
方案详情
文
MXene量子点(MXene quantum dots, mqd)由于其优异的光学性能、良好的生物相容性、天然的亲水性和现成的功能化等特点,在荧光材料领域引起了广泛的关注[1-3]。mqd在许多领域都有潜在的应用,特别是在生物医学[2,4,5]、传感[6-11]、光电器件[12,13]和催化[14,15]。近年来,自荧光量子阱的首次报道以来,改进量子阱合成策略的研究越来越多。mqd的合成主要有两种方法:从本体前体的自上而下切割法和从分子前体的自下而上方法。迄今为止,获得mqd的典型方法为自上而下的方法(以Ti3C2量子点为主),包括水热/溶剂热法、超声法、球磨法、插层法和组合法[2,4,14,16 - 19]。在自顶向下合成mqd时,最常用的方法是在高浓度酸性溶液中蚀刻MAX相(“M”表示过渡金属,“A”表示- IIIA/IVA族元素,X表示C和/或N元素)后的水热法。而水热法需要6-12 h才能完成反应[2,8]。因此,开发快速、直接、方便的荧光量子点合成工艺将是该领域的一个重大突破。微波辅助合成可缩短反应时间的量子点的研究较少。次氯酸/次氯酸盐(HOCl/ClO−),进口活性活性氧(reactive oxygen species, ROS)在生物体内起着重要的保护作用[20,21]。体内ClO−水平异常与创伤愈合受损、癌症、关节炎、神经元变性和心血管疾病等慢性和退行性疾病的发展高度相关[22-24]。因此,快速、灵敏地检测ClO−在环境和生活领域都具有重要意义。到目前为止,许多荧光探针都被用来检测ClO−,因为它们具有高稳定性、高灵敏度和高选择性等优点[25-28]。与普通荧光法相比,比值荧光法可以最大限度地减少来自背景的假信号,对ClO−的检测具有更好的灵敏度[29,30]。在用于检测ClO−的比率荧光探针领域,仍然需要精心的设计和合成。因此,构建和制备一种简单、高效的ClO−检测比荧光探针仍然面临着巨大的挑战。本文采用姜黄素荧光共振能量转移(FRET)技术设计了基于Ti3C2 MQDs的比例荧光探针(方案1)。首先在微波辅助照射下刻蚀MAX相5 min后合成Ti3C2 MQDs。与其他自底向上合成Ti3C2 mqd的方法相比,微波辅助合成大大缩短了反应时间。此外,微波加热更均匀,允许更快的反应和更少的副产物形成。在姜黄素存在的情况下,Ti3C2 MQDs在430 nm处的荧光发射被FRET猝灭,而姜黄素在540 nm处的荧光发射被增强。加入ClO−后,姜黄素的酚和甲氧基被氧化成醌,在540 nm处荧光发射逐渐猝灭,在430 nm处荧光发射逐渐恢复。在365 nm紫外灯下,加入ClO−后,溶液颜色由黄绿色变为蓝色,肉眼可见。在此基础上,设计了比例荧光和“裸眼”探针检测姜黄素和ClO−。本工作不仅为Ti3C2 mqd的合成提供了一种新的方法,而且拓展了Ti3C2 mqd在生物和化学领域的应用环境检测。
方案详情

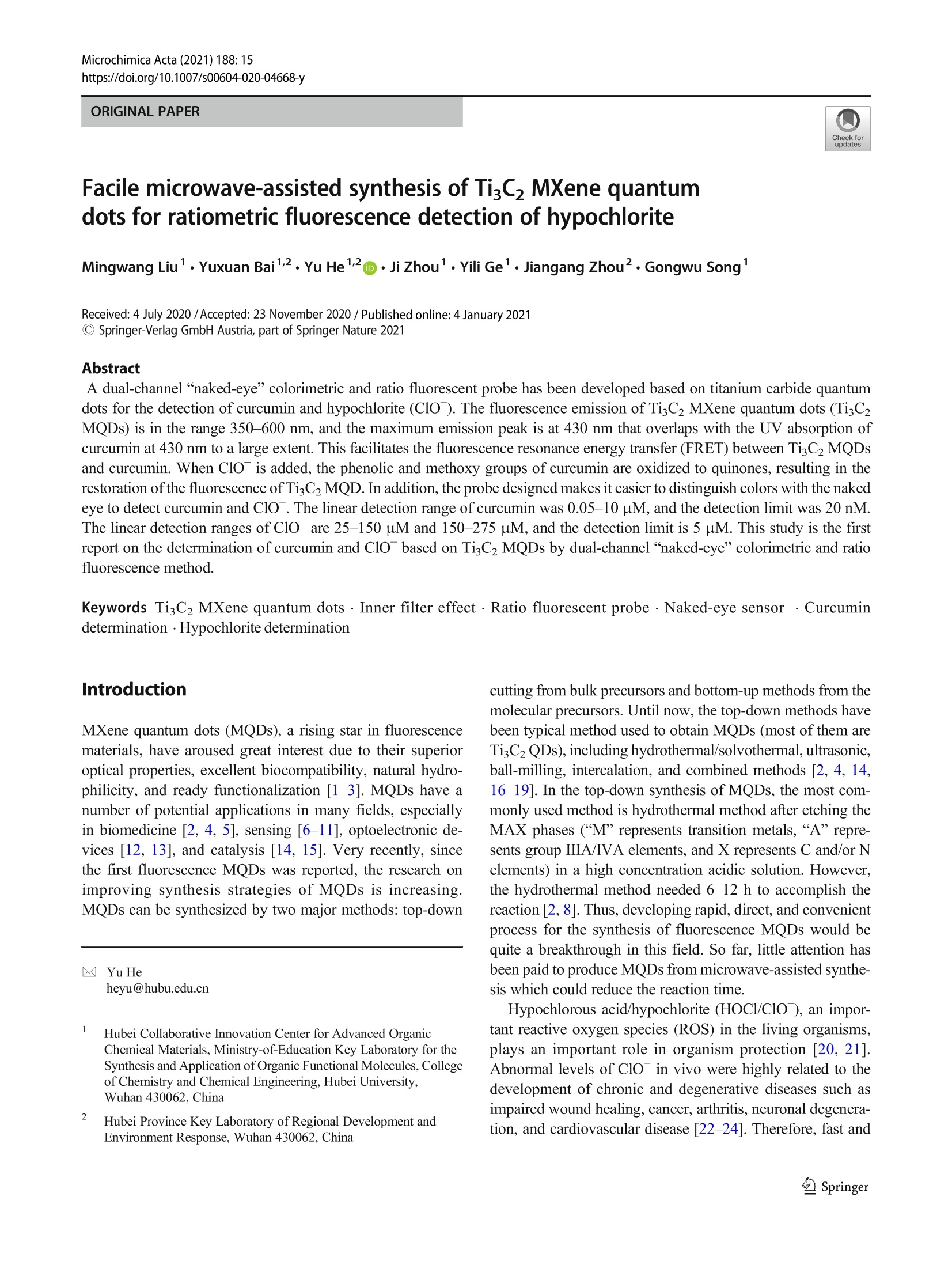
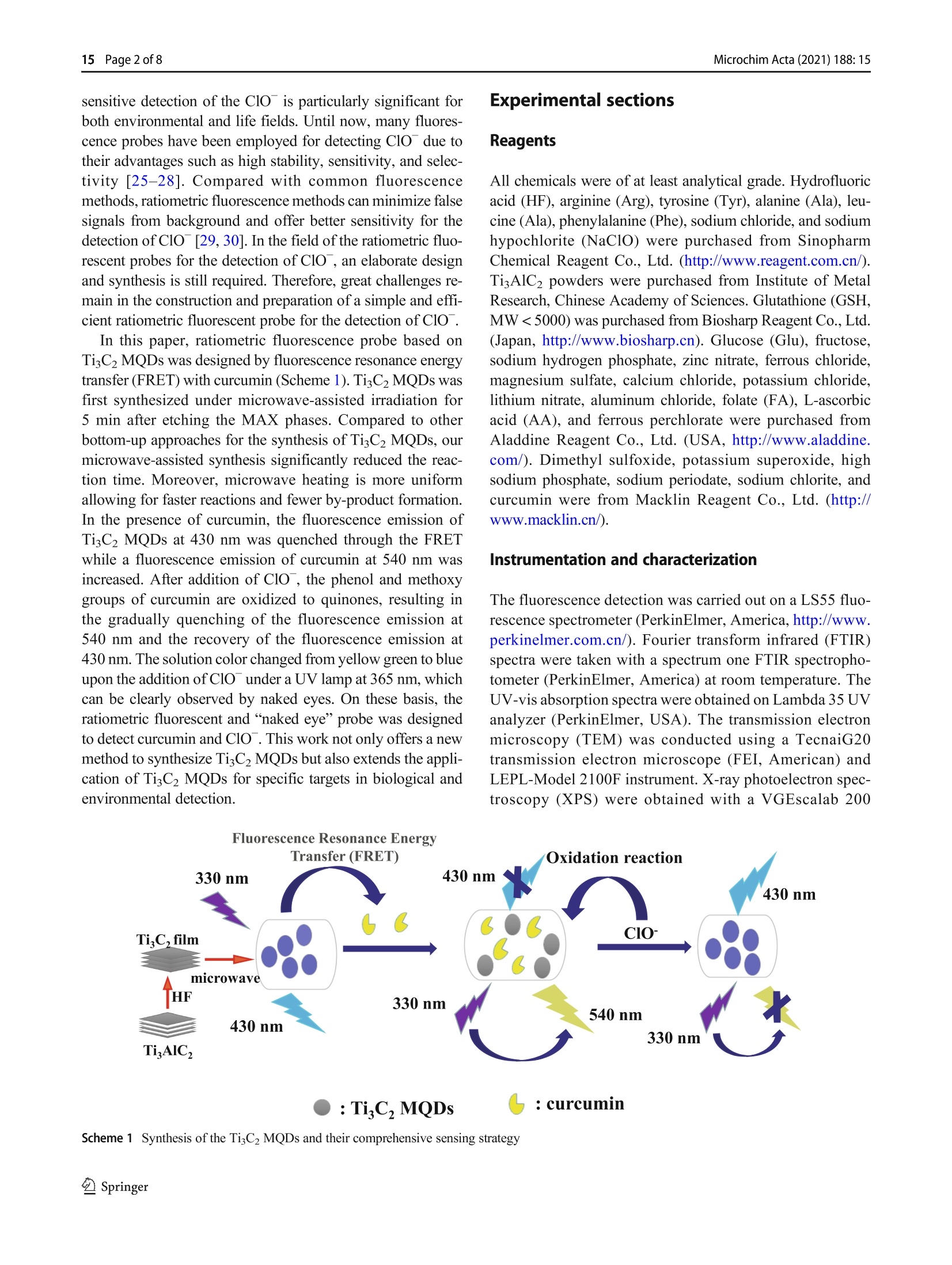
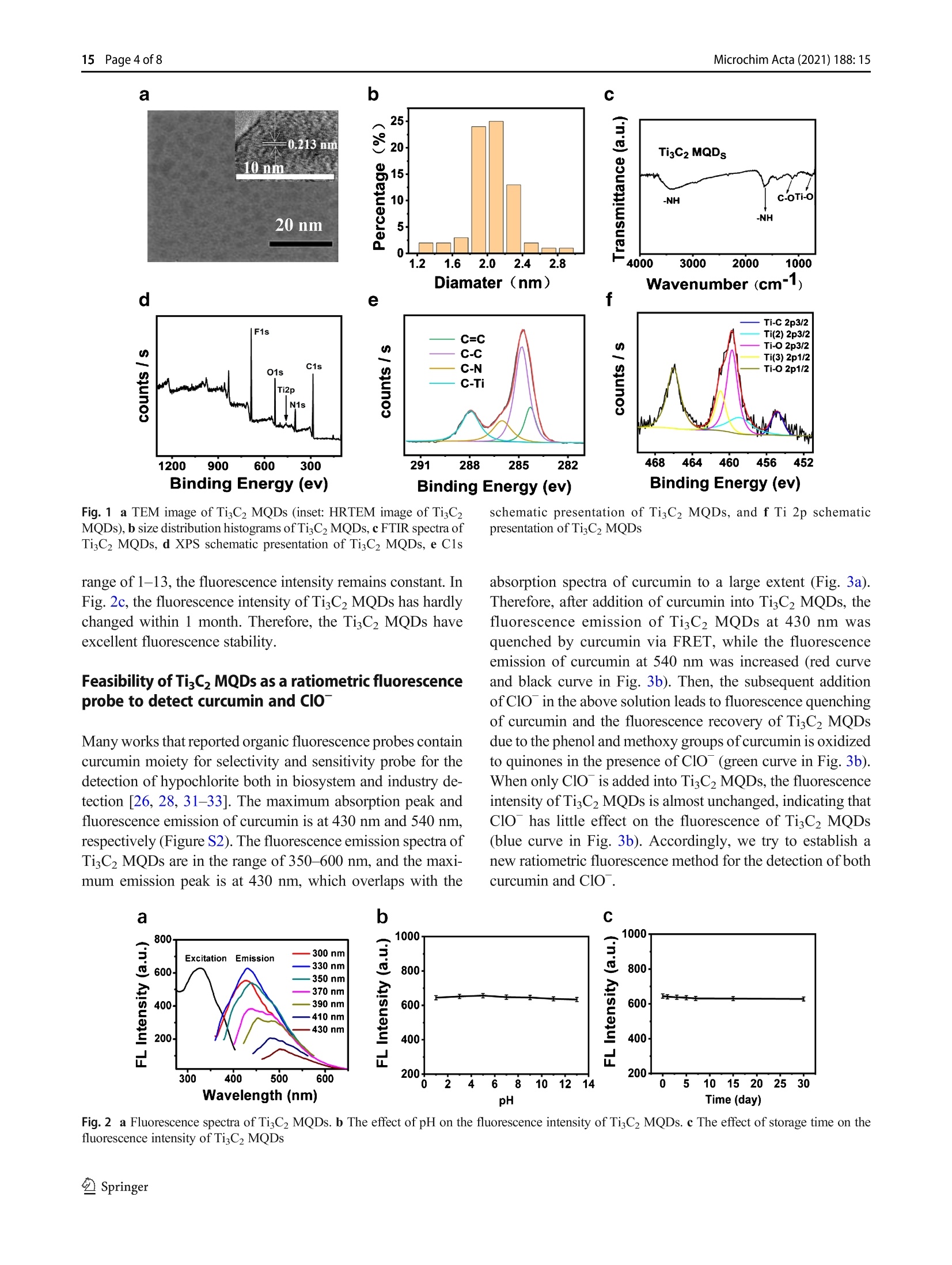
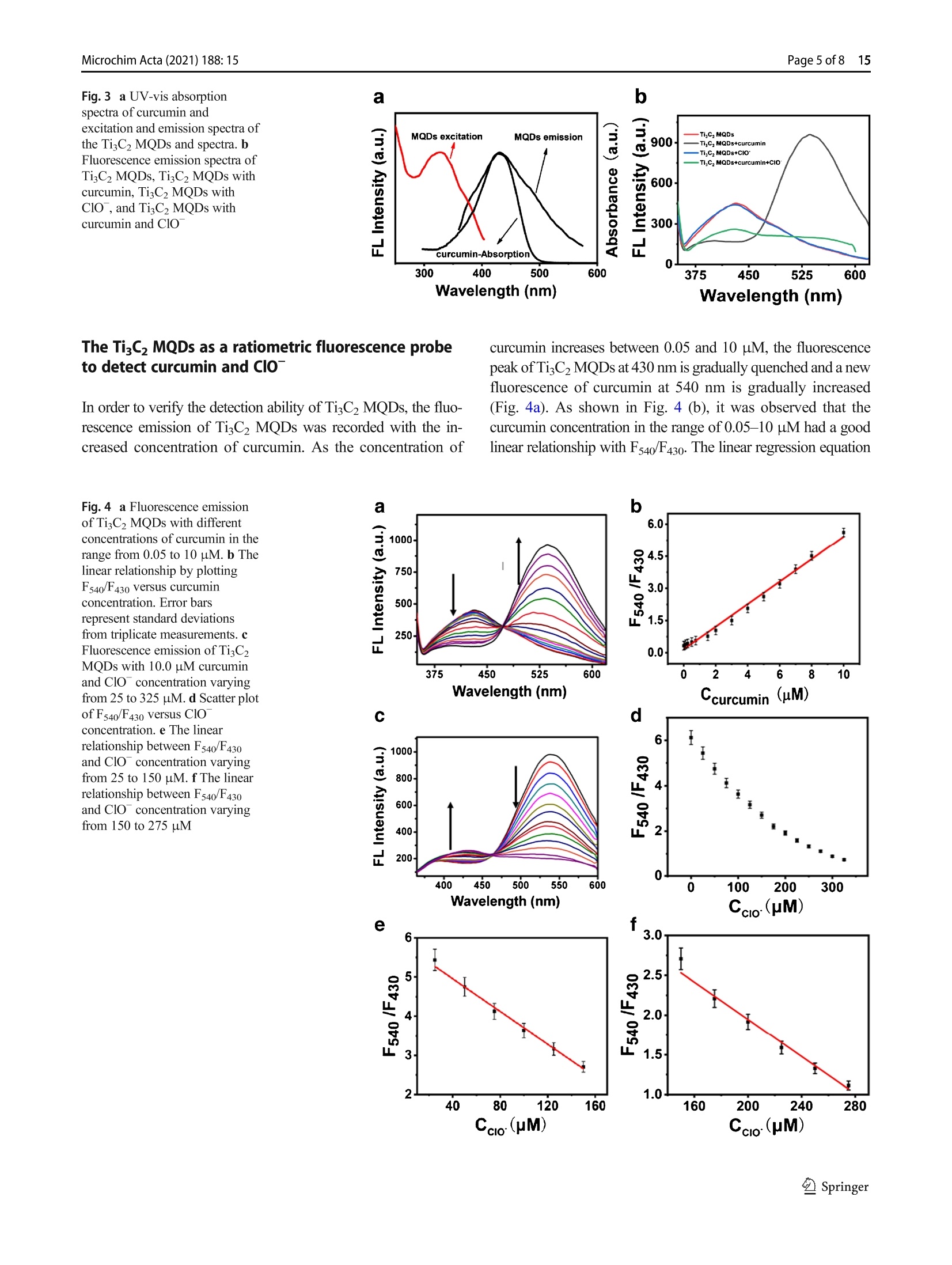
Microchimica Acta (2021) 188: 15https://doi.org/10.1007/s00604-020-04668-yORIGINAL PAPER Microchim Acta (2021) 188:1515 Page 2 of 8 Facile microwave-assisted synthesis of TigC2 MXene quantumdots for ratiometric fluorescence detection of hypochlorite Mingwang Liu1· Yuxuan Bai12. Yu He12D). Ji Zhou1. Yili Ge1. Jiangang Zhou2. Gongwu Song1 Received: 4 July 2020 /Accepted: 23 November 2020 / Published online: 4 January 2021 CSpringer-Verlag GmbH Austria, part of Springer Nature 2021 Abstract A dual-channel“naked-eye”colorimetric and ratio fluorescent probe has been developed based on titanium carbide quantumdots for the detection of curcumin and hypochlorite (ClO). The fluorescence emission of TizC2 MXene quantum dots (TisC2MQDs) is in the range 350-600 nm, and the maximum emission peak is at 430 nm that overlaps with the UV absorption ofcurcumin at 430 nm to a large extent. This facilitates the fluorescence resonance energy transfer (FRET) between TigC2 MQDsand curcumin. When ClO is added, the phenolic and methoxy groups of curcumin are oxidized to quinones, resulting in therestoration of the fluorescence ofTisC2MQD. In addition, the probe designed makes it easier to distinguish colors with the nakedeye to detect curcumin and ClO. The linear detection range of curcumin was 0.05-10 uM, and the detection limit was 20 nM.The linear detection ranges of ClO are 25-150 pM and 150-275 uM, and the detection limit is 5 uM. This study is the firstreport on the determination of curcumin and ClO based on TiC2 MQDs by dual-channel“naked-eye”colorimetric and ratiofluorescence method. Keywords TisC2 MXene quantum dots·Inner filter effect· Ratio fluorescent probe·Naked-eye sensor · Curcumindetermination·Hypochlorite determination Introduction MXene quantum dots (MQDs), a rising star in fluorescencematerials, have aroused great interest due to their superioroptical properties, excellent biocompatibility, natural hydro-philicity, and ready functionalization [1-3]. MQDs have anumber of potential applications in many fields, especiallyin biomedicine [2, 4, 5], sensing [6-11], optoelectronic de-vices [12, 13], and catalysis [14, 15]. Very recently, sincethe first fluorescence MQDs was reported, the research onimproving synthesis strategies of MQDs is increasing.MQDs can be synthesized by two major methods: top-down ( 区 Yu He heyu@hubu.edu.cn ) Hubei Collaborative Innovation Center for Advanced OrganicChemical Materials, Ministry-of-Education Key Laboratory for theSynthesis and Application ofOrganic Functional Molecules, Collegeof Chemistry and Chemical Engineering, Hubei University,Wuhan 430062, China 2 Hubei Province Key Laboratory of Regional Development andEnvironment Response, Wuhan 430062,China cutting from bulk precursors and bottom-up methods from themolecular precursors. Until now, the top-down methods havebeen typical method used to obtain MQDs (most of them areTizC2 QDs), including hydrothermal/solvothermal, ultrasonic,ball-milling, intercalation, and combined methods [2, 4, 14,16-19]. In the top-down synthesis of MQDs, the most com-monly used method is hydrothermal method after etching theMAX phases (“M”represents transition metals,“A”repre-sents group IIIA/IVA elements, and X represents C and/or Nelements) in a high concentration acidic solution. However,the hydrothermal method needed 6-12 h to accomplish thereaction [2,8]. Thus, developing rapid, direct, and convenientprocess for the synthesis of fluorescence MQDs would bequite a breakthrough in this field. So far, little attention hasbeen paid to produce MQDs from microwave-assisted synthe-sis which could reduce the reaction time. Hypochlorous acid/hypochlorite (HOCl/ClO), an impor-tant reactive oxygen species (ROS) in the living organisms,1_plays an important role in organism protection [20, 21].Abnormal levels of ClO in vivo were highly related to thedevelopment of chronic and degenerative diseases such asimpaired wound healing, cancer, arthritis, neuronal degenera-tion, and cardiovascular disease [22-24]. Therefore, fast and In this paper, ratiometric fluorescence probe based onTi3C2MQDs was designed by fluorescence resonance energytransfer (FRET) with curcumin (Scheme 1). TisC2 MQDs wasfirst synthesized under microwave-assisted irradiation for5 min after etching the MAX phases. Compared to otherbottom-up approaches for the synthesis of TiC2 MQDs, ourmicrowave-assisted synthesis significantly reduced the reac-tion time. Moreover, microwave heating is more uniformallowing for faster reactions and fewer by-product formation.In the presence of curcumin, the fluorescence emission ofTisC2 MQDs at 430 nm was quenched through the FRETwhile a fluorescence emission of curcumin at 540 nm wasincreased. After addition of ClO , the phenol and methoxygroups of curcumin are oxidized to quinones, resulting inthe gradually quenching of the fluorescence emission at540 nm and the recovery of the fluorescence emission at430 nm. The solution color changed from yellow green to blueupon the addition of ClO under a UV lamp at 365 nm, whichcan be clearly observed by naked eyes. On these basis, theratiometric fluorescent and "naked eye” probe was designedto detect curcumin and ClO. This work not only offers a newmethod to synthesize TiC2 MQDs but also extends the appli-cation of TigC2 MQDs for specific targets in biological andenvironmental detection. Experimental sections Reagents All chemicals were of at least analytical grade. Hydrofluoricacid (HF), arginine (Arg), tyrosine (Tyr), alanine (Ala), leu-cine (Ala), phenylalanine (Phe), sodium chloride, and sodiumhypochlorite (NaClO) were purchased from SinopharmChemical Reagent Co., Ltd. (http://www.reagent.com.cn/).TiAlC2 powders were purchased from Institute of MetalResearch, Chinese Academy of Sciences. Glutathione (GSH,MW<5000) was purchased from Biosharp Reagent Co., Ltd.