方案详情
文
据报道,许多微观生理系统成功地模拟了器官微环境。然而,目前只有少数系统专注于实时生理监测,用于候选药物的临床前细胞毒性评估。本文中介绍一个多传感器肺癌芯片平台,用于基于跨上皮电阻抗(TEER)的候选药物细胞毒性评估。ITO电极的优异透明度允许使用3D打印数字显微镜对芯片上的细胞进行视觉监测,在此之前从未报道过。采用光学pH传感器在线监测培养基pH值。作为概念验证,将癌症NCI-H1437细胞培养在玻璃基微流控芯片上,实时获取生物传感器数据。然后使用TEER阻抗传感器实时监测不同浓度药物阿霉素(DOX)和多西他赛的毒性。根据细胞指数(CI)评估TEER阻抗响应,而在实验结束时进行活/死测定以比较细胞活力。细胞指数评估表明,阿霉素浓度的增加导致的细胞死亡率高于多西他赛。药物治疗期间还记录了pH反应和显微镜图像。本文中开发的平台是一个很有前途的工具,用于未来微观生理系统的新药化合物的细胞毒性评估和特殊药物的开发。
方案详情

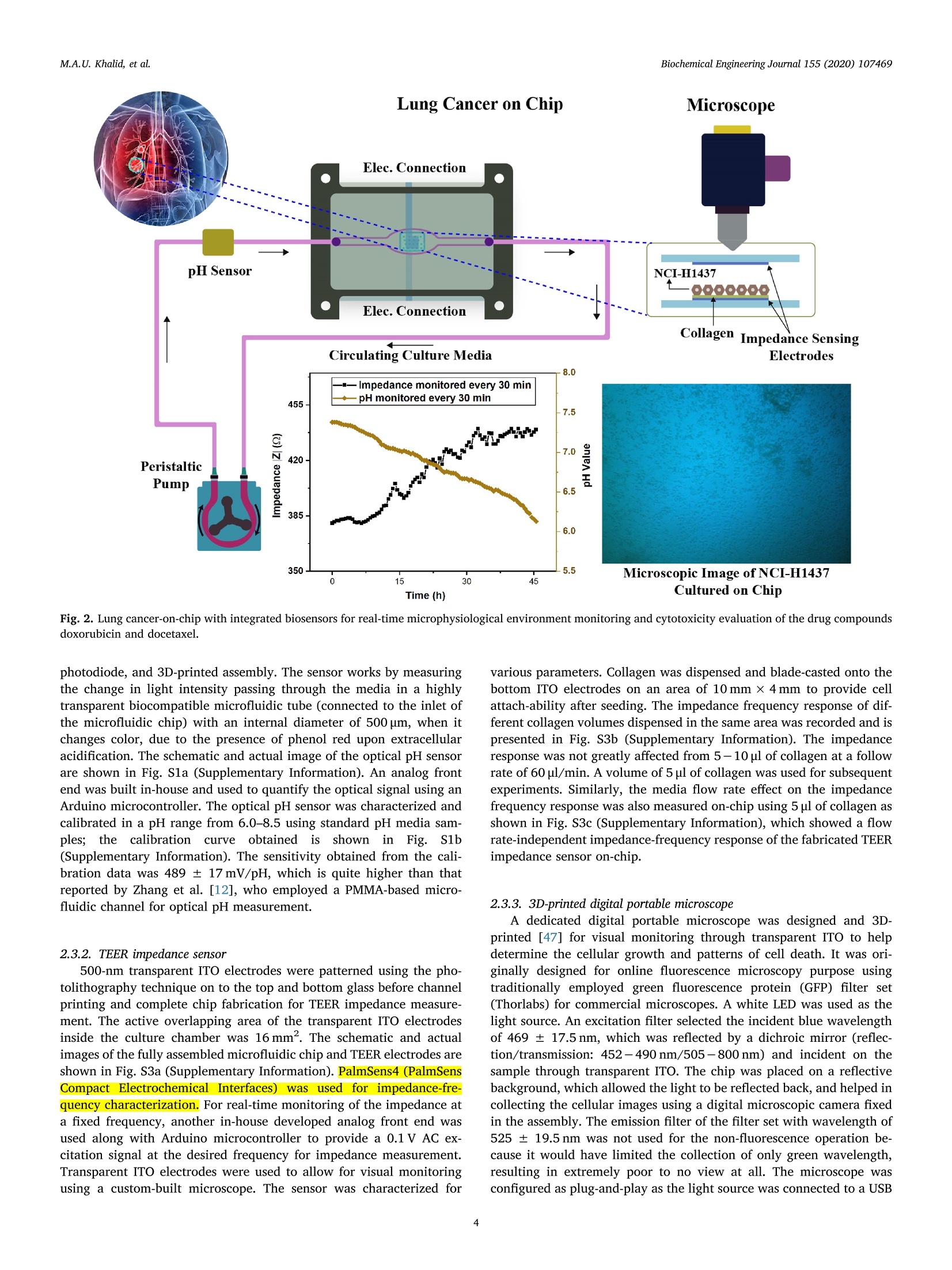
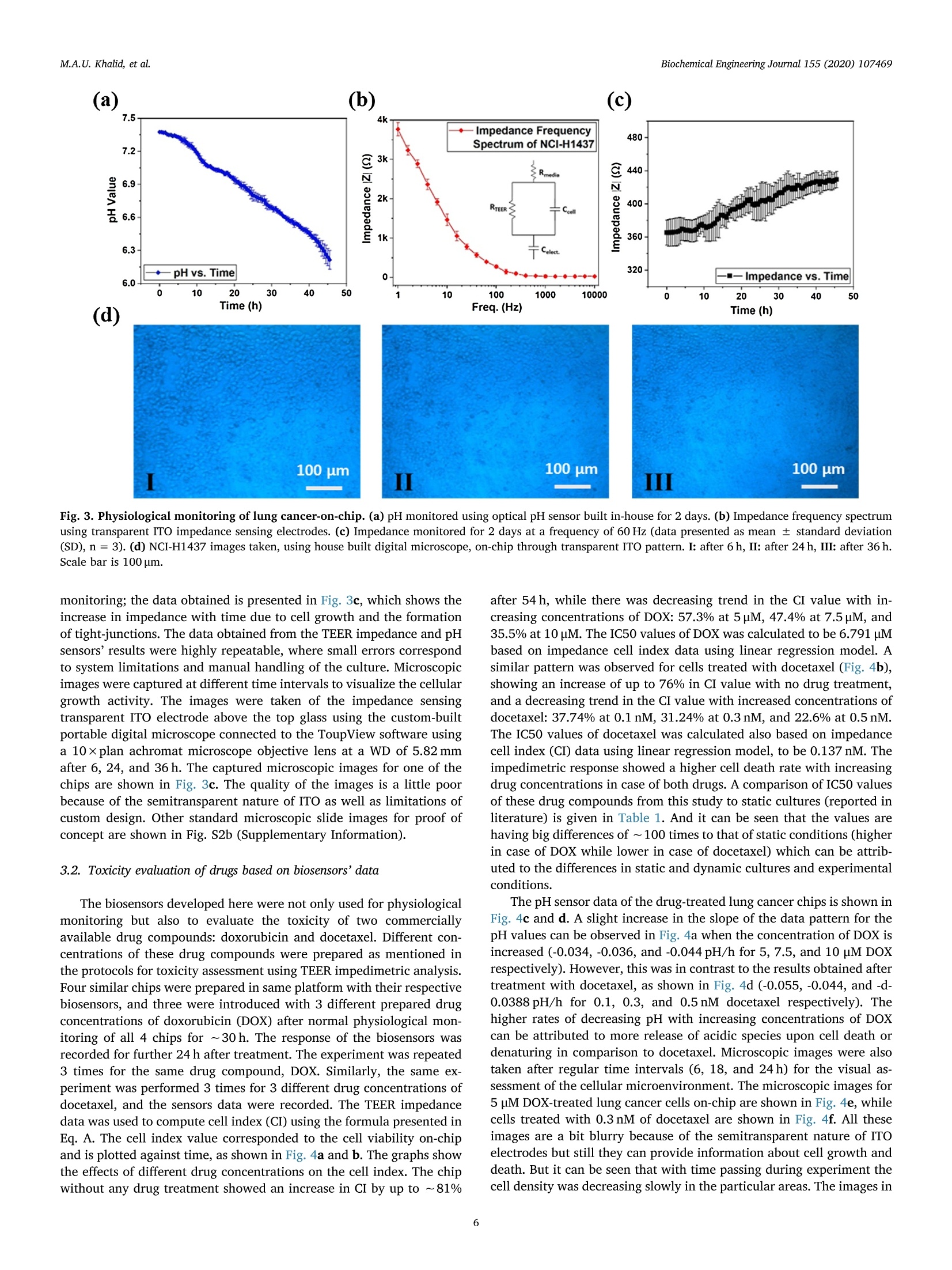
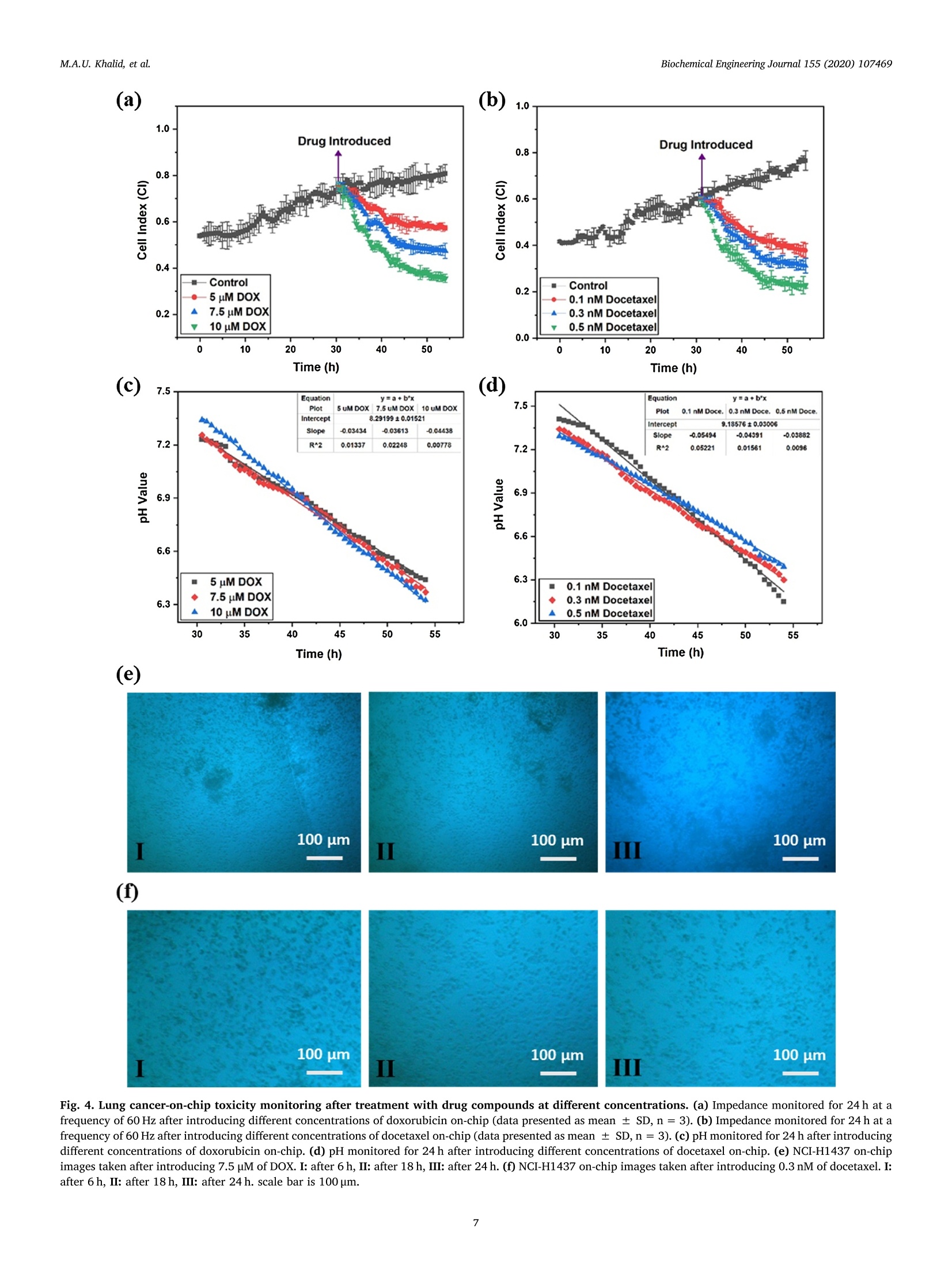
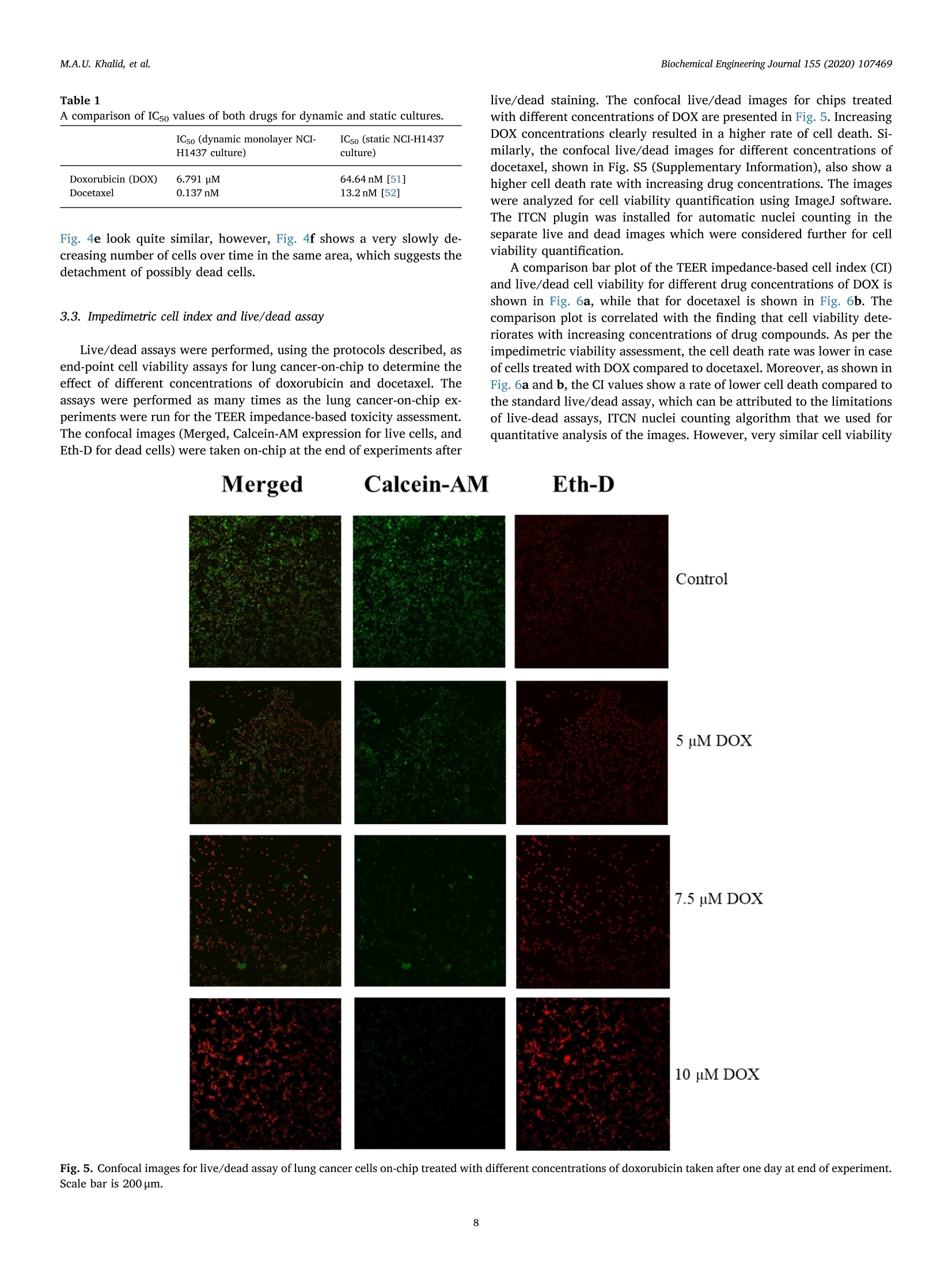
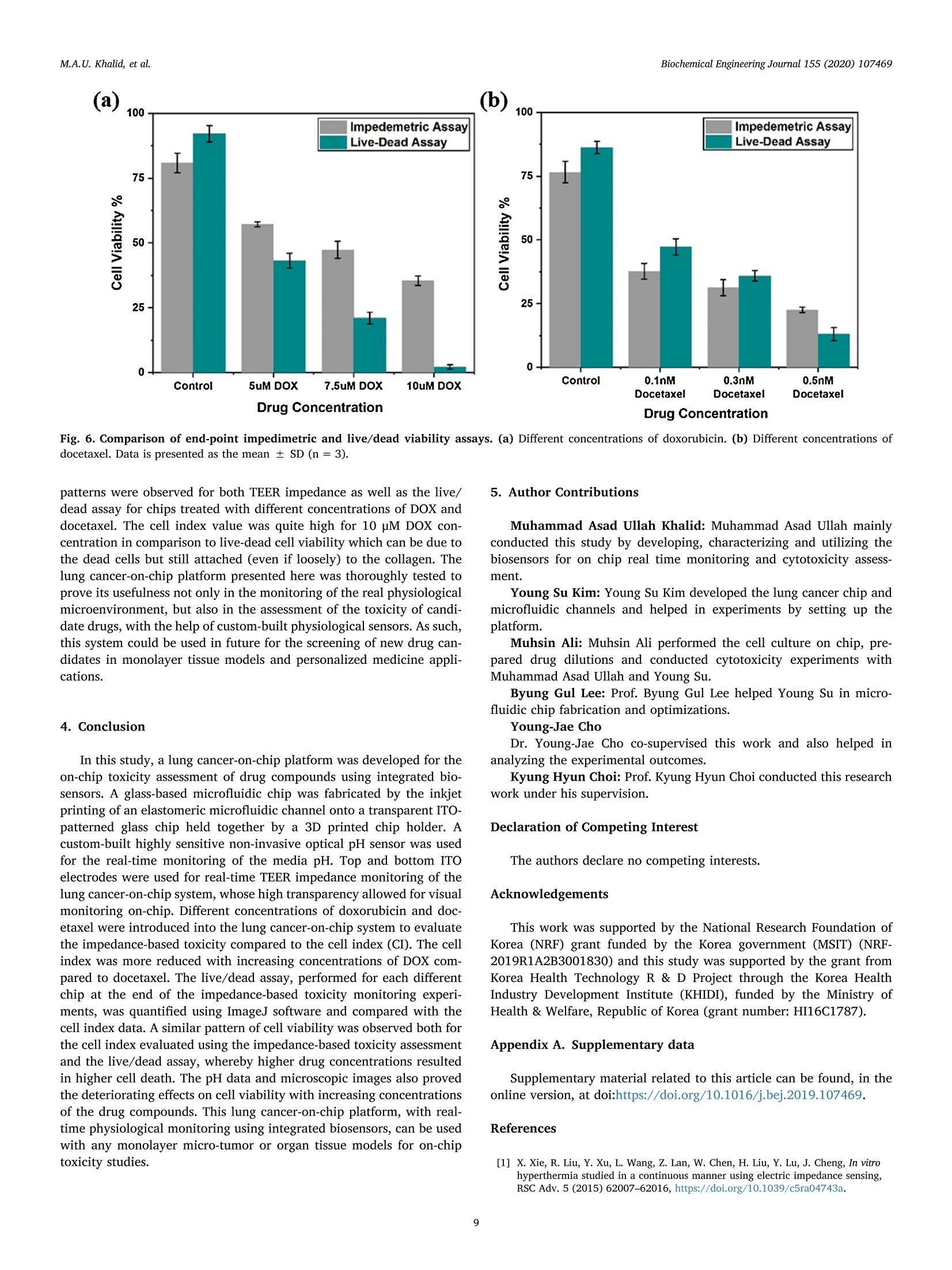
据报道,许多微观生理系统成功地模拟了器官微环境。然而,目前只有少数系统专注于实时生理监测,用于候选药物的临床前细胞毒性评估。本文中介绍一个多传感器肺癌芯片平台,用于基于跨上皮电阻抗(TEER)的候选药物细胞毒性评估。ITO电极的优异透明度允许使用3D打印数字显微镜对芯片上的细胞进行视觉监测,在此之前从未报道过。采用光学pH传感器在线监测培养基pH值。作为概念验证,将癌症NCI-H1437细胞培养在玻璃基微流控芯片上,实时获取生物传感器数据。然后使用TEER阻抗传感器实时监测不同浓度药物阿霉素(DOX)和多西他赛的毒性。根据细胞指数(CI)评估TEER阻抗响应,而在实验结束时进行活/死测定以比较细胞活力。细胞指数评估表明,阿霉素浓度的增加导致的细胞死亡率高于多西他赛。药物治疗期间还记录了pH反应和显微镜图像。本文中开发的平台是一个很有前途的工具,用于未来微观生理系统的新药化合物的细胞毒性评估和特殊药物的开发。 原文链接https://doi.org/10.1016/j.bej.2019.107469本文中进行电化学测试的仪器为荷兰PalmSens,型号:PalmSens4多通道电化学分析仪。需要了解更多电化学方面的知识,请关注我们或进入PalmSens中文网站:http://www.palmsens.cn/Biochemical Engineering Journal 155 (2020)107469journal homepage: www.elsevier.com/locate/bej M.A.U. Khalid, et alBiochemical Engineering Journal 155 (2020) 107469 Contents lists available at ScienceDirect Biochemical Engineering Journal Regular article A lung cancer-on-chip platform with integrated biosensors for physiological monitoring and toxicity assessment Muhammad Asad Ullah Khalid , Young Soo Kim , Muhsin Ali , Byung Gul Lee", Young-Jae Cho *, Kyung Hyun Choi ,** #Department of Mechatronics Engineering, Jeju National University, Republic of Korea Department of Civil Engineering, Jeju National University, Republic of Korea Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Bundang Hospital, Republic of Korea “BioSpero, Inc., Jeju-do, Republic of Korea HIGHLIGHTS · A lung cancer-on-chip platform was developed for real-time physiologica l monitoring. · All the biosensors were developed in-house including pH, and TEER impedance sensors. ·Transparent ITO electrode allowed for visual monitoring using 3D-printed microscope. · The toxicity of anticancer drugs doxorubicin and docetaxel was evaluated. · A comparison of impedimetric and live/dead cell viabilities was also analyzed. ARTICLEINFO ABSTRACT Keywords: Biosensors Real -time monitoring TEER impedance Lung cancer Drug tox i c i ty Cell index Numerous micro-physiological systems have been reported to successfully mimic the organ microenvironment .However, there are currently only a few systems that focus on r eal-time physiological monitoring for preclinical cytotoxicity assessment of drug candidates. We developed a multi -sensor lung cancer-on-chip platform for trans-epithelial electr i cal (TEER) impedance based cytotoxicity evaluation of drug candidates. The excellent trans-parency of ITO electrodes allowed for visua l monitoring of cells on chip using a 3D-printed digital microscope,which has not been previously reported. An optical pH sensor was used for online monitoring of media pH. As a proof of concept, lung cancer NCI-H1437 cells were cultured on glass-based microfluidic chip and biosensors data were obtained in real-time. The toxicity of different concentrations of drugs doxorubicin (DOX) and doc-etaxel was then monitored in real -time using the TEER impedance sensor. The TEER impedance response was evaluated in terms of cell index (CI), whereas a live/dead assay was performed for the comparison of cell viability at the end of the experiments. The cell index assessment suggested that the increasing concentrations of doxorubicin resulted in a higher cell death rate than docetaxel. The pH response and microscopic images were also recorded during drug treatment. The platform we developed here, i s a promising tool for the cytotoxicity evaluation of novel drug compounds for future micro-physiological systems and development of personalized medicine. Micro-physiological systems are constantly evolving thanks to the emergence of advanced technologies able to accurately mimic the in vivo microenvironment. However, to maintain a suitable environment for in-vivo l i ke growth and functionality of these systems , there is a great need to monitor their physiological state in real-time. Real-time monitoring is not only useful to determine the status of the micro-physiological systems, but can also provide information to evaluate its response to different external stimuli, such as deteriorating physiolo-gical parameters or the cytotoxicity evaluation of drug compounds [1-12]. Traditional drug development studies involve animal testing in *Co-corresponding author. **Corresponding author at: Department of Mechatronics Engineering, Jeju National University, Republic of Korea E-mail address: amm@je j unu.ac.kr (K.H. Choi ). https://doi.org /10.1016/j .bej .2019.107469 1369-703X/ C 2019 Published by Elsevier B.V. order to analyze the cytotoxicity and efficacy of drug candidates which haven’t served the purpose well due to their dissimilarities with the actual human physiology as well as growing ethical concerns. Animal testing of cosmetics has recent l y been banned on ethical grounds in Europe [13]. Cytotoxicity assays are an indispensable prerequisite for clinical use, where t he evaluation of the toxicity of drug candidates i n micro-physiological systems using integrated biosensors for high throughput real-t i me analysis is necessary before they can be used for clinical purposes. Zhang et al. reported a multi-sensor multi organ-on-a-chip platform used for real-time monitoring and also demonstrated the evaluation of toxicity using electrochemical biosensors. [12] However trans-epithelial electrical (TEER) i mpedance and electrical cell substrate impedance sensing (ECIS) are important parameters that have been used for real time bio-i mpedance monitoring in addition to cell viabi l i ty assessment [1-3,6-8,11,12,14-22]. Trans-epithelial elec-trical resistance or impedance quanti f ies the tightness of junctions or barriers between cells that can be affected by the passage number of cells and flow rate, among others [23]. Most of the reported literature [1,4,6,15,18,22-25]have ut i lized commercial systems, including EVOM2, Mi ll icell ERS, AutoLab potentiostat , and Roche xCELLigence real-time cell analysis (RTCA) system, for the measurement of TEER impedance or ECIS. TEER impedance and ECIS have been fairly well developed in recent decades for use in non-invasive real-time mon-itoring of cell viability and growth, as well as for the assessment of the toxicity of drug compounds [2,4,6,10,22], in comparison to costly and laborious end-point viability assays, such as l ive/dead and MTT assays,which require fluorescence staining and confocal microscopy. Baba-hosseini et al . have reported using single cell bioelectrical impedance to characterize the effects of sphingosine kinase inhibitors (SphKIs) on MDA-MB-231 (human epithelia l breast cancer) cell line, and compared the effectiveness of drug delivery using poly lactic-co-glycolic acid (PLGA) nanoparticles (NPs) loaded with SphKIs