方案详情
文
相比于索氏提取,时间更短,产率更高。
Abstract:
The longan (Dimocarpus Longan Lour.) peel was extracted with 95% ethanol employing microwave-assisted extraction and Soxhlet extraction method, the total phenolic content of microwave-assisted extract of Langan peel (MEL) and Soxhlet extract of Langan peel (SEL) reached 96.78 mg/g and 90.35 mg/g dry weight, respectively, expressed as pyrocatechol equivalents, which were quanti?ed using Folin–Ciocalteu reagent. Subsequently, antioxidant properties of two extracts were investigated employing various established systems in vitro including 2,20-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, hydroxyl radical scavenging assay using a new resonance scattering (RS) method, reducing power and total antioxidant capacity. MEL and SEL showed excellent antioxidant in all test systems compared to synthetic antioxidant 2,6-di-ter-butyl-4-methylphenol (BHT) and the antioxidant activities of MEL were all superior to those of SEL. Furthermore, the suitability of MEL and SEL as substitute of BHT were determined in peanut oil, and the decrease of lipid oxidation were monitored using thiobarbituric acid-reactive substances (TBARS) assay. MEL and SEL treatment signi?cantly (P < 0.05) reduced lipid oxidation in peanut oil compared to the control. No signi?cant differences (P ? 0:05T in lipid oxidation were detected between MEL, SEL and BHT samples of peanut oil.
方案详情

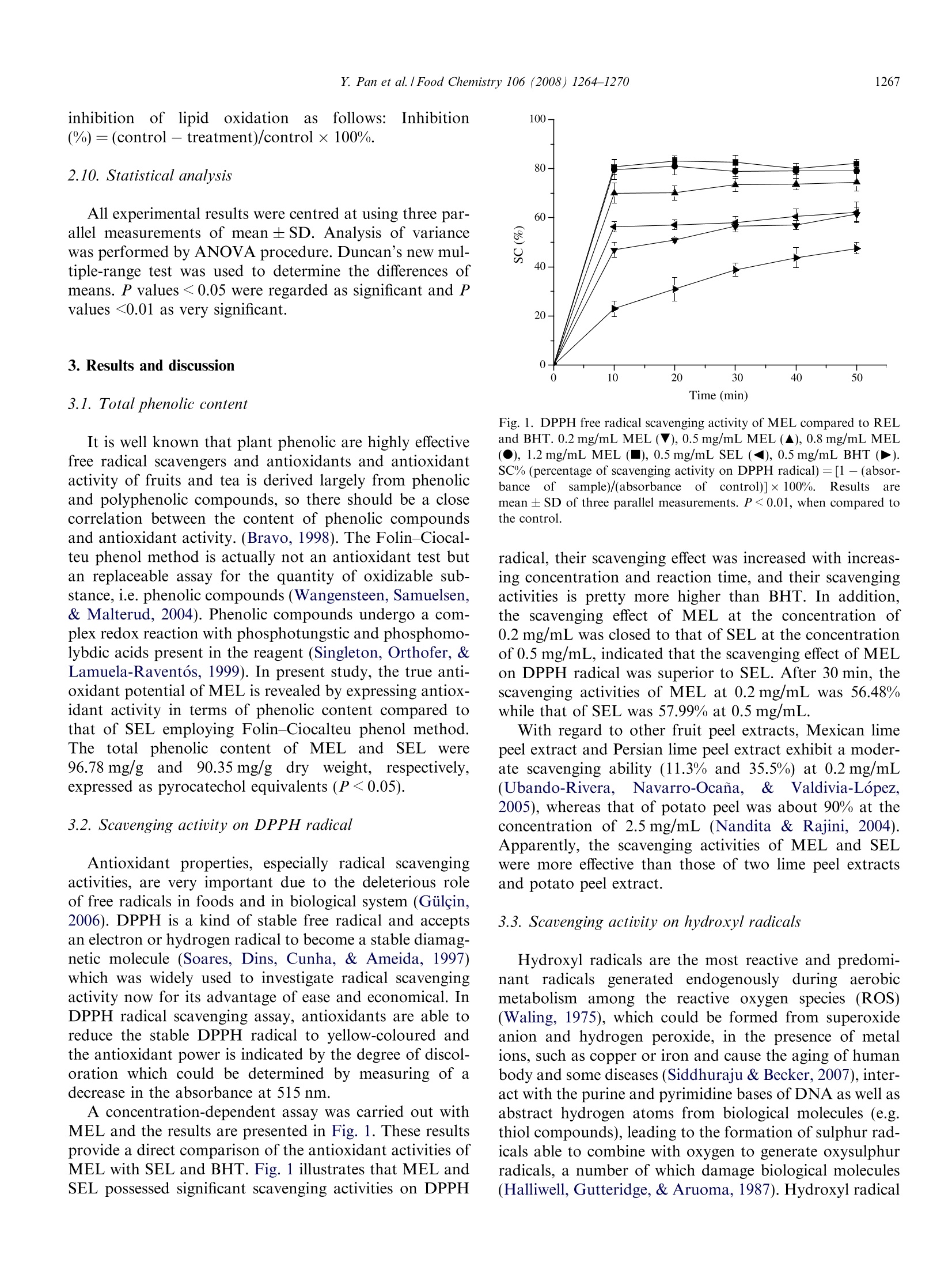
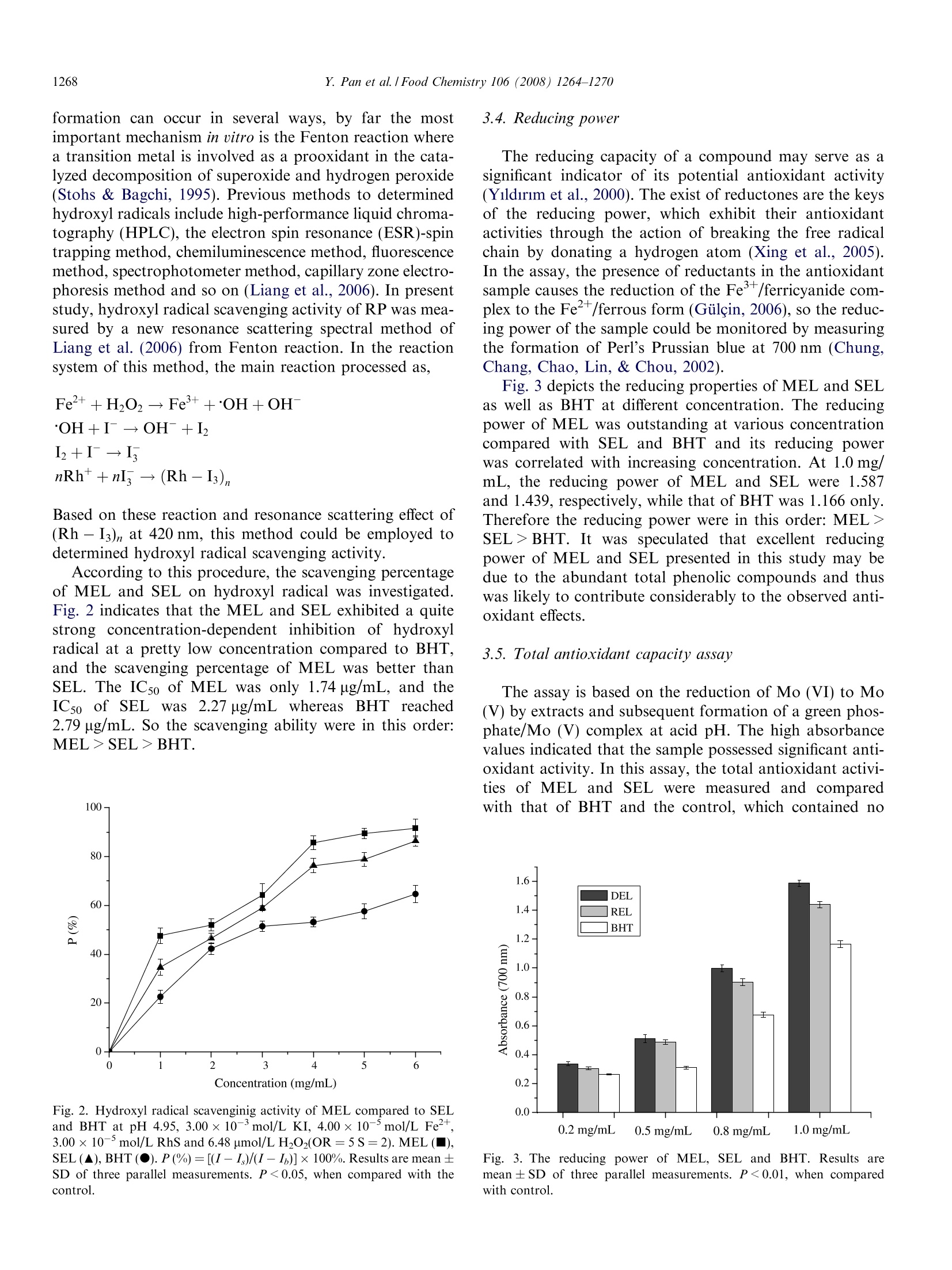
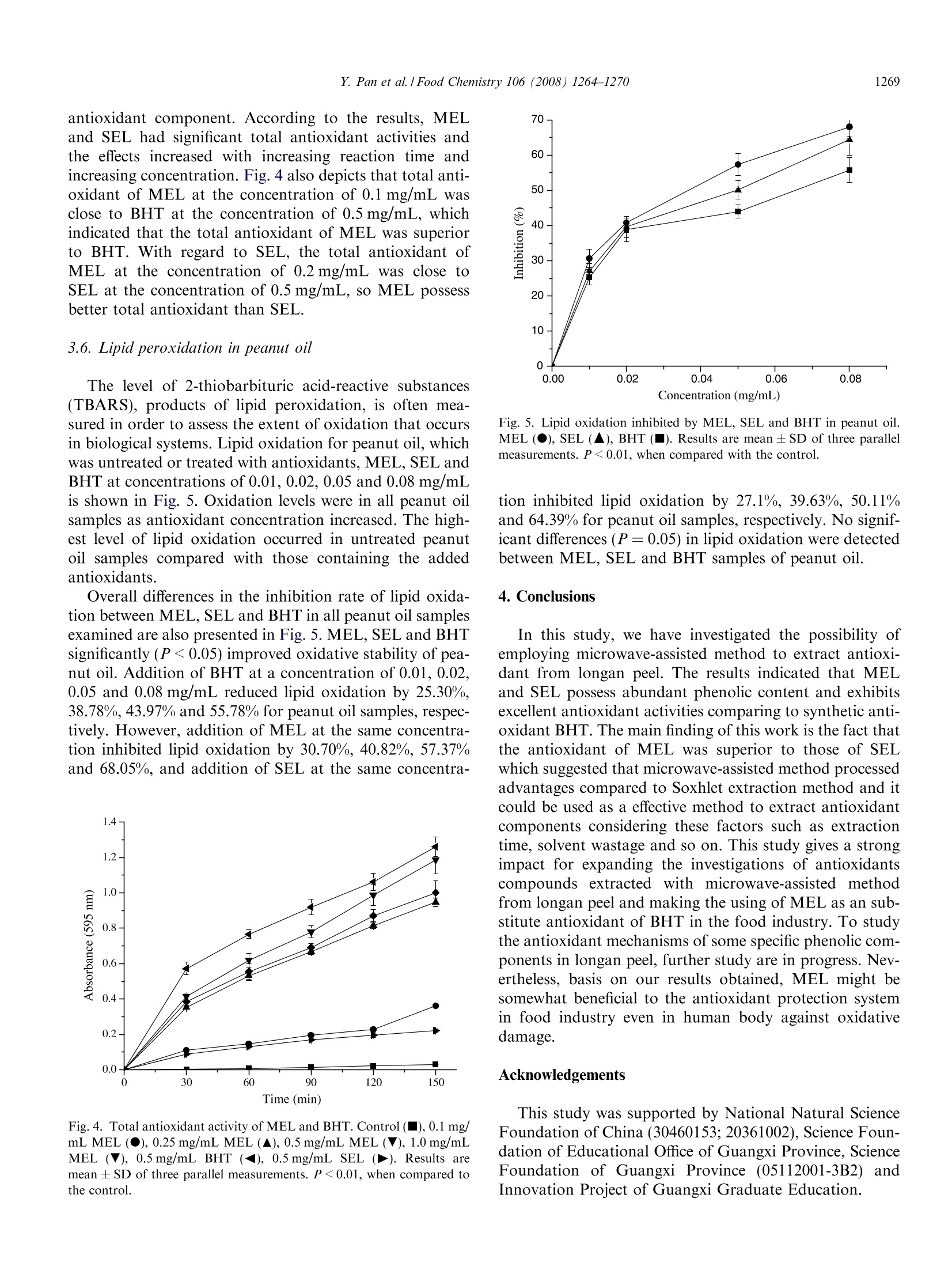
Available online at www.sciencedirect.comFoodChemistry 1265Y. Pan et al. / Food Chemistry 106 (2008)1264-1270 Food Chemistry 106 (2008) 1264-1270 www.elsevier.com/locate/foodchem Analytical, Nutritional and Clinical Methods Antioxidant activity of microwave-assisted extractof longan (Dimocarpus Longan Lour.) peel Yingming Pan*, Kai Wang, Siqin Huang, Hengshan Wang *, Xiaomei Mu,Chunhuan He, Xiaowen Ji, Jie Zhang, Fujuan Huang School of Chemistry and Chemical Engineering, Guangxi Normal University, Guilin 541004, PR ChinaReceived 8 December 2006; received in revised form 25 June 2007; accepted 16 July 2007 Abstract The longan (Dimocarpus Longan Lour.) peel was extracted with 95% ethanol employing microwave-assisted extraction and Soxhletextraction method, the total phenolic content of microwave-assisted extract of Langan peel (MEL) and Soxhlet extract of Langan peel(SEL) reached 96.78 mg/g and 90.35 mg/g dry weight, respectively, expressed as pyrocatechol equivalents, which were quantified usingFolin-Ciocalteu reagent. Subsequently, antioxidant properties of two extracts were investigated employing various established systemsin uitro including 2,2'-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, hydroxyl radical scavenging assay using a new reso-nance scattering (RS) method, reducing power and total antioxidant capacity. MEL and SEL showed excellent antioxidant in all testsystems compared to synthetic antioxidant 2,6-di-ter-butyl-4-methylphenol (BHT) and the antioxidant activities of MEL were all supe-rior to those of SEL. Furthermore, the suitability of MEL and SEL as substitute of BHT were determined in peanut oil, and the decreaseof lipid oxidation were monitored using thiobarbituric acid-reactive substances (TBARS) assay. MEL and SEL treatment significantly(P <0.05) reduced lipid oxidation in peanut oil compared to the control. No significant differences (P=0.05) in lipid oxidation weredetected between MEL, SEL and BHT samples of peanut oil. @ 2007 Elsevier Ltd. All rights reserved. Keywords: Longan peel; Microwave-assisted extraction; Total phenolic content; DPPHradical; Hydroxyl radical; Reducing power; Total antioxidantcapacity 1. Introduction Active oxygen and free radicals exist in human body inthe form of superoxide anion (O), hydrogen peroxide(H202) and hydroxyl radical (OH) and so on. As normalmetabolic action going on in human body, active oxygenand free radicals are constantly formed. If they reach highlevels, oxidative stress in human body would be created,which leads to a variety of biochemical and physiologicallesions and often results in metabolic impairment and celldeath (Ames, 1998). On the other hand, high levels ofactive oxygen and free radicals could also cause lipid oxi- ( * C orresponding authors. Tel.: + 8 6 773 5 8 54037; fax: +867735803930 (Y. Pan). ) ( E-mail