本研究评估了红曲霉米醋在固态发酵过程中口感结构、口感活性指标和抗氧化活性的变化,以及它们之间的潜在关系。研究结果可为通过关键口感活性指标调控红曲霉米醋的抗氧化特性提供参考,并为进一步探索开发新型醋基功能食品提供新思路。
方案详情

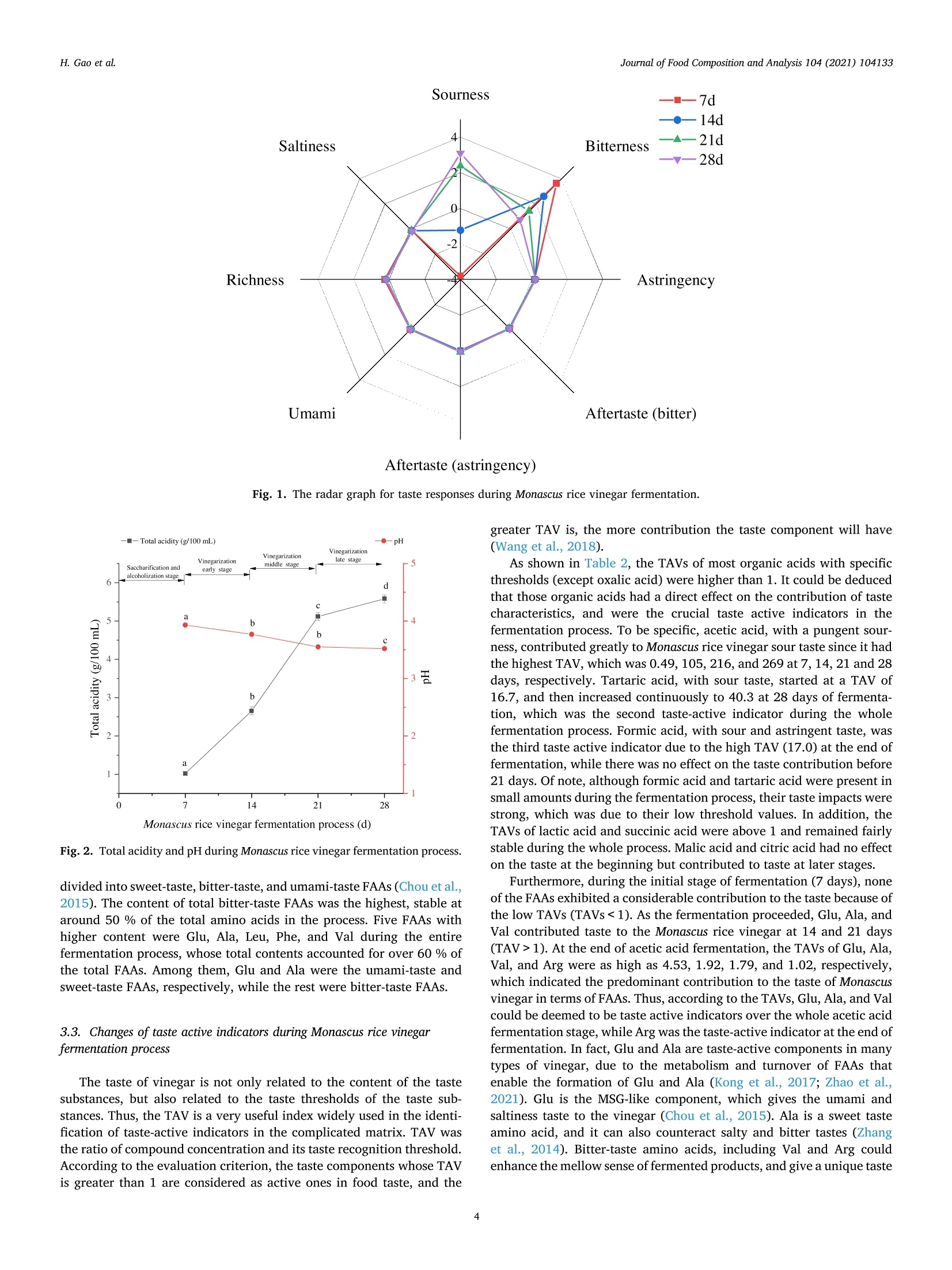
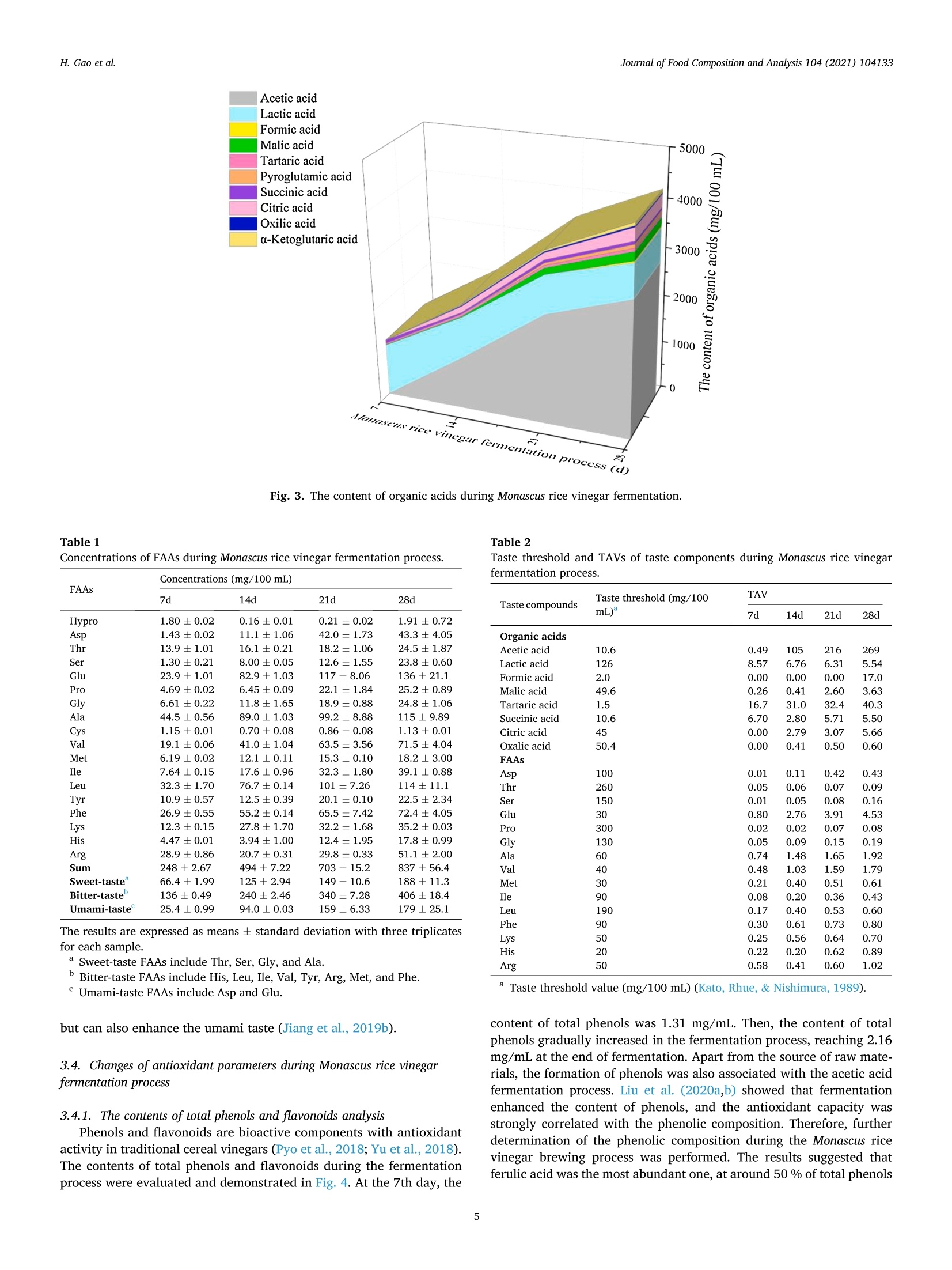
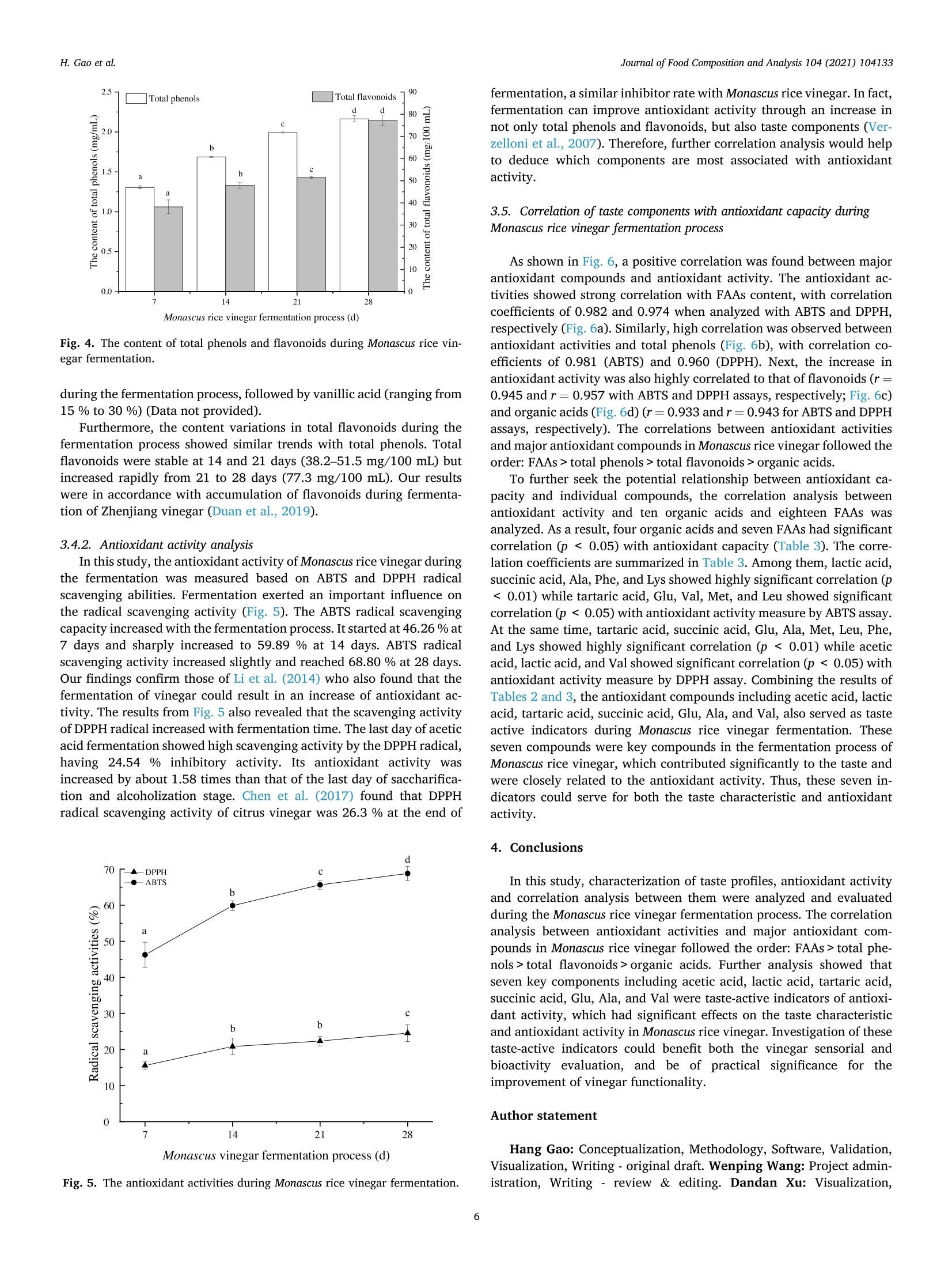
Journal of Food Composition and Analysis 104 (2021) 104133 H. Gao et al.Journal of Food Composition and Analysis 104 (2021) 104133 Contents lists available at ScienceDirect Journal of Food Composition and Analysis journal homepage: www.elsevier.com/locate/jfca Original Research Article Taste-active indicators and their correlation with antioxidant ability duringthe Monascus rice vinegar solid-state fermentation process Hang Gao, Wenping Wang , Dandan Xu , Peng Wang, Yan Zhao, German Mazzab Xin Zhang'a,* Beijing Academy of Food Sciences, Beijing, PR China "Institute for Research and Development in Process Engineering, Biotechnology and Alternative Energies, (PROBIEN, CONICET-UNCo), 1400 Buenos Aires St., 8300,Neuquen, Argentina ARTICLEINFO A BSTRACT Keywords:Monascus rice vinegarSolid-state fermentation processTaste active indicatorsAntioxidant componentsAntioxidant capacityCorrelation The present study evaluated the changes of taste profiles, taste active indicators, and antioxidant activity inMonascus rice vinegar during the solid-state fermentation process, as well as their potential relationships. Theresults showed that the contents of antioxidant components and antioxidant activity increased with fermentationtime. According to the taste active value (TAV) evaluation, seven organic acids and four free amino acids (FAAs)with high TAVs were considered as taste active indicators. The correlation analysis between antioxidant activityand major antioxidant compounds in Monascus rice vinegar followed the order: FAAs>total phenols>totalflavonoids> organic acids. Further analysis revealed that seven key components including acetic acid, lactic acid,tartaric acid, succinic acid, Glu, Ala, and Val were taste active indicators of antioxidant activity, which hadsignificant effects on the taste characteristic and antioxidant activity in Monascus rice vinegar. Thus, our findingswould provide references to regulate the antioxidant characteristic of Monascus rice vinegar through the key tasteactive indicators, and would provide new insights to further explore the development of new vinegar-basedfunctional foods. Vinegar is a worldwide traditional fermented seasoning, which hasbeen produced and consumed as a commercial product with a history ofapproximately 3000 years (Budak et al., 2014).Recently, vinegar hasattracted much attention due to its health benefits, especially the anti-oxidant activity (Aykin et al., 2015; Chiu et al., 2017). Antioxidant ac-tivity benefits prevent the formation of free radicals, which aredeleterious for health or their neutralization after formation; thus, ef-fects of antioxidant activity present significance in disease risk reduction(Fernando and Isabel, 2020; Jiang et al., 2019a,b). Chou et al. (2015)found that black vinegar contributed the antioxidant effects onhigh-fat/cholesterol-diet fed hamsters. Krusong et al. (2019) evaluatedcorn silk vinegars for their antioxidant activity toward scavenging rad-icals of 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2, -azino-bis(3-eth-ylbenzothiazoline-6-sulfonic acid) (ABTS). The results showed that theradical scavenging activity highly related to the high phenolic contents.In fact, several components in vinegar have positive effects on the antioxidant activity. Polyphenols and flavonoids were recognized aseffective antioxidants originate from raw materials (Martillanes et al.,2018). Xie et al. (2017) found that catechins, chlorogenic acid, andgallic acid serve as the principal antioxidant ingredients in Shanxi agedvinegar. Besides, solid-state fermentation could improve antioxidantactivity through the release and accumulation of polyphenol com-pounds, FAAs, organic acids, and others (Dey et al., 2016). Zhang et al.(2019) suggested that organic acids and FAAs exhibited antioxidantactivity in Zhenjiang aromatic vinegar, and FAAs showed synergisticeffect on the antioxidant activity of phenolic compounds. Monascus vinegar is one of the most popular Chinese vinegars for itsunique flavor. However, compared with other popular vinegars,research on Monascus vinegar is limited. Red yeast rice, a kind of riceproduct fermented by Monascus sp, has been confirmed to possesspharmacologically effects mainly due to its secondary metabolites, suchas pigment, Monacolin K (Huang et al., 2006). Thus, red yeast rice haslong been extensively used as a traditional functional food ingredient inChina (Maciej et al., 2019). Monascus rice vinegar is made from red yeast ( E-mail address: z x .zhang @ hot m ail. c om (X. Zhang). ) ( https: // doi.org / 10 . 