versus conventional delivery [4]. Caviglia et al. demonstrated the cytotoxic effect of antic-ancer drugs doxorubicin (DOX), oxaliplatin (OX) as well as OX-loaded liposomes us i ng real- time impedance monitoring on HeLa (i mmortal)and HT1080 (fibrosarcoma) cel l l ines [26]. Li et al. reported the drug (digoxin as model drug combined with colestyramine or Verapami l )absorption related assessment of nephrotoxicity in intestine-kidney chip model using TEER analysis [27]. Hondroulis et al. confirmed the low toxicity of graphene nanomater i als in i n-vitro blood brain barrier (BBB)model and its individual cel l components with the help of TEER as-sessments [28]. Xiao and Luong developed an ECIS for measuring the concentration and time response function of fibroblastic V79 cells ex-posed to mercury chloride and 1,3,5-trinitrobenzene (TNB) [29].Rothbauer et al. have demonstrated the cytotoxicity effect of silica nanoparticles using impedimetric analysis on the vitality and re-generative capabi l ity of human lung adenocarcinoma cells upon acute and chronic exposure scenarios [16]. In most of the studies, ECIS is mostly used with interdigitated electrodes (IDEs) or microelectrode arrays (MEAs) excited at higher frequency (in kHz) AC signals on which cells are cultured for impedi-metric measurement, while for TEER impedance, the top and bottom electrode pair or set of pairs were used for impedance measurement using low frequency (10 s of Hz) AC signals. Depending on the chip and electrodes design, any of these techniques can provide similar in-formation on cell viability. Media pH is also an important parameter for determining the physiological state of the culture, measuring the levels of extracel l ular acidification when the ce l ls are metabolizing [30].Various electrochemical and optica l microf l uidic pH sensors have been developed for micro-physiological systems [31-34]. Combined with pH and TEER impedance sensor, on-chip visual monitoring can also pro-vide useful information. By observing the detaching dead or metastatic cells due to loss or rearrangement of intercellular tight junctions [3,35-41], which are also responsible for a confluent monolayer cul-ture, the integrity of the cellular barriers can be assessed qualitatively.For example, Chawla et al . [42] used and proved the usefulness of an integrative approach to i mpedance and visual growth rate monitoring of a microfluidic cell culture. While there are certain limitations in-volved in using TEER impedance and visual monitoring together, this problem can be solved using transparent impedance sensing electrodes,such as the transparent ITO electrodes, which have already been used for ECIS measurement [5,9] in microfluidic devices for cytotoxicity assessments. In this study, we present a micro-physiological system with in-tegrated biosensors for lung cancer-on-chip real-time monitoring and cytotoxicity evaluation of drug compounds in a dynamic micro-en-vironment using state-of-the-art organ-on-a-chip approach . The glass -based microfluidic chip with embedded top and bottom indium t i n oxide (ITO)-based TEER i mpedance sensing electrodes was prepared in-house by inkjet pr i nting the elastomeric microfluidic channel onto the lower side of the top glass. NCI-H1437 lung cancer cel l s were cultured on the bottom glass ITO area using a customized seeding kit after dis-pensing collagen on it for cel l adhesion. A highly sensi t ive non-invasive optical pH sensor, along with a TEER impedance sensor, and a 3D-printed dedicated digital fluorescence type microscope were used for real-t i me monitoring. The optical pH sensor and ITO-based impedance sensor with their respective analog front ends were developed in-house.The 3D-printed digital microscope was also custom developed for the visual monitoring of the cel l s on the chip. ITO was used for the im-pedance sensing electrodes due to i ts excellent transparency, which allowed for the visual monitor i ng of the cel l monolayer from the top us i ng a custom-bui l t digital microscope, which has not been previously reported for use in lung cancer cytotoxicity assessments. Doxorubicin and docetaxel were used in the cytotoxicity studies of our lung-on-chip platform as they were readily available and have been previously used as anticancer drugs for lung cancer treatment [43-45].Different drug concentrations were prepared and introduced into mul-tiple chips prepared using same protocols and the timely response of the biosensors was recorded. The data obtained from the pH sensors showed a rapid extracellular acidification due to increasing cell death and an increased release of acidic molecules wi t h increasing drug concentrations. The TEER impedance data converted to the cel l index (CI) (normalized impedance values) showed that an increase in the drug concentrations caused higher cell death rates. Increasing doxorubicin concentrations were found to be more toxic in comparison to docetaxel,according to the cel l index measurements. The hal f maximal inhibitory concentrations (ICso) were also calculated for both drugs using linear regression model of cell index data. The CI response was complemented by the standard l ive/dead assay, performed at the end of the on-chip experiments. The comparison between the two different viabi l i ty assays suggested that real-t ime impedance-based CI could be used as the technique of choice for future drug screening systems as an ini t ial cy-totoxicity evaluation. The system developed here could also be used for different organ-on-a-chip toxicology studies and personalized medicine in future. 2. Materials and methods 2.1. Reagent and chemicals ·Cel l cul t ure: NCI-H1437 lung cancer cell l i ne was obtained from Korea Cell Line Bank. RPMI 1640 cell culture media (cat#11875093), fetal bovine serum (FBS) (cat# 16000044), Dulbecco's phosphate buffered sal i ne (DPBS) (cat# 14190144), 0.05% Trypsin-EDTA (cat# 25300054), and penic i llin/streptomycin (P/S) for cell culture (cat # 15070063) were purchased from ThermoFisher (USA). Collagen, type I solution from rat tail (cat# C3867-1VL) was purchased from Sigma-Aldrich (South Korea). · Cell assay: LIVE/DEAD Z-CYTOX Live/dead viabi l ity/cytotoxicity kit for mammalian cells (cat# ez-1000) was purchased from DAEI-LLAB Service, South Korea. Trypan blue stain 0.4% (cat# T13001)was supplied by Logo Biosystems (South Korea). Fig. 1. Microfluidic glass chip fabrication. (a) Expanded schematic view (b) Schematic assembly-top view and cross sec t ion view (c) 3D channel pr i nting system and print head (d) Origina l microfluidic chip images - separate parts and assembled chip. ·Drugs: Doxorubicin hydrochloride CAY-15007-3 (cat# A62690)manufactured by Cayman Chemical Company, sales and delivery of Life Technologies Korea LLC was purchased from Thermo Fisher Scient i fic (Korea). Docetaxel (cat#2232) was bought from BioVision (USA). Dimethyl sulfoxide (DMSO) for molecular biology (cat# 472,301) was bought from Sigma-Aldrich (South Korea). 