方案详情
文
o-Dichlorobenzene was dried over anhydrous sodium sulfate and distilled prior to use.All reactions were performed in a commercial microwave reactor(XH-100A,100-1000W,Beijing Xianghu Science and Technology Development Co.Ltd,Beijing,P.R.China).The temperature of the reaction mixture was measured by an immersed platinum resistance thermometer.Melting points were measured on a melting point apparatus and are uncorrected.H NMR(200 or 300 MHz)and C NMR(50 or75MHz)spectra were obtained on an EI mass spectrometer.IR spectra were recorded on a FT-IR spectrometer with an OMNI sampler.TLC separations were performed on silica gel GF plates ,and the plates were visualized with UV light.
方案详情

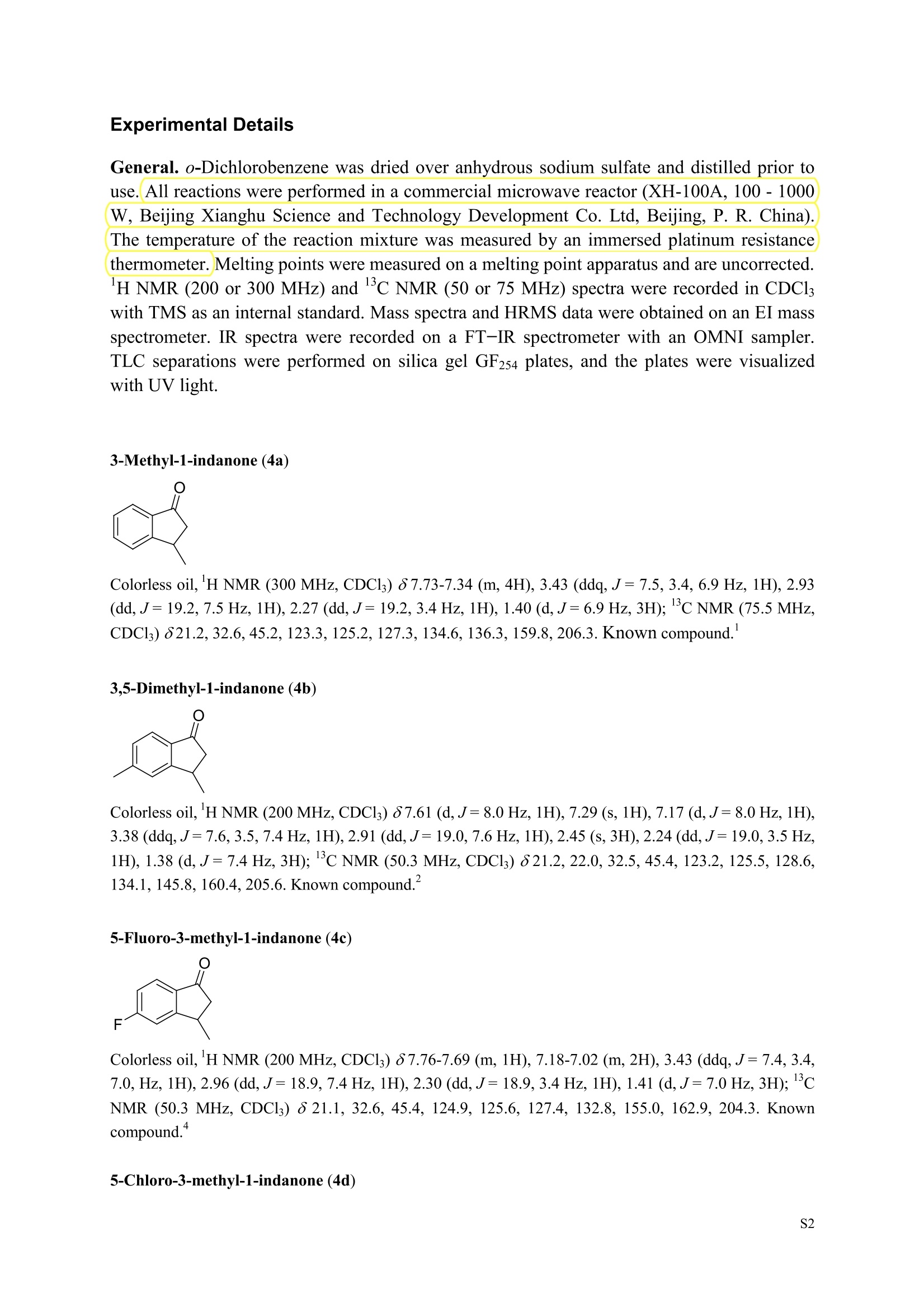
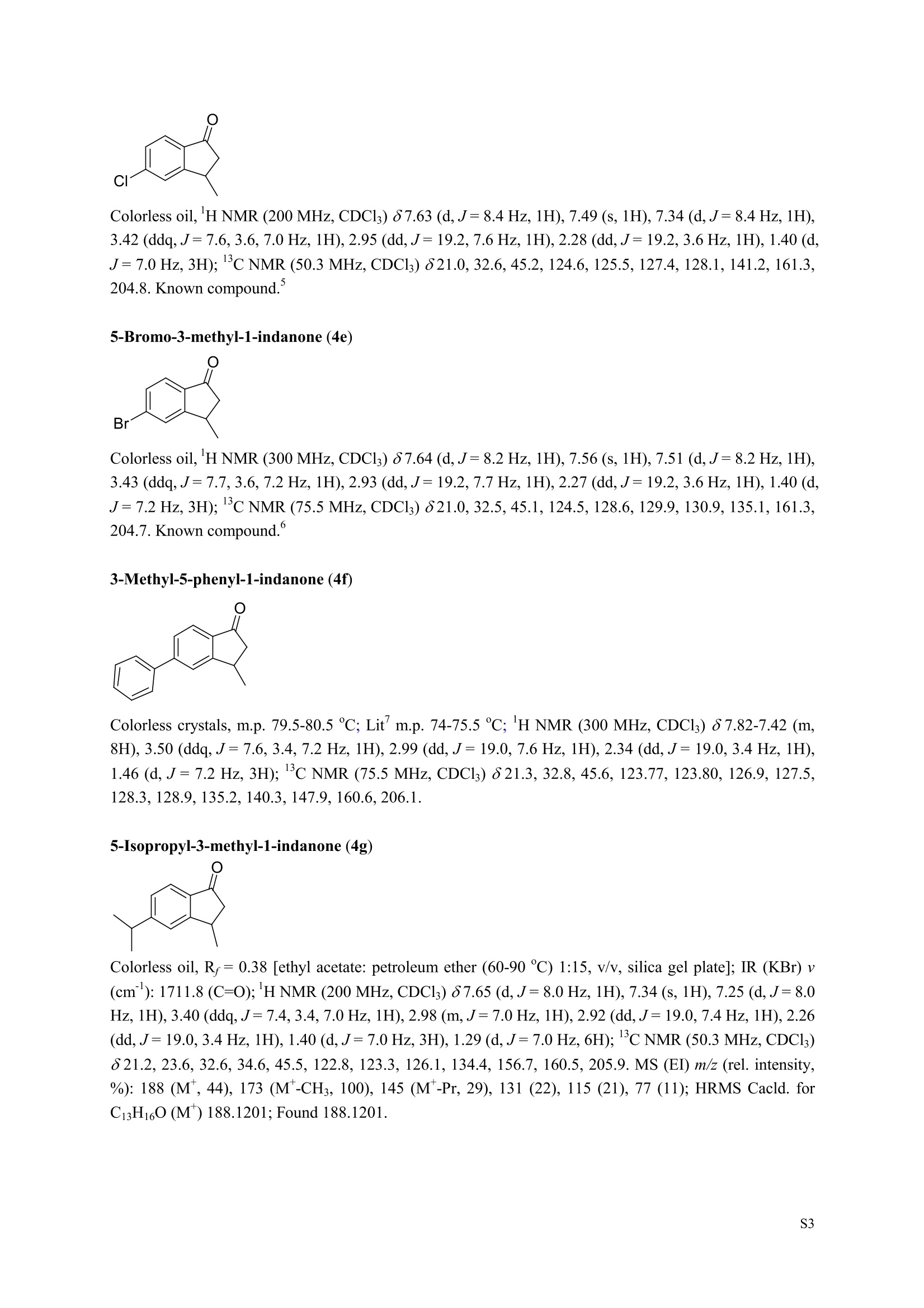
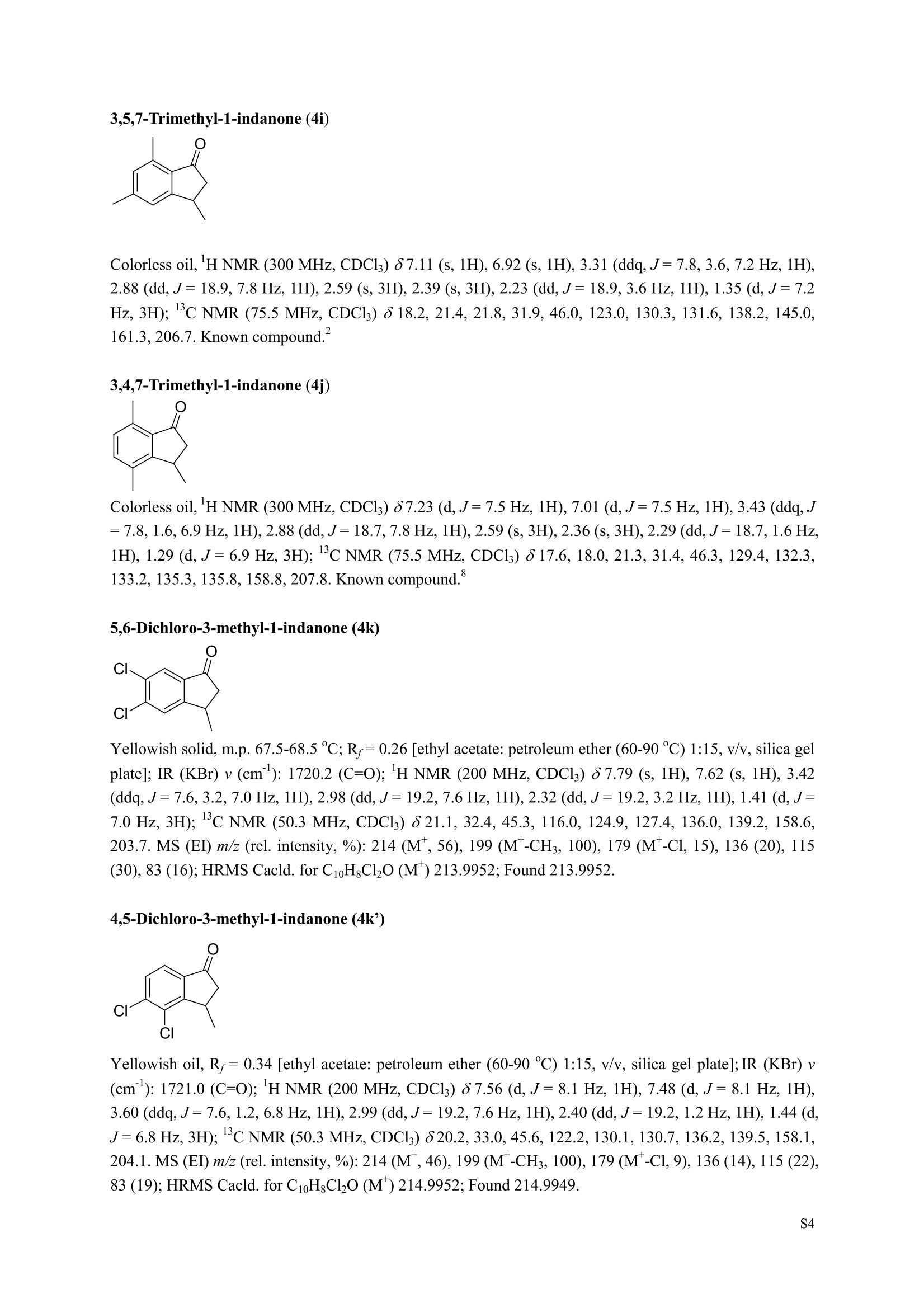
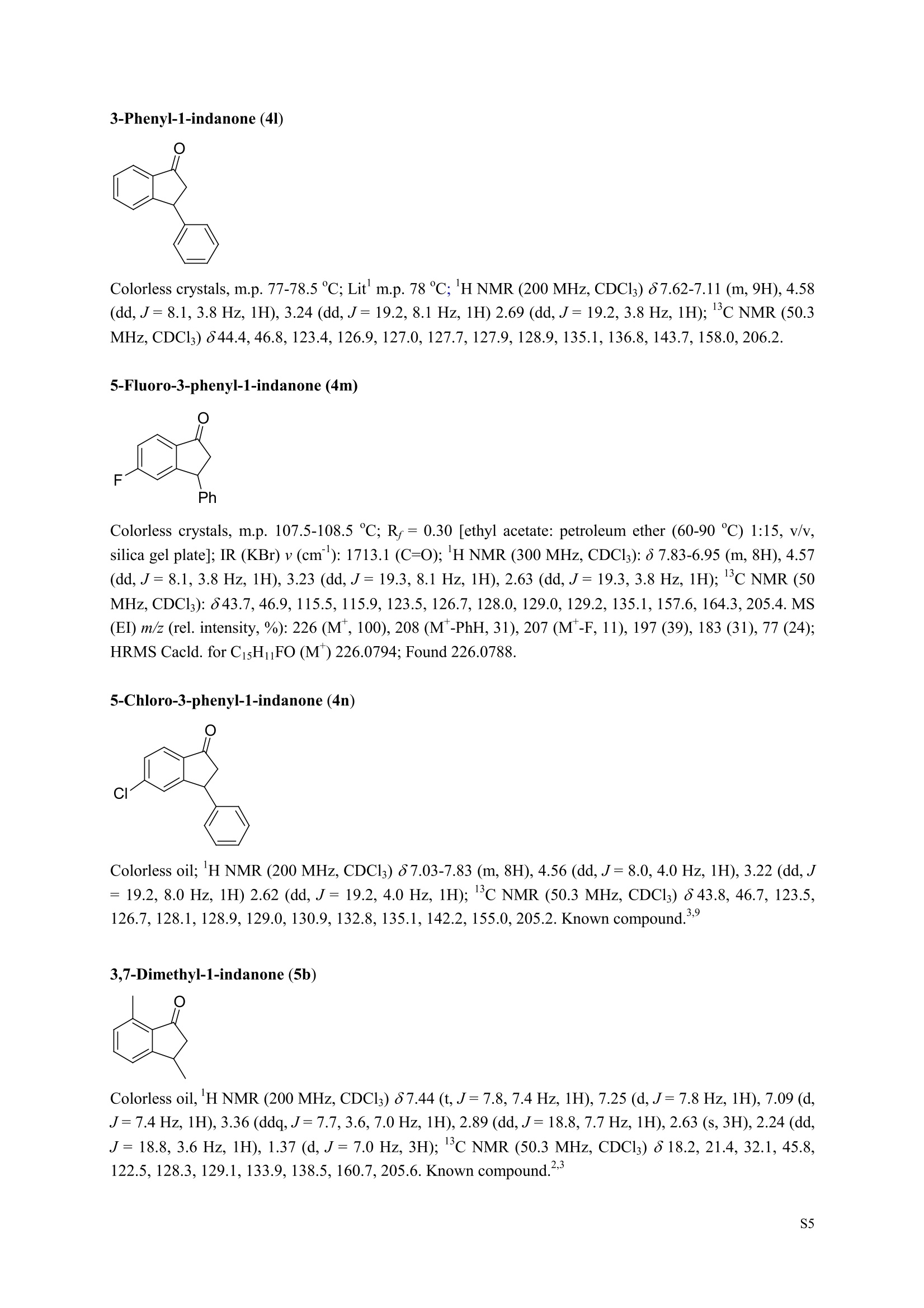
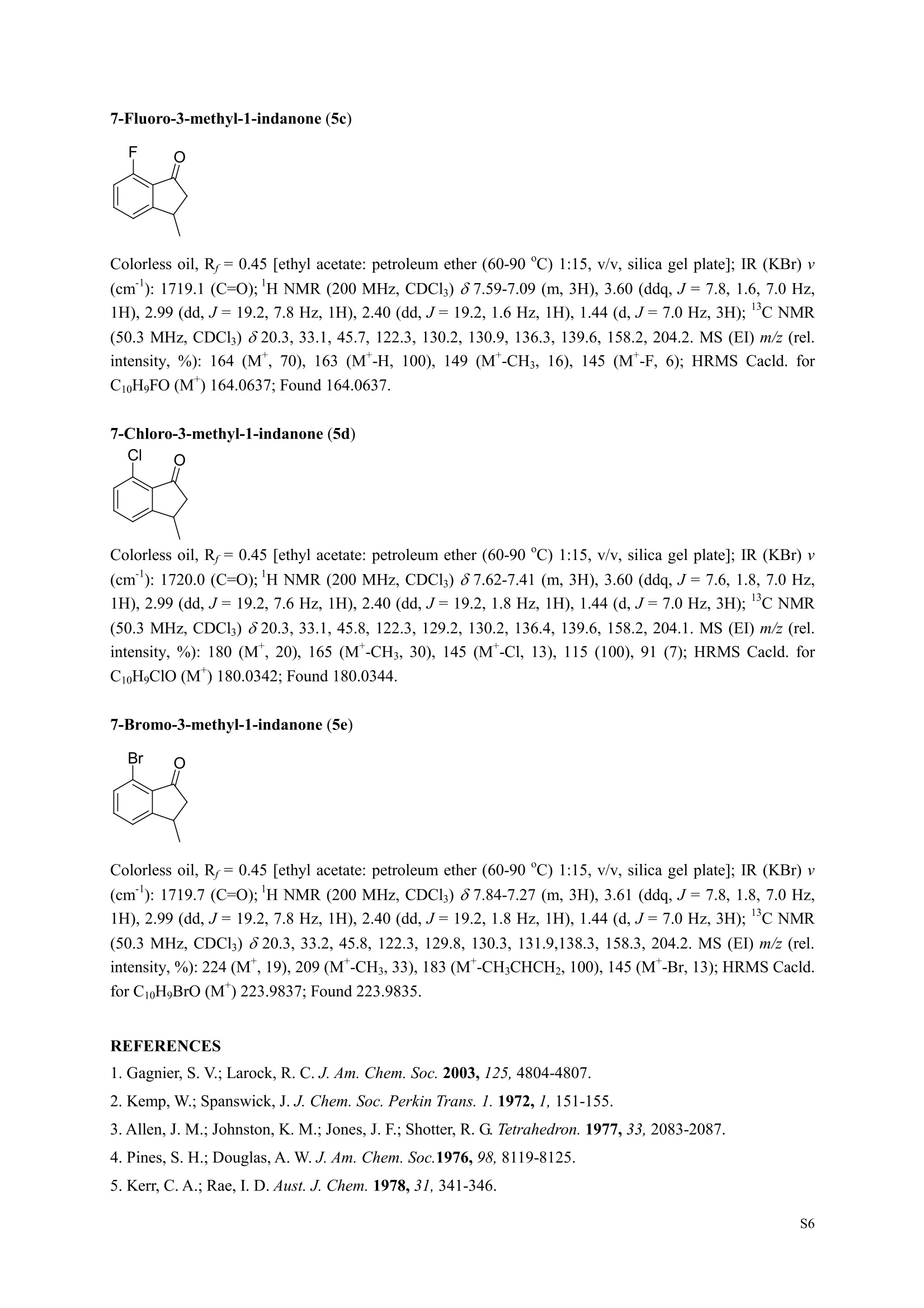
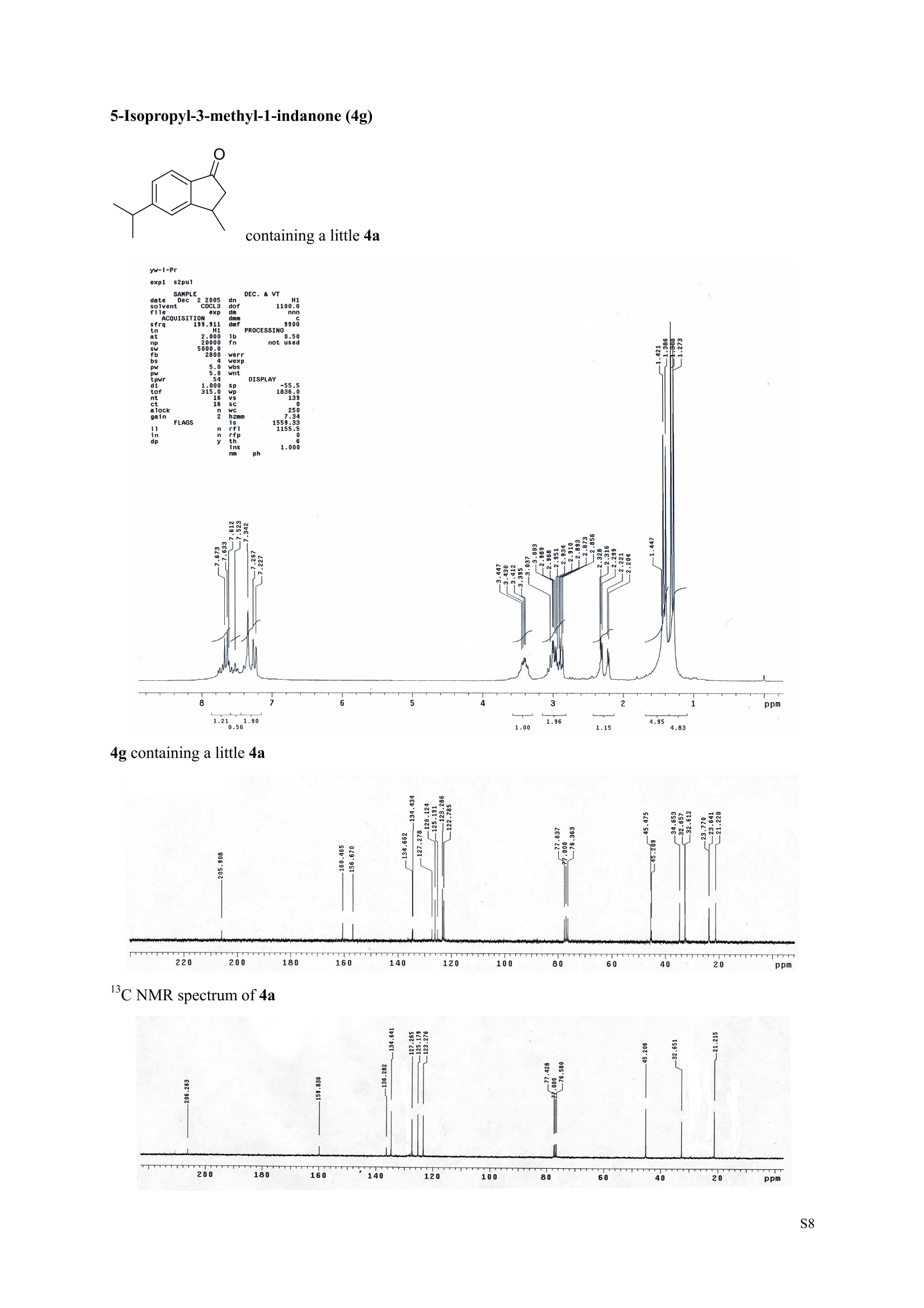
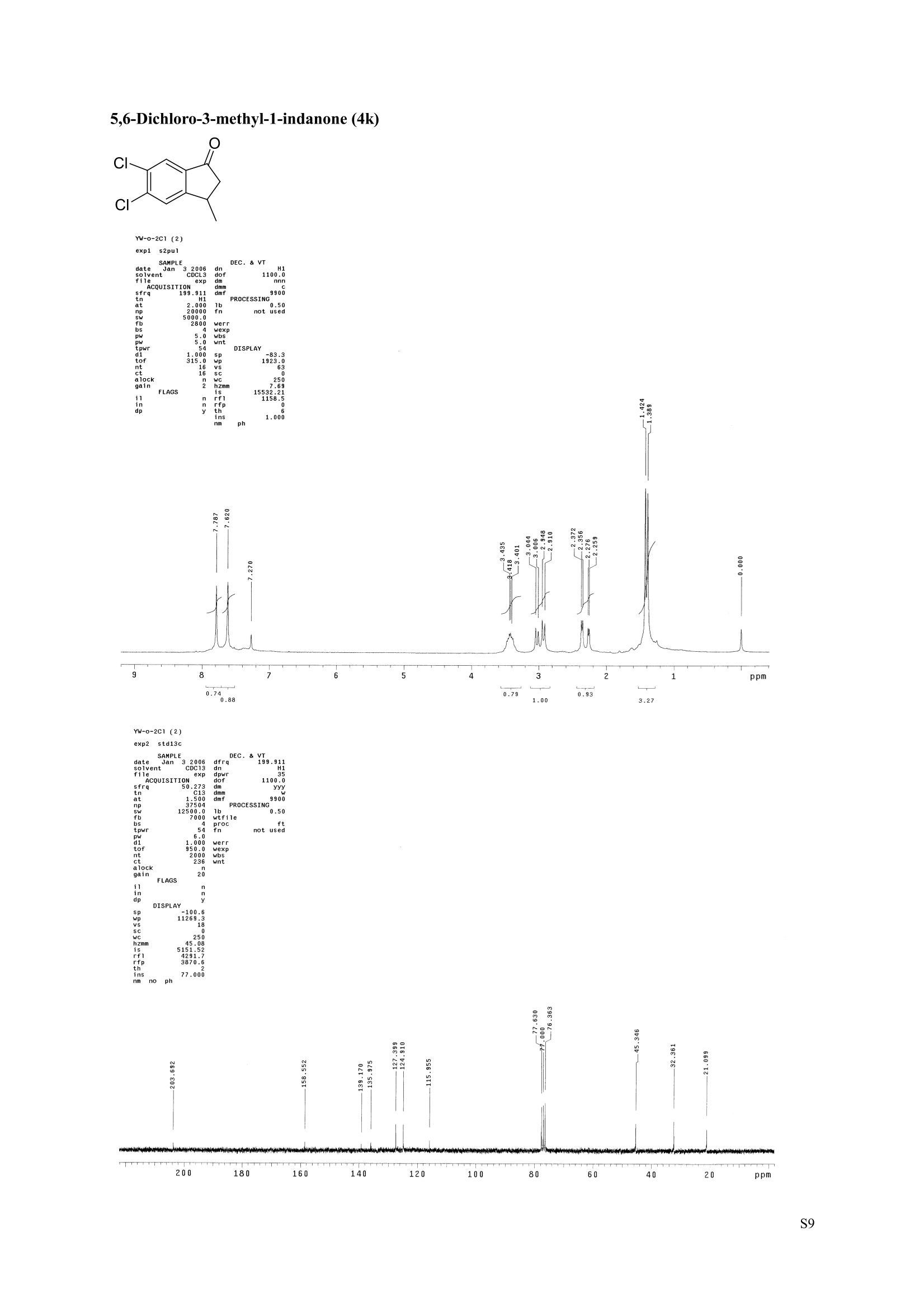
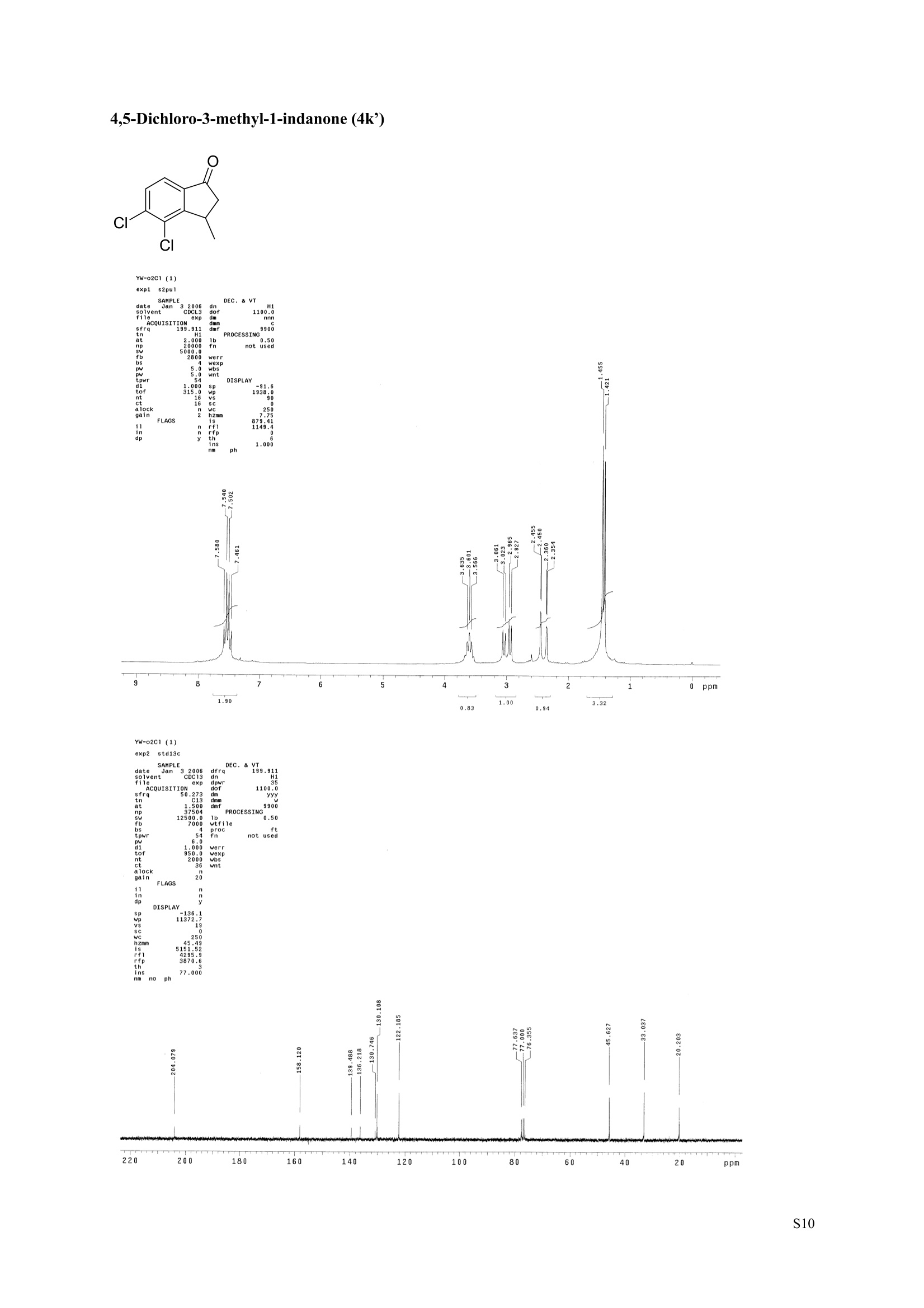
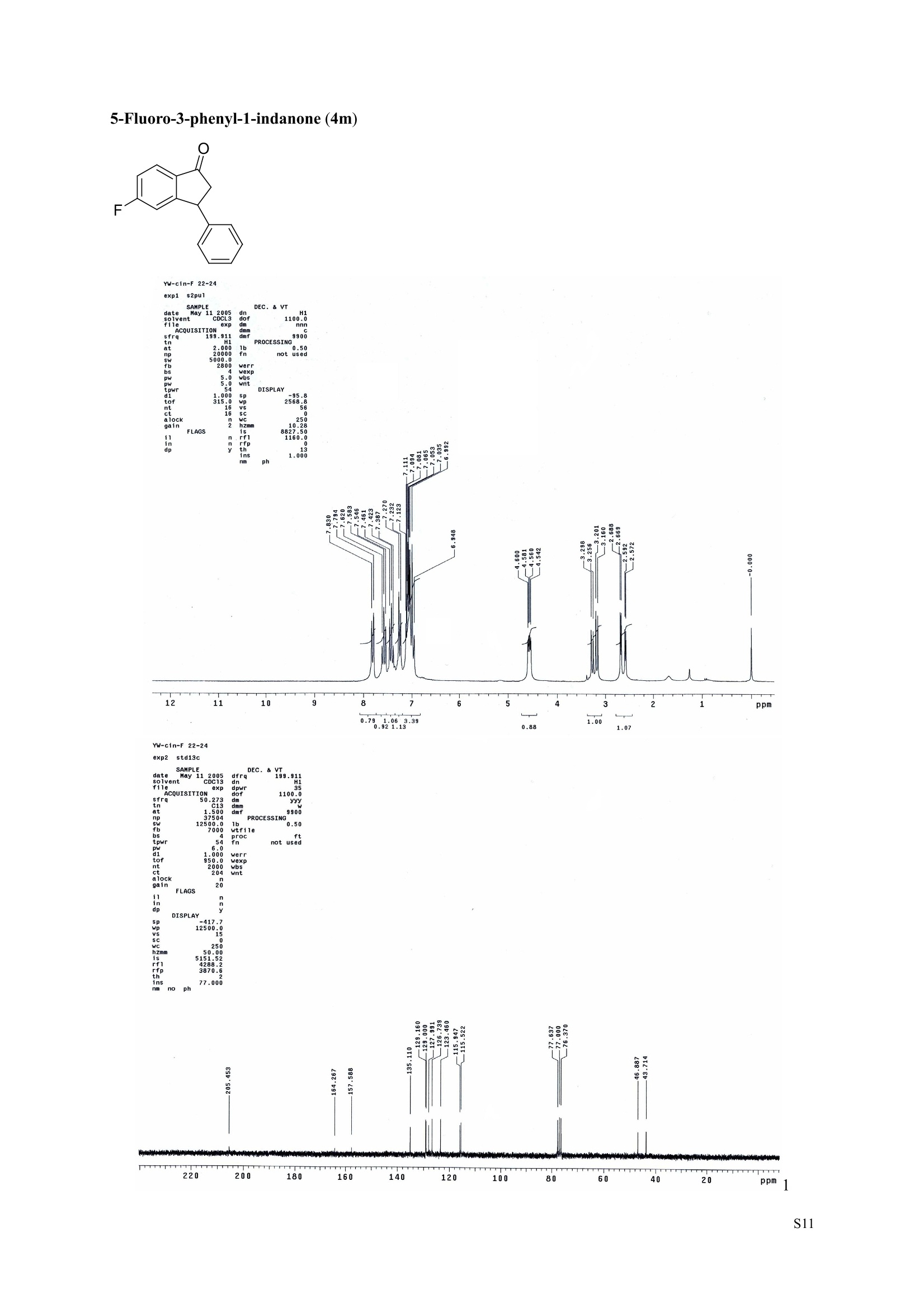
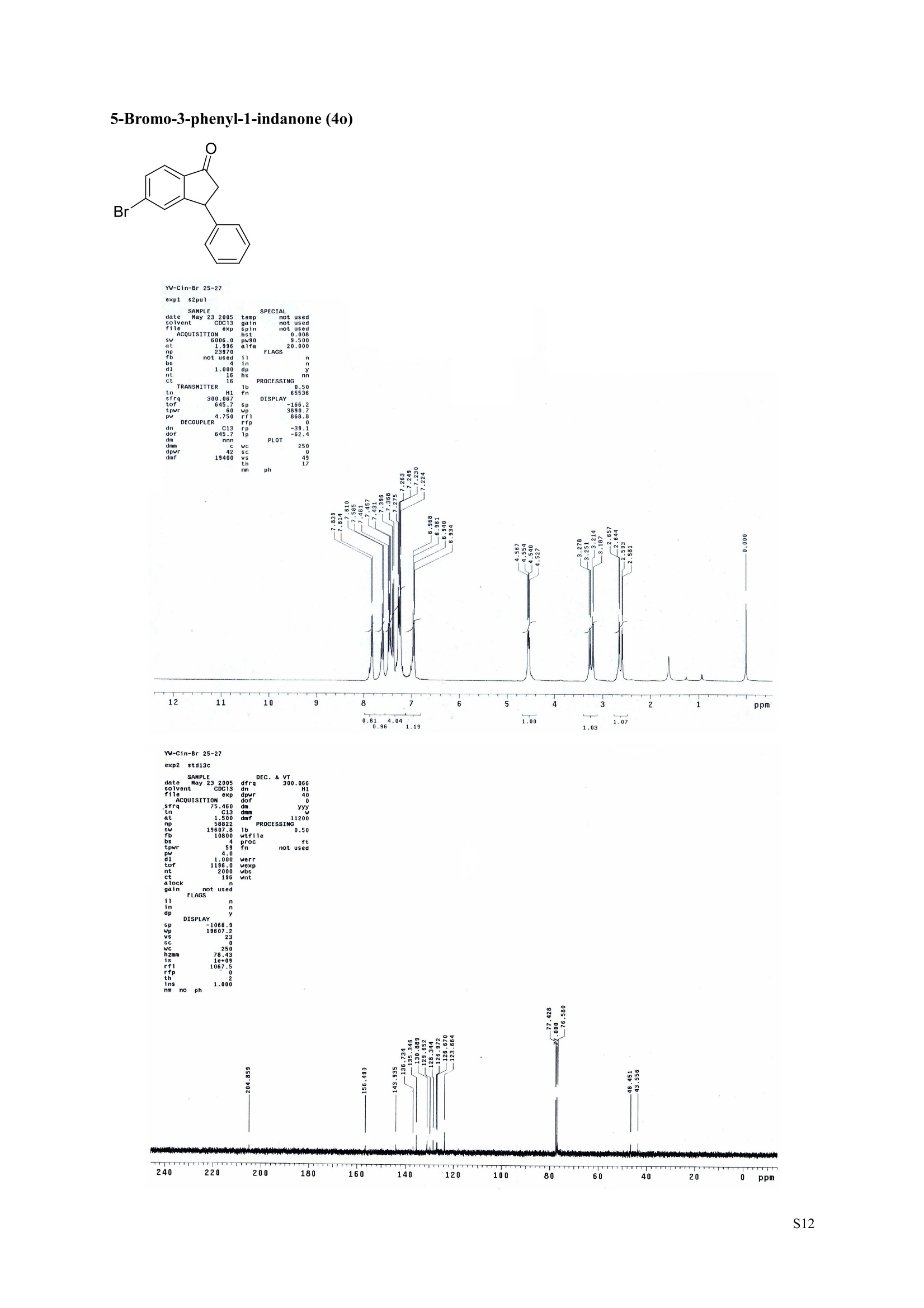
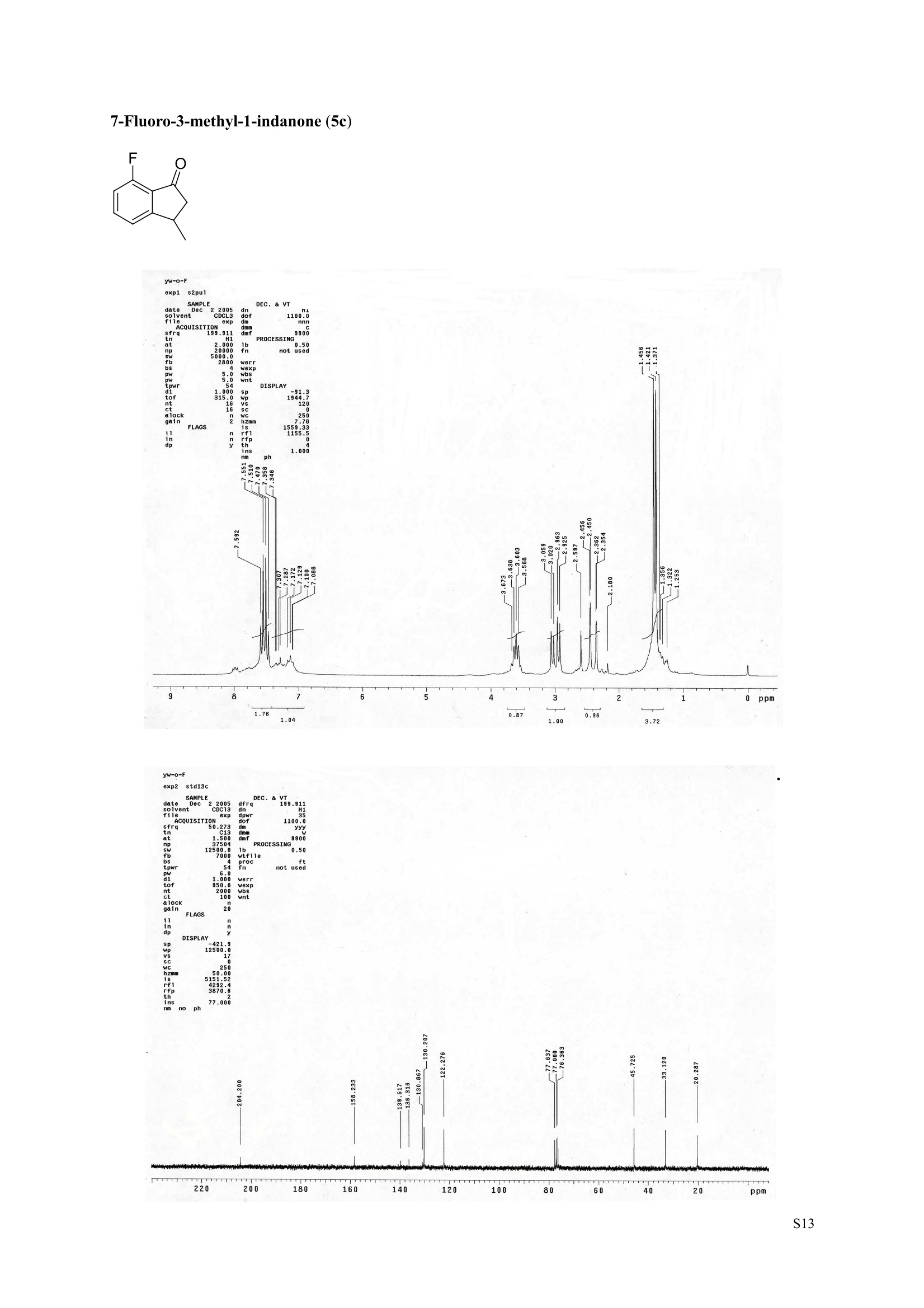
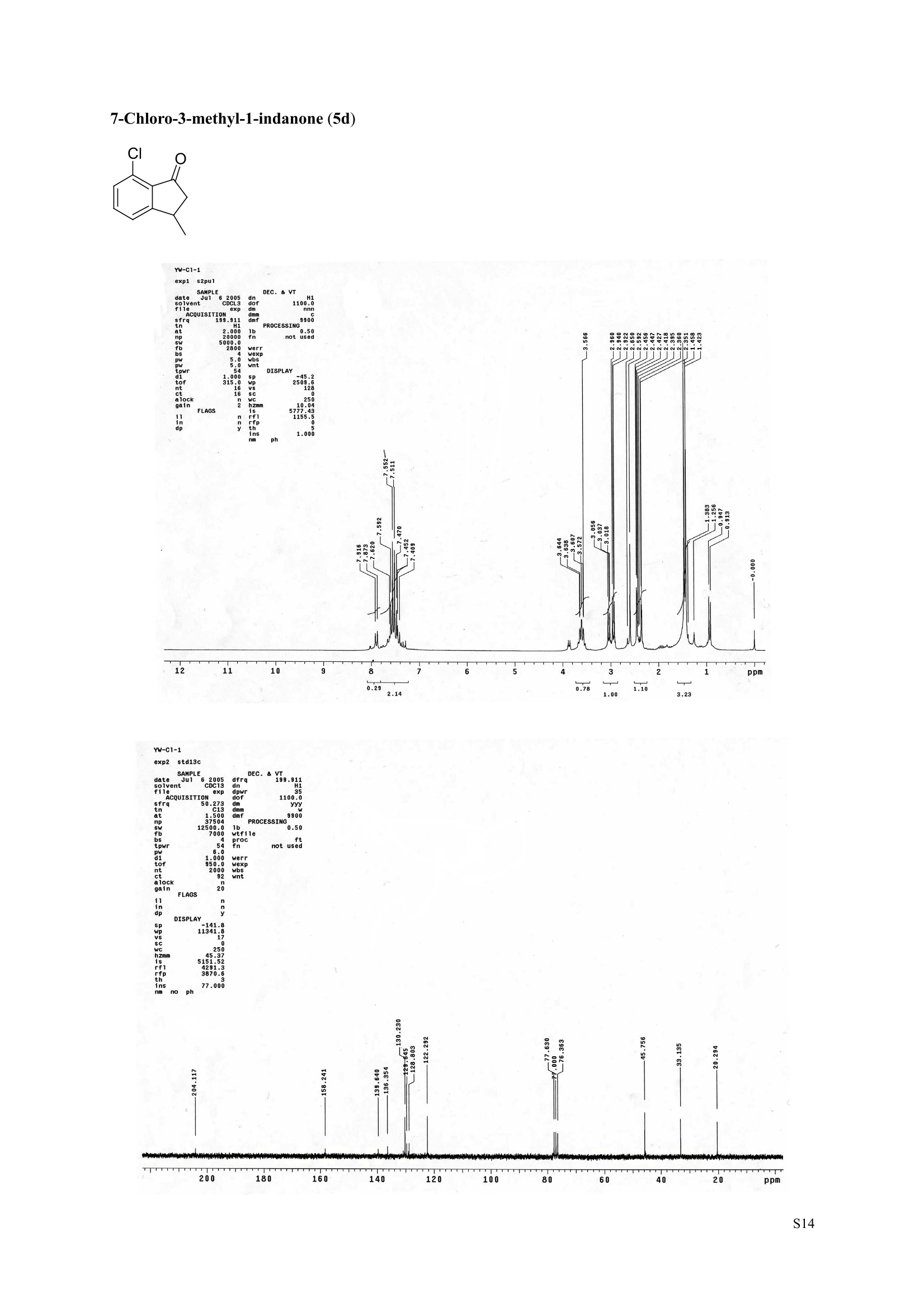
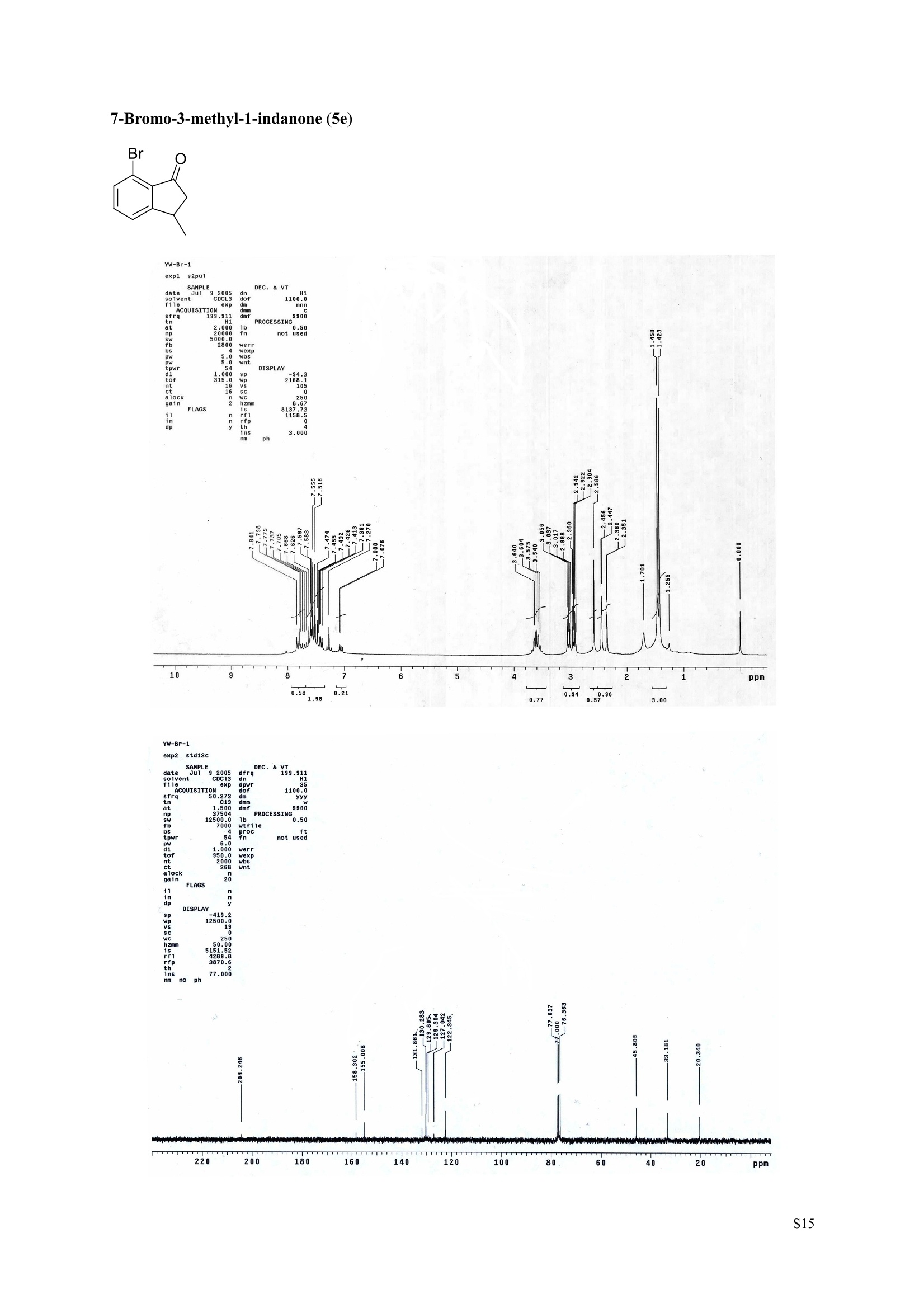
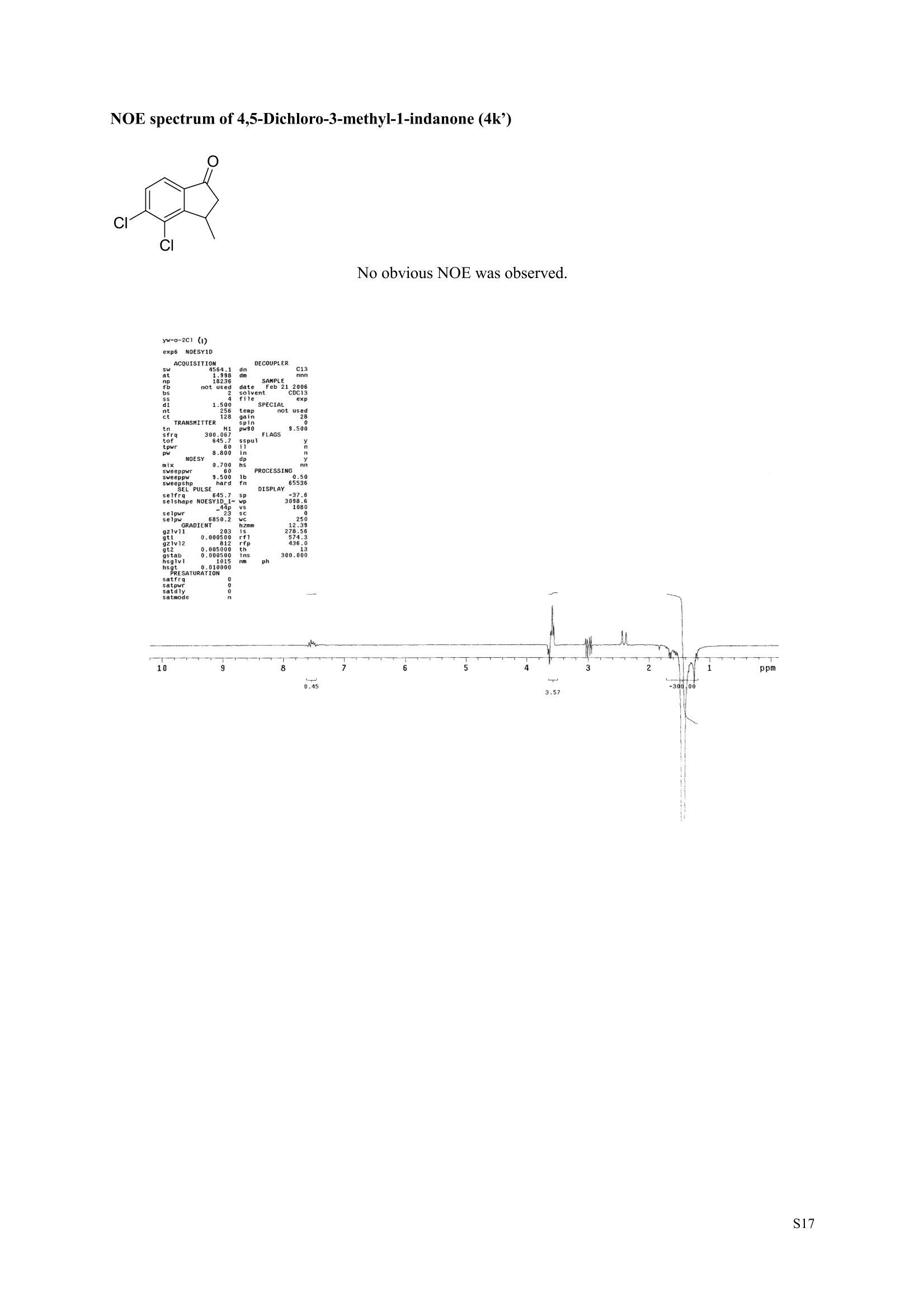
3,5,7-Trimethyl-1-indanone (4i) 7-Fluoro-3-methyl-1-indanone (5c) Microwave-Assisted One-Pot Synthesis of 1-Indanones from Arenesand a,B-Unsaturated Acyl Chlorides Wei Yin", Yuan Ma",* Jiaxi Xu', and Yufen Zhao" “Key Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology,Ministry ofEducation, Department of Chemistry, Tsinghua University, Beijing 100084, People’sRepublic of China. "Key Laboratory