1016/j.jfc a .202 1 .10 4 1 33 ) ( Received 8 June 2021; R e ceived in revised f o rm 20 July 2021; Accepted 24 August 2021Available online 26 August 2021 ) ( 0889-1575/C 2021 Els e vier Inc. Al l rights reserved. ) rice with the addition of Da qu as starter based on a spontaneoussolid-state fermentation technique. The production of Monascus ricevinegar involves these stages: the degradation of starch from the rice tofermentable sugars by molds, the conversion of fermentable sugars toethanol by yeast, and the oxidation of ethanol to acetic acid by bacteria.However, every stage is not completely independent but interacting as amultilateral fermentation process. The diverse microbial metabolismsand complicated chemical reactions jointly contributed to the flavor,taste and bioactivity. Mostly notably, some metabolites that contributeto taste are quite crucial because they greatly influence not only tastecharacteristic but also antioxidant activity (Kim et al., 2012). Thus,analyzing the taste profiles and antioxidant activity, then clarifying thetaste active indicators and the potential correlation with antioxidantability during the Monascus rice vinegar fermentation process wouldprovide references to control the antioxidant characteristic of vinegarthrough regulating the main compositions, and further improve thefunctional vinegar production. Nevertheless, insufficient informationexists about those above mentioned issues. Thus, further key taste andantioxidant related components need to be elucidated as crucialindicators. This study aimed to figure out the taste-active indicators and revealthe potential correlations with antioxidant activity during Monascus ricevinegar fermentation. Thus, the taste characteristics, taste components,and antioxidant activity during Monascus rice vinegar production werequantified by electronic nose (e-nose), automatic amino acid analyzer,high-performance liquid chromatography (HPLC), and scavenging freeradicals test methods. Then, the TAV analysis was used to reveal thetaste active indicators that make significant contribution to the taste.Furthermore, Pearson’s correlation analysis was employed to furtherelucidate the potential correlation between antioxidant ability and tasteactive indicators. These findings would be helpful for providing theo-retical basis on functional control for the development of nutritional andhealthy vinegar products. 2. Materials and methods 2.1. Samples collection At first, the rice and red yeast rice (w/w, 10:1) without steamingwere subjected to 5 lots of water wetting. Amyloglucosidase (0.1 %, wt)was subsequently added for the saccharification step. Subsequently,Daqu and yeast were incubated into the tank for the alcoholization step.Later 5 % of bran and rice husk (w/w,1:1) were mixed into the tanktogether with old Pei (containing acetic bacteria) for the acetic acidfermentation. The mixture was completely turned over every day tokeep it ventilated. In the current study, samples at different fermentation stages ofMonascus rice vinegar including saccharification and alcoholizationstage (7 days), vinegarization early stage (14 days), vinegarizationmiddle stage (21 days) and vinegarization late stage (28 days) werecollected. In order to obtain the most unbiased samples, the sampleswere collected from the top, middle, and bottom of the tank, and thenmixed thoroughly. Then, each sample was centrifuged at 1700 g for 20min and the supernatant was collected and stored at -80°C for furtheranalysis. 2.2. Reagents Ten organic acid standards were purchased from Dr. EhrenstorferGmbH Co., Ltd. The mixed standard solution of amino acids (AA) (typeH) was purchased from Wako Pure Chemical Industries, Ltd. Gallic acid,Folin-Ciocalteau, rutin, and ascorbic acid were purchased from SigmaAldrich Co., Ltd. All other chemicals used were either of analytical gradeor of the highest purity commercially available. The INSENT SA402B electronic taste-sensing system (INSENT,Tokyo, Japan)consisting of eight metallic electrodes was applied fortaste measurements. Samples were diluted to 1:10 with distilled water,and each sample of 30 mL was poured into the measuring glass fordetection. The electrodes were rinsed in a buffer (30 mmol/L KCl +0.3mmol/L tartaric acid) between each measurement to prevent any cu-mulative effect. Based on results of preliminary experiments for a sta-bility system,e-tongue measurement was performed four times for eachsample and the data obtained on the latter three occasions were appliedfor subsequent analysis (Jo et al., 2016). All experiments were imple-mented at room temperature and ambient atmosphere. 2.4. Organic acid analysis Samples were pretreated before injection; 500 pL of sample super-natant (as indicated in Section 2.1) were mixed with 200 pL 10.6%(w/v) potassium ferrocyanide and 200 pL 30 % (w/v) zinc sulfate. Themixture was adjusted to 10 mL with ultra-pure water and then filtered(20 um) for the organic acid analysis (Scherer et al., 2012). Organic acids were analyzed using Waters e2695 HPLC (WatersCorporation, Milford,MA) equipped with a2998 UV detector, a columnoven and an Atlantis T3 C18 reversed-phase column (4.6 mm ×250 mmi.d., 5um) (Waters). The mobile phase consisted of 0.1 % phosphoricacid solution and methanol (95:5, v/v) with isocratic elution (0.80 mL/min) for 20 min. Identification and quantification were conducted at210 nm (Lima et al., 2014). For the quantification of organic acids,calibration curves for each compound were constructed using purestandards at different concentrations (acetic acid: y=389.6x-8328,5--5000 ug/mL, R²=0.999; lactic acid: y=350.3x-5469, 34.16-3416ug/mL, R²= 0.999; formic acid, y=574.9x+478.1, 4.93-493 ug/mL,R2= 0.999; malic acid: y=606.0x-704.8, 5.54-554 ug/mL, R²=0.999; tartaric acid: y=1000x-9234, 5.80-580 pg/mL,R2= 0.999;pyroglutamic acid: y=4068x-1596, 4.53-453 pg/mL, R²=0.999;succinic acid: y= 407.9x-1896,5.38-538 ug/mL, R2= 0.999; citricacid: y =772.2x-2466, 5.03-503 ug/mL, R²=0.999; oxalic acid: y=8599x+ 22960, 5.66-566 ng/mL,R²=0.999; o-ketoglutaric acid: y=4474x-21218,5.99-599ug/mL, R²=0.999). 