2.2. Microfluidic chip fabrication and platform setup A microfluidic chip was fabricated using two soda lime glass layers (with pre-patterned ITO electrodes for TEER impedance measurement ),at the top and bottom each 1.1 mm thick, 56-mm long, and 41-mm wide. Nusil medical grade silicone (MED-6033) elastomer was used to print the microfluidic channel on the bottom of the top glass using an inkjet printing system. The 3D printing system actually uses pneumat i c dispensing to discharge the biocompatible sil i cone elastomer through a nozzle to make microfluidic patterns onto the glass substrate. The si l i-cone elastomer was prepared using the company suggested composition and degassed before loading into the syringe of the printing head. An expanded and assembled schematic view of the fabricated microfluidic chip is shown in F ig. l a and b. The channel printing parameters of the 3D print i ng system (as shown in Fig. 1c) were adjusted as follows:nozzle inner diameter - 200 um, standoff distance between nozzle and substrate - 100 um, x-y stage velocity -2 mm/s, and pneumatic pres-sure - 140kPa. The fabricated microfluidic channel had a width of ~500 um, and a culture chamber width of ~6.5 mm, while the pattern width and height were 800 um and 300 um respectively. The micro-fluidic channel was printed on the lower side of the top glass, while the microfluidic connectors were glued on t he upper side of the top glass to provide external fluidic routings. A 3D-pr i nted chip holder was de-signed to hold the top and bottom glasses together. Initial l y there were leakage problems nearthee microfluidic connectors so poly-dimethylsiloxane (PDMS) gaskets were printed around the holes in top glass before gluing the microfluidic connectors which provided better sealing. Actual images of the prepared top and bottom glass and the microfluidic chip assembly are provide i n Fi g. 1d. Cleaning procedures were carried out before using the microfluidic chip. Soda lime glass was used over traditional PDMS and poly-methyl-methacrylate (PMMA) for microfluidic chip fabrication because of its non-permeabil i ty to outer environmental gas and chemical molecules in comparison. The fabri-cated microfluidic chip was seeded with lung cancer cells and run by connecting external fluidics using a peristaltic pump with a media flow rate of 60 pl/min. Usually the media flow rates for micro-physiological systems are usually set in the range of 10-100 ml/h with channel thickness: 100-300 um [46]. However the selected flow rate was opti-mized for proper functioning of our microfluidic chip. The system was enclosed in a custom-bui l t incubator whose CO2 and temperature were regulated at 5% and 37℃, respectively, by a feedback controller. In-house bui l t integrated sensors provided real-time monitoring informa-tion of the microphysiological environment. A schematic representation of the system is shown in F ig . 2. An image of the actual representative lung cancer on-chip-platform, with all the system components high-lighted, i ncluding peristaltic micro-pump, media reservoir, microfluidic chip, incubator platform, pH sensor, and digita l microscope, is shown i n Fig. S4 (Supplementary Information). 2.3. Integrated biosensors Various biosensors can provide information about the micro-physiological environment, however, pH and impedimetric biosensors were chosen as the most suitable for real -time monitoring and toxicity assessment [30] because of their ease of implementation. All the bio-nsors were developed in-house, including the digital portable mi -croscope for microenvironment real-time monitoring of lung cancer-on-chip, and were later used for the toxicity assessment of the commer-cially available drug compounds doxorubicin and docetaxel. 2.3.1. pH sensor An optical pH sensor was developed for non-invasive pH monitoring of the culture media. The sensor was developed us i ng commercial l y available electronic components, a white LED, an optical filter, Fig.2. Lung cancer-on-chip with integrated biosensors for real -time microphysiological environment monitoring and cytotoxicity evaluation of the drug compounds doxorubicin and docetaxel. photodiode, and 3D-printed assembly. The sensor works by measur i ng the change in light intensity passing through the media in a highly transparent biocompatible microfluidic tube (connected to the inlet of the microfluidic chip) with an internal diameter of 500 um, when it changes color , due to the presence of phenol red upon extracellular acidification. The schematic and actual i mage of the optical pH sensor are shown in Fig. Sla (Supplementary Information). An analog front end was built in-house and used to quantify the optical signal using an Arduino microcontroller. The optical pH sensor was characterized and calibrated in a pH range from 6.0-8.5 using standard pH media sam-ples; the calibration ncurve obtained isshown in F Fig. S1b (Supplementary Information). The sensitivity obtained from the cali-bration data was 489 ± 17 mV/pH, which is quite higher than that reported by Zhang et al. [12], who employed a PMMA-based micro-fluidic channel for optical pH measurement. 2.3.2. TEER impedance sensor 500-nm transparent ITO electrodes were patterned using the pho-tolithography technique on to the top and bottom glass before channel printing and complete chip fabrication for TEER impedance measure-ment. The active overlapping area of the transparent ITO electrodes inside the culture chamber was 16 mm. The schematic and actual images of the fully assembled microfluidic chip and TEER electrodes are shown in Fig. S3a (Supplementary Information). PalmSens4 (PalmSens Compact t Electrochemical1 Interfaces)) was used for impedance-fre-quency characterization. For real-time monitoring of the i mpedance at a fixed frequency , another in-house developed analog front end was used along with Arduino microcontroller to provide a 0.1V AC ex-citation signal at the desired frequency for impedance measurement.Transparent ITO electrodes were used to allow for visual monitoring using a custom-bui l t microscope. The sensor was characterized for various parameters. Collagen was dispensed and blade-casted onto the bottom ITO electrodes on an area of 10 mm × 4mm to provide cell attach-ability after seeding. The impedance frequency response of dif-ferent collagen volumes dispensed in the same area was recorded and is presented in Fig. S3b (Supplementary Information). The impedance response was not greatly affected from 5-10 ul of collagen at a follow rate of 60 ul/min. A volume of 5 ul of collagen was used for subsequent experiments. Simi l arly, the media flow rate effect on the impedance frequency response was also measured on-chip using 5 pl of collagen as shown in Fig. S3c (Supplementary Information), which showed a flow rate-independent impedance-frequency response of the fabricated TEER impedance sensor on-chip. 2.3.3. 