address: panym2004@ ya hoo . com . cn (Y. Pa n ). ) ( 0308-8146/$ - s ee front matter @ 2007 Elsevier Ltd. A l l rights reserved.doi:10.1016/j.foodchem.2007.07.033 ) plant has been become the focus of the research cofCantioxidant. The preservative effect of many plant spices and herbssuggests the presence of antioxidative and antimicrobialconstituents in all parts of the plants (tree bark, stalks,leaves, fruits, roots,flowers, pods and seeds) (Hirasa &Takemasa, 1998; Kim, Kim, Kim, & Heo, 1997). Actually,plants contain a diverse group of phenolic compounds,including simple phenolics, phenolic acids, anthocyanins,hydroxycinnamic acid derivatives, and flavonoids. All thephenolic classes have the structural requirements of freeradical scavengers and have potential as food antioxidants(Bandoniene & Murkovic, 2002), so phenolic compoundsare attracting considerable interest in the field of chemistry,food and medicine (Okuda, Valentine, Shapiro, & Down-ing, 1994). Longan (Dimocarpus longan Lour.) is a member of theSapindaceae family which is a highly attractive subtropicalfruit widely distributed in the south of China, previousstudy of its biochemical and physiological activities focusedon longan seeds mostly. Morton (1987) depicted that lon-gan seeds are traditional used as a folklore medicine, whichare administered to counteract heavy sweating and the pul-verized kernel serves as a styptic. Specially, longan seedshas previously been shown to possess potent antioxidantactivities which could be ascribed to their phenolic contents(Soong & Barlow, 2004). However, there is a little study onlongan peel which usually regards as a waste material, espe-cially no previous study on the antioxidant property of lon-gan peel so far as we know. Different extraction techniques, such as dispersed-sol-ids, percolation, Soxhlet and supercritical fluid extractionhave been used to isolate antioxidants from the plants,however none of them can be considered as an optimalmethod for this purpose (Grigonis, Venskutonis, Sivik,Sandahl, & Eskilsson, 2005). Recently, microwave-assistedmethod has been used as an alternative laboratory scaleextraction method, which proved to be considerably moreeffective and economical. It provides higher recoveries,requires considerable less time and the smaller solventconsumption compared to conventional extraction (Marti-no, Ramaiola, Urbano, Bracco, & Collina, 2006). It wasalso reported that phenolic compounds can be easilyextracted with microwave-assisted method (Pan, Niu, &Liu, 2003). So microwave-assisted method should havesignificant implications for extraction of antioxidant com-pounds in plants. In present study, the possibility of using microwave-assisted method as a rapid and effective method for extractingantioxidant compounds from longan peel was investigatedfor the first time, and the total phenolic content and theantioxidant activities of MEL was compared to those ofSEL to confirm the advantage of microwave-assistedmethod. Subsequently, the suitability of MEL and SELas substitute antioxidants of BHT were also determinedin all test systems in vitro as well as their using in peanutoil. 2.1. Materials Folin-Ciocalteu reagent, 2,2'-diphenyl-1-picrylhydrazyl(DPPH),2,6-di-ter-butyl-4-methylphenol (BHT), thiobar-bituric acid (TBA) and trichloroacetic acid (TCA) werepurchased from Sigma Chemicals Co. (St. Louis, MO,USA). Other chemicals;Vwere purchased from ChinaNational Medicine Group Shanghai Corporation (Shang-hai, China). All chemicals and solvents used were of analyt-ical grade. The peanut oil, which was stripped, was bought fromBeijing Chemical Company (Beijing, China). It containedvery low o-tocopherol (2.0 mg kg-) and no syntheticantioxidants. 2.2. Equipment and apparatus The following instruments were used: UV-1100 spectro-photometer (Beijing Rayleigh Analytical Instrument Cor-poration,RE-52AA China); rotavapour (ShanghaiYarongBiochemistryInstrumentt Factory, Shanghai,China); DZF-1B vacuum drier (Shanghai Yuejin MedicalInstrument CO., Ltd., Shanghai, China); SHB-BA water-circulation multifunction vacuum pump (Zhengzhou GreatWall Scientific Industry and Trade CO., Ltd., Zhengzhou,China); XH-100A microwave extraction and synthesisapparatus (Beijing XiangHu Science and TechnologyDevelopment Co., Ltd., Beijing, China); Rayleigh scatter-ing and synchronous fluorescence (SF) spectra wererecorded with a model RF-540 spectrofluorophotometer(Shimadzu, Japan), using the synchronous scanning tech-nique that the excited wavelength Aex was equal to the emis-sion wavelength Aem (2ex = 1em), a model of TU-1901 dualm:beams spectrophotometer (Puxi Com., China) were usedfor recording the absorption spectra. 