2.5. Total acidity and pH analysis The pH of each sample was determined using a PB-10 digital pH-meter (Sartorius, Germany). The total acidity was determined by titri-metric method. Briefly, 2 mL sample were diluted to 20 mL in a volu-metric flask with water. Following the addition of 60 mL distilled water,the sample was titrated with 0.05 mol/L sodium hydroxide (NaOH)standard solution to pH 8.20. A blank control was performed in the samemanner as for the determination of samples (Kelebek et al., 2017). Re-sults were expressed in grams of acetic acid per 100 mL of sample. Totalacidity was calculated: Where, Vi is the volume of NaOH standard solution consumed by thesample, mL; V2 is the volume of NaOH standard solution consumed by the blankcontrol,mL; V3 is the volume of the sample, mL. 2.6. FAAs analysis FAAs analysis was performed based on a modified method of Huaet al. (2014). Sample (400 pL) was placed in a 10-mL volumetric flaskand filled with 0.02 M HCl. The mixture was added with an equal volume of 5 % sulfosalicylic acid solution. Then, the supernatant wasfiltered prior to determination. The samples were analyzed by an automatic amino acid analyzer (L-8900 System; Hitachi, Tokyo, Japan) equipped with a visible detector.Chromatographic separations were performed on an ion-exchangechromatographic column (4.6 mm x 60 mm) with the elution oflithium buffer. After reacting with ninhydrin, the resultant derivativeswere measured at 440 nm and 570 nm simultaneously. A standard FAAsmixture (type H, Wako) was used for identification and quantification(external standard method). 2.7. Major antioxidant components analysis 2.7.1. Total phenols analysis Total phenols were measured by Folin-Ciocalteu method (Kappelet al., 2008). In brief, a total of 50 pL 1:4 diluted sample was added to 1mL of 20 mg/mL Na2CO3. Mixture was left to stand for 2 min, then 450uL of Folin-Ciocalteu reagent (previously diluted with water 1:1, v/v)were added. The absorbance was determined at 750 nm after 30 min ofreaction using a Synergy H4 spectrophotometer (BioTek Instruments,Winooski, VT). The results were calculated using a standard curve andexpressed as mg equivalent of gallic acid per mL of sample (mg/mL). 2.7.2. Total flavonoids analysis Total flavonoids were measured by the described colorimetricmethod (Hussain et al., 2019). Briefly, 400 uL of water and 50 pL of 50g/L NaNO2 solution were added to 100 uL of test sample. After mixingfor 6 min, 50 uL of 100 g/L Al(NO3)3 solution were added and left tostand for another 6 min. Finally, 200 uL of NaOH at 200 g/L concen-tration and distilled water were added to obtain a final volume of 1.25mL. After standing for 15 min in the dark, sample absorbance was read at510 nm. Total flavonoid contents were calculated using a standard curveand expressed as mg equivalent of rutin per milliliter of sample (mg/100mL). 2.8. Antioxidant capacity analysis 2.8.1. ABTS radical scavenging capacity analysis The free radical scavenging capacity was determined using modifiedABTS decolorization assay (Ilyasov et al., 2018). The method consists inadding 100 pL of methanol extract from the sample or ascorbic acid(positive control) to the freshly prepared ABTS radical solution to a totalvolume of 2 mL. The blank was prepared by mixing 1900 pL of ABTSradical solution with 100 uL of double distilled water. After reacting for30 min in the dark, the absorbance of the mixture was measured at 405nm. The inhibition percentage of ABTS discoloration was calculated: Where, Ablank is the absorbance of control; Asample is the absorbance of the sample. 2.8.2. DPPH radical scavenging capacity analysis DPPH radical scavenging activities analysis was performed accord-ing to the previously described method with slight modifications (Kru-song et al., 2019). For the DPPH assay, 100 uL of methanol extract fromthe sample or ascorbic acid (positive control) with varying concentra-tions were added to 1900 uL of freshly prepared DPPH radical solution,and left in the dark for 30 min. A decrease in absorbance was measuredat 515 nm against a blank of ethanol. The percentage results of scav-enging activity were calculated as % inhibition using Eq. (2). 2.9. Statistical analysis significant differences among different fermentation processes weredetermined by one-way analysis of variance. A value of p <0.05 wasconsidered as statistically significant. Correlations among variableswere assessed by means of the Pearson’s correlation test. Statisticalanalysis was performed using the SPSS version 22.0 software packagefor Windows. Graphs were constructed by Origin 2019b software. 