3D-printed digital portable microscope A dedicated digital portable microscope was designed and 3D-printed [47] for visual monitoring through transparent ITO to help determine the cellular growth and patterns of cell death. It was ori-ginally designed for online f l uorescence microscopy purpose using traditionally employed green f l uorescence protein (GFP) filter set (Thorlabs) for commercial microscopes . A white LED was used as the light source. An excitation filter selected the incident blue wavelength of 469 ±17.5 nm, which was reflected by a dichroic mirror (reflec-tion/transmission: 452-490 nm/505-800nm) and incident on the sample through transparent ITO. The chip was placed on a ref l ective background, which allowed the light to be reflected back, and helped in col l ecting the cellular images using a digital microscopic camera fixed in the assembly. The emission f i l ter of the f i lter set with wavelength of 525±19.5nm was not used for the non-fluorescence operation be-cause it would have limited the collection of only green wavelength,resulting in extremely poor to no view at all. The microscope was configured as plug-and-play as t he light source was connected to a USB connect i ng the camera to the PC. ToupView software was used to take the on-chip images of the lung cancer after certain ti me intervals. The 3D design and original microscope images are shown in Fig. S2a (Supplementary Information). We originally employed a 10× plan achromat microscopic objective as magnification lens for viewing at a working distance (WD) of 5.82 mm from the sample for our application,however, it was also able to take images at 20 ×resolution at a 1.13 mm working distance. The 10 ×lens was used for ease of larger WD and to cover a wider field of view of the lung cancer cells on-chip. 2.4. Experimental protocols 2.4.1. Cell culture and real-time monitoring NCI-H1437 lung cancer cells were cultured in freshly prepared ali-quots of RPMI 1640 cel l culture medium supplemented with 10% FBS v/v and 1% v/v Pen/Strep antibiotic solution. NCI -H1437 cells were cultured in a humidi f ied cell culture incubator with 5% CO2 at 37℃.NCI-H1437 cells were expanded by up to three passages before seeding on a microfluidic chip. Ce l ls were washed every 48 h with pre-warmed DPBS solution for the removal of cellular metabolites and cell debris,including dead cells. Cells at 90% confluence were trypsinized using 0.05% Trypsin-EDTA solution. The cel l pellet was re-suspended in an appropriate amount of 10% RPMI medium for seeding on-chip. The ITO-coated glass chip was sterilized using iso-propyl alcohol, then rinsed three times with deionized water. The chip was air-dried and UV-radiated i n a biosafety cabinet for 3 h. The cell cul t ure area of the ITO glass chip was coated with 5 pl of collagen type I using a micro pipette,then blade casted on 40 mm² culture area above the printed ITO pat-tern, before allowing the collagen to polymerize for 20 min. Although any biocompatible material for cell attachment , such as PLGA, used for sensing applicat i ons [48,49], as well as dielectric onto the ITO, could have been used, collagen was preferred due to its inherent matrix structures. The chip was assembled in a customized cell seeding kit where 1000 ul of 1.5 × 10 cells/ml NCI-H1437 cell suspension was seeded. The seeding kit was stored overnight in a humidified cell cul-ture incubator wi t h 5% CO2 at 37℃ for cel l attachment to the collagen-coated surface of the ITO glass chip. On the following day, the media was removed and the chip was placed on rubber bands of a customized metallic chip holder. The top ITO glass chip was placed on the upper side of the previous chip. The second chip had an inkjet-printed elas-tomeric microfluidic channel and microfluidic connectors for cel l cul-ture media routing. The screws of the chip holder were tightened and the chip was placed inside the microphysiological platform for further experiments. T he tubing was attached to the channel after pass i ng through the pH sensors assembly for media flow through microfluidic connectors. The standard cell culture parameters were set for cell monolayer formation in the chip and the TEER electrodes were con-nected to an externa l analog front end for real -t i me monitoring. All of the chips used in further experiments were prepared following the same protocol. The chips were periodically monitored for monolayer for-mation using the in-house built microscope. The normal culture was observed for 2 days using pH and TEER impedance sensors, with re-sponses recorded along with microscopic images. The pH was mon-itored every 30 min, as was TEER impedance at a 60 Hz AC excitation frequency, while the microscopic images were taken after longer in-tervals of time, namely 6, 24, and 36 h, to observe any significant changes. The frequency of the TEER excitation source was fixed low at 60 Hz because parameters were measured using TEER impedance ap-proach and not ECIS, the latter of which is usually measured at higher frequencies. 2.4.2. Drug treatment and toxicity assessment Doxorubicin hydrochloride powder was weighed using a precision weighing balance (OHAUS Adventurer model #AR2140; OHAUS, USA)and a standard solution of 1 mM was prepared i n double disti l led water.Briefly, 1 mM of standard solution of doxorubicin was further di l uted in 5,7.5 and 10 uM concentrations i n RPMI media. Docetaxel was dis-solved in DMSO and a standard solution of 1 uM was prepared. This standard solution of docetaxel was further diluted in RPMI media and 0.1,0.3, and 0.5 nM concentrations were prepared. Different con-centrations of doxorubicin and docetaxel were introduced in the chip via cel l culture media reservoir (discarding the previous media) after ~30 h of normal culture monitoring. Cells were observed for toxicity for 24 h. Impedance at a fixed low excitation frequency of 60 Hz and pH were monitored every 30 min for every chip treated with each different drug concentrations . The normalized impedance response was recorded as cell index (CI) [2,10] for the of impedance-based cel l viabi l ity eva-luations. The cell index was calculated as follows: Where, I Z|t = Impedance magnitude of the chamber with cells at t i me t |Z|。= Impedance magnitude of the chamber without cellsimp The microscopic images were taken after 6, 18, and 24 h for visual assessment of the lung cancer-on-chip after treatment wi t h different concentrations of doxorubicin and docetaxel . 2.4.3. Live-dead assay The manufacturer's instructions were followed and the cell culture area was covered with 300 pl of EZ-Cytox solution after thoroughly washing with DPBS. The chip was then incubated in a humidified cell culture incubator with 5% CO2 at 37℃ for 60 min. The chip was ex-amined under confocal laser scanning microscopy (CLSM) at an ex-citation of 530~560nm and emission of 590~620nm (model #FV1200; Olympus, Japan) at 10×resolution. Chips treated with dif-ferent drug concentrations (5, 7.5, and 10 uM of doxorubicin and 0.1,0.3, and 0.5 nM of docetaxel) were assessed at the end of the real-time TEER impedance monitoring experiments and analyzed in live-dead assays. The images were analyzed using ImageJ software for cell via-bi l ity quantification by instal l ing the ITCN ImageJ plug-in for nuclei counting. The resulting outcomes were compared to the impedimetric cell index (CI). 3. Results and discussion 3.1. Real time monitoring of lung cancer-on-chip A lung cancer-on-chip platform (Fig. 2) was developed and mon-itored for its microphysiological environment in real-t i me using the custom-bui l t biosensors already described. The optical pH sensor,shown in Fig . 