方案详情
文
有机发光体因其独特的光物理特性而具有广泛的应用。近年来,非共轭有机发光体,与传统的结合的发光体,由于其方便的制备、环境的影响,已经获得了很多关注。在这项研究中,一种新的基于非传统发光体的首次报道了在动态共价交联的聚氨酯上出现的16种新的化学反应。该新发光体不仅在固体状态下表现出固有的强荧光发射,而且还拥有高的机械性能以及良好的形状记忆和自修复性能。特别是,新的发光体是由脂肪族多官能环状碳酸酯合成的方法更为直接,避免了使用有毒的异氰酸酯。
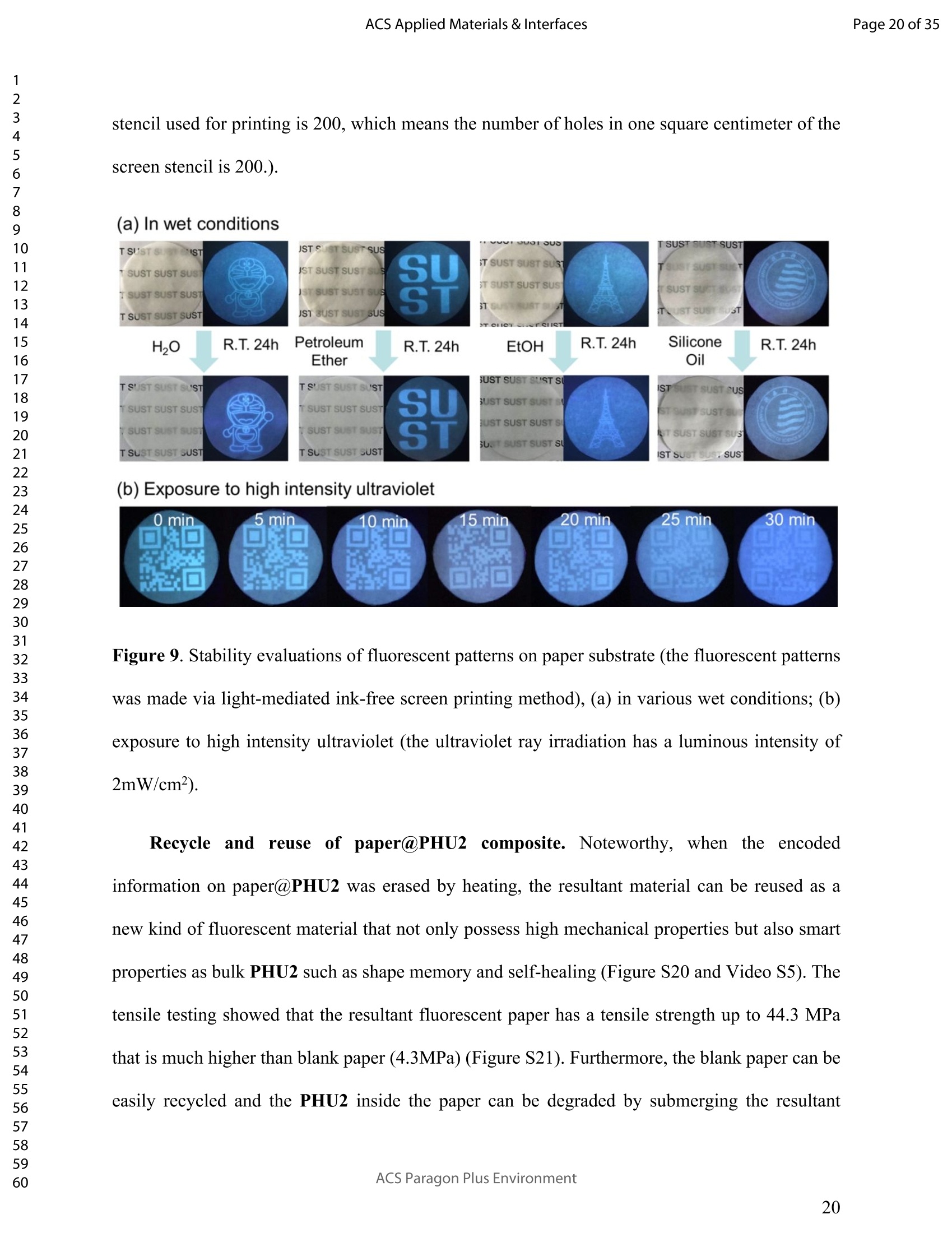
调查表明,所产生的发光体的内在发光是由诱导的通过聚合物链的交联,并可以通过控制聚合物链的程度来很好地调整。交联。通过利用所产生的聚合物的独特特性。我们进一步开发了一种简便的方法,命名为 "光介导的无墨屏幕打印",用于防伪纸的制作。与传统的油墨印刷技术不同,新方法使用紫外光而不是昂贵的安全墨水来编码。天然纤维素纸的防伪信息。防伪信息是在各种潮湿条件下都是稳定的,显示出在快速增长的药品、包装和食品行业的假冒。
方案详情

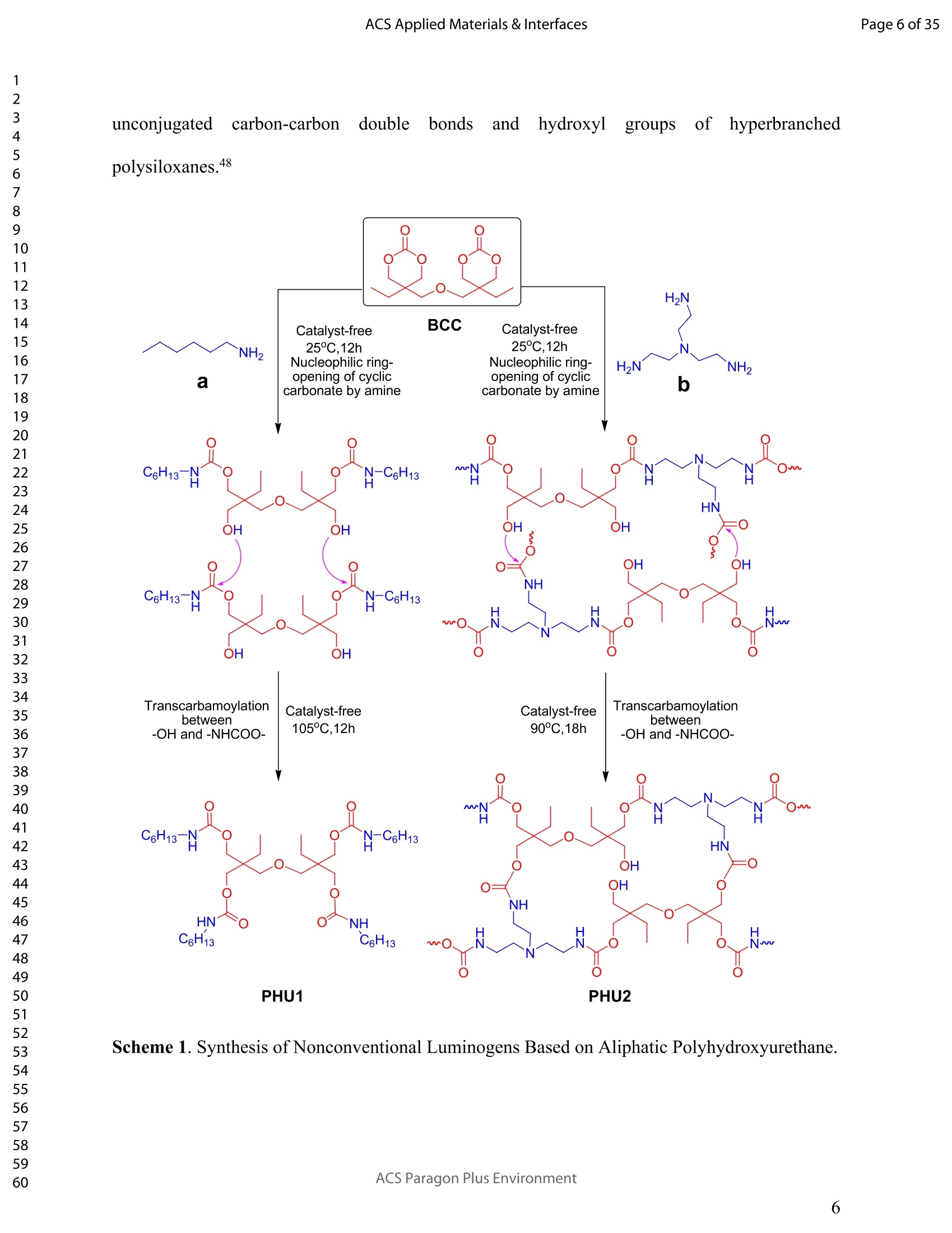
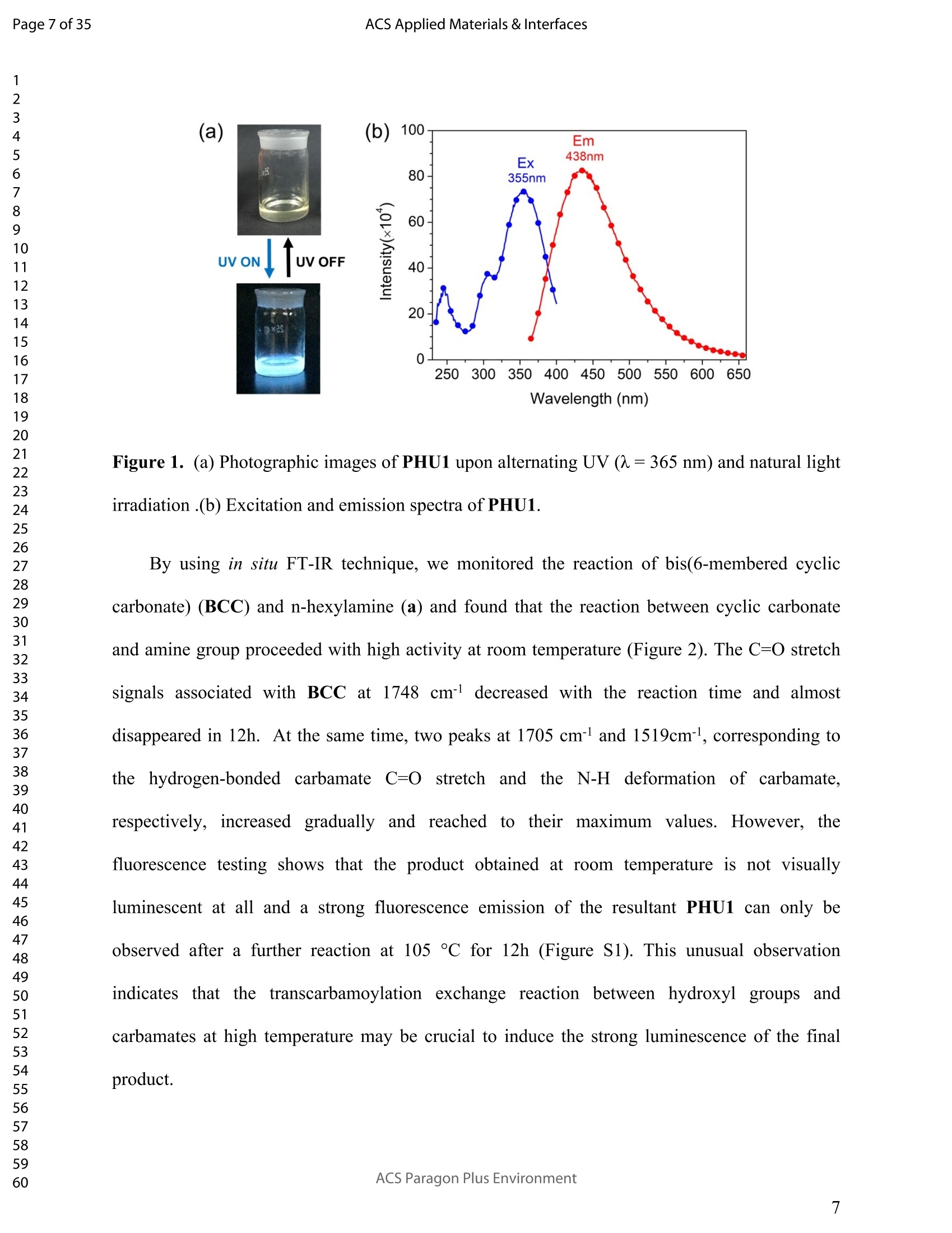
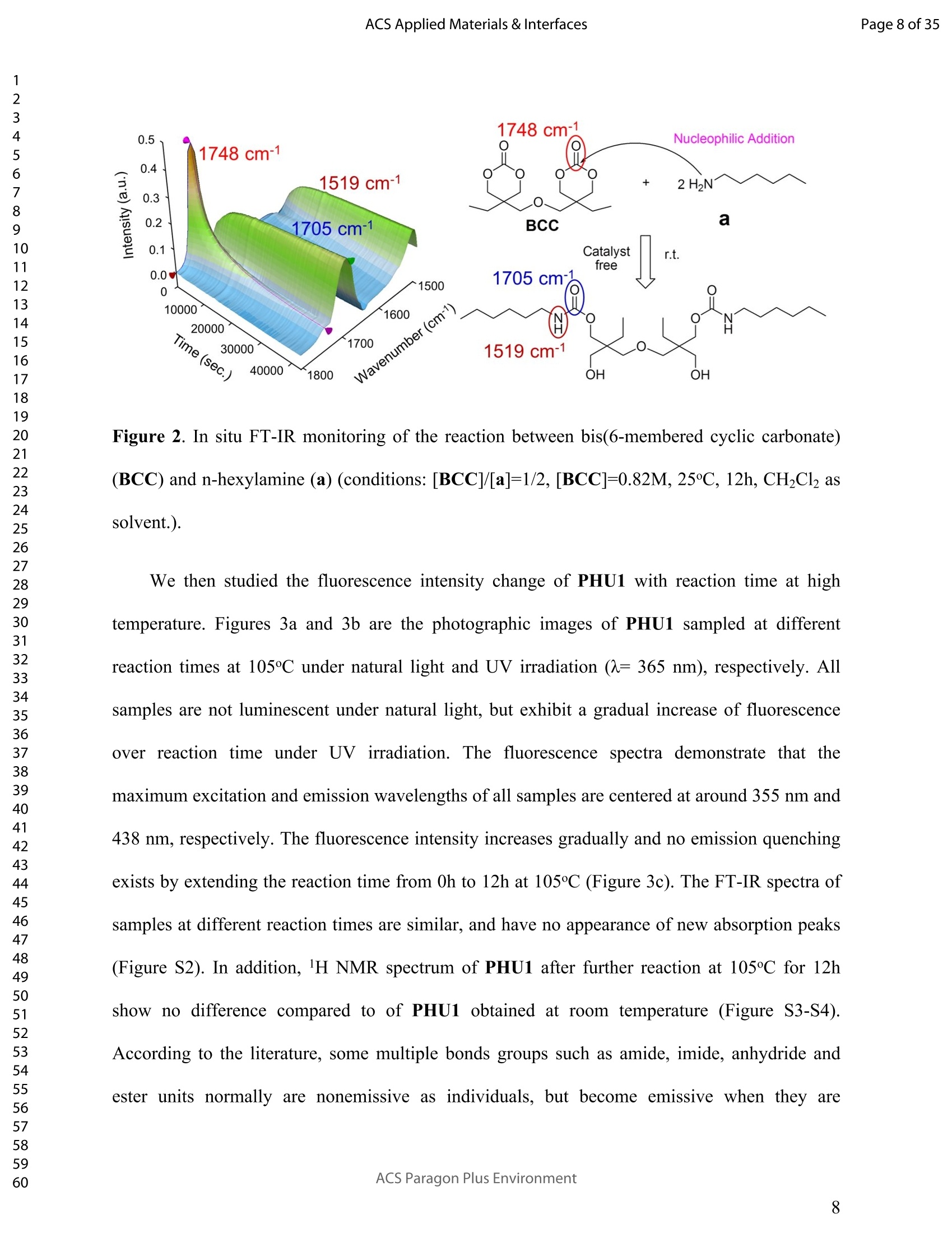
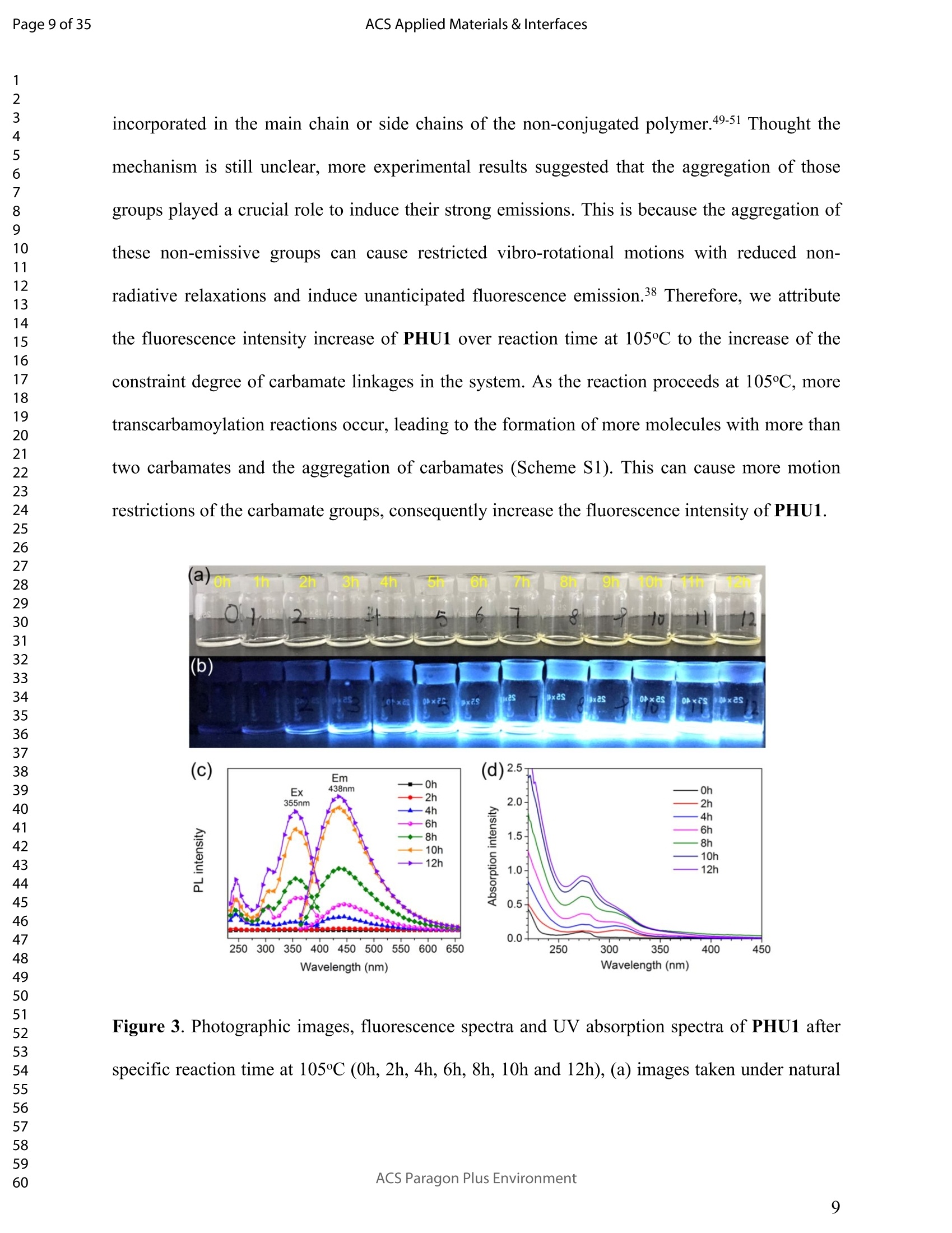
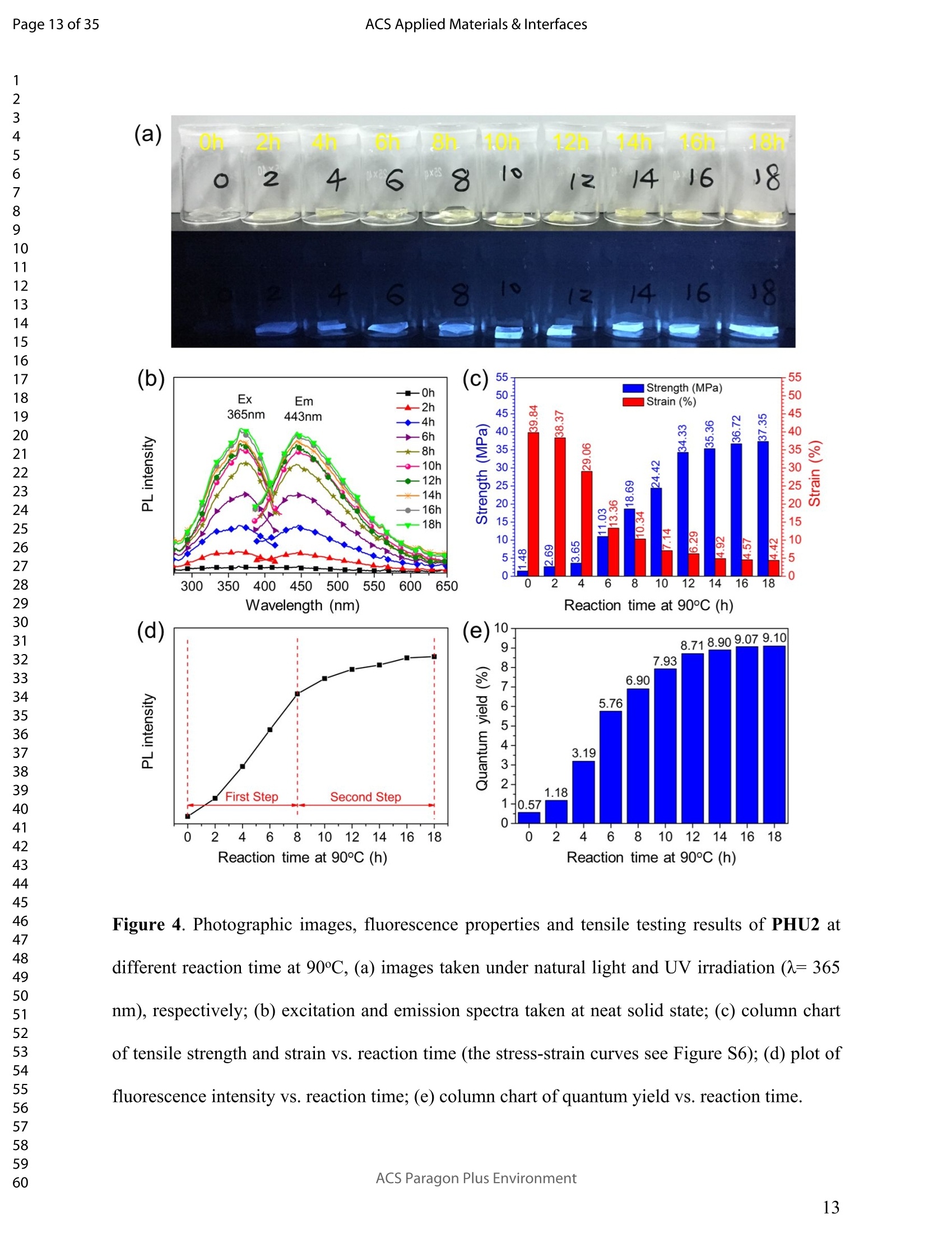
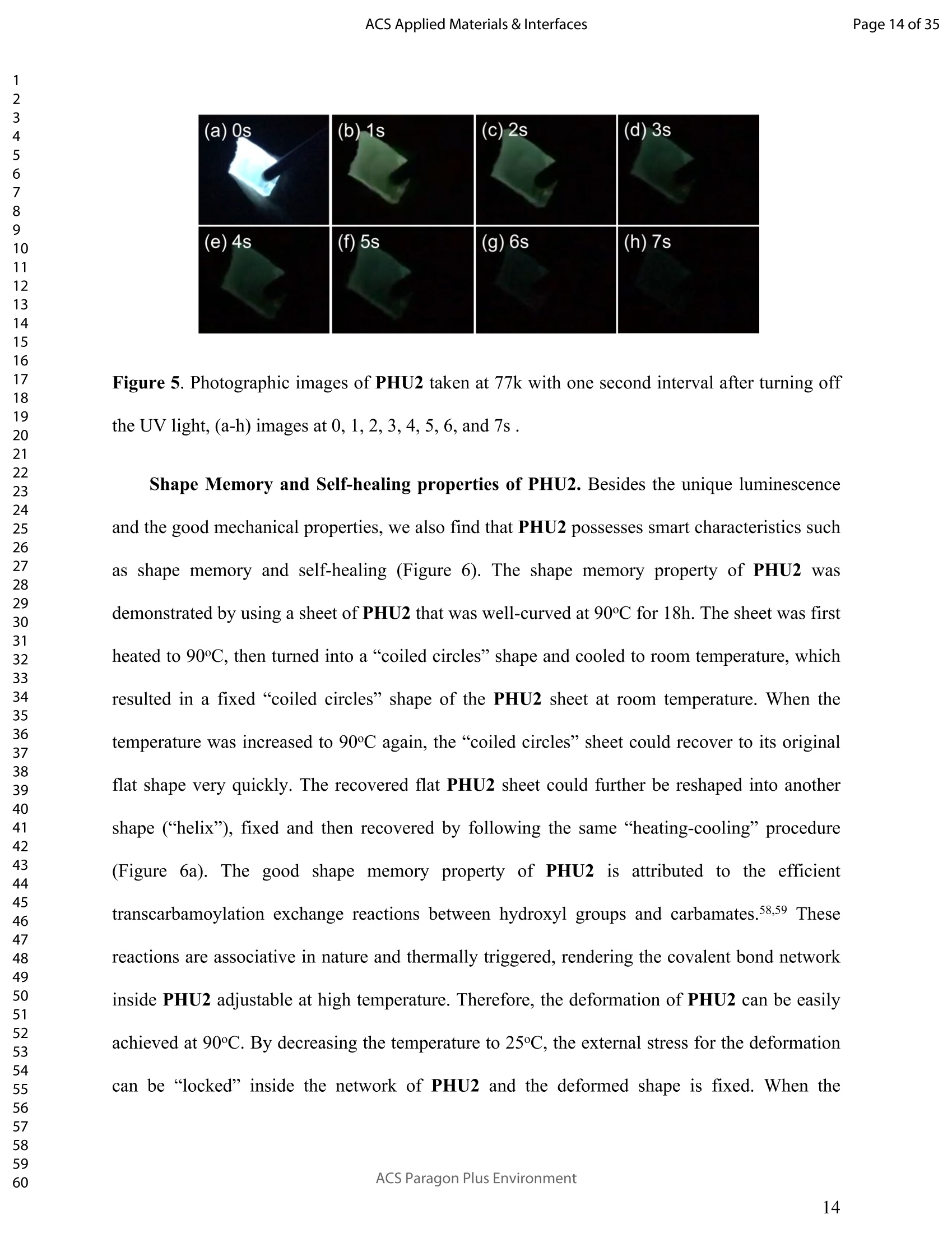
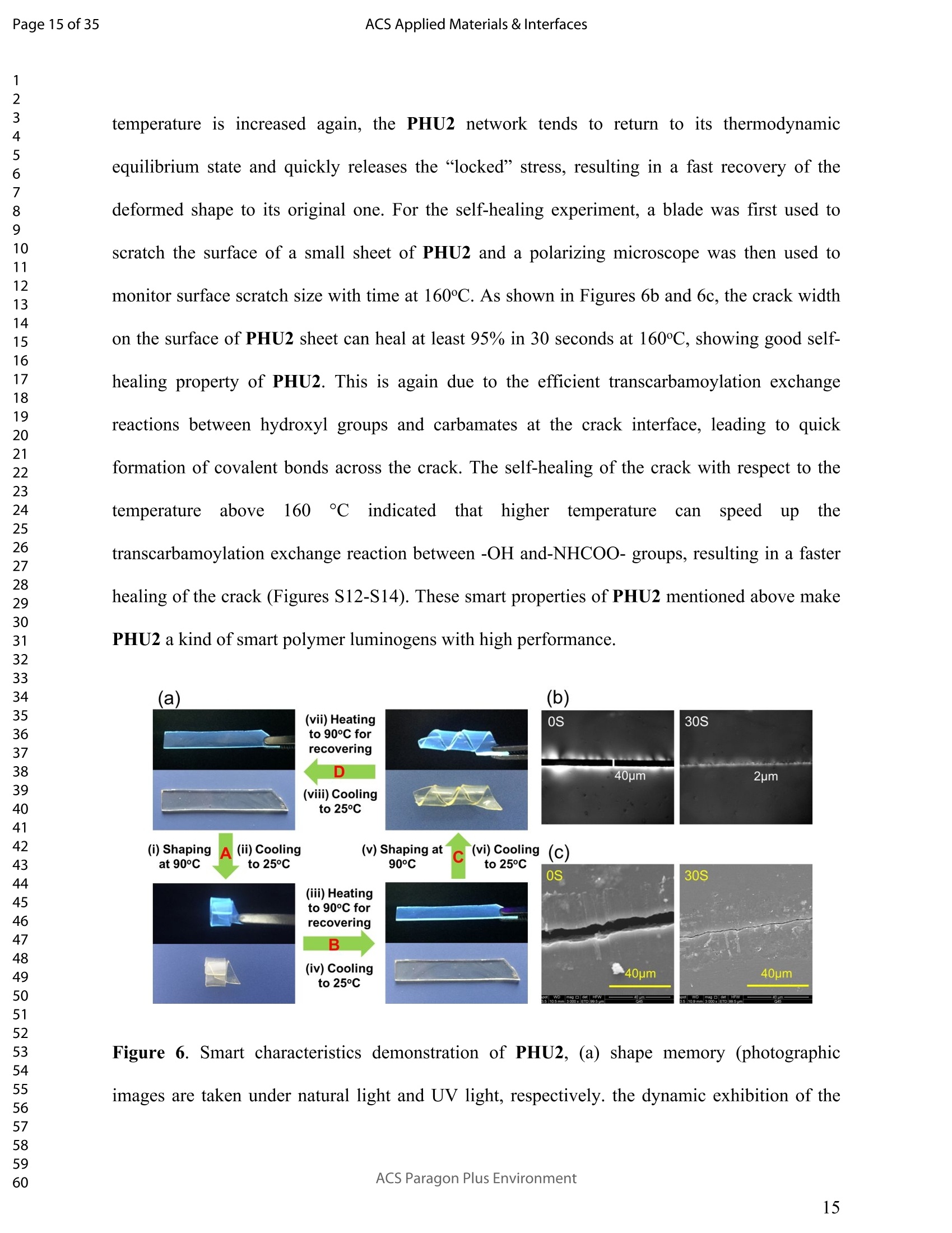
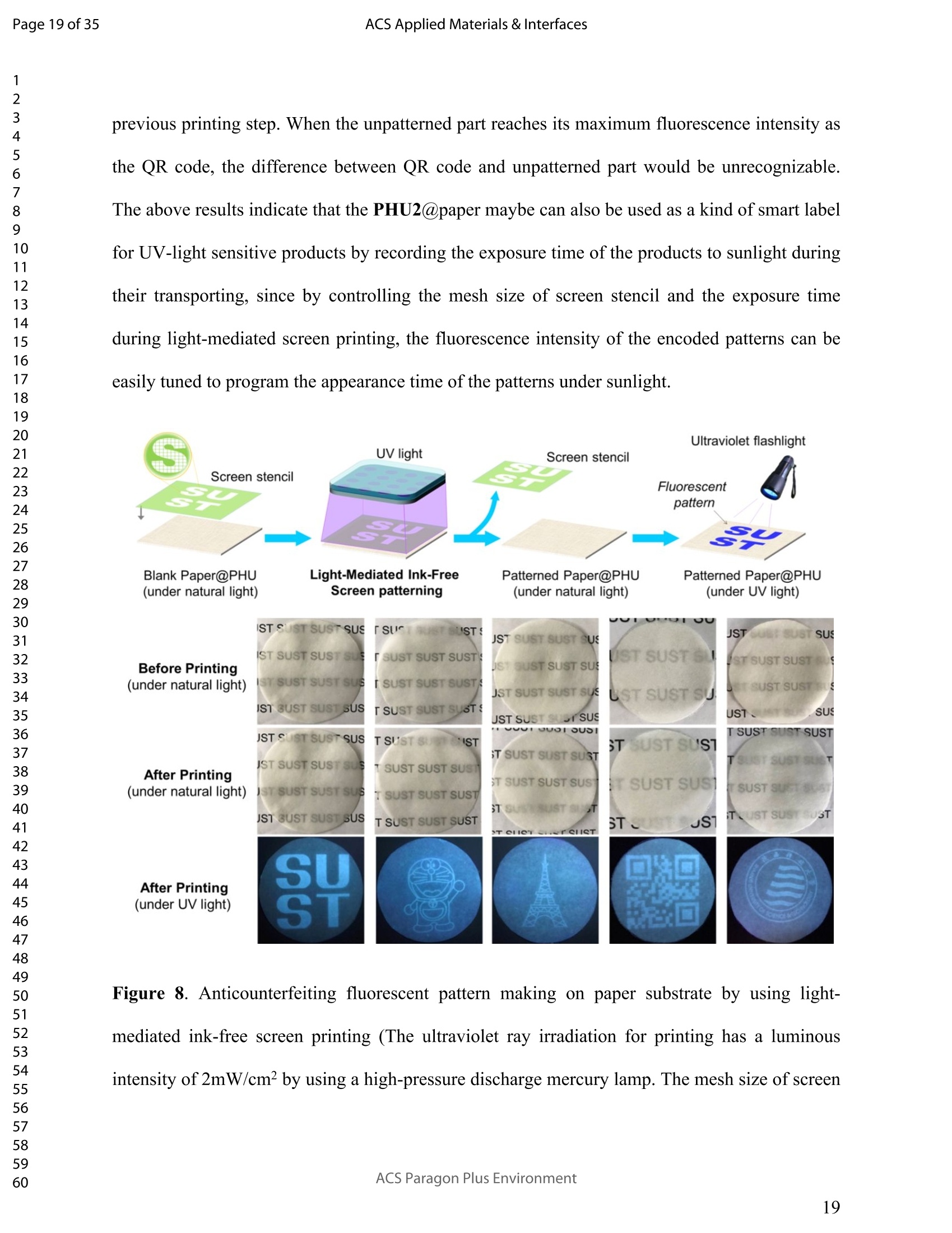
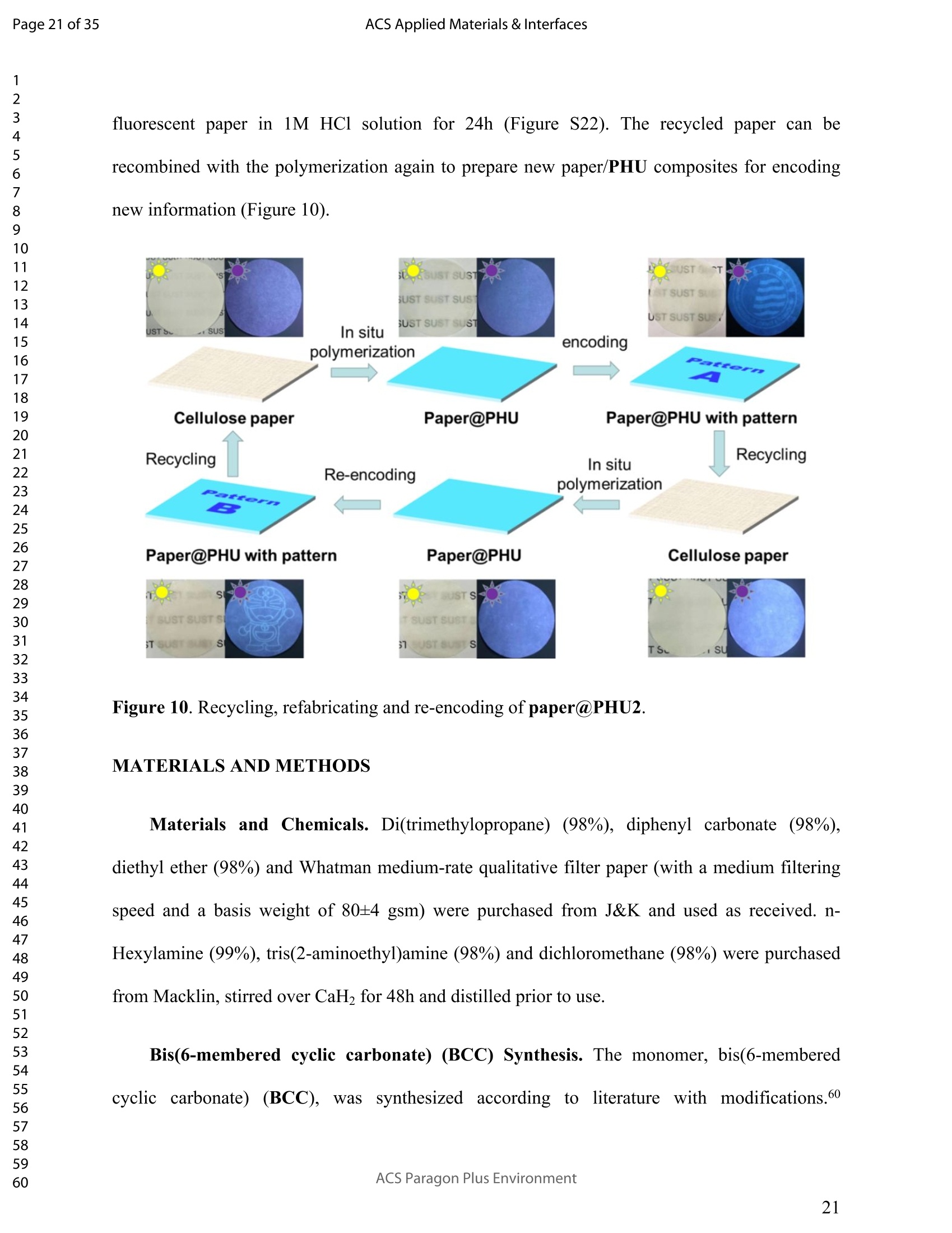
ACSAPPLIEDMATERIALSCINTERFACES ACS Applied Materials & InterfacesPage 1 of 35 Subscriber access provided by Murdoch University Library Applications of Polymer, Composite, and Coating Materials A New Kind of Nonconventional Luminogens Basedon Aliphatic Polyhydroxyurethane and its PotentialApplication in Ink-Free Anticounterfeiting Printing Zihao Feng, Wei Zhao, Zhenhua Liang, Yanfeng Lv, Fukang Xiang, Deqiang Sun, Chuanyin Xiong, Chao Duan, Lei Dai, and Yonghao Ni ACS Appl. Mater. Interfaces,Just Accepted Manuscript·DOI:10.1021/acsami.9b22475·Publication Date (Web): 18 Feb 2020 Downloaded from pubs.acs.org on February 18, 2020 Just Accepted "Just Accepted”manuscripts have been peer-reviewed and accepted for publication. They are postedonline prior to technical editing, formatting for publication and author proofing. The American ChemicalSociety provides “Just Accepted" as a service to the research community to expedite the disseminationof scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear infull in PDF format accompanied by an HTML abstract. “Just Accepted”manuscripts have been fullypeer reviewed, but should not be considered the official version of record. They are citable by theDigital Object Identifier (DOIQ). “Just Accepted"is an optional service offered to authors. Therefore,the “Just Accepted" Web site may not include all articles that will be published in the journal. Aftera manuscript is technically edited and formatted, it will be removed from the “Just Accepted" Website and published as an ASAP article. Note that technical editing may introduce minor changesto the manuscript text and/or graphics which could affect content, and all legal disclaimers andethical guidelines that apply to the journal pertain. ACS cannot be held responsible for errors orconsequences arising from the use of information contained in these “Just Accepted”manuscripts. ( i s published by the American Chemical S o ciety. 1155 Sixteenth S t reet N.W.,Washington, DC 20036 ) Published by American Chemical Society. Copyright C American Chemical Society. ( However, n o copyright claim is made to original U.S. Government works, or worksproduced by employees o f any Commonwealth rea l m Crown government in the cou r se of their duties. ) A New Kind of Nonconventional Luminogens Based on Aliphatic Polyhydroxyurethane and its Potential Application in Ink-Free AnticounterfeitingPrinting Zihao Feng,t,#.s.Wei Zhao,*,t,#,s,LZhenhua Liang,t Yanfeng Lv,t Fukang, Xiang,Deqiang Sun,tChuanyin Xiong, Chao Duan, Lei Dai and Yonghao Ni* t College of Bioresources Chemical and Materials Engineering, Shaanxi University of Scienceand Technology, Xi’an 710021, People’s Republic of China. #Key Laboratory of Paper based Functional Materials, China National Light Industry, Xi’an710021,People’s Republic of China. Shaanxi Provincial Key Laboratory of