(Japan, http://www.biosharp.cn). Glucose (Glu), fructose,sodium hydrogen phosphate, zinc nitrate, ferrous chloride,magnesium sulfate, calcium chloride, potassium chloride,lithium nitrate, aluminum chloride, folate (FA), L-ascorbicacid (AA), and ferrous perchlorate were purchased fromAladdine Reagent Co., Ltd. (USA, http://www.aladdine.com/).Dimethyl sulfoxide, potassium superoxide, highsodium phosphate, sodium periodate, sodium chlorite, andcurcumin were from Macklin Reagent Co., Ltd. (http://www.macklin.cn/). Instrumentation and characterization The fluorescence detection was carried out on a LS55 fluo-rescence spectrometer (PerkinElmer, America, http://www.perkinelmer.com.cn/). Fourier transform infrared (FTIR)spectra were taken with a spectrum one FTIR spectropho-tometer (PerkinElmer, America) at room temperature. TheUV-vis absorption spectra were obtained on Lambda 35 UVanalyzer (PerkinElmer, USA). The transmission electronmicroscopy (TEM) was conducted using a TecnaiG20transmission electron microscope (FEI, American) andLEPL-Model 2100F instrument. X-ray photoelectron spec-troscopy (XPS) were obtained with a VGEscalab 200 :TiCz MQDs : curcumin Scheme 1 Synthesis of the TigC2 MQDs and their comprehensive sensing strategy spectrometer using an aluminum anode (AlKα) operating at510 W with a background pressure of 2×10- mbar(Thermo Scientific, USA, https://www.thermofisher.com.cn/). The XD-100A computer microwave catalytic synthe-sis extraction instrument was used to synthesize TisC2MQDs (Beijing Xianghu Technology Co., Ltd., China,http://www.xianghukeji.com/). Synthesis of TigC2 MQDs One gram of TiAlC2 powder was slowly added to 16 mL ofHF solution and stirred at 40°C for 24 h. The mixture wasthen centrifuged and washed 4-6 times with distilled wateruntil the pH of the supernatant was close to 6. The super-natant was removed and the black precipitate (Ti3C2MXene) was dried under vacuum at 60 °C for 12 h to obtainTiC2 MXene powder. A total of 100 mg of TiC2 MXenepowder was added to a 100-mL round bottom flask contain-ing 50 mL of deionized water. The pH was adjusted to 9with ammonia water, and the suspension was homogenizedby ultrasonic treatment for 3 min. The round bottom flaskwas placed in a microwave reactor under microwave-assisted irradiation (800 W, 90 °C) for 5 min. The reactionsuspension was filtered through a 0.22-um microporousmembrane to obtain TisC2 MQDs solution. After treatmentwith a rotary evaporator, vacuum drying was performed at60 ℃ for 12 h to obtain TizC2 MQDs powder, which wasstored in a refrigerator at 2-8 °C for use. Ratiometric fluorescent detection of curcumin andCIoT Thirty-seven milligram of curcumin was dissolved in100 mL of ethanol to obtain a 1-mM curcumin ethanolsolution for use. Curcumin solutions of different concen-trations (final concentration 0.05-10 uM) were mixedwith TigC2 MQDs (1 mg mL, 200 uL) solution anddiluted to 2 mL with deionized water. The fluorescencespectrum was then recorded at an excitation wavelengthof 330 nm with a slit width of 15/20 nm and a voltage of780 V. In order to achieve the detection of C1O , TigC2MQDs (1 mg mL,200 pL), 20 uL of 1 mM curcumin,and various concentrations of ClO (25-325 uM) werethoroughly mixed and diluted to 2 mL. The slit width isset to 15/20 nm and the photomultiplier tube voltage is780 V. The fluorescence spectrum was recorded at anexcitation wavelength of 330 nm. In addition, the colorchanges of the detection system were observed, respec-tively, which proved the possibility of TigC2 MQDs as anaked-eye sensor for semi-quantitative detection ofcurcumin and ClO . Results and discussion Preparation and characterization of TigC2 MQDs After etching the MAX phases by HF, Ti,C2 MQDs were syn-thesized by a fast microwave-assisted method. TigC2 nanosheetwas placed in a microwave reactor under microwave-assistedirradiation (800 W, 90°℃) from 5 min to 1 h. The fluorescenceintensity of TiC2 MQDs kept consistent with the change ofreaction time (Figure S1). Therefore, 5 min was selected as theoptimal