2, was implemented at the inlet of the microfluidic chip.The data of the culture media pH was recorded every 30 min for 2 days.The pH sensor data is presented in Fi g. 3a for three similar chips shown using areas of different colors (± standard error close to average re-sponse in blue). The data clearly shows a decrease in the media pH over time due to extracel l ular acidi f ication [30,31,50]. Similarly, the im-pedance frequency response was recorded for three different lung cancer chips and showed a typical exponential response when the im-pedance vs. f requency (logio) data was plotted. The frequency response is shown in Fi g. 3b , along with the equivalent circuit of measured i m-pedance values. The equivalent circuit describes the model parameters like transepithelial electrical resistance (RTEER), cell capacitance (Ccel l ),solution resistance (Rmedia) and electrode capacitance (Ce l ect). At low frequencies the RTEER is dominant while at high frequencies the Rmedia*The background impedance (comprising of Rmedia and Celect ) is sub-tracted from overall impedance obtained to calculate the actual cell monolayer impedance [18]. The parameters of the monolayer im-pedance (RTEER and Ccell) were not evaluated individually in this study as the in house built device had limitation of measuring overall im-pedance magnitude only. The TEER impedance sensor data at a fi xed frequency of 60 Hz was recorded along with pH for real -t i me Fig. 3. Physiological monitoring of lung cancer-on-chip. (a) pH monitored using optical pH sensor built in-house for 2 days. (b) Impedance frequency spectrum using transparent ITO impedance sensing electrodes. (c) Impedance monitored for 2 days at a frequency of 60 Hz (data presented as mean ± standard deviation (SD), n=3). (d) NCI-H1437 images t aken, using house built digital microscope, on -chip through transparen t ITO pattern. I: after 6 h, II: after 24 h, III: after 36 h.Scale bar is 100 um. monitoring; the data obtained is presented in F i g. 3c, which shows the increase in impedance with time due to cell growth and the formation of tight-junctions. T he data obtained from the TEER impedance and pH sensors’results were highly repeatable, where small errors correspond to system limitations and manual handling of the culture. Microscopic images were captured at di f ferent time intervals to visualize the cel l ular growth activity. The i mages were taken of the impedance sens i ng transparent ITO electrode above the top glass using the custom-bui l t portable digital microscope connected to the ToupView software using a 10×plan achromat microscope objective lens at a WD of 5.82 mm after 6, 24, and 36 h. The captured microscopic images for one of the chips are shown in Fi g. 3c. The quality of the images is a little poor because of the semitransparent nature of ITO as well as limitations of custom design. Other standard microscopic slide images for proof of concept are shown in Fig. S2b (Supplementary Information). 3.2. Toxicity evaluation of drugs based on biosensors’data The biosensors developed here were not only used for physiological monitoring but also to evaluate the toxicity of two commercially available drug compounds: doxorubicin and docetaxel. Different con-centrations of these drug compounds were prepared as mentioned in the protocols for toxicity assessment using TEER impedimetric analysis.Four similar chips were prepared in same platform with thei r respective biosensors, and three were introduced with 3 different prepared drug concentrations of doxorubicin (DOX) after normal physiological mon-itoring of all 4 chips for ~30 h. The response of the biosensors was recorded for further 24 h after treatment. The experiment was repeated 3 times for the same drug compound, DOX. Similarly, the same ex-periment was performed 3 times for 3 different drug concentrations of docetaxel, and the sensors data were recorded. The TEER impedance data was used to compute cell i ndex (CI) using the formula presented in Eq. A. The cell index value corresponded to the cell viability on-chip and i s plotted against time, as shown in Fig. 4a and b. The graphs show the effects of different drug concentrations on the ce ll i ndex. The chip without any drug treatment showed an increase in CI by up to ~81% after 54 h, while there was decreasing trend in the CI value with in-creasing concentrations of DOX: 57.3% at 5 pM, 47.4% at 7.5 pM, and 35.5% at 10 uM. The IC50 values of DOX was calculated to be 6.791 uM based on impedance cell index data using linear regression model . A similar pattern was observed for cells treated with docetaxel (Fig . 4b),showing an increase of up to 76% in CI value with no drug treatment,and a decreasing trend in the CI value with increased concentrations of docetaxel: 37.74% at 0.1 nM, 31.24% at 0.3 nM, and 22.6% at 0.5 nM.The IC50 values of docetaxel was calculated also based on impedance cell index (CI ) data using linear regression model, to be 0.137 nM. The impedimetric response showed a higher cel l death rate with increasing drug concentrations i n case of both drugs. A comparison of IC50 values of these drug compounds from this study to static cultures (reported in l i terature) is given in Table 1. And it can be seen that the values are having big differences of ~100 times to that of static conditions (higher in case of DOX while lower in case of docetaxel) which can be attrib-uted to the differences in static and dynamic cultures and experimental conditions. The pH sensor data of the drug-treated lung cancer chips is shown i n F i g. 4c and d. A slight increase in the slope of the data pattern for the pH values can be observed in F ig. 4a when the concentration of DOX is increased (-0.034,-0.036, and -0.044 pH/h for 5, 7.5, and 10 uM DOX respectively ). However, this was in contrast to the results obtained after treatment with docetaxel, as shown in Fig. 4d (-0.055,-0.044, and-d-0.0388 pH/h for 0.1, 0.3, and 0.5 nM docetaxel respectively). The higher rates of decreasing pH with increasing concentrations of DOX can be attributed to more release of acidic species upon cel l death or denaturing in comparison to docetaxel. Microscopic images were also taken after regular time intervals (6, 18, and 24 h) for the visual as-sessment of the cellular microenvironment . The microscopic images for 5 uM DOX-treated lung cancer cel l s on-chip are shown in Fig. 4e, while cells treated with 0.3 nM of docetaxel are shown in Fig. 4f. All these images are a bit blurry because of the semitransparent nature of ITO electrodes but st i ll they can provide information about cell growth and death. But it can be seen that with time passing dur i ng experiment the cell density was decreasing slowly in the particular areas. The i mages i n (a) (b)1.0 1.0· Fig. 4. Lung cancer-on-chip toxicity monitoring after treatment with drug compounds at different concentrations. (a) Impedance monitored for 24 h at a frequency of 60 Hz after introducing different concentrations of doxorubicin on -chip (data presented as mean ± SD,n= 3). (b) Impedance monitored for 24 h at a frequency of 60 Hz after introducing different concentrations of docetaxel on-chip (data presented as mean ± SD,n = 3). (c) pH monitored for 24 h after introducing di f ferent concentrations of doxorubicin on-chip. (d) pH monitored for 24 h after introducing different concentrations of docetaxel on-chip. (e) NCI-H1437 on-chip images taken after introducing 7.5 uM of DOX. I : after 6 h, II: after 18 h, ⅢI: after 24 h. (f) NCI-H1437 on-chip images taken afte r i ntroduc i ng 0.3 nM of docetaxel. I:after 6 h, II: after 18 h, ⅢI: after 24 h. scale bar is 100 um. Table 1 A comparison of IC s o values of both drugs for dynamic and static cultures. ICso (dynamic monolayer NCI-H1437 culture) IC5o (static NCI-H1437 culture) Doxorubicin (DOX) Docetaxel 6.791 uM 64.64nM [51] 13.2nM [52] 0.137nM Fig . 