Papermaking Technology and Specialty PaperDevelopment, Xi'an 710021, People’s Republic of China. -National Demonstration Centre for Experimental Light Chemistry Engineering Education,Shaanxi University of Science and Technology, Xi’an 710021, People’s Republic of China.Department of Chemical Engineering, University of New Brunswick, Fredericton E3B 5A3,New Brunswick, Canada KEYWORDS Polyhydroxyurethane, Luminescence, Ink-Free, Anticounterfeiting, Printing ABSTRACT Organic luminogens have extensive applications due to their unique photophysicalproperties. In recent years, non-conjugated organic luminogens, in contrast to traditionalconjugated luminogens, have gained much attention due to their facile preparation, environment-friendliness and biocompatibility. In this study, a new kind of nonconventional luminogens basedon dynamic covalent cross-linked polyhydroxyurethane is reported for the first time. The newluminogens not only exhibit intrinsic strong fluorescent emissions at solid states, but also possesshigh mechanical properties alongwith good shape memory and self-healing properties.Especially, the new luminogens are synthesized from aliphatic polyfunctional cyclic carbonateand amines via a much more straightforward way, avoiding the use of toxic isocyanates.Investigations indicated that the intrinsic luminescence of the resultant luminogens was inducedby the crosslinking of polymer chains and could be well tuned by controlling the degree ofcrosslinking. By taking advantage of the unique characteristics of the resultant polymerluminogens, we further developed a facile method, named “light-mediated ink-free screenprinting”, for anticounterfeiting paper fabrication. Different from traditional ink-based printingtechnology, the new method used UV-light instead of expensive security ink to enceanticounterfeiting information