of Bioorganic Chemistry and Molecular Engineering ofMinistry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, People’s Republic of China mayuan@mail.tsinghua.edu.cn Supplemental Materials Content.. .S1 Experimental details: General... .S2 The analytic data of1-indanones 4 and 5.. .S2 Copies of 'H NMR and I’c NMR spectra of the unknown compounds 4 and 5. .S8 Copy of HNMR NOE spectrum of unknown compound 4k’. .S17 Experimental Details General. o-Dichlorobenzene was dried over anhydrous sodium sulfate and distilled prior touse.c(All reactions were performed in a commercial microwave reactor (XH-100A, 100-1000W, Beijing Xianghu Science and Technology Development Co. Ltd, Beijing, P. R. China).The temperature of the reaction mixture was measured by an immersed platinum resistancethermometer.JlMelting points were measured on a melting point apparatus and are uncorrected.'H NMR (200 or 300 MHz) and c NMR (50 or 75 MHz) spectra were recorded in CDClwith TMS as an internal standard. Mass spectra and HRMS data were obtained on an EI massspectrometer. IR spectra were recorded on a FT-IR spectrometer with an OMNI sampler.TLC separations were performed on silica gel GF254 plates, and the plates were visualizedwith UV light. 3-Methyl-1-indanone (4a) O Colorless oil, 'H NMR (300 MHz, CDCl;)87.73-7.34 (m, 4H), 3.43 (ddq, J= 7.5, 3.4, 6.9 Hz, 1H), 2.93(dd, J=19.2, 7.5 Hz, 1H), 2.27 (dd, J= 19.2, 3.4 Hz, 1H), 1.40 (d,J=6.9 Hz, 3H); CNMR (75.5 MHz,CDCl3) 821.2,32.6,45.2,123.3,125.2,127.3,134.6,136.3,159.8,206.3.Known compound. 