reaction time for the preparation of Ti,C2MQDs. The TEM image of Fig. la shows that the TizC2MQDs arespherical and well dispersed in an aqueous medium, with uni-form size and distribution. The diameter of the TigC2 MQDs isabout 2 nm with the clear crystalline structure and the latticespacing is about 0.213 nm corresponding to the (0110) facet ofTigC2 MXenes (inset of Fig. 1a). The average size of the distri-bution histogram is showed in the inset of Fig. 1b, which con-firms that the TigC2 MQDs have an average diameter of 2.0±0.33 nm. FTIR spectra were used to analyze the possible groupson the surface ofTisC2 MQDs. As shown in Fig. 1c, two strongpeaks were observed at 1650 cmand 3400 cm,correspond-ing to N-H stretching vibration and the amide band stretchingvibration, respectively. The FTIR band at 1067 cmcorre-sponds to the stretching vibration of C-O. The peak at720 cmmay be the stretching vibration of the Ti-O bond [2,4]. As shown in Fig. 1d, X-ray photoelectron spectroscopy(XPS) further indicates that TigC2 MQDs contains elementssuch as carbon, nitrogen, titanium, oxygen, and fluorine. Thehigh-resolution XPS spectrum of C1s shows four deconvolutedpeaks (Fig. 1e), corresponding to C=C (284.3 eV), C-C(284.8eV),C-Ti(288.0eV), and C-N (286.1 eV), respectively.In addition, the high-resolution XPS spectrum of Ti 2p showsfive deconvoluted peaks (Fig. 1f). The Ti 2p spectrum showsthe Ti-C and Ti-O in Ti 2p3/2 peaks at 454.9 and 459.7 eV,which are the typical characteristic peaks of TisC2 MXenenanosheets [8, 10]. This was further demonstrated that TizC2MQDs keep the primary structure of TigC2 MXenes. Stability and fluorescence properties of the TisC2MQDs As shown in Fig. 2a, similar to the previously reported TisC2MQDs, the fluorescence emission spectrum of the TisC2MQDs depends on the excitation wavelength. When the exci-tation wavelength gradually changed from 300 to 430 nm, theemission peak gradually shifted red from 420 to 510 nm. At anexcitation wavelength of 330 nm, TisC2 MQDs showedstrong emission centered at 430 nm. To evaluate the fluores-cence stability of TisC2 MQDs, we investigated the changesof the fluorescence intensity of TigC2 MQDs with pH andtime. Figure 2b shows the effect of pH on the fluorescenceintensity of TigC2 MQDs at 430 nm. When the pH is in the Fig. 1 a TEM image ofTizC2 MQDs (inset: HRTEM image of TiC2MQDs), b size distribution histograms ofTiC2 MQDs, c FTIR spectra ofTigC2 MQDs, d XPS schematic presentation of TisC2 MQDs, e C1s schematic presentation of TigC2 MQDs, and f Ti 2p schematicpresentation of TisC2MQDs range of 1-13, the fluorescence intensity remains constant. InFig. 2c, the fluorescence intensity ofTigC2 MQDs has hardlychanged within 1 month. Therefore, the TiC2 MQDs haveexcellent fluorescence stability. Feasibility of TisC2 MQDs as a ratiometric fluorescenceprobe to detect curcumin and ClO" Many works that reported organic fluorescence probes containcurcumin moiety for selectivity and sensitivity probe for thedetection of hypochlorite both in biosystem and industry de-tection [26, 28, 31-33]. The maximum absorption peak andfluorescence emission of curcumin is at 430 nm and 540 nm.respectively (Figure S2). The fluorescence emission spectra ofTigC2 MQDs are in the range of 350-600 nm, and the maxi-mum emission peak is at 430 nm, which overlaps with the absorption spectra of curcumin to a large extent (Fig. 3a).Therefore, after addition of curcumin into TigC2 MQDs, thefluorescence emission of TisC2 MQDs at 430 nm wasquenched by curcumin via FRET, while the fluorescenceemission of curcumin at 540 nm was increased (red curveand black curve in Fig. 3b). Then, the subsequent additionof ClO in the above solution leads to fluorescence quenchingof curcumin and the fluorescence