2.3. Preparation ofextracts Longan was obtained from Guilin PharmaceuticalsGroup of China (Zhongshan Road, Guilin City, China).The peel of Longan were ground (max particle size0.4 mm) after dried in oven at 60±0.5°C, then wasextracted with1 microwave-assisted extraction. Soxhletextraction was also employed for comparation. Micro-wave-assisted extraction were performed in a three neckflask with a temperature detector, ground material (5 g)were extracted with 50 mL ethanol (95%) at 80℃ for30 min, the microwave power was 500 W and the micro-wave frequency was 2450 MHz. For Soxhlet extraction,ground material (5g) were placed in a Soxhlet apparatusand extracted with 80 mL of ethanol (95%) for 2 h. Solventof two extracts were evaporated using a RE-52AA rotava-pour at 50 °C and a SHB-BA water-circulation multifunc-tion vacuum pump. Extracts were finally dried in a DZF-1B vacuum drier at 30°C and 0.07 MPa and were stored in a freezer until use. The yield of MEL and SEL were12.8% and 10.9%, respectively. 2.4. Determination of total phenolic compounds Total soluble phenolics in MEL and SEL were deter-mined using Folin-Ciocalteu reagent according to themethod of Slinkard and Singleton (1977) using pyrocate-chol as a standard. Briefly, extract solution (1 mL, 1 mg/mL) in a volumetric flask was diluted with glass-distilledwater (46 mL). Folin-Ciocalteu reagent (1 mL) was addedand the contents of flask were mixed thoroughly. After3 min, sodium carbonate solution (3 mL, 2%) were added,and then the mixture was allowed to stand for 2 h withintermittent shaking. The absorbance was measured at760 nm. The concentration of total phenolic compoundsin the extract was determined as micrograms of pyrocate-chol equivalents by using an equation that was obtainedfrom standardpyrocatecholgraphgivenas:absor-bance=0.22 ug pyrocatechol+ 0.0571. 2.5. Scavenging activity on DPPH radical To evaluate the free radical scavenging activity, MELwas allowed to react with a stable free radical, 2,2'-diphe-nyl-1-picrylhydrazyl radical (DPPH) (Brand, Cuvelier, &Berset, 1995; Sanchez-Moreno, Larrauri, & Saura-Calixto,1998; Blois, 1958). Briefly, MEL or SEL solution (0.2 mL)in 95% ethanol at different concentrations (0.2, 0.5, 0.8,1.2 mg/mL) was added to 8 mL 0.004% (w/v) solution ofDPPH in 95% ethanol. The reaction mixture was incubatedat 28C. The scavenging activity on DPPH radical wasdetermined by measuring the absorbance at 515 nm each10 min until the reaction reached the steady state. The anti-oxidant activity was expressed as a percentage of scaveng-ing activity on DPPH radical: SC%=[1 -(absorbance ofsample)/(absorbance of control)]×100%. The control con-tains all reagents except the extract. The DPPH radicalscavenging activity of BHT (0.5mg/mL)) and SEL(0.5 mg/mL) were also assayed for comparison. All testswere performed in triplicate and mean were centred. 2.6. Hydroxyl radical scavenging activity Hydroxyl radical scavenging activity of MEL, SEL andBHT was estimated through the new method of Liang,Zhou, andJJiang (2006). HCl-NaAc buffer solution(pH=4.95, 1.0 mL), KI solution (0.020 mol/L, 0.7 mL),Fe(II) solution (4.00×10-mol/L, 0.1 mL) and H202standard solution (6.48 umol/L, 0.4 mL) were piped in a10mLgraduated tube.tthen rhodamine B(RhB,1.50×10-4mol/L, 1.4 mL) was mixed. The mixed solutionwas diluted to 5 mL with water and mixed thoroughly. Theresonance scattering spectra was obtained by using the syn-chronous scanning technique in a model RF-540 spectro-fluorophotometer. The Is which presents the RS intensityfor the system containing H202 and scavenger, I presents the RS intensity for the system containing H2O2 and Ithe RS intensity in the absence of H202 were measuredat 420 nm. The scavenging percentage (P) could be calcu-lated as P (%)=[(I-I )/(I-I)]×100 (%). The IC5o(defined as the concentration of sample at which 50% ofhydroxyl radical were scavenged) was calculated for eachsample. 