3. Results and discussion 3.1. Changes of taste properties evaluated by E-tongue during Monascusrice vinegar fermentation process E-tongue equipped with sensor probes that simulate the human tasteis widely used in taste evaluation, which can well exclude the subjec-tivity of sensory evaluation (Jiang et al., 2018). The radar graph for thechanges in response of eight taste sensors to Monascus rice vinegar overthe fermentation period is displayed in Fig. 1. As the fermentation timeprogressed, the sourness gradually increased, the bitterness graduallydecreased, while the signal values of the other taste sensors did notchange obviously. These results implied that the variation of tastecharacteristics was affected by the process and that might be mainlyascribed to the accumulation of taste components category and con-centration during the fermentation process. Thus, measurement in thechanges of taste components including organic acids and FAAs wascarried out. 3.2. Changes of taste components during Monascus rice vinegarfermentation process 3.2.1. Organic acids The total acidity is an important quality parameter of vinegar, and isstrongly affected by organic acids (Gong et al., 2021). As shown in Fig. 2,the total acidity increased rapidly during Monascus rice vinegarfermentation process. At the end of fermentation, the total acidityreached 5.58 g/100 mL, which was significantly higher than that on the7th day (1.02 g/100 mL). Meanwhile, pH decreased from 3.93 to 3.52within 28 days of fermentation. Ten organic acids in the fermentation process of Monascus rice vin-egar were quantified with calibration curves and their variations arepresented in Fig. 3. The total level of organic acids started from 1193mg/100 mL at 7 days,rapidly reached 2223 mg/100 mL, 3563 mg/100mL and 4308 mg/100 mL at 14, 21 and 28 days, respectively. In addi-tion, acetic acid started at 5.21 mg/100 mL in 7 days of Monascus ricevinegar fermentation, subsequently increasing to 2855 mg/100 mL invinegarization late stage. On the contrary, the content of lactic acid was1079 mg/100 mL at 7 days, but its content gradually dropped to 699mg/100 mL at the corresponding late stage. The results showed that thepredominant organic acids during the entire fermentation process wereacetic acid and lactic acid, accounting for over 80 % of the total organicacids. These findings were in line with previous studies (Nie et al.,2013). At the same time, other organic acids with less amount exhibiteddifferent variation tendencies. The contents of malic acid, tartaric acid,citric acid, oxalic acid, and o-ketoglutaric acid continuously increasedduring the whole fermentation process. Formic acid and pyroglutamicacid were produced in the middle and late stage of acetic acid fermen-tation; their contents also showed an upward trend. However, the con-tent of succinic acid fluctuated until the 14th day, and then tended toremain stable. 3.2.2. FAAs The changes in FAAs contents during Monascus rice vinegarfermentation process are described in Table 1. The contents of total FAAsaccumulated during the fermentation process: 248 mg/100 mL, 494 mg/100 mL, 703 mg/100 mL, and 837 mg/100 mL at 7, 14,21 and 28 days,respectively. The contents and species of FAAs were relatively moreabundant at the later stages of acetic acid fermentation. FAAs were Aftertaste (astringency) Fig. 1. The radar graph for taste responses during Monascus rice vinegar fermentation. Monascus rice vinegar fermentation process (d) Fig. 2. Total acidity and pH during Monascus rice vinegar fermentation process. divided into sweet-taste, bitter-taste,and umami-taste FAAs (Chou et al.,2015). The content of total bitter-taste FAAs was the highest, stable ataround 50 % of the total amino acids in the process. Five FAAs withhigher content were Glu, Ala, Leu, Phe, and Val during the entirefermentation process, whose total contents accounted for over 60 % ofthe total FAAs. Among them, Glu and Ala were the umami-taste andsweet-taste FAAs, respectively, while the rest were bitter-taste FAAs. 3.3. Changes of taste active indicators during Monascus rice vinegarfermentation process The taste of vinegar is not only related to the content of the tastesubstances, but also related to the taste thresholds of the taste sub-stances. Thus, the TAV is a very useful index widely used in the identi-fication of taste-active indicators in the complicated matrix. TAV wasthe ratio of compound concentration and its taste recognition threshold.According to the evaluation criterion, the taste components whose TAVis greater than 1 are considered as active ones in food taste, and the greater TAV is, the more contribution the taste component will have(Wang et al., 2018). As shown in Table 2, the TAVs of most organic acids with specificthresholds (except oxalic acid) were higher than 1. It could be deducedthat those organic acids had a direct effect on the contribution of tastecharacteristics, and were the crucial taste active indicators in thefermentation process. To be specific, acetic acid, with a pungent sour-ness, contributed greatly to Monascus rice vinegar sour taste since it hadthe highest TAV, which was 0.49, 105,216,and 269 at 7, 14,21 and 28days, respectively. Tartaric acid, with sour taste, started at a TAV of16.7, and then increased continuously to 40.3 at 28 days of fermenta-tion, which was the second taste-active indicator during the wholefermentation process. Formic acid, with sour and astringent taste, wasthe third taste active