recovery of TisC2 MQDsdue to the phenol and methoxy groups of curcumin is oxidizedto quinones in the presence of ClO (green curve in Fig.3b).When only ClOis added into TiC2 MQDs, the fluorescenceintensity of TisC2 MQDs is almost unchanged, indicating thatClO has little effect on the fluorescence of TigC2 MQDs(blue curve in Fig. 3b). Accordingly, we try to establish anew ratiometric fluorescence method for the detection of bothcurcumin and Clo. a c 600 The TisC2 MQDs as a ratiometric fluorescence probeto detect curcumin and Clo In order to verify the detection ability of Ti,C2MQDs, the fluo-rescence emission of TigC2 MQDs was recorded with the in-creased concentration of curcumin. As the concentration of curcumin increases between 0.05 and 10 uM, the fluorescencepeak of TigC2 MQDs at 430 nm is gradually quenched and a newfluorescence of curcumin at 540 nm is gradually increased(Fig. 4a). As shown in Fig. 4 (b), it was observed that thecurcumin concentration in the range of 0.05-10 pM had a goodlinear relationship with F540/F430. The linear regression equation Fig.5 a Selectivity of TisC2MQDs towards curcumin.bSelectivity of TiC2 MQDs/curcumin towards ClO is expressed as F540/F430=0.525 Ccurcumin+0.168 (R²=0.991).The detection limit is calculated to be 20 nM based on 3o/m(where m is the slope of the calibration plot and o is the standarddeviation of the blank). Table S1 shows some of the reportedcurcumin assays compared to our method. After addition ofClO with concentration varying from 25 to 325 uM intoTigC2 MQDs/curcumin solution, the fluorescence quenching ofcurcumin and the fluorescence recovery of the TigC2 MQDshappened, because phenol and methoxy groups of curcuminare oxidized to quinones in the presence of ClO (Fig. 4c).Figure 4d shows the scatter diagram between F540/F430 andClOconcentration. Figure 4e and are linear relationship be-tween F540/F430 and ClO concentration. There is a good linearrelationship between F540F430 and ClO concentration between25 and 275 uM, and its linear range is 25-150 pM and 150-275 uM, respectively. The linear regression equations were F540/F430=-0.021C cio +5.79 and F540/F430=-0.0117C cio +4.29, respectively. The detection limit is calculated to be 5 uM.Compared with the reported nanomaterial-based optical methodsfor the determination of hypochlorite (Table S2). The selectivity In order to evaluate the selectivity ofthe TisC2 MQDs as aratiometric fluorescence probe for the detection of curcumin(4uM), the response to various substances in the same con-dition was investigated (Fig. 5a). We added the followingsubstances instead of curcumin to TizC2 MQDs solutions,such as 200-uM interfering ions (K *, Na *, Fe’+, Al’+,Ca²+, Mg?+, So4, Fe?+,Zn²+,Cr, and HPO4),100 uMvarious amino acids, glutathione (GSH), folic acid (FA), andL-ascorbic acid (AA), two carbohydrates (fructose and glu-cose). Only curcumin has significantly influence on F540/F430of TigC2 MQDs, demonstrating the superior selectively to-wards curcumin. In order to evaluate the selectivity of TigC2 MQDs/curcumin as a ratiometric fluorescence probe to detect CIO(150 uM), the response of various substances was investigat-ed. As shown in Fig. 5b, various oxidized anions and freeradicals 200 uM (including H202,02,02, HO·, P207,IO4,ONOO, Cl02, S203 ) were added to the TigC2 Fig.6 The TisC2 MQDs withdifferent concentration ofcurcumin under (a) sunlight and(b) 365-nm UV lamp. The TiC2MQDs/curcumin with differentconcentration of ClO under (c)sunlight and (d) 365 nm UV lamp MQDs fluorescent probe with curcumin (10 pM). The super-oxide solution (O2) was prepared by adding KO2(1.0mg) tothe dried dimethyl sulfoxide (1.0 mL) and stirring vigorouslyfor 10 min. The fenton reaction of ferrous perchlorate (5 mM)and H202 