on natural cellulose paper. The anticounterfeiting information isstable under various wet conditions, showing promising applications in the fast-growingcounterfeiting of pharmaceuticals, packaging and food industry. INTRODUCTION Organic luminogens have attracted increasing attention owing to their unique photophysicalproperties and extensive applications in organic light-emitting diodes,l2 organic lasers,3.4 bio/chemosensors,5-8 optical waveguide, and so on. The conventional organic luminogensnormally contain fused aromatic rings in their molecular structures, forming remarkable n-electronic conjugation systems and ensuring strong emissions.9-11 Many of the conventionalorganic luminogenss are eminently irradiative in dilute solutions, but demonstateweakluminescence or are totally nonluminescent in their concentrated solutions or solid states,resulting in concentration quenching and aggregation-caused quenching (ACQ) problems.12,13This is a shortcoming of the conventional organic luminogens and limits their use as luminescentmaterials for solid-state devices. In 2001, Tang and co-workers found that hexaphenylsilole bearing conjugated electronicstructure was nonluminescent in solution state, but exhibited strong emission in aggregation orsolid state. This finding is in contrast to the aggregation-caused quenching effect observed forthe conventional luminogens.The term of “aggregation-induced emission(AIE)”wassubsequently coined for this unique phenomenon, demonstrating that the aggregation in somecases can also play a constructive role, rather than destructive role, for the emissions ofluminescent materials. 