, Sol-gel immobilization of lactate oxidase from organic solvent: toward the advanced lactate biosensor, Anal. Chem.82 (2010) 1601–1604, https://doi.org/10.1021/ac9027615. [46] E. Daboss, E. Shcherbacheva, M. Komkova, A. Eliseev, A. Karyakin, Core –Shell Nanozymes “Artifciial Peroxidase ”: Stability with Superior Catalytic Properties, J. Phys. Chem. Lett. 12 (n.d.) 5547–5551. (https://doi.org/10.1021/acs.jpclett.1c 01200). [47] E.V. Karpova, A.A. Karyakin, Noninvasive monitoring of diabetes and hypoxia by wearable folw-through biosensors, Curr. Opin. Electrochem. 23 (2020), https://doi.org/10.1016/j.coelec.2020.02.018. Elena V. Daboss is senior research fellow at Chemistry faculty of M.V. Lomonosov Moscow State University (MSU). She received Ph.D. at MSU in 2019 under the supervision of Prof. Arkady A. Karyakin. E.V. Daboss was awarded a scholarship of the President of the Russian Federation for young scientists and graduate students i n 2019–2021 and the Moscow Government Prize for Young Scientists in 2022. Her current research is focused on the development of advanced electrochemical (bio) sensors and nanozymes based on iron and nickel hex-acyanoferrates and elaboration of wearable electrochemical devices for noninvasive diagnostics. Elizaveta V. Shcherbacheva graduated from M.V. Lomonosov Moscow State University in 2021 with a degree in Fundamental and Applied Chemistry. In 2016, she joined the Laboratory of Electrochemical Methods where she worked with Dr. E.V. Daboss (Karpova) and Prof. A.A. Karyakin. In 2022, Elizaveta was awarded the gold medal for young scientists of the Russian Academy of Sciences. Her interests include synthesis of nano-zymes and their application in biosensorics. Arkady A. Karyakin is professor of chemistry, head of the Electrochemical Methods Laboratory, Chemistry faculty of M. V. Lomonosov Moscow State University (MSU). He graduated from MSU in 1981, receiving his Ph.D. and D.Sc. in 1986 and 1996, respectively. His research areas involve electrocatalysis and bioelectrocatalysis, conductive and electroactive poly-mers, electroanalysis (including the use of nano-electrode ar-rays) and biosensors, biofuel cells. In 2010–2014A.A. Karyakin served as Associate Editor for Electroanalysis (Wiley-VCH). From January, 1, 2015A.A. Karyakin became Associate Editor of Electrochemistry Communications (Elsevier). In 2011A.A. Karyakin was elected as a member of Academia Europaea. In 2012A.A. Karyakin was awarded with Bioelectrochemistry Prize of the International So-ciety of Electrochemistry (ISE), in 2014 became ISE Fellow.
确定





还剩5页未读,是否继续阅读?
雷迪美特中国有限公司为您提供《【PalmSens4电化学应用】核壳型纳米酶-氧化酶生物传感器,用于无创同时监测糖尿病和缺氧》,该方案主要用于其他中生化检验检测,参考标准--,《【PalmSens4电化学应用】核壳型纳米酶-氧化酶生物传感器,用于无创同时监测糖尿病和缺氧》用到的仪器有PalmSens4便携式电化学工作站
推荐专场
该厂商其他方案
更多
