3,5-Dimethyl-1-indanone (4b) Colorless oil, H NMR (200 MHz, CDCl;) 87.61 (d,J=8.0 Hz, 1H), 7.29 (s, 1H), 7.17 (d,J=8.0 Hz, 1H).3.38 (ddq, J=7.6, 3.5, 7.4 Hz, 1H), 2.91 (dd,J=19.0, 7.6 Hz, 1H), 2.45 (s, 3H), 2.24 (dd,J=19.0, 3.5 Hz.1H), 1.38(d, J=7.4Hz, 3H); 1c NMR (50.3MHz, CDCl;) 821.2, 22.0, 32.5, 45.4,123.2,125.5, 128.6,134.1,145.8,160.4,205.6. Known compound. 5-Fluoro-3-methyl-1-indanone (4c) Colorless oil, H NMR (200 MHz, CDCl3) 87.76-7.69 (m, 1H), 7.18-7.02 (m, 2H), 3.43 (ddq, J= 7.4, 3.4,7.0, Hz, 1H), 2.96 (dd, J=18.9, 7.4 Hz, 1H), 2.30 (dd,J=18.9, 3.4 Hz, 1H), 1.41 (d,J=7.0 Hz,3H);cNMR (50.3 MHz, CDCl;) 8 21.1,32.6,45.4,124.9,125.6,127.4,132.8,155.0,162.9,204.3. Knowncompound. Colorless oil, H NMR (200 MHz, CDCl;) 87.63 (d,J=8.4 Hz,1H), 7.49 (s, 1H), 7.34 (d, J=8.4 Hz, 1H).3.42 (ddq,J=7.6, 3.6,7.0 Hz, 1H), 2.95 (dd,J=19.2, 7.6 Hz, 1H), 2.28 (dd,J=19.2,3.6 Hz,1H), 1.40 (d,J=7.0 Hz,3H); CNMR (50.3 MHz, CDCl;) 821.0, 32.6, 45.2,124.6,125.5,127.4,128.1, 141.2,161.3,204.8. Known compound.' 5-Bromo-3-methyl-1-indanone (4e) Colorless oil,H NMR (300 MHz, CDCl;) 87.64 (d,J=8.2Hz, 1H), 7.56 (s, 1H), 7.51 (d,J=8.2 Hz, 1H).3.43 (ddq,J=7.7, 3.6, 7.2 Hz,1H), 2.93 (dd,J=19.2, 7.7 Hz, 1H), 2.27 (dd,J=19.2, 3.6 Hz, 1H), 1.40 (d,J=7.2 Hz,3H); 'C NMR (75.5 MHz, CDCl;) 621.0, 32.5, 45.1, 124.5,128.6,129.9, 130.9,135.1,161.3,204.7. Known compound. 3-Methyl-5-phenyl-1-indanone (4f) Colorless crystals, m.p. 79.5-80.5C; Lit’m.p. 74-75.5℃; 'HNMR (300 MHz, CDCl;) 8 7.82-7.42(m,8H), 3.50 (ddq,J= 7.6, 3.4, 7.2 Hz, 1H), 2.99 (dd,J=19.0, 7.6 Hz, 1H), 2.34 (dd,J=19.0, 3.4 Hz,1H),1.46 (d, J= 7.2 Hz, 3H); C NMR (75.5 MHz, CDCl;) 821.3, 32.8, 45.6, 123.77,123.80,126.9,127.5,128.3,128.9,135.2,140.3,147.9,160.6,206.1. 