2.7. Reducing power The determination of reducing power was performed asdescribed by Oyaizu (1986). One millilitre of various MELor SEL solution (0.05, 0.08, 0.1 and 0.2 mg/mL) weremixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) andpotassium ferricyanide (2.5mL, 1%). After the mixturewas incubated at 50°C for 20 min, trichloroacetic acid(2.5 mL, 10%) was added,and the mixture was centrifugedat 3000 rpm for 10 min. The upper layer of solution(2.5 mL) was mixed with distilled water (2.5 mL) and ferricchloride (0.5 mL, 0.1%), then the absorbance was measuredat 700 nm against a blank. Increasing absorbance of thereaction mixture indicates increasing reducing power. Thereducing power of BHT (0.5 mg/mL) was also determinedfor comparation. 2.8. Determination oftotal antioxidant capacity The total antioxidant capacity of MEL was determinedaccording to the method of Prieto, Pineda, and Aguilar(1999). MEL or SEL solution (0.1 mL) was combined with0.3 mL of reagent solution (0.6 M sulphuric acid, 28 mMsodium phosphate and 4mM ammonium molybdate).The reaction mixture was incubated at 95°C for 150 min.After the mixture had cooled to room temperature, theabsorbance of the mixture was measured at 695 nm againsta blank. The readings were taken each 30 min. The antiox-idant activity was expressed as the absorbance of the sam-ple. The antioxidant activity of BHT (0.5 mg/mL) and SEL(0.5 mg/mL) were also assayed for comparison. 2.9. Antioxidant potential of MEL and SEL in peanut oil Calculated amounts of MEL and SEL (0.01, 0.02, 0.05and 0.08 mg/mL of the oil) were added to 50 mL of peanutoil. The additive was mixed into the oil with a magneticstirrer. Synthetic antioxidant BHT was used as a referencefor comparison. The oxidative deterioration of sampleswas studied using Schaal oven test method as describedby Economou, Oreopoulou, and Thomopoulos (1991).The oil samples (50 mL each)were placed in open100 mL beakers, and placed in 60±0.5°℃ oven for 24 h.A blank sample was prepared under the same conditions,without adding any additives. The rate of antioxidationof peanut oil was estimated according to the increase of2-thiobarbituric acid-reactive substances (TBARS) usingthe classical TBAprocedure. The TBARS values ofuntreated and treated samples were used to calculate the inhibition of lipid oxidationnasfollows: Inhibition(%)=(control - treatment)/control×100%. 2.10. Statistical analysis All experimental results were centred at using three par-allel measurements of mean ± SD. Analysis of variancewas performed by ANOVA procedure. Duncan’s new mul-tiple-range test was used to determine the differences ofmeans. P values <0.05 were regarded as significant and Pvalues <0.01 as very significant. 3. Results and discussion 3.1. Total phenolic content It is well known that plant phenolic are highly effectivefree radical scavengers and antioxidants and antioxidantactivity of fruits and tea is derived largely from phenolicand polyphenolic compounds, so there should be a closecorrelation between the content of phenolic compoundsand antioxidant activity.(Bravo, 1998). The Folin-Ciocal-teu phenol method is actually not an antioxidant test butan replaceable assay for the quantity of oxidizable sub-stance, i.e. phenolic compounds (Wangensteen, Samuelsen,& Malterud,2004). Phenolic compounds undergo a com-plex redox reaction with phosphotungstic and phosphomo-lybdic acids present in the reagent (Singleton, Orthofer, &Lamuela-Raventos, 1999). In present study, the true anti-oxidant potential of MEL is revealed by expressing antiox-idant activity in terms of phenolic content compared tothat of SEL employing Folin-Ciocalteu phenol method.The totall phenolic content of MEL and SELwere96.78mg/gaand990.35 mg/g dry weight, respectively,expressed as pyrocatechol equivalents (P <0.05). 3.2. Scavenging activity on DPPH radical Antioxidant properties, especially radical scavengingactivities, are very important due to the deleterious roleof free radicals in foods and in biological system (Giilcin,2006). DPPH is a kind of stable free radical and acceptsan electron or hydrogen radical to become a stable diamag-netic molecule (Soares, Dins, Cunha, & Ameida,1997)which was widely used to investigate radical scavengingactivity now for its advantage of ease and economical. InDPPH radical scavenging assay, antioxidants are able toreduce the stable DPPH radical to yellow-coloured andthe antioxidant power is indicated by the degree of discol-oration which could be determined by