produces hydroxyl radical (HO·). H202 was mixedwith NaClO (5 mM) in order to form02. Only ClO havesignificantly influence on F540/F430 of Ti3C2MQDs/curcumin, demonstrating the superior selectively ofthe system towards ClO . "Naked-eye" detection of curcumin and CIo Photograph of the TiC2 MQDs with different concentrationof curcumin under sunlight and a 365-nm UV lamp aredisplayed in Figs. 6a and 6b. The solution colorchanged from colorless to yellow under sunlight andfrom blue to yellow under a 365-nm UV lamp, respec-tively. As shown in Figs. 6c and 6d, the solution colorrecovered to colorless under sunlight and blue under365 nm UV lamp by Clo, respectively, which can beclearly observed by naked eyes. Conclusions In conclusion, we successfully prepared TisC2 MQDs by mi-crowave synthesis for the first time, greatly reducing thesynthesis time. The TisC2 MXene quantum dots (TisC2MQDs) exhibit excitation wavelength-dependent bluephotoluminescence with excitation/emission peaks at330/430 nm. The FRET between Ti3C2 MQDs andcurcumin can quench the fluorescence of TisC2 MQDs.Hypochlorite (ClO) can oxidize curcumin’s phenol andmethoxy group to quinone, causing the fluorescence in-tensity of Ti3C2 MQDs to recover. And the color of themixed system changed significantly during the detectionprocess. Therefore, we have established a dual-channel“naked-eye”colorimetric and ratio fluorescent probe forthe detection of curcumin and ClO . Supplementary Information The online version contains supplementarymaterial available at https://doi.org/10.1007/s00604-020-04668-y. Funding This work was financially supported by Supported by NationalNatural Science Foundation of China (21707030) and Wuhan YouthScience and technology plan (2016070204010133). Compliance with ethical standards Conflict of interest The authors declare that they have no com-peting interests. ( 1. Shao BB, Liu ZF, Zeng GM, Wang H, Liang QH, He QY, ChengM, Zhou CY, Jiang LB, Song B (2020) Two-dimensional transition metal c arbide and nitride (MXene) derived quantum dots (QDs): synthesis, properties, applications and prospect s . J Mater Chem A Mater Energy Sustain 8:7508-7535 ) ( 2. Xue Q, Zhang H J, Zhu M S, Pei ZX, Li HF, Wang ZF, H uang Y, Deng Q H, Z hou J, Du S Y, H uang Q, Zhi CY (2017)Photoluminescent TigC2 M X ene quantum dots for multicolor cel-lular imaging. Adv Mater 29:1604847 ) ( 3. Lu S Y, Sui L Z, L iu Y, Yong X, X iao GJ, Y uan KJ, Liu Z Y, LiuBZ, Zou B , Yang B (2019) White photoluminescent TisC2 MXenequantum dots with two-photon fluorescence. Adv Sci 6:1801470 ) ( 4. Yu X H, Cai X K, Cui H D, Lee SW, Yu X F , L i u BL ( 2 017) F luorine-free preparation of titanium carbide MXene quantum dotswith h igh near-infrared photothermal performances for cancer ther-apy. Nanoscale 9:17859-17864 ) ( 5. Rafieerad A, Yan W, Sequiera GL, Sareen N, Abu-El-Rub E,Moudgil M , Dhingra S ( 2019) Application of TizC2 MXene quan-tum dots for immunomodulation and regenerative medicine. AdvHealthcare Mater 8 (16):1900569 ) ( 6. Xu Q, Ding L , Wen Y Y, Yang WJ,Zhou HJ, Ch e n XZ , Jason S, Z hou AG, Wee-Jun O, L i N (2018) H igh photoluminescence quan- t um yield of 18.7% by nitrogen-doped TisC2 MXene quantum dots.J Mater Chem 6:6360 - 6369 ) ( 7. Liang Z, Kang M , Payne GF, W a ng X, Sun R (2016) Probing energy and electron transfer mechanisms in fluorescence quenching of biomass carbon quantum dots. ACS Appl Mater Interface 8(27):17478-17488 ) ( 8. Guo Z, Zhu X, Wang S, Lei C, Huang Y, Ni e Z, Ya o S (2018) F luorescent TigC2 MXene q u antum dots f o r an a lkaline phospha- tase assay and embryonic stem cell ide n tification based on the inne r f ilter effec t . Nanoscale 10:19579- 1 9585 ) ( 9. Chen X, Li J, Pan G, Xu W , Z hu J , Zhou D , L i D , Chen C, Lu