5-Isopropyl-3-methyl-1-indanone(4g) Colorless oil, Rr= 0.38 [ethyl acetate: petroleum ether (60-90℃) 1:15, v/v, silica gel plate]; IR (KBr) v(cm*): 1711.8(C=O); HNMR (200MHz, CDCl;) 87.65 (d,J=8.0 Hz, 1H), 7.34 (s, 1H), 7.25 (d,J=8.CHz, 1H), 3.40 (ddq,J=7.4, 3.4, 7.0 Hz, 1H), 2.98 (m,J=7.0Hz, 1H), 2.92 (dd,J= 19.0, 7.4 Hz, 1H), 2.26(dd,J=19.0, 3.4 Hz, 1H), 1.40 (d,J=7.0 Hz, 3H),1.29 (d, J=7.0 Hz, 6H);CNMR(50.3 MHz, CDCl;)821.2,23.6,32.6,34.6,45.5,122.8,123.3,126.1,134.4,156.7,160.5,205.9. MS (EI) m/z (rel. intensity,%): 188 (M , 44), 173 (M*-CH, 100), 145 (M*-Pr, 29), 131 (22),115 (21), 77 (11); HRMS Cacld. forC13H16O (M) 188.1201; Found 188.1201. Colorless oil, H NMR (300 MHz, CDCl;) 87.11 (s, 1H), 6.92 (s, 1H), 3.31 (ddq,J= 7.8, 3.6, 7.2 Hz,1H).2.88 (dd,J=18.9, 7.8 Hz, 1H), 2.59 (s, 3H), 2.39 (s, 3H), 2.23 (dd,J= 18.9, 3.6 Hz, 1H), 1.35 (d,J=7.2Hz, 3H); C NMR (75.5 MHz, CDCl;) 818.2,21.4,21.8,31.9,46.0,123.0,130.3, 131.6,138.2,145.0,161.3,206.7.Known compound. 3,4,7-Trimethyl-1-indanone (4j) Colorless oil, H NMR (300 MHz, CDCl;) 87.23 (d,J=7.5Hz, 1H), 7.01 (d,J=7.5 Hz, 1H), 3.43 (ddq, J=7.8,1.6,6.9 Hz,1H),2.88 (dd,J=18.7,7.8 Hz,1H),2.59 (s,3H),2.36 (s,3H), 2.29 (dd,J=18.7,1.6 Hz,1H), 1.29(d, J= 6.9 Hz, 3H); 1'c NMR (75.5MHz, CDCl;) 817.6, 18.0, 21.3,31.4,46.3,129.4, 132.3,133.2,135.3,135.8,158.8,207.8. Known compound.Q 5,6-Dichloro-3-methyl-1-indanone (4k) Yellowish solid, m.p. 67.5-68.5℃; R=0.26 [ethyl acetate: petroleum ether (60-90℃)1:15, v/v, silica gelplate]; IR (KBr) v (cm*'): 1720.2(C=O); 'H NMR (200 MHz, CDCl;) 8 7.79 (s, 1H), 7.62 (s, 1H), 3.42(ddq,J=7.6,3.2, 7.0 Hz, 1H), 2.98 (dd, J=19.2, 7.6 Hz, 1H), 2.32 (dd,J=19.2,3.2 Hz, 1H), 1.41 (d,J=7.0 Hz, 3H); c NMR (50.3 MHz, CDCl;) 8 21.1, 32.4, 45.3, 116.0,124.9,127.4, 136.0,139.2, 158.6,203.7. MS (EI) m/z (rel. intensity, %): 214 (M, 56), 199 (M*-CH3, 100), 179 (M*-Cl, 15), 136 (20),115(30), 83 (16); HRMS Cacld. for C10HgCl2O(M )213.9952; Found 213.9952. 