measuring of adecrease in the absorbance at 515 nm. A concentration-dependent assay was carried out withMEL and the results are presented in Fig. 1. These resultsprovide a direct comparison of the antioxidant activities ofMEL with SEL and BHT. Fig. 1 illustrates that MEL andSEL possessed significant scavenging activities on DPPH 100- Fig. 1. DPPH free radical scavenging activity of MEL compared to RELand BHT. 0.2 mg/mL MEL(V), 0.5 mg/mL MEL (▲), 0.8 mg/mL MEL(●), 1.2 mg/mL MEL (), 0.5mg/mL SEL (), 0.5 mg/mL BHT ().SC% (percentage of scavenging activity on DPPH radical)=[1 -(absor-bance oofsample)/(absorbance of control)]×100%. Resultsaremean ± SD of three parallel measurements. P <0.01, when compared tothe control. radical, their scavenging effect was increased with increas-ing concentration and reaction time, and their scavengingactivities is pretty more higher than BHT. In addition,the scavenging effect of MEL at the concentration of0.2 mg/mL was closed to that of SEL at the concentrationof 0.5 mg/mL, indicated that the scavenging effect of MELon DPPH radical was superior to SEL. After 30 min, thescavenging activities of MEL at 0.2 mg/mL was 56.48%while that of SEL was 57.99% at 0.5 mg/mL. With regard to other fruit peel extracts, Mexican limepeel extract and Persian lime peel extract exhibit a moder-ate scavenging ability (11.3% and 35.5%) at 0.2 mg/mL(Ubando-Rivera.,Navarro-Ocana.,&VValdivia-Lopez,2005), whereas that of potato peel was about 90% at theconcentration of 2.5 mg/mL (Nandita & Rajini, 2004).Apparently, the scavenging activities of MEL and SELwere more effective than those of two lime peel extractsand potato peel extract. 3.3. Scavenging activity on hydroxyl radicals Hydroxyl radicals are the most reactive and predomi-nant radicalsgenerated endogenously during aerobicmetabolism among the reactive oxygen species (ROS)(Waling, 1975), which could be formed from superoxideanion and hydrogen peroxide, in the presence of metalions, such as copper or iron and causas coppe1 O1.e the aging of humanbody and some diseases (Siddhuraju & Becker, 2007),inter-act with the purine and pyrimidine bases of DNA as well asabstract hydrogen atoms from biological molecules (e.g.thiol compounds), leading to the formation of sulphur rad-icals able to combine with oxygen to generate oxysulphurradicals, a number of which damage biological molecules(Halliwell, Gutteridge, & Aruoma, 1987). Hydroxyl radical formation can occur in severa ways, by far the mostimportant mechanism in uitro is the Fenton reaction wherea transition metal is involved as a prooxidant in the cata-lyzed decomposition of superoxide and hydrogen peroxide(Stohs & Bagchi, 1995). Previous methods to determinedhydroxyl radicals include high-performance liquid chroma-tography (HPLC), the electron spin resonance (ESR)-spintrapping method, chemiluminescence method,fluorescencemethod, spectrophotometer method, capillary zone electro-phoresis method and so on (Liang et al., 2006). In presentstudy, hydroxyl radical scavenging activity of RP was mea-sured by a new resonance scattering spectral method ofLiang et al. (2006) from Fenton reaction. In the reactionsystem of this method, the main reaction processed as, Based on these reaction and resonance scattering effect of(Rh-I3)n at 420 nm, this method could be employed todetermined hydroxyl radical scavenging activity. According to this procedure, the scavenging percentageof MEL and SEL on hydroxyl radical was investigated.Fig.2 indicates that the MEL and SEL exhibited a quitestrong concentration-dependent inhibition of hydroxylradical at a pretty low concentration compared to BHT,and the scavenging percentage of MEL was better thanSEL. The IC5o of MEL was only 1.74 ug/mL, and theIC50 of SEL was2.27 ug/mL whereas BHT reached2.79 ug/mL. So the scavenging ability were in this order:MEL> SEL> BHT. Fig. 2. Hydroxyl radical scavenginig activity of MEL compared to SELand BHT at pH 4.95, 3.00×10-mol/L KI, 4.00×10-5mol/L Fe+,3.00×10-5 mol/L RhS and 6.48 umol/L H202(OR=5S=2).MEL(■),SEL(▲), BHT (●).P(%)=[(I-I )/(I-I)]×100%. Results are mean ±SD of three parallel measurements. P<0.05, when compared with thecontrol. The reducing capacity of a compound may serve as asignificant indicator of its potential antioxidant activity(Yildirim et al.,2000). The exist of reductones are the keysof the reducing power, which