G, Song H (2019) T isC2 MXene q u antum dots/TiO2 i n verse opal heterojunction electrode platform for superiorphotoelectrochemical biosensing. Sensors Actuators B C hem 289:131-137 ) ( 10. Cai GN, Yu ZZ, Tong P, Tang DP (2019) TisC2 MXene quantumdots-encapsulated liposome for photothermal immunoassay using aportable near-infrare d imaging camera on smartphone. Nanoscale 11:15659-15667 ) ( 11. Liu MW, He Y, Zhou J , Ge YL, Zhou J G , Song GW (2020) A “naked-eye”colorimetric a n d r a tiometric f luorescence p robe foruric acid based on Ti3C2 MXene quantum dots. Anal C h im Ac t a1103:134-142 ) 12. Xu Q, Yang W, Wen Y, Liu S, Liu Z, Ong W-J, Li N (2019)Hydrochromic full-color MXene quantum dots through hydrogenbonding toward ultrahigh-efficiency white light-emitting diodes.ApplMater Today 16:90-101 13.5.Huang D, Xie Y, Lu D, Wang Z, Wang J, Yu H, Zhang H (2019)Demonstration of a white laser with V2C MXene-based quantumdots. Adv Mater 31:1901117 14. Zeng Z, Yan Y, ChenJ, Zan P, Tian Q, Chen P (2019) Boosting thephotocatalytic ability of CuzO nanowires for CO2 conversion byMXene quantum dots. Adv Funct Mater 29:1806500 ( 15 T ang R, Zhou S, Li C, C hen R, Zhang L, Z h ang Z, Yi n L ( 2 020) Janus-structured co-TisC2 MXene quantum dots as a Schottky cat- alyst for high-performance p h otoelectrochemical water oxi d ation. Adv F unct Mater 2000637 ) ( 16. Xu G , Niu Y, Yang X , Jin Z , Wang Y, X u Y, N i u H (2018) Preparation of TigC2Tx MXene-derived quantum dots with white/ blue-emitting photoluminescence and Electrochemiluminescence. Adv Opt Mater 6:1800951 ) 17.Zhou L, Wu FM, Yu JH, Deng QH, Zhang FA, Wang G (2017)Titanium carbide (TisC2Tx) MXene: a novel precursor to amphi-philic carbide-derived graphene quantum dots for fluorescent ink,light-emitting composite and bioimaging. Carbon 118:50-57 ( 18. Zhang T , Jiang X, Li G, Yao Q, Lee JY ( 2018) A red phosphorous-assisted ball-milling synthesis of fe w layered TigC2Tx (MXene) nanodot composite. Chem Nano Mat 4:56-60 ) 19. Desai ML, Basu H, Singhal RK, Saha S, Kailasa SK (2018)Microwave-assisted synthesis of water-soluble Eu’hybrid carbondots with enhanced fluorescence for the sensing of Hgions andimaging of fungal cells. New J Chem 42(8):6125-6133 20. Yang G, WanX, Su Y, Zeng X, Tang J (2016)Acidophilic S-dopedcarbon quantum dots derived from cellulose fibers and their fluo-rescence sensing performance for metal ions in an extremely strongacid environment. J Mater Chem A 4(33):12841-12849 ( 21. L ou Z, Li P , H an K (2015) Redox-responsive fl u orescent p r obeswith d ifferent desig n strategies. Acc Che m Res 48:1358-1368 ) ( 22. Xu Q L, H eo C H, K im G, L ee HW, Kim HM, Y o on J ( 2015) Development of i m idazoline-2-thiones b ased t wo-photon f l uores- cence probes for imaging h y pochlorite g e neration i n a co-culture system. Angew C hem Int E d 54:4890- 4 894 ) 23. Chen XQ, Wang F, Hyun JY, Wei TW, Qiang J, Ren XT, Shin I,Yoon J (2016) Recent progress in the development of fluorescent,luminescent and colorimetric probes for detection of reactive oxy-gen and nitrogen species. Chem Soc Rev 45:2976-3016 ( 24. Zhang PS, Wang H, Hong YX, Yu ML,Zeng RJ, Long YF, Chen J (2018) Selective visualization of endogenous hypochlorous acid inzebrafish d uring lipopolysaccharide-induced acute liver injuryusing a p olymer mi c elles-based rat i ometric fluorescent pro b e. Biosens Bioelectron 99:318 - 324 ) 25. Guo JQ, Ye S, Li H, Song J, Qu JL (2020) One-pot synthesizednitrogen-fluorine-codoped carbon quantum dots for ClO-ions de-tection in water samples. Dyes Pigments 175:108178 26. Yue YK, Yin CX, Huo FJ, Chao JB, Zhang YB (2014) The appli-cation of natural drug-curcumin in the detection hypochlorous acid of real sample and its bioimaging. Sensors Actuators