4,5-Dichloro-3-methyl-1-indanone (4k') Yellowish oil, Rr= 0.34 [ethyl acetate: petroleum ether (60-90℃) 1:15, v/v, silica gel plate];IR (KBr) v(cm*): 1721.0 (C=O); H NMR (200 MHz, CDCl;) 8 7.56 (d, J= 8.1 Hz, 1H), 7.48 (d, J= 8.1 Hz, 1H).3.60 (ddq,J=7.6, 1.2, 6.8 Hz, 1H), 2.99 (dd,J=19.2, 7.6 Hz, 1H), 2.40 (dd,J=19.2, 1.2 Hz, 1H), 1.44 (d,J=6.8 Hz,3H); C NMR (50.3 MHz, CDCl;) 820.2, 33.0, 45.6,122.2,130.1,130.7,136.2, 139.5, 158.1,204.1. MS (EI) m/z (rel.intensity,%): 214(M , 46), 199(M -CH,100), 179 (M -Cl, 9), 136 (14),115(22),83 (19); HRMS Cacld. for CioHgClO (M") 214.9952; Found 214.9949. 3-Phenyl-1-indanone (4l) Colorless crystals, m.p. 77-78.5℃; Lit m.p. 78℃; H NMR (200 MHz, CDCl;) 87.62-7.11 (m, 9H),4.58(dd, J=8.1, 3.8 Hz, 1H), 3.24 (dd, J=19.2, 8.1 Hz, 1H) 2.69 (dd, J= 19.2, 3.8 Hz, 1H); 1'c NMR (50.3MHz,CDCl3) 844.4,46.8,123.4,126.9,127.0,127.7,127.9,128.9,135.1,136.8,143.7,158.0,206.2. 5-Fluoro-3-phenyl-1-indanone (4m) Colorless crystals, m.p. 107.5-108.5℃; Rr=0.30 [ethyl acetate: petroleum ether (60-90℃) 1:15, v/v,silica gel plate]; IR (KBr)v (cm*'): 1713.1 (C=O); 'H NMR (300 MHz, CDCl;): 8 7.83-6.95 (m, 8H), 4.57(dd, J=8.1, 3.8 Hz, 1H), 3.23 (dd, J=19.3, 8.1 Hz, 1H), 2.63 (dd, J= 19.3, 3.8 Hz, 1H); 'cNMR (50MHz, CDCl3): 843.7,46.9,115.5,115.9,123.5,126.7,128.0,129.0,129.2,135.1,157.6,164.3,205.4.MS(EI) m/z (rel. intensity,%): 226 (M,100),208 (M -PhH, 31), 207 (M"-F, 11), 197 (39), 183 (31),77 (24);HRMS Cacld. for CisHuFO(M )226.0794; Found 226.0788. 