indicator due to the high TAV (17.0) at the end offermentation, while there was no effect on the taste contribution before21 days. Of note, although formic acid and tartaric acid were present insmall amounts during the fermentation process, their taste impacts werestrong, which was due to their low threshold values. In addition, theTAVs of lactic acid and succinic acid were above 1 and remained fairlystable during the whole process. Malic acid and citric acid had no effecton the taste at the beginning but contributed to taste at later stages. Furthermore, during the initial stage of fermentation (7 days), noneof the FAAs exhibited a considerable contribution to the taste because ofthe low TAVs (TAVs <1). As the fermentation proceeded, Glu, Ala, andVal contributed taste to the Monascus rice vinegar at 14 and 21 days(TAV>1). At the end of acetic acid fermentation, the TAVs of Glu, Ala,Val, and Arg were as high as 4.53, 1.92, 1.79, and 1.02, respectively,which indicated the predominant contribution to the taste of Monascusvinegar in terms of FAAs. Thus, according to the TAVs, Glu, Ala, and Valcould be deemed to be taste active indicators over the whole acetic acidfermentation stage, while Arg was the taste-active indicator at the end offermentation. In fact, Glu and Ala are taste-active components in manytypes of vinegar, due to the metabolism and turnover of FAAs thatenable the formation of Glu and Ala (Kong et al., 2017; Zhao et al.,2021). Glu is the MSG-like component, which gives the umami andsaltiness taste to the vinegar (Chou et al., 2015). Ala is a sweet tasteamino acid, and it can also counteract salty and bitter tastes (Zhanget al., 2014). Bitter-taste amino acids, including Val and Arg couldenhance the mellow sense of fermented products, and give a unique taste Fig. 3. The content of organic acids during Monascus rice vinegar fermentation. Table 1Concentrations of FAAs during Monascus rice vinegar fermentation process. Table 2 Taste threshold and TAVs of taste components during Monascus rice vinegarfermentation process. Concentrations (mg/100 mL) FAAs 7d 14d 21d 28d Hypro 1.80±0.02 0.16±0.01 0.21±0.02 1.91±0.72 Asp 1.43±0.02 11.1±1.06 42.0±1.73 43.3±4.05 Thr 13.9±1.01 16.1±0.21 18.2±1.06 24.5±1.87 Ser 1.30±0.21 8.00±0.05 12.6±1.55 23.8±0.60 Glu 23.9±1.01 82.9±1.03 117±8.06 136±21.1 Pro 4.69±0.02 6.45±0.09 22.1±1.84 25.2±0.89 Gly 6.61±0.22 11.8±1.65 18.9±0.88 24.8±1.06 Ala 44.5±0.56 89.0±1.03 99.2±8.88 115±9.89 Cys 1.15±0.01 0.70±0.08 0.86±0.08 1.13±0.01 Val 19.1±0.06 41.0±1.04 63.5±3.56 71.5±4.04 Met 6.19±0.02 12.1±0.11 15.3±0.10 18.2±3.00 Ile 7.64±0.15 17.6±0.96 32.3±1.80 39.1±0.88 Leu 32.3±1.70 76.7±0.14 101±7.26 114±11.1 Tyr 10.9±0.57 12.5±0.39 20.1±0.10 22.5±2.34 Phe 26.9±0.55 55.2±0.14 65.5±7.42 72.4±4.05 Lys 12.3±0.15 27.8±1.70 32.2±1.68 35.2±0.03 His 4.47±0.01 3.94±1.00 12.4±1.95 17.8±0.99 Arg 28.9±0.86 20.7±0.31 29.8±0.33 51.1±2.00 Sum 248±2.67 494±7.22 703±15.2 837±56.4 Sweet-taste° 66.4±1.99 125±2.94 149±10.6 188±11.3 Bitter-taste 136±0.49 240±2.46 340±7.28 406±18.4 Umami-taste° 25.4±0.99 94.0±0.03 159±6.33 179±25.1 The results are expressed as means ± standard deviation with three triplicatesfor each sample. Sweet-taste FAAs include Thr, Ser, Gly, and Ala. Taste compounds Taste threshold (mg/100 TAV mL) 7d 14d 21d 28d Organic acids Acetic acid 10.6 0.49 105 216 269 Lactic acid 126 8.57 6.76 6.31 5.54 Formic acid 2.0 0.00 0.00 0.00 17.0 Malic acid 49.6 0.26 0.41 2.60 3.63 Tartaric acid 1.5 16.7 31.0 32.4 40.3 Succinic acid 10.6 6.70 2.80 5.71 5.50 Citric acid 45 0.00 2.79 3.07 5.66 Oxalic acid 50.4 0.00 0.41 0.50 0.60 FAAs Asp 100 0.01 0.11 0.42 0.43 Thr 260 0.05 0.06 0.07 0.09 Ser 150 0.01 0.05 0.08 0.16 Glu 30 0.80 2.76 3.91 4.53 Pro 300 0.02 0.02 0.07 0.08 Gly 130 0.05 0.09 0.15 0.19 Ala 60 0.74 1.48 1.65 1.92 Val 40 0.48 1.03 1.59 1.79 Met 30 0.21 0.40 0.51 0.61 Ile 90 0.08 0.20 0.36 0.43 Leu 190 0.17 0.40 0.53 0.60 Phe 90 0.30 0.61 0.73 0.80 Lys 50 0.25 0.56 0.64 0.70 His 20 0.22 0.20 0.62 0.89 Arg 50 0.58 0.41 0.60 1.02 Bitter-taste FAAs include His, Leu, Ile, Val, Tyr, Arg, Met, and Phe.Umami-taste FAAs include Asp and Glu. Taste threshold value (mg/100 mL) (Kato, Rhue, & Nishimura, 1989). but can also enhance the umami taste (Jiang et al., 2019b). 3.4. Changes of antioxidant parameters during Monascus rice vinegarfermentation process 3.4.1. The contents of total phenols and flavonoids analysis Phenols and flavonoids are bioactive components with antioxidantactivity in traditional cereal vinegars (Pyo et al., 2018; Yu et al., 2018).The contents of total phenols and flavonoids during the fermentationprocess were evaluated and demonstrated in Fig. 4. At the 7th day, the content of total phenols was 1.31 mg/mL. Then, the content of totalphenols gradually increased in the fermentation process, reaching 2.16mg/mL at the end of fermentation. Apart from the source of raw mate-rials, the formation of phenols was also associated with the acetic acidfermentation process. Liu et al. (2020a,b) showed that fermentationenhanced the content of phenols, and the antioxidant capacity wasstrongly correlated with the phenolic composition. Therefore, furtherdetermination of the phenolic composition during the Monascus ricevinegar brewing process was performed. The results suggested thatferulic acid was the most abundant one, at around 50 % of total phenols Fig. 4. The content of total phenols and flavonoids during Monascus rice vin-egar fermentation. during the fermentation process, followed by vanillic acid (ranging from15 % to 30 %) (Data not provided). Furthermore, the content variations in total flavonoids during thefermentation process showed similar trends with total phenols. Totalflavonoids were stable at 14 and 21 days (38.2-51.5 mg/100 mL) butincreased rapidly from 21 to 28 days (77.3 mg/100 mL). Our resultswere in accordance with accumulation of flavonoids during fermenta-tion of Zhenjiang vinegar (Duan et al., 2019). 