4e look quite similar, however, Fig . 4f shows a very slowly de-creasing number of cells over t ime in the same area, which suggests the detachment of possibly dead cel l s. 3.3. Impedimetric cell index and live/dead assay Live/dead assays were performed, using the protocols described, as end-point cell viability assays for lung cancer-on-chip to determine the effect of different concentrations of doxorubicin and docetaxel. The assays were performed as many times as the lung cancer-on-chip ex-periments were run for the TEER impedance-based toxicity assessment .The confocal images (Merged, Calcein-AM expression for live cells, and Eth-D for dead cells) were taken on-chip at the end of experiments after l i ve/dead staining. The confocal live/dead images for chips treated with different concentrations of DOX are presented in Fi g. 5. Increasing DOX concentrations clearly resulted in a higher rate of cell death. Si -milarly, the confocal live/dead images for different concentrations of docetaxel, shown in Fig. S5 (Supplementary Informat i on), also show a higher cell death rate with increasing drug concentrations. The images were analyzed for cell viabi l ity quantification using ImageJ software.The ITCN plugin was instal l ed for automatic nuclei count i ng in the separate live and dead images which were considered further for cell viability quanti f ication. A comparison bar plot of the TEER impedance-based cell index (CI)and l ive/dead ce l l viabi l ity for different drug concentrations of DOX is shown in Fig. 6a, while that for docetaxel is shown in Fig. 6b. The comparison plot is correlated with the finding that cell viability dete-riorates with increasing concentrations of drug compounds. As per the impedimetric viabil i ty assessment , the cell death rate was lower in case of cells treated with DOX compared to docetaxel. Moreover, as shown in Fi g.6a and b, the CI values show a rate of lower cell death compared to the standard l ive/dead assay, which can be attributed to the l i mitations of live-dead assays, ITCN nuclei counting algorithm that we used for quant i tative analysis of the images. However, very similar cel l viability Fig. 6. Comparison of end-point impedimetric and live/dead viability assays. (a) Different concentrations of doxorubicin. (b) Different concentrations of docetaxel. Data is presented as the mean ± SD (n=3). patterns were observed for both TEER impedance as well as the live/dead assay for chips treated with different concentrations of DOX and docetaxel. The cell index value was quite high for 10 uM DOX con-centration in comparison to l ive-dead cell viabi li ty which can be due to the dead cel l s but st i ll attached (even if loosely) to the collagen. The lung cancer-on-chip platform presented here was thoroughly tested to prove i ts usefulness not only i n the monitoring of the real physiological microenvironment, but also in the assessment of the toxicity of candi-date drugs, with the help of custom-built physiological sensors. As such,this system could be used in future for the screening of new drug can-didates in monolayer tissue models and personalized medicine appl i -cations. 4. Conclusion In t his study, a lung cancer-on-chip platform was developed for the on-chip toxicity assessment of drug compounds using integrated bio-sensors. A glass-based microfluidic chip was fabricated by the inkjet printing of an elastomeric microf l uidic channel onto a transparent ITO-patterned glass chip held together by a 3D printed chip holder. A custom-buil t highly sensitive non-invasive optical pH sensor was used for the real-t i me monitoring of the media pH. Top and bottom ITO electrodes were used for real-time TEER impedance monitoring of the lung cancer-on-chip system, whose high transparency allowed for visual monitoring on-chip. Different concentrations of doxorubicin and doc-etaxel were introduced into the lung cancer-on-chip system to evaluate the impedance-based toxicity compared to the cell index (CI). The cell index was more reduced with increasing concentrations of DOX com-pared to docetaxel. The live/dead assay, performed for each different chip at the end of the impedance-based toxicity monitoring experi-ments, was quantified using ImageJ software and compared with the cell i ndex data. A similar pattern of cell viabi li ty was observed both for the cell index evaluated using the impedance-based toxicity assessment and the live/dead assay, whereby higher drug concentrations resulted in higher cell death. The pH data and microscopic images also proved the deteriorating effects on cell viabi l ity with increas i ng concentrations of the drug compounds. This lung cancer-on-chip platform, with real-time physiological monitoring using integrated biosensors, can be used with any monolayer micro-tumor or organ tissue models for on-chip toxicity studies. 5. Author Contributions Muhammad Asad Ullah Khalid: Muhammad Asad Ullah mainly conducted this study by developing, characterizing and utilizing the biosensors for on chip real time monitoring and cytotoxicity assess-ment. Young Su Kim: Young Su Kim developed the lung cancer chip and microf l uidic channels and helped in experiments by set t ing up the platform. Muhsin Ali: Muhsin Ali performed the cell culture on chip, pre-pared drug dilutions and conducted cytotoxicity experiments with Muhammad Asad Ullah and Young Su. Byung Gul Lee: Prof . Byung Gul Lee helped Young Su in micro-fluidic chip fabrication and optimizations. Young-Jae Cho Dr. Young-Jae Cho co-supervised this work and also helped in analyzing the experimental outcomes. Kyung Hyun Choi: Prof. Kyung Hyun Choi conducted this research work under his supervision. Declaration of Competing Interest The authors declare no competing interests. Acknowledgements This work was supported by the Nationa l Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1A2B3001830) and this study was supported by the grant from Korea Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C1787). Appendix A. Supplementary data Supplementary material related to this article can be found, in the onl i ne version, at doi:h ttps://doi.or g/10.1016/j .bej .2019.107469. References [1] X. Xie, R. Liu, Y. Xu, L. Wang, Z. Lan, W. Chen, H. Liu, Y. Lu, J . Cheng, In vitro hyperthermia studied i n a continuous manner us i ng electric impedance sensing ,RSC Adv. 5 (2015) 62007-62016, https://doi .or g/10.1039/c5ra04743a . [2] Y. Pan, N. Hu, X. Wei, L. Gong, B. Zhang, H. Wan, P. Wang, 3D cell-based biosensor for cell viabi li ty and drug assessment by 3D electric cell/matrigel -substrate im-pedance sensing, Biosens. Bioelectron. 