B Chem 202:551-556 27.Zhou Z, Gu JP, Qiao XG, Wu HX, Fu HR, Wang L, Li HY, Ma LF(2019) Double protected lanthanide fluorescence core@shell col-loidal hybrid for the selective and sensitive detection of ClO-.Sensors Actuators B Chem 282:437-442 ( 28. Wang L, L i WX, Zhi WJ, Ye D D , Zhang WL, Ni L (2018) Ra p id detection ofhypochlorite by a coumarin-based hydrazide inaqueoussolution and its application in live-cell imaging. S e nsors Actuators B Chem 255:1112-1118 ) 29. Tan HL, Wu XY, Weng YH, Lu YJ, Huang ZZ (2020) A self-assembled FRET nanoprobe with metal-organic framework as ascaffold for ratiometric detection of hypochlorous acid. AnalChem 92(4):3447-3454 30. Wei ZN, Li HQ, Liu SB, Wang W, Chen HL, Xiao LH, Ren CL,Chen XG (2019) Carbon dots as fluorescent/colorimetric probe forreal time detection of hypochlorite and ascorbic acid in body fluidand cell. Anal Chem 91(24):15477-15483 31. Lan JS, Liu L, Zeng RF, Qin YH, Liu Y, Jiang XY, Aihemaiti A,Ding Y, Zhang T, Ho RJY (2020) Rational modulation ofcoumarin-hemicyanine platform based on OH substitution forhigher selective detection of hypochlorite. Chem Commun 56(8):1219-1222 32.2.Zhon XL, Yang Q, Chen YS, Jiang YL, Wang BX, Shen J(2019) Amitochondria-targeted fluorescent probe based on coumarin-pyridine derivatives for hypochlorite imaging in living cells andzebrafish. J Mater Chem B 7(46):7332-7337 ( 33. Shiraishi Y, Yamada C, H irai T(2019) A coumarin-dihydroperimidine dye as a fluorescent chemosensor f or hypochlo-rite in 99% water . RSC Adv 9(49):28636-28641 ) ( Publisher's n ote Springer Nature re m ains neutral with reg a rd to jurisdic-tional claims in p ublished maps and institutional affiliations. ) Springer 微波辅助合成Ti3C2 MXene量子点用于次氯酸盐的比率荧光检测介绍 MXene量子点(MXene quantum dots, mqd)由于其优异的光学性能、良好的生物相容性、天然的亲水性和现成的功能化等特点,在荧光材料领域引起了广泛的关注[1-3]。mqd在许多领域都有潜在的应用,特别是在生物医学[2,4,5]、传感[6-11]、光电器件[12,13]和催化[14,15]。近年来,自荧光量子阱的首次报道以来,改进量子阱合成策略的研究越来越多。mqd的合成主要有两种方法:从本体前体的自上而下切割法和从分子前体的自下而上方法。迄今为止,获得mqd的典型方法为自上而下的方法(以Ti3C2量子点为主),包括水热/溶剂热法、超声法、球磨法、插层法和组合法[2,4,14,16 - 19]。在自顶向下合成mqd时,最常用的方法是在高浓度酸性溶液中蚀刻MAX相(“M”表示过渡金属,“A”表示- IIIA/IVA族元素,X表示C和/或N元素)后的水热法。而水热法需要6-12 h才能完成反应[2,8]。因此,开发快速、直接、方便的荧光量子点合成工艺将是该领域的一个重大突破。微波辅助合成可缩短反应时间的量子点的研究较少。次氯酸/次氯酸盐(HOCl/ClO−),进口活性活性氧(reactive oxygen species, ROS)在生物体内起着重要的保护作用[20,21]。体内ClO−水平异常与创伤愈合受损、癌症、关节炎、神经元变性和心血管疾病等慢性和退行性疾病的发展高度相关[22-24]。因此,快速、灵敏地检测ClO−在环境和生活领域都具有重要意义。到目前为止,许多荧光探针都被用来检测ClO−,因为它们具有高稳定性、高灵敏度和高选择性等优点[25-28]。与普通荧光法相比,比值荧光法可以最大限度地减少来自背景的假信号,对ClO−的检测具有更好的灵敏度[29,30]。在用于检测ClO−的比率荧光探针领域,仍然需要精心的设计和合成。因此,构建和制备一种简单、高效的ClO−检测比荧光探针仍然面临着巨大的挑战。本文采用姜黄素荧光共振能量转移(FRET)技术设计了基于Ti3C2 MQDs的比例荧光探针(方案1)。首先在微波辅助照射下刻蚀MAX相5 min后合成Ti3C2 MQDs。与其他自底向上合成Ti3C2 mqd的方法相比,微波辅助合成大大缩短了反应时间。此外,微波加热更均匀,允许更快的反应和更少的副产物形成。在姜黄素存在的情况下,Ti3C2 MQDs在430 nm处的荧光发射被FRET猝灭,而姜黄素在540 nm处的荧光发射被增强。加入ClO−后,姜黄素的酚和甲氧基被氧化成醌,在540 nm处荧光发射逐渐猝灭,在430 nm处荧光发射逐渐恢复。在365 nm紫外灯下,加入ClO−后,溶液颜色由黄绿色变为蓝色,肉眼可见。在此基础上,设计了比例荧光和“裸眼”探针检测姜黄素和ClO−。本工作不仅为Ti3C2 mqd的合成提供了一种新的方法,而且拓展了Ti3C2 mqd在生物和化学领域的应用环境检测。方案1 Ti3C2 mqd的合成及其综合传感策略图1 Ti3C2 MQDs的TEM图像(插入Ti3C2 MQDs的HRTEM图像),b Ti3C2 MQDs的尺寸分布直方图,c Ti3C2 MQDs的FTIR光谱,d Ti3C2 MQDs的XPS示意图,e C1sTi3C2 mqd的原理图,以及Ti3C2 mqd的Ti 2p原理图在1-13范围内,荧光强度保持不变。从图2c中可以看出,Ti3C2 mqd在1个月内荧光强度几乎没有变化。因此,Ti3C2 mqd具有良好的荧光稳定图2 Ti3C2 mqd的荧光光谱。b pH对Ti3C2 mqd荧光强度的影响。c储存时间对Ti3C2 mqd荧光强度的影响结论 综上所述,我们首次成功地用米crowave合成了Ti3C2 MQDs,大大缩短了合成时间。Ti3C2 MXene量子点(Ti3C2 MQDs)表现出激发波长依赖的蓝色光致发光,激发/发射峰位于330/430 nm。Ti3C2 MQDs与姜黄素之间的荧光猝灭反应可以猝灭Ti3C2 MQDs的荧光。次氯酸盐(ClO -)能氧化姜黄素的酚和酚甲氧基转化为醌,使Ti3C2 mqd的荧光强度恢复。在检测过程中,混合系统的颜色发生了明显的变化。因此,我们建立了一种双通道“裸眼”比色和比例荧光探针用于姜黄素和ClO−的检测。
确定




还剩6页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《次氯酸盐中比率荧光检测方案(微波萃取仪)》,该方案主要用于其他中其他检测,参考标准--,《次氯酸盐中比率荧光检测方案(微波萃取仪)》用到的仪器有电脑微波催化合成萃取仪 祥鹄XH-100A、微波固液相合成萃取仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