exhibit their antioxidantactivities through the action of breaking the free radicalchain by donating a hydrogen atom (Xing et al., 2005).In the assay, the presence of reductants in the antioxidantsample causes the reduction of the Fe+/ferricyanide com-plex to the Fe/ferrous form (Giilcin, 2006), so the reduc-ing power of the sample could be monitored by measuringthe formation of Perl’s Prussian blue at 700 nm (Chung,Chang, Chao,Lin, & Chou,2002). Fig. 3 depicts the reducing properties of MEL and SELas well as BHT at different concentration. The reducingpower of MEL was outstanding at various concentrationcompared with SEL and BHT and its reducing powerwas correlated with increasing concentration. At 1.0 mg/mL, the reducing power of MEL and SEL were 1.587and 1.439, respectively, while that of BHT was 1.166 only.Therefore the reducing power were in this order: MEL>SEL > BHT. It was speculated that excellent reducingpower of MEL and SEL presented in this study may bedue to the abundant total phenolic compounds and thuswas likely to contribute considerably to the observed anti-oxidant effects. 3.5. Total antioxidant capacity assay The assay is based on the reduction of Mo (VI) to Mo(V) by extracts and subsequent formation of a green phos-phate/Mo (V) complex at acid pH. The high absorbancevalues indicated that the sample possessed significant anti-oxidant activity. In this assay, the total antioxidant activi-ties of MEL and SEL. were measured and comparedwith that of BHT and the control, which contained no Fig. 3. The reducing power of MEL, SEL and BHT. Results aremean ± SD of three parallel measurements. P<0.01, when comparedwith control. antioxidant component. According to the results, MELand SEL had significant total antioxidant activities andthe effects increased with increasing reaction time andincreasing concentration. Fig. 4 also depicts that total anti-oxidant of MEL at the concentration of 0.1 mg/mL wasclose to BHT at the concentration of 0.5 mg/mL, whichindicated that the total antioxidant of MEL was superiorto BHT. With regard to SEL, the total antioxidant ofMEL at the concentration of 0.2 mg/mL was close toSEL at the concentration of 0.5 mg/mL, so MEL possessbetter total antioxidant than SEL. 3.6.Lipid peroxidation in peanut oil The level of 2-thiobarbituric acid-reactive substances(TBARS), products of lipid peroxidation, is often mea-sured in order to assess the extent of oxidation that occursin biological systems. Lipid oxidation for peanut oil, whichwas untreated or treated with antioxidants, MEL, SEL andBHT at concentrations of 0.01,0.02, 0.05 and 0.08 mg/mLis shown in Fig. 5. Oxidation levels were in all peanut oilsamples as antioxidant concentration increased. The high-est level of lipid oxidation occurred in untreated peanutoil samples compared with those containing the addedantioxidants. Overall differences in the inhibition rate of lipid oxida-tion between MEL, SEL and BHT in all peanut oil samplesexamined are also presented in Fig. 5. MEL, SEL and BHTsignificantly (P<0.05) improved oxidative stability of pea-nut oil. Addition of BHT at a concentration of 0.01, 0.02,0.05 and 0.08 mg/mL reduced lipid oxidation by 25.30%,38.78%,43.97% and 55.78% for peanut oil samples, respec-tively. However, addition of MEL at the same concentra-tion inhibited lipid oxidation by 30.70%,40.82%, 57.37%and 68.05%, and addition of SEL at the same concentra- Fig. 4. Total antioxidant activity of MEL and BHT. Control(■), 0.1 mg/mL MEL (●), 0.25 mg/mL MEL (▲), 0.5 mg/mL MEL (V), 1.0 mg/mLMEL (V), 0.5 mg/mL BHT (), 0.5 mg/mL SEL (D). Results aremean ±SD of three parallel measurements. P<0.01, when compared tothe control. Fig. 5. Lipid oxidation inhibited by MEL, SEL and BHT in peanut oil.MEL (●), SEL (), BHT (). Results are mean ± SD of three parallelmeasurements. P <0.01, when compared with the control. tion inhibited lipid oxidation by 27.1%, 39.63%, 50.11%and 64.39% for peanut oil samples, respectively. No signif-icant differences (P=0.05) in lipid oxidation were detectedbetween MEL, SEL and BHT samples of peanut oil. 4. Conclusions In this study, we have investigated the possibility ofemploying microwave-assisted method to extract antioxi-dant from longan peel. The results indicated that MELand SEL possess abundant phenolic content and exhibitsexcellent antioxidant activities comparing to synthetic