14,15 Since then, this pioneer work has inspired the exploration of manyorganic liminogens with AIE effect.16-21 IMost AIE luminogens reported so far are smallmolecules with conjugated electronic structure. For practical applications, expensive processingtechniques such as vacuum sublimation and vapor deposition are employed to fabricate thesematerials into thin solid films, which limit these material applications for large-area flat-paneldevices. Fortunately, this disadvantage can be alleviated by synthesizing AIE polymers. The AIEpolymers can be easily fabricated into large-area thin solid films and devices by using facileprocessing techniques such as spin-coating, static casting, and ink-jet printing.22More recently,the intrinsic luminescence was observed for a variety nonconventional luminogens without any type of proverbial chromophores,23-27 and (hetero)cyclic aromatic segments.27 Among theseemerging luminogens, polymers based nonconventional luminogens have attracted great interestsdue to their chain flexibility, structure tenability, facile preparation, environment-friendliness andbiocompatibility, which show significant fundamental importance and promising technicalapplications.28-37 Most of these nonconventional polymer luminogens explored so far, possesslinear or hyperbranched structures, which shows limitations for the fabrications of solid-statedevices that desire forhigh mechanical properties.38,39Polyurethanes (PUs) are a class ofimportant polymers with a variety of applications such as adhesives, coatings, foams, andsealants. Conventional PUs synthesis involves the use of highly toxic isocyanates, which areharmful for human health.40 Compared to conventional PUs, polyhydroxyurethanes (PHUs)prepared via the reaction of cyclic carbonates with primary amines have attracted considerableinterest in recent years due to their isocyanate-free synthetic routes, enhanced properties (vs. thatof PU) and promising applications.41-47 Herein, we report a new kind of nonconventionalluminogens based on aliphatic polyhydroxyurethane for