130 (2019) 344-351, https ://doi .org /10.1016/j .bios.2018.09.046. [3] A. Mansoorifar, A. Koklu, A. Beskok, Quant i fication of cel l death using an im-pedance-based microfluidic device, Anal . Chem. 91 (2019) 4140-4148, h ttps ://do i .org /10.1021/ac CS s ..a a n alc h em.8b05890. [4] H. Babahosseini , V. Srinivasaraghavan, Z. Zhao, F. Gil l am, E. Childress, J.S. Strobl,W.L . Santos, C . Zhang, M. Agah, The impact of sphingosine kinase inhibitor-loaded nanoparticles on bioelectrical and biomechanical properties of cancer cells, Lab Chip 16 (2016) 188-198, https://doi .org/10.1039/c 5lc01201e . [5] Y.T. Kang, M.J. Kim, Y.H. Cho, A cel l i mpedance measurement device for the cy-totoxicity assay dependent on the velocity of supplied toxic fluid, J.Micromechanics Microengineering. 28 (2018), ht t p s://d o i .org /10.1088/1361- 6439/aaab39. [6]D. Bovard, A. Sandoz, K. Luettich, S . Frentzel , A. Iskandar, D. Marescot t i , K. Trivedi,E. Guedj, Q. Dutertre, M.C. Peitsch, J. Hoeng, A lung/liver-on-a -chip platform for acute and chronic toxicity studies , Lab Chip 18 (2018) 3814-3829,h ttps ://doi .o r g /10.1039/c8lc 01029c . [7] R. Lee, J. Kim, S.Y . Kim, S.M. Jang, S.M. Lee, I.H. Choi, S.W. Park, J.S. Shin,JdI K.H. Yoo, Capac i tance-based assay for real -t i me monitoring of endocytosis and cell v i ability, Lab Chip 12 (2012) 2377-2384, h ttps ://d o i .org /10.1039/c2lc 21236f . [8]Y.T. Lai, Y.S. Chu, J .C . Lo, Y.H. Hung, C.M. Lo, Effects of electrode diameter on the detection sensitivity and frequency characteri s tics of electric ce l l-substrate im-pedance sensing, Sensors Actuators, B Chem. (2019) 707-715, h t tps ://doi .org /10.1016/j .s n b.2019.02.098. [9] Y. An, T. Jin, F. Zhang , P. He, Electric cell -substrate impedance sensing (ECIS) for profi l ing cytotoxicity of cigarette smoke, J. E l ectroanal . Chem. 834 (2019)180-186, h ttps://doi .org /10.1016/j .jelechem.2018.12.047. [10] T.B. Tran, C . Baek, J. Min, Electric cell-substrate impedance sensing (ecis) with microelectrode arrays for i nvestigation of cancer Cel l -Fibroblasts i nteraction , PLoS One 11 (2016)1-12, htt p s://doi.org /10.1371/j ournal .pone .0153813. [11] A.T . Young, K.R. Rivera, P.D. Erb, M.A. Daniele, Monitoring of microphysiological systems: i ntegrat i ng sensors and real-t ime data analysis toward autonomous deci-sion-making, ACS Sens. 4 (2019) 1454-1464, https ://doi .org /10.1021/acssensors.8b01549. [12] Y.S. Zhang, J . Aleman, S.R. Shin, T. Kilic , D. Kim, S .A.M. Shaegh, S. Massa, R. Riahi,S. Chae, N. Hu, H. Avci, W. Zhang, A. Silvestri, A.S . Nezhad , A. Manbohi, F. De Ferrar i , A. Polini, G. Calzone, N. Shaikh, P. Ale r asool , E. Budina, J. Kang, N. Bhise,J. Riba s , A. Pourmand, A. Skarda l , T. Shupe, C.E. Bishop, M.R. Dokmeci, A. Atala,A. Khademhosseini , Multisensor-integrated organs -on-chips platform for automated and continual in si t u monitoring of organoid behav i ors, Proc. Nat l. Acad. Sci . U . S.A. 114 (2017) E2293-E2302, https://doi .org /10.1073/pnas.1612906114. [13] M. Leist, N . Hasiwa, C. Rovida, M. Daneshian, D. Basketter, I. Kimber, H. Clewel l ,T. Gocht, A. Goldberg, F. Busquet, A.M. Rossi, M. Schwarz, M. Stephens,R. Taalman, T.B. Knudsen, J . McKim, G. Harris, D. Pamies, T. Hartung, Consensus report on the future of animal-free systemic toxicity testing, A LTEX. 31 (2014)341-356, h t tps://doi .org /10.14573/a lte x .1406091. [14] I. Park, T. Nguyen, J. Park , A.Y. Yoo, J.K. Park, S. Cho, Impedance characterization of chitosan cytotoxicity to MCF-7 breas t cancer cells us i ng a mul t idisc i ndium tin oxide microelectrode array, J . Electrochem. Soc. 165 (2018) B55-B59, h ttps://doi .org /10.1149/2.1201802jes. [15] M.W. van der Helm, O.Y.F. Henry, A. Bein, T. Hamkins-Indik, M.J . Cronce,W.D. Leineweber, M. Odijk, A.D. van der Meer, L .I . Segerink, D.E. Ingber, Non-invasive sensing of transepithelia l barrier function and tissue differentiation in organs-on-chips us i ng impedance spectroscopy, Lab Chip 19 (2019) 452-463,https://d o i .org /10.1039/c8lc00129d . [16] M. Rothbauer, I. Praisler, D. Docter, R.H. Stauber, P. Ertl , Microfluidic impedimetric cel l regeneration assay to monitor the enhanced cytotoxic effect of nanomaterial perfusion, Biosensors 5 (2015) 736-749, https://d oi.org /10.3390/bios 5040736. [17]M.S. Nikshoar, S. Khosravi, M. Jahangiri, A. Zandi, Z.S. Miripour, S . Bonakdar,M. Abdolahad, Distinguishment of popul a ted metastatic cancer cells from primary ones based on their i nvasion to endothelial barrier by biosensor arrays fabricated on nanoroughened poly(methyl methacrylate), Biosens. Bioelectron. 118 (2018)51-57, https://doi .org /10.1016/j.bios.2018.07.036. [18]O.Y.F. Henry, R. Villenave, M.J . Cronce, W.D. Leineweber, M.A. Benz, D.E. Ingbe r ,Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function, Lab Chip 17 (2017)2264-2271, https://doi.or g/10.1039/c7lc00155j . [19] S.K. Krishnan, E. Singh , P. Singh, M. Meyyappan, H.S . Nalwa, A review on gra-phene-based nanocompos i tes for electrochemical and f luorescent biosensors, RSC Q1Adv. 9 (2019) 8778-8781, h t tps ://doi.org /10.1039/c8ra09577a . [20]C. Canali, A. Heiskanen, H.B. Muhammad, P. Hoyum, F.J. Pettersen,M. Hemmingsen, A. Wolff, M. Dufva, O.G. Martinsen, J. Emneus, Bioimpedance monitoring of 3D cell cu l turing-Complementary electrode configurations for en-hanced spatia l sensi t ivity, Biosens. Bioelectron. 63 (2015) 72-79, https://d o i .org /10.1016/j .bios .2014.07.020. [21] R.A. Schramm, M.H. Koslow, D.A. Nelson, M. Larsen, J. Castracane, A novel im-pedance biosensor for measurement of trans-epithelia l resistance i n cel l s cultured on nanofiber scaffolds, Biosensors 7 (2017) 1-10, https ://doi .org /10.3390/bios7030035. [22]E. Oztiirk, A.K. Karaboga Arslan, A.H. Dokumaci , M.B. Yerer, Real-time analysis of impedance alterations by the effects of vanadium pentoxide on severa l carcinoma cel l lines, Turkish J. Pharm. Sci . 15 (2018) 1-6, https ://doi .org /10.4274/t j p s .80764. [23] B. Srinivasan, A.R. Kolli, M.B. Esch, H.E. Abaci , M.L. Shuler, J .J. Hickman, TEER measurement techniques for in vitro barrier model systems, J. Lab. Autom. 20(2015) 107-126, https://doi.org /10.1177/2211068214561025. [24] B.M. Maoz, A. Herland, O.Y.F. Henry, W.D. Leineweber, M. Yadid, J . Doyle,R. Mannix, V.J. Kujala, E.A. Fitzgerald , K.K. Parker, D.E. Ingber, Organs-on-Chips with combined mult i-electrode array and transepi t helial electrica l res i stance mea -surement capabi li ties, Lab Chip 17 (2017) 2294-2302, h t tps ://d oi.org /10.1039/c7lc00412e . [25] N . Ferrell , R.R. Desai, A.J. Fleischman, S. Roy , H.D. Humes, W.H. Fissell , A mi-crofluidic bioreactor with i ntegrated transepithelial electrica l resi s tance (TEER)measurement electrodes for evaluation of renal epithe l ial cel ls , Biotechnol. Bioeng.107 (2010) 707-716, https://doi .org /10.1002/bi t.22835. [26] C. Caviglia, K. Zor, L. Montini , V. Til l i , S. Canepa, F. Melander, H.B. Muhammad,M. Carminat i , G . Ferrar i , R. Raiteri , A. Heiskanen, T.L. Andresen, J . Emneus,I mpedimetric toxic i ty assay in microfluidics using free and l iposome-encapsulated anticancer drugs, Anal. Chem. 87 (2015) 2204-2212, https://doi .org /10.1021/ac503621d . [27] Z. Li , W. Su, Y. Zhu, T . Tao, D. Li , X. Peng, J. Qin, Drug absorption related ne-phrotoxic i ty assessment on an intestine-kidney chip, Biomicrofluidics. 