3.4.2. Antioxidant activity analysis In this study, the antioxidant activity of Monascus rice vinegar duringthe fermentation was measured based on ABTS and DPPH radicalscavenging abilities. Fermentation exerted an important influence onthe radical scavenging activity (Fig. 5). The ABTS radical scavengingcapacity increased with the fermentation process. It started at 46.26 % at7 days and sharply increased to 59.89 % at 14 days. ABTS radicalscavenging activity increased slightly and reached 68.80 % at 28 days.Our findings confirm those of Li et al. (2014) who also found that thefermentation of vinegar could result in an increase of antioxidant ac-tivity. The results from Fig. 5 also revealed that the scavenging activityof DPPH radical increased with fermentation time. The last day of aceticacid fermentation showed high scavenging activity by the DPPH radical,having 24.54 % inhibitory activity. Its antioxidant activity wasincreased by about 1.58 times than that of the last day of saccharifica-tion and alcoholization stage. Chen et al. (2017) found that DPPHradical scavenging activity of citrus vinegar was 26.3 % at the end of Fig. 5. The antioxidant activities during Monascus rice vinegar fermentation. fermentation, a similar inhibitor rate with Monascus rice vinegar. In fact,fermentation can improve antioxidant activity through an increase innot only total phenols and flavonoids, but also taste components (Ver-zelloni et al., 2007). Therefore, further correlation analysis would helpto deduce which components are most associated with antioxidantactivity. 3.5. Correlation oftaste components with antioxidant capacity duringMonascus rice vinegar fermentation process As shown in Fig. 6, a positive correlation was found between majorantioxidant compounds and antioxidant activity. The antioxidant ac-tivities showed strong correlation with FAAs content, with correlationcoefficients of 0.982 and 0.974 when analyzed with ABTS and DPPH,respectively (Fig.6a). Similarly, high correlation was observed betweenantioxidant activities and total phenols (Fig. 6b), with correlation co-efficients of 0.981 (ABTS) and 0.960 (DPPH). Next, the increase inantioxidant activity was also highly correlated to that of flavonoids (r=0.945 and r= 0.957 with ABTS and DPPH assays, respectively; Fig.6c)and organic acids (Fig.6d) (r=0.933 andr= 0.943 for ABTS and DPPHassays, respectively). The correlations between antioxidant activitiesand major antioxidant compounds in Monascus rice vinegar followed theorder: FAAs> total phenols > total flavonoids>organic acids. To further seek the potential relationship between antioxidant ca-pacity and individual compounds, the correlation analysis betweenantioxidant activity and ten organic acids and eighteen FAAs wasanalyzed. As a result, four organic acids and seven FAAs had significantcorrelation (p <0.05) with antioxidant capacity (Table 3). The corre-lation coefficients are summarized in Table 3. Among them, lactic acid,succinic acid, Ala, Phe, and Lys showed highly significant correlation (p<0.01) while tartaric acid, Glu, Val, Met, and Leu showed significantcorrelation (p <0.05) with antioxidant activity measure by ABTS assay.At the same time, tartaric acid, succinic acid, Glu, Ala, Met, Leu, Phe,and Lys showed highly significant correlation (p<0.01) while aceticacid, lactic acid, and Val showed significant correlation (p <0.05) withantioxidant activity measure by DPPH assay. Combining the results ofTables 2 and 3, the antioxidant compounds including acetic acid, lacticacid, tartaric acid, succinic acid, Glu, Ala, and Val, also served as tasteactive indicators during Monascus rice vinegar fermentation. Theseseven compounds were key compounds in the fermentation process ofMonascus rice vinegar, which contributed significantly to the taste andwere closely related to the antioxidant activity. Thus, these seven in-dicators could serve for both the taste characteristic and antioxidantactivity. 