5-Chloro-3-phenyl-1-indanone (4n) Colorless oil; H NMR (200 MHz, CDCl;) 87.03-7.83 (m, 8H), 4.56 (dd, J=8.0, 4.0 Hz, 1H), 3.22 (dd, J=19.2, 8.0 Hz, 1H) 2.62 (dd, J= 19.2, 4.0 Hz, 1H);1’c NMR (50.3 MHz, CDCl;) 8 43.8, 46.7, 123.5,126.7,128.1,128.9,129.0,130.9,132.8,135.1,142.2,155.0,205.2. Known compound. 3,7-Dimethyl-1-indanone (5b) Colorless oil, H NMR (200 MHz, CDCl;) 87.44 (t, J=7.8,7.4 Hz, 1H), 7.25 (d, J=7.8 Hz, 1H), 7.09 (d.J=7.4 Hz,1H),3.36 (ddq,J=7.7,3.6,7.0 Hz, 1H), 2.89 (dd,J=18.8, 7.7Hz, 1H), 2.63 (S,3H), 2.24 (dd,J=18.8,3.6 Hz, 1H), 1.37 (d, J= 7.0 Hz, 3H); 3C NMR (50.3 MHz, CDCl;) 8 18.2, 21.4, 32.1, 45.8,122.5,128.3,129.1,133.9,138.5,160.7,205.6. Known compound. Colorless oil, Rr=0.45 [ethyl acetate: petroleum ether (60-90℃) 1:15, v/v, silica gel plate]; IR (KBr) v(cm*): 1719.1 (C=O);'HNMR (200 MHz, CDCl;) 87.59-7.09 (m, 3H), 3.60 (ddq, J= 7.8, 1.6, 7.0 Hz,1H), 2.99 (dd, J=19.2, 7.8 Hz, 1H), 2.40 (dd,J=19.2, 1.6 Hz, 1H), 1.44 (d,J=7.0 Hz, 3H);C NMR(50.3 MHz, CDCl3) 820.3,33.1, 45.7,122.3,130.2,130.9,136.3,139.6,158.2,204.2. MS (EI) m/z (rel.intensity, %): 164 (M, 70), 163 (M-H, 100), 149 (M*-CH, 16), 145 (M-F, 6); HRMS Cacld. forC10H,FO (M ) 164.0637; Found 164.0637. 7-Chloro-3-methyl-1-indanone (5d) Colorless oil, Rr=0.45 [ethyl acetate: petroleum ether (60-90℃) 1:15, v/v, silica gel plate]; IR (KBr) v(cm'): 1720.0 (C-O);'H NMR (200 MHz, CDCl;) 8 7.62-7.41 (m, 3H), 3.60 (ddq, J=7.6, 1.8, 7.0 Hz,1H), 2.99 (dd, J=19.2, 7.6 Hz, 1H), 2.40 (dd, J=19.2, 1.8 Hz, 1H), 1.44 (d,J= 7.0 Hz, 3H);CNMR(50.3 MHz, CDCl3) 820.3,33.1,45.8,122.3,129.2,130.2,136.4,139.6,158.2,204.1. MS (EI) m/z (rel.intensity, %): 180 (M , 20), 165 (M*-CH3, 30), 145 (M*-CI, 13), 115 (100), 91 (7); HRMS Cacld. forC10HgClO (M) 180.0342; Found 180.0344. 