the first time. The new luminogens areprepared from polyfunctional aliphatic cyclic carbonate and amines via a simple method,avoiding the use of toxic isocyanates. In contrast to the nonconventional polymer luminogensexplored so far, the new luminogens possess dynamic covalent cross-linked networks anddemonstrate high mechanical properties along with smart properties such as shape memory andself-healing. The intrinsic luminescence of the new luminogens was induced by the crosslinkingof polymer chains and can be easily tuned by the crosslink density of polymers, providing afacile process concept to fine-tune the luminescence strength. By taking advantage of this uniquecharacteristic, we further developed a new screen printing method, named "light-mediated ink-free screen printing”, relying on UV-light, instead of expensive security ink, to encode anticounterfeiting information on the substrate. The encoded anticounterfeiting information isprogrammable and stable under various conditions, which shows promising applications inmodern anticounterfeiting field. RESULTS AND DISCUSSION Polyhydroxyurethane Synthesisand Fluorescence Investigation. The synthesisSoofpolyhydroxyurethane is based on the chemistry between bis(6-membered cyclic carbonate) andamines and a catalyst-free transcarbamoylation exchange reactions at high temperature betweenhydroxyl groups and carbamates (Scheme 1). The synthetic procedure is much straightforwardand economical. The two reactants, bis(6-membered cyclic carbonate) and amine were firstdissolved in dichloromethane separately and then mixed together at room temperature for thenucleophilic ring-opening ofcyclic carbonate by amine. After 12 hours, the solvent was removedby heating the reaction mixture to a high temperature above 90℃ to alloww aa furthertranscarbamoylation exchange reactions at this