4. Conclusions In this study, characterization of taste profiles, antioxidant activityand correlation analysis between them were analyzed and evaluatedduring the Monascus rice vinegar fermentation process. The correlationanalysis between antioxidant activities and major antioxidant com-pounds in Monascus rice vinegar followed the order: FAAs> total phe-nols> total flavonoids >organic acids. Further analysis showed thatseven key components including acetic acid, lactic acid, tartaric acid,succinic acid, Glu, Ala, and Val were taste-active indicators of antioxi-dant activity, which had significant effects on the taste characteristicand antioxidant activity in Monascus rice vinegar. Investigation of thesetaste-active indicators could benefit both the vinegar sensorial andbioactivity evaluation, and be of practicalSs1ignificance for theimprovement of vinegar functionality. Author statement Hang Gao: Conceptualization, Methodology, Software, Validation,Visualization, Writing-original draft. Wenping Wang: Project admin-istration, Writing - review & editing. Dandan Xu: Visualization, Total flavonoids (mg/100 mL) Fig. 6. Correlations between antioxidant activities and total phenols (a), total flavonoids (b), organic acids (c), and FAAs (d) of Monascus rice vinegar. Table 3 Pearson’s correlation coefficients between antioxidant activities and maincomponents in Monascus rice vinegar. Pearson’s correlation coefficients Compounds ABTS radical scavenging DPPH radical scavenging activities activities Acetic acid 0.830 0.960* Lactic acid 0.998** 0.987* Tartaric acid 0.987 0.999* Succinic acid 0.996** 0.9977** Glu 0.984* 0.992** Ala 0.995** 0.999** Val 0.954* 0.971* Met 0.976* 0.994** Leu 0.987* 0.993** Phe 0.997** 0.995** Lys 1.000** 0.992** Indicates significance at the 0.05 probability level." Indicates significance at the 0.01 probability level. Investigation. Peng Wang: Visualization, Investigation. Yan Zhao: Su-pervision, Project administration, Funding acquisition. German Mazza:Visualization. Xin Zhang: Supervision, Conceptualization, Writing-Reviewing and Editing, Project administration. Funding This work was supported by the National Key R&D Program of China(2016YFD0400500). Organic acids (mg/100 mL) Declaration of Competing Interest The authors report no declarations of interest. References Aykin, E., Budak, N.H., Gizel-Seydim, Z.B., 2015. Bioactive components of mothervinegar. J. Am. Coll. Nutr. 34 (1), 80-89. Budak,N.H., Aykin, E., Seydim, A.C., Greene,A.K., Guzel-Seydim, Z.B., 2014. Functionalproperties of vinegar. J. Food Sci. 79 (5), 757-764. Chen, Y., Huang, Y., Bai, Y., Fu, C., Zhou, M., Gao, B., et al., 2017. Effects of mixedcultures of Saccharomyces cerevisiae and Lactobacillus plantarum in alcoholicfermentation on the physicochemical and sensory properties of citrus vinegar. LWT-Food Sci. Technol. 84, 753-763. Chiu, H.F., Cheng, Y., Lu, Y.Y., Han, Y.C., Shen, Y.C., Venkatakrishnan, K., et al., 2017.Anti-mutagenicity,hypouricemic and antioxidant activities of alkaloids from vinegarand mei vinegar. J. Food Biochem. 41 (4), 12373-12382. Chou, C.H., Liu, C.W., Yang, D.J., Wu, Y., Chen, Y.C., 2015. Amino acid, mineral, andpolyphenolic profiles of black vinegar, and its lipid lowering and antioxidant effectsin vivo. Food Chem. 168, 63-69. Dey, T.B., Chakraborty, S., Jain, K.K., Sharma, A., Kuhad, R.C., 2016. Antioxidantphenolics and their microbial production by submerged and solid state fermentationprocess: a review. Trends Food Sci. Technol. 53,60-74. Duan, W.H., Xia, T., Zhang, B., Li, S.P., Zhang, C.W., Zhao, C.Y., et al., 2019. Changes ofphysicochemical, bioactive compounds and antioxidant capacity during the brewingprocess of Zhenjiang Aromatic Vinegar. Molecules (Basel, Switzerland) 24 (21),3935-3947. Fernando, T.A., Isabel, E., 2020. Antioxidant characteristics of honey from Mozambiquebased on specific flavonoids and phenolic acid compounds. J.Food Compos. Anal. 86(8-9),103377. Gong,M., Zhou, Z.L., Liu, S.P., Zhu, S.H., Mao, J., 2021. Formation pathways andprecursors of furfural during Zhenjiang aromatic vinegar production. Food Chem.354(2),129503. Hua, Z., Wang, Z.Y., Xin,Y., Zhao, H.T., Zhang, Y.C., Dong, A.J., et al., 2014.Determination of free amino acids and 18 elements in freeze-dried strawberry andblueberry fruit using an Amino Acid Analyzer and ICP-MS with micro-wavedigestion. Food Chem. 147 (15), 189-194. Huang,H.N., Hua, Y.Y., Bao, G.R., Xie, L.H., 2006. The quantification of monacolin K insome red yeast rice from Fujian province and the comparison of the other product.Chem. Pharm. Bull. 54 (5), 687-689. Hussain, T., Al-Attas, O.S., Alamery, S., Ahmed, M., Alrokayan, S., 2019. The plantflavonoid, fisetin alleviates cigarette smoke-induced oxidative stress, andinflammation in Wistar rat lungs. J. Food Biochem. 43 (3), 12962-12973. Ilyasov,I.R., Beloborodov, V.L., Selivanova, I.A., 2018. Three ABTS + radical cation-based approaches for the evaluation of antioxidant activity: fast- and slow-reactingantioxidant behavior. Chem. Pap. 72, 1917-1925. Jiang, H.Y., Zhang, M., Bhandari, B., Adhikari,B., 2018. Application of electronic tonguefor fresh foods qualityy (evaluation: a review. Food Rev. Int. 1-24. Jiang, H., Yu, F., Qin, L., Zhang, N.,Cao, Q., Li, D.X., Song, C., 2019a. Dynamic change inamino acids, catechins, alkaloids, and gallic acid in six types of tea processed fromthe same batch of fresh tea (Camellia sinensis L.) leaves. J. Food Compos. Anal. 77,:28-38. Jiang, Y.J., Lv, X.C., Zhang, C., Zheng, Y.M., Zheng, B.D., Xu, D., et al., 2019b. Microbialdynamics and flavor formation during the traditional brewing of Monascus vinegar.Food Res. Int. 125 (C), 108531. Jo, Y.H., Chung, N.H., Park, S.W., Noh, B.S., Jeong, Y.J., Kwon, J.H., 2016. Applicationof E-tongue, E-nose, and MS-E-nose for discriminating aged vinegars based on tasteand aroma profiles. Food Sci. Biotechnol. 