anti-oxidant BHT.The main finding of this work is the fact thatthe antioxidant of MEL was superior to those of SELwhich suggested that microwave-assisted method processedadvantages compared to Soxhlet extraction method and itcould be used as a effective method to extract antioxidantcomponents considering these factors such as extractiontime, solvent wastage and so on. This study gives a strongimpact for expanding the investigations of antioxidantscompounds extracted with microwave-assisted methodfrom longan peel and making the using of MEL as an sub-stitute antioxidant of BHT in the food industry. To studythe antioxidant mechanisms of some specific phenolic com-ponents in longan peel, further study are in progress. Nev-ertheless, basis on our results obtained, MEL might besomewhat beneficial to the antioxidant protection systemin food industry even in human body against oxidativedamage. Acknowledgements This study was supported by National Natural ScienceFoundation of China (30460153;20361002), Science Foun-dation of Educational Office of Guangxi Province, ScienceFoundation of Guangxi Province (05112001-3B2)andInnovation Project of Guangxi Graduate Education. ( Ames, B . ( 1 998). Micronutrients p revent tC cancer and delay aging. Toxicology Letters, 1 02, 5 -18. ) ( Bandoniene, D . , & M urkovic, M. (2002). On-line HPLC-DPPH screening method for evaluation o f radical s cavenging phenols e x tracted f r om apples (Malus domestica L .). Journal o f A gricultural and Food Chemistry, 50, 2482 - 2487. ) ( Blois, M. S. (1958). Antioxidant determinations by t h e use of a stable free radical. Nature, 26, 1 1 99-1200. ) ( Brand, W .W., Cuvelier,M. E., & Ber s et, C. (1 9 95).Use of a free ra d icalmethod t o evaluate antioxidant activity. Lebensmittel-Wissenschaft und Technologie, 20, 25-30. ) ( Bravo, L . (1998). P o lyphenols: chemistry, dietary so u rces, me t abolism, and nutritional significance. Nutrition Reuiews, 56(11), 3 1 7-333. ) ( Chung, Y . C., C hang, C. T . , Chao, W . W., Li n , C. F., & Chou, S. T. (2002). Antioxidant activity a nd s a fety of the 50% et h anolic extractfrom r ed bean f ermented b y Bacillus subtilis IMR-NKI. Journal of Agriculture and Food Chemistry,50, 2454 - 2458. ) ( Economou, K .D . , O r eopoulou, V., & T h omopoulos, C. D. (1991).Antioxidant a ctivity o f some p l ant e x tracts of t he family L a biatae.Journal of the American Oil Chemists’ S o ciety, 68, 109-115. ) ( Grigonis, D . , V enskutonis, P. R., Sivik, B ., Sandahl, M ., & E s kilsson, C. S . ( 2005). C omparison of different ex t raction techniques for isolationof a ntioxidants from s weet grass (Hierochloe o d orata). Journal of Supercritical Fluids, 33, 2 23-2 3 3. ) ( G ulcin, I (2006). Antioxidant an d ant i radical activities of L- ca rnitine. Life Sciences, 78, 803-811. ) ( Halliwell, B . , & Gutteridge, J. M. C . ( 1999). Free radicals in biology andmedicine. Oxford: Oxford University Press. ) Halliwell, B., Gutteridge, J. M. C., & Aruoma, O. I. (1987). Thedeoxyribose method: a simple “test tube" assay for determination ofrate constants for reactions of hydroxyl radicals. Analytical Biochem-istry, 165,215-219. ( Hirasa, K., & T akemasa, M . (1998). Spice science a nd technology. New York: Marcel Dekker. ) ( Kim, B., K im,J., K i m, H., & He o , M. (1997). Biological screening of 100 plants f or cosmetic us e (I I ): antioxidative activity and fre e radical scavenging a ctivity. International Journal of Cosmetic S cience, 19, 299-307. ) ( Koleva, I . I., Linssen, J . P . H., Beek, T. A. V., Evstatieva, L. N., K ortenska, V., & Handjieva, N. (2003). A ntioxidant activity screeningof extracts from S ideritis species (Labiatae) grown in Bulgaria. Journalof the Science of Food and Agriculture, 83,809-819. ) ( Liang, A. H., Zhou, S . M ., & Jiang, Z. L . (2006). A simple and s ensitiveresonance s cattering spectra l method for determination of hydroxyl radical in Fenton s ystem using rhodamine S a nd its application toscreening the antioxidant. Talanta, 7 0 , 444 4 4 8. ) ( Martino, E., R amaiola, I . , Urbano, M.,Bracco, F., & Co l lina, S. ( 2 006).Microwave-assisted extractio n of coumarin and relate d compounds from Melilotus officinalis (L.) Pallas a s an alternativ e t o Soxhlet and ultrasound-assisted extraction. Journal o f Chromatography A, 1 125,147-151. ) ( M orton, J. ( 1 987). I n fruits