temperature for a period of time. We first carriedout the reaction between bis(6-membered cyclic carbonate) (BCC) and n-hexylamine (a) byfollowing the above procedure and surprisingly found that the resultant polyhydroxyurethane(PHU1) exhibited bright blue fluorescence under UV irradiation (A=365 nm) (Figure la). Thefluorescence spectrum of PHU1 showed the maximum excitation and emission wavelengths ataround 355 nm and 438 nm, respectively (Figure 1b). Because PHU1, prepared from aliphaticpolyfunctional cyclic carbonate and amine, has no bulky aromatic or conjugated segments (suchas n-aromatic benzene rings) in its molecular structure, we speculate that the newly generatedcarbamates (-NHCOO-) may play a crucial role for the strong luminescence of the resultantmaterials. In an early study, Niu et al. reported unanticipated fluorescence produced from 1 unconjugatedcarbon-carbondouble bonds and hydroxylgroupsoff hhyperbranchedpolysiloxanes.48 Scheme 1. Synthesis of Nonconventional Luminogens Based on Aliphatic Polyhydroxyurethane. 1 Figure 1. (a) Photographic images of PHU1 upon alternating UV (A=365 nm) and natural lightirradiation .(b) Excitation and emission spectra of PHU1. By using in situ FT-IR technique, we monitored the reaction of bis(6-membered cycliccarbonate) (BCC) and n-hexylamine (a) and found that the reaction between cyclic carbonateand amine group proceeded with high activity at room temperature (Figure 2). The C=0 stretchsignalsassociated with BCC at 1748 cm decreased with the reaction time and almos)stdisappeared in 12h. At the same time, two peaks at 1705 cm- and 1519cm, corresponding tothe hydrogen-bonded carbamate C=O :stretchandthe N-H deformation of carbamate.respectively, increased gradually and reachedto their maximutm values. However, thefluorescence testing shows that the product obtained at room temperature