25 (5), 1313-1318. Kato, H., Rhue, M.R., Nishimura, T., 1989. Role of free amino acids and peptides in foodtaste.ACS Symp Ser Am Chem Soc 388, 158-174. Kappel, V.D., Costa, G.M., Scola, G., Silva, F.A., Landell, M.F., Valente, P., et al., 2008.Phenolic content and antioxidant and antimicrobial properties of fruits of Capsicumbaccatum L. var. pendulum at different maturity stages. J. Med. Food 11 (2),267-274. Kelebek, H., Kadirolu, P., Demircan, N.B., Selli,S., 2017. Screening of bioactivecomponents in grape and apple vinegars: antioxidant and antimicrobial potential.J. Inst. Brew. 123 (3), 407-416. Kim, S.H., Cho, H.K., Shin, H.S., 2012. Physicochemical properties and antioxidantactivities of commercial vinegar drinks in Korea. Food Sci. Biotechnol. 21 (6),1729-1734. Kong, Y., Zhang, L.L., Sun, Y., Zhang, Y.Y., Sun, B.G., Chen, H.T., 2017. Determination ofthe free amino acid, organic acid, and nucleotide in commercial vinegars.J. FoodSci.82 (5),1116-1123. Krusong, W.,Sriphochanart, W.,Suwapanich, R., Mekkerdchoo, O., Massa, S., 2019.Healthy dried baby corn silk vinegar production and determination of its mainorganic volatiles containing antimicrobial activity. LWT- Food Sci. Technol. 117,11108620. Li,T., Lo, Y.M., Moon, B.K., 2014. Feasibility of using Hericium erinaceus as the substratefor vinegar fermentation. LWT -Food Sci. Technol. 55 (1), 323-328. Lima, M.D.S., Silani, I.D.S.V., Toaldo, I.M., Correa, L.C., Biasoto, A.C.T., Pereira, G.E.,et al., 2014. Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the northeast region ofBrazil. Food Chem. 161 (15), 94-103. Liu, A.P., Peng, Y., Ao, X.L., Pan, W.S., Chen, S.J., Li, H., et al., 2020a. Effects of Aspergillus niger biofortification on the microbial community and quality of Baoningvinegar. LWT- Food Sci. Technol. 131,109728-109734. Liu, D.C., He, Y.L., Xiao, J.B., Zhou, Q., Wang, M.F., 2020b. The occurrence and stabilityof Maillard reaction products in various traditional Chinese sauces -ScienceDirect.Food Chem. 342,128319-128327. Maciej, B., Eric, B., Olivier, S.D., Lars, E., Marat, E., Zlatko, F., et al., 2019. The role of redyeast rice (RYR) supplementation in plasma cholesterol control: a review and expertopinion. Atheroscler. Suppl. 39. Martillanes, S., Ayuso-Yuste, M.C., Gil, M.V., Manzano-Duran, R., Delgado-Adamez, J.,2018. Bioavailability, composition and functional characterization of extracts fromOryza sativa L. bran. Food Res. Int. 111, 299-305. Nie,Z.Q., Yu, Z., Min, W., Yue, H., Wang, Y.N., Luo, J.M., et al., 2013. Exploringmicrobial succession and diversity during solid-state fermentation of Tianjin duliumature vinegar. Bioresour. Technol. 148C (8), 325-333. Pyo, Y.H., Hwang, J.Y., Seong, K.S., 2018. Hypouricemic and antioxidant effects of soyvinegar extracts in hyperuricemic mice. J. Med. Food 21 (12), 1299-1305. Scherer, R., Rybka, A.C.P., Ballus, C.A., Meinhart, A.D., Filho, J.T., Godoy, H.T., 2012.Validation of a HPLC method for simultaneous determination of main organic acidsin fruits and juices. Food Chem. 135 (1), 150-154. Verzelloni, E.,Tagliazucchi,D., Conte, A., 2007. Relationship between the antioxidantproperties and the phenolic and flavonoid content in traditional balsamic vinegar.Food Chem. 105 (2),564-571. Wang, J.B., Li, W., Li, Z.P., Wu, W.H., Tang, X.M., 2018. Analysis and evaluation of thecharacteristic taste components in Portobello mushroom. J. Food Sci. 83 (6),1542-1551. Xie, X.L., Zheng, Y., Liu, X., Cheng, C., Zhang, X.L., Xia, T., et al., 2017. Antioxidantactivity of Chinese Shanxi aged vinegar and its correlation with polyphenols andflavonoids during the brewing process. J. Food Sci. 82 (10), 2479-2486. Yu, X., Yang, M., Dong, J.L., Shen, R.L., 2018. Comparative analysis of the antioxidantcapacities and phenolic compounds of oat and buckwheat vinegars duringproduction processes. J. Food Sci. 83 (3), 844-853. Zhang, H., Wang, Z.Y., Yang, X., Zhao, H.T., Zhang, Y.C., Dong, A.J., et al., 2014.Determination of free amino acids and 18 elements in freeze-dried strawberry andblueberry fruit using an Amino Acid Analyzer and ICP-MS with micro-wavedigestion. Food Chem. 147 (15), 189-194. Zhang, B., Xia, T., Duan, W.H., Zhang, Z.J., Li, Y., Fang, B., et al., 2019. EZlldinffects oforganicacids, amino acids and phenolic compounds on antioxidant characteristic ofZhenjiang aromatic vinegar. Molecules 24 (20),3799-3811. Zhao, J., Guo, L.C., Chun, W., Zhi, M.H., Xi,B.X., Guang, F.X., et al., 2021. Profiling thekey metabolites produced during the modern brewing process of Chinese rice wine.Food Res. Int. 139 (6877),109955. 本研究评估了红曲霉米醋在固态发酵过程中口感结构、口感活性指标和抗氧化活性的变化,以及它们之间的潜在关系。结果表明,发酵时间越长,抗氧化成分含量越高,抗氧化活性越强。根据口感活性值(TAV)评价,认为TAV高的7种有机酸和4种游离氨基酸(FAAs)是口感活性指标。红曲霉米醋抗氧化活性与主要抗氧化物质的相关性分析顺序为:FAAs>总酚>总黄酮>有机酸。进一步分析发现,乙酸、乳酸、酒石酸、琥珀酸、谷氨酸、Ala和Val等7种关键成分是红曲米醋抗氧化活性的味觉活性指标,对红曲米醋的口感特性和抗氧化活性有显著影响。本研究结果可为通过关键口感活性指标调控红曲霉米醋的抗氧化特性提供参考,并为进一步探索开发新型醋基功能食品提供新思路。如需了解更多详情请参照原文献:Taste-active indicators and their correlation with antioxidant ability during the Monascus rice vinegar solid-state fermentation process
确定





还剩6页未读,是否继续阅读?
产品配置单
北京盈盛恒泰科技有限责任公司为您提供《红曲霉米醋中味觉指标检测方案(感官智能分析)》,该方案主要用于食醋中理化分析检测,参考标准--,《红曲霉米醋中味觉指标检测方案(感官智能分析)》用到的仪器有电子舌、日本INSENT味觉分析系统(电子舌)
推荐专场
感官智能分析系统(电子鼻/电子舌)
相关方案
更多
该厂商其他方案
更多
