of warm climates. Florida: Creative Re s ource Systems, Inc. ) ( Nandita, S., & Rajini, P. S. ( 2004). F ree radical scavenging activity of anaqueous e x tract of potato peel. F ood C h emistry, 85, 611-61 6 . ) ( Okuda, T., V alentine, V. A., Shapiro, D. N., & D owning, J.R . (1994).Cloning o f genomic loci a nd c h romosomal lo c alization o f h u man PATAIRE-1 and -3 protein kinase genes. Genomics, 21, 217-221 . ) Oyaizu, M. (1986). Studies on product of browning reaction preparedfrom glucose amine. Japanese Journal of Nutrition, 44, 307-315. Pan, Y. M., Liang, Y., Wang, H. S., & Liang, M. (2004). Antioxidantactivities of several Chinese medicine herbs. Food Chemistry, 88,347-350. ( Pan, X., Niu, G., & Liu, H.(2003). Microwave-assisted e xtraction of tea polyphenols an d t ea c affeine from green t tea leaves. ChemicalEngineering P rogress, 4 2, 1 29- 13 3. ) Pan, Y. M., Zhang, X. P., Wang, H. S., Liang, Y., Zhu, J. C., Li, H. Y.,et al. (2007). Antioxidant potential of ethanolic extract of Polygonumcuspidatum and application in peanut oil. Food Chemistry.doi:10.1016/i.foodchem.2007.05.03. ( Pan, Y. M., Z hu, J. C ., Wang, H . S . , Zhang, X. P., Zhang, Y., He, C. H., et al. (2007). Antioxidant activity of ethanolic extract of Cortex fraxini and u se in p eanut oil. Food Chemistry, 103, 913-9 1 8. ) ( Prieto , P. , Pineda , M., & A guilar, M . ( 1 999). S pectrophotometric quantitation of antioxidant capacity through the formation of aphosphomolybdenum com p lex: Specific application to th e determina- tion of vitamin E. Analytical Biochemistry, 269, 337-341. ) Sanchez-Moreno, C., Larrauri, J. A., & Saura-Calixto, Fulgencio. (1998).A procedure to measure the antiradical efficiency of polyphenols.Journal of Food Science and Agriculture, 76, 270-276. Siddhuraju, P.,& Becker, K. (2007). The antioxidant and free radicalscavenging activities of processed cowpea (Vigna unguiculata (L.)Walp.) seed extracts. Food Chemistry, 101, 10-19. Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999).Analysis of total phenols and other oxidation substrates and antiox-idants by means of folin-ciocalteu reagent. Methods in Enzymology,299,152-178. Slinkard, K., & Singleton,V.L. (1977). Total phenol analyses: automationand comparison with manual methods. American Journal of Enologyand Viticulture, 28, 49-55. Soares, J. R., Dins, T. C. P., Cunha, A. P., & Ameida, L.M. (1997).Antioxidant activity of some extracts of Thymus zygis. Free RadicalResearch, 26, 469-478. Soong, Y. Y., &Barlow, P. J. (2004). Antioxidant activityy andphenolicc content of selected fruit seeds. Food Chemistry, 88,411-417. ( Stohs, S . J., & Bagchi, D . (1995). Oxidative mechanism in t h e toxicity of m etal i ons. Free Radical Biology and Medicine, 18, 321-336. ) Ubando-Rivera, J., Navarro-Ocana, A., & Valdivia-Lopez,M. A. (2005).Mexican lime peel: comparative study on contents of dietary fibre andassociated antioxidant activity. Food Chemistry, 89, 57-61. Valentao, P., Fernandes, E., Carvalho, F., Andrade, P. B., Seabra, R. M.,& Bastos, M. L. (2002). Antioxidative properties of cardoon (Cynaracardunculus L.) infusion against superoxide radical,hydroxyl radicaland hypochlorous acid. Journal of Agricultural and Food Chemistry,50、4989-4993. Waling, C.(1975). Fenton’s regent revisited. Accounts of ChemicalResearch, 8, 125-131. Wangensteen, H., Samuelsen, A. B., & Malterud, K. E. (2004). Antiox-idant activity in extracts from coriander. Food Chemistry, 88, 293-297. Xing, R., Yu, H. H., Liu, S., Zhang, W. W., Zhang, Q. B., Li, Z. E.,et al.(2005). Antioxidant activity of differently regioselective chitosansulfates in vitro. Bioorganic & Medicinal Chemistry, 13, 1387-1392. ( Yildirim, A.,Mavi, A., O k tay, M., Ka r a, A. A ., Algur, F., & Bilaloglu, V.(2000). Comparison of antioxidant and an t imicrobial activities o f Tilia (Tilia Argentea D esf Ex D C ), Sa g e (Saluia tr i loba L.), and B l ack Tea (Camellia sinensis) extracts. Journal of Agriculture and Food Chemistry, 48,5030-5034. )
确定




还剩5页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《Antioxidant activity of microwave-assisted extract of longan (Dimocarpus Longan Lour.) peel(微波萃取)》,该方案主要用于其他中--检测,参考标准--,《Antioxidant activity of microwave-assisted extract of longan (Dimocarpus Longan Lour.) peel(微波萃取)》用到的仪器有祥鹄微波合成仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