11 (2017),https://doi.o rg /10.1063/1.4984768. [28] E. Hondroulis, Z. Zhang, C. Chen, C.Z. Li , Impedance based nanotoxicity assessment of graphene nanomaterials at the cellular and tissue l evel , Anal . Lett. 45(2012)272-282, h ttps ://do i.or g/10.1080/00032719.2011.633184. [29](C. Xiao, J.H.T . Luong, On-line monitoring of cell growth and cytotoxicity using electric cel l -substrate impedance sensing (ECIS), Biotechnol. Prog. 19 (2003)1000-1005, https://doi.org /10.1021/bp025733x . [30] F.A. Alexander, S. Eggert , J . Wiest, Skin-on-a-chip: transepithelial electrical re-sistance and extracellular acidification measurements through an automated air-l iquid interface, Genes (Basel ). 9 (2018), https://doi .org /10.3390/g e nes 9020114. [31] S.A.M. Shaegh, F . De Ferrari, Y.S. Zhang, M. Nabavinia, N.B. Mohammad, J. Ryan,A. Pourmand, E. Laukaitis, R .B. Sadeghian, A. Nadhman, S.R. Shin, A.S. Nezhad,A. Khademhosseini , M.R. Dokmeci, A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactor s and organ-on-chip devices,Biomicrofluidics 10 (2016), h ttps://doi .org /10.1063/1.4955155. [32] A. Weltin, K. Slotwinski, J . Kieninger, I . Moser, G. Jobst, M. Wego, R. Ehret,G.A. Urban, Cel l culture monitoring for drug screening and cancer research: a transparent, microfluidic, mult i -sensor microsystem, Lab Chip 14 (2014) 138-146,https ://d o i .org /10.1039/c3lc 50759a . [33] D. Kattipparambil Rajan , M. Patrikoski, J . Verho, J. Sivula, H. Ihalainen,S. Mie t tinen, J. Lekkala, Optical non-contact pH measurement in cell culture with ster il izable, modular parts, Talanta 161 (2016) 755-761,ht tps ://doi .org /10.1016/i .t a lant a .2016.09.021. [34] E. Tanumihardja, W. Olthuis, A. van den Berg, Ruthenium oxide nanorods as po-tentiometric ph sensor for organs-on-chip purposes, Sens. (Switzerland) 18(2018),20(ht t ps://doi .org /10.3390/s18092901. [35] B.R. Briickner, A. Janshoff , I mportance of integrity of cell-cell junc t ions for the mechanics of conf l uent MDCK I I cel l s, Sci . Rep. 8 (2018) 1-11, https://doi .org /10.1038/s41598-018-32421-2. [36] T.A. Mar t in, W.G. Jiang, Loss of t ight junction barrier function and i ts role in cancer metastasis, Biochim. Biophys. Acta Biomembr . 1788 (2009) 872-891, https ://doi .org /10.1016/j.bbamem.2008.11.005. [37] K. Brennan, G. Offiah, E.A. McSherry, A.M. Hopkins, Tight junctions: a barrier to the initiation and progression of breast cancer? J . Biomed. Biotechnol . (2010)(2010), https://doi.org /10.1155/2010/460607. [38] S. Chen, R. Einspanier, J. Schoen, Transepithelial electrical resistance (TEER): a functional parameter to monitor the qual i ty of oviduct epithelia l ce l ls cultured on f i lter supports, Histochem. Cel l Biol . 144 (2015) 509-515, h ttps ://doi .o r g /10.1007/s00418-015-1351-1. [39]Y. Wu, M.R.K. Al i , B. Dong, T . Han, K. Chen, J. Chen, Y. Tang , N. Fang, F. Wang,M.A. El -Sayed, Gold Nanorod Photothermal Therapy Alters Cel l Junctions and Actin Network i n Inhibit i ng Cancer Cell Collective Migration, ACS Nano 12 (2018)9279-9290, https://doi .org /10.1021/acsnano.8b04128. [40] R. Port i llo-Lara, N. Annabi , Microengineered cancer-on-a-chip platforms t o study the metastatic microenvironment , Lab Chip 16 (2016) 4063-4081, h t tps ://doi.org /10.1039/c6lc00718j . [41] M. Ruzycka, M.R. Cimpan, I. Rios-Mondragon, I.P. Grudzinski, Microfluidics for studying metastatic patterns of lung cancer, J. Nanobiotechnology 17 (2019)1-30,ht t ps ://doi .org/10.1186/s12951-019-0492-0. [42] K. Chawla, S.C. Biirgel , G.W. Schmidt, H.-M. Kaltenbach, F. Rudol f , O. Frey,A. Hierlemann, Integrat i ng impedance-based growth-rate monitoring into a mi -crofluidic ce l l culture platform for live -cel l microscopy, Microsystems Nanoeng. 4(2018), https://doi .org /10.1038/s 41378-018-0006-5. [43]A. Pircher, I .K. Wasle , M. Mian, G. Gamer i th, E . Ulsper g er , M. S tudnicka, A. Mohn-S taudner, W. Hilb e , M. Fiegl, Docet a xe l i n the treatment of non-small ce l l lung ca n cer (NSCLC) - An observational study focusing on symptom i mprovement ,Ant i ca n cer Res . 33 (2013)3831-3836. [44]A. Montero, F . Fossella, G. Hortobagyi, V . Valero, Docetaxel for treatment of solid tumours: A systematic review of cl i nical data, Lancet Oncol.6 (2005) 229-239,h t t ps://doi .o r g /10.1016/S1470-2045(05)70094-2. [45]I N.M. Meghani, H.H. Amin, C. Park, J .B. Park, J .H. Cui , Q.R. Cao, B.J . Lee, Design and evaluation of clickable gelatin-oleic nanoparticles using fattigation-platform for cancer therapy, Int . J. Pharm. 545 (2018) 101-112, htt p s://d o i .org /10.1016/j i jpharm.2018.04.047. [46]G.S. Jeong, J. Oh, S.B . Kim, M.R. Dokmec i , H. Bae, S .H. Lee, A. Khademhosseini,Siphon-driven microfluidic passive pump with a yarn f low res i stance controller, Lab Chip 14 (2014) 4213-4219, https://doi .o r g /10.1039/c 4l c 00510d. [47] M. Ali , M.A.U. Khalid, I. Shah, S.W. Kim, Y.S. Kim, J.H. Lim, K.H. Choi, Paper-based selective and quant i tative detection of uric acid using citrate-capped Pt nano-particles (PtNPs) as a colorimetric sensing probe through a s i mple and remote-based device, New J . Chem. 43 (2019) 7636-7645, https://d o i .org /10.1039/c9nj01257e . [48]] M.A.U . Khalid, M. Al i , A.M. Soomro, S.W. Kim, H.B. Kim, B .G. Lee, K.H. Choi, A highly sensitive biodegradable pressure sensor based on nanofibrous dielectric,Sensors ActuatorsA Phys. 294 (2019) 140-147,https://doi .org /10.1016/j .s n a.2019.05.021. [49] A.M. Soomro, F. Jabbar, M. Ali, J.W. Lee, S.W. Mun, K.H. Choi, All-range flexible and biocompatible humidity sensor based on poly lactic glycolic acid (PLGA) and its application in human breathing for wearable health monitoring, J. Mater. Sci.Mater. Electron. 30 (2019) 9455-9465, h t tps://doi .org /10.1007/s10854-019-01277-1. [50] J. Marzioch, J. Kieninger , A. We l t i n, H. F l amm, K. Aravind a lochanan, J.A. Sandvik,E.O. Pettersen, Q. Peng, G.A. Urban, On-chip photodynamic therapy -monitoring cell metabolism using electrochemical microsensors, Lab Chip 18 (2018)3353-3360, https://doi .org /10.1039/C8LC00799C . [51]N National Center for Biotechnology Information. PubChem Database.Source=ChEMBL, Bioactivity=AID 742409 -SID 123097261, ht tps ://pu b chem .ncbi .nlm.nih .g ov /bioass a y /742409#sid =123097261 (accessed on Dec . 4, 2019). [52] K. Stock, M.F. Estrada, S . Vidic, K. Gjerde, A. Rudisch, V.E. Santo, M. Barbier,S. Blom, S .C. Arundkar , I. Selvam, A. Osswald, Y. Stein, S. Gruenewald, C. Brito,W. Van Weerden, V. Rotter, E . Boghaert , M. Oren, W. Sommergruber, Y . Chong ,R. De Hoogt , R. Graeser , Captur i ng tumor complexity in vitro: comparative analysis of 2D and 3D tumor models for drug discovery, Sci . Rep. 6 (2016)1-15, h t tps://doi .org /10.1038/srep28951.
确定






还剩9页未读,是否继续阅读?
雷迪美特中国有限公司为您提供《【PalmSens4电化学应用】集成生物传感器的肺癌芯片平台,用于生理监测和毒性评估》,该方案主要用于内脏器官中生化检验检测,参考标准--,《【PalmSens4电化学应用】集成生物传感器的肺癌芯片平台,用于生理监测和毒性评估》用到的仪器有PalmSens4便携式电化学工作站
推荐专场
该厂商其他方案
更多
