7-Bromo-3-methyl-1-indanone (5e) Colorless oil, R=0.45 [ethyl acetate: petroleum ether (60-90C) 1:15, v/v, silica gel plate]; IR (KBr) v(cm*): 1719.7 (C=O); 'H NMR (200 MHz, CDCl;) 8 7.84-7.27 (m, 3H), 3.61 (ddq, J=7.8, 1.8, 7.0 Hz.1H), 2.99 (dd, J=19.2, 7.8 Hz, 1H), 2.40(dd, J=19.2, 1.8 Hz, 1H), 1.44 (d, J=7.0 Hz, 3H); 1cNMR.(50.3 MHz, CDCl3) 820.3,33.2,45.8,122.3,129.8,130.3,131.9,138.3,158.3, 204.2. MS (EI) m/z (rel.intensity, %): 224 (M ,19), 209 (M"-CH,33), 183 (M-CH;CHCH2,100), 145 (M -Br, 13); HRMS Cacldfor C10HgBrO(M) 223.9837; Found 223.9835. REFERENCES 1.Gagnier, S. V; Larock, R. C. J. Am. Chem. Soc. 2003, 125, 4804-4807. 2. Kemp, W.; Spanswick, J. J. Chem. Soc. Perkin Trans. 1.1972,1,151-155. 3. Allen, J. M.; Johnston, K. M.; Jones, J. F.; Shotter, R. G. Tetrahedron. 1977,33,2083-2087. 4. Pines, S. H.; Douglas, A. W. J. Am. Chem. Soc.1976,98, 8119-8125. 5. Kerr, C. A.; Rae, I. D. Aust. J. Chem. 1978,31, 341-346. 6. Sircar, I; Duell, B. L.; Cain, M. H.; Burke, S. E.; Bristol, J. A. J. Med. Chem. 1986, 29,2142-2148. 7. Smith, C. E.; Williamson, W. R. N.; Cashin, C. H.; Kitchen,E. A. J.Med. Chem. 1979,22,1464-1469. 8. Boykin,D. W.; Hertzler,R. L.;Delphon, J. K.; Eisenbraun, E. J.J. Org. Chem. 1989, 54, 1418-23. 9. Boegesoe, K. P.; Arnt, J.; Hyttel,J.; Pedersen, H. J. Med. Chem. 1993, 36,2761-2770. yw-1-Pr 8 寸O寸 NN 88N 8 6 5 4 2 pp 1.21 1.90 1.96 4.95 1.0100 1.15 4.83 4g containing a little 4a 寸r‘r的NN 导 寸 o 220 200 180 160 140 120 100 80 60 40 20 Ppm 13c NMR spectrum of 4a YW-0-2C1 (2 200 180 160 140 120 100 80 60 40 20 ppm 品 8 的NiggO 的 的m 9 8 7 6 5 4 3 2 1 0ppm 1.90 1.00 3.32 0.83 0.94 N N : 28088- 62002 NW的 is & 220 180 160 140 120 100 80 60 40 20 PP YW-cin-F 22-24 ph :88g: 卜 57l 寸89的的 8 898 8 gu : S r 12 11 10 8 5 3 2 ppm 0.79 1.06 3.39 1.00 21.13 0.88 1.07 YW-c1n-F 22-24 exp2 std13c SAMPLE date .May 112005 dfrrqso1vent DECC.899.91 DC13 dr H1 f1116 H1 f11 CQUISITION 1100.0 frq 50 yyy .500 9900 317591 PF 00B PROCESSIN( LO 0..550 7000 tf11le prcoc not used 999 0.0 20008wt 204 wn1 a1ock 20 FIFLAGS 20 DISPLAAY 417.7 12L2500.0 2 50.0 0 5151.52 4288.2 tp 3870.6 77909 im:no Br YW-Cin-Br 25-27 m38rrl 寸 l 寸 寸 Ne 6s pp" 12 11 10 9 8 7 6 5 1.00 1.01 o.814.04 1.03 YW-Cin-Br 25-27 exp2std13c SAMPLE DEC. &300.066 date May 23 2005C13 dfrq solvent fi1e cp AACQUISITION sfifrc 460 yyy Ci. .5 111200 58822 PROCESSING 19607.8 1b 00.50 10800 wtf11e 59 not used 4.0 1.000 werr tof 1196.0 wexp 2000 wb: nt ct 196 wnt a1ocK gain not used FLAGS dp DISPLAY -1066.9 19607:2 23 21250 78.43 1e+09 1067. th 1n 1.00( ph 06p9sr- DSEEZTS-E96EETb-T Er- t T40 2080 60 TTTT PP 220 200 180 160 140 120 100 7-Chloro-3-methyl-1-indanone (5d) TT 220 200 180 160 140 120 100 80 60 40 20 ppm 导号: 35 RG 8 寸 人 12 11 10 ppm 0.884.77 0.93 1.02 1.011.84 10 "SO N &gr :8 em号林 TT 240 220 200 180 160 140 120(0 100 80 60 40 20 ppm No obvious NOE was observed. S17 S Chloro--methyl--indanone (d)S
确定



还剩15页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《Microwave-Assisted One-Pot Synthesis of 1-Indanones from Arenes and α β-Unsaturated Acyl Chlorides》,该方案主要用于其他中--检测,参考标准--,《Microwave-Assisted One-Pot Synthesis of 1-Indanones from Arenes and α β-Unsaturated Acyl Chlorides》用到的仪器有祥鹄电脑微波催化合成/萃取仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