is not visuallyluminescent at all and a strong fluorescence emission of the resultant PHUP1HU1 can only beobserved after a further reaction at 105 ℃ for 12h (Figure S1). This unusual observationindicatesthatt theiettranscarbamoylation exchange reaction between hydroxyl groupsandcarbamates at high temperature may be crucial to induce the strong luminescence of the finalproduct. 1 Figure 2. In situ FT-IR monitoring of the reaction between bis(6-membered cyclic carbonate)(BCC) and n-hexylamine (a) (conditions: [BCC]/[a]=1/2, [BCC]=0.82M, 25C, 12h,CH2Cl2 assolvent.). We then studied the fluorescence intensity change of PHU1 with reaction time at hightemperature. Figures 3a and 3b are the photographic images of PHU1 sampled at differentreaction times at 105°C under natural light and UV irradiation (1= 365 nm), respectively. Allsamples are not luminescent under natural light, but exhibit a gradual increase of fluorescenceover reaction time under UV irradiation. The fluorescencespectra demonstrate that themaximum excitation and emission wavelengths of all samples are centered at around 355 nm and438 nm, respectively. The fluorescence intensity increases gradually and no emission quenchingexists by extending the reaction time from Oh to 12h at 105℃ (Figure 3c). The FT-IR spectra ofsamples at different reaction times are similar, and have no appearance of new absorption peaks(Figure S2). In addition, H NMR spectrum of PHU1 after further reaction at 105°℃ for 12hshow no difference compared to of PHU1 obtained at room temperature (Figure S3-S4).According to the literature, some multiple bonds groups such as amide, imide, anhydride andester units normally are nonemissive as individuals, but become emissive when they are incorporated in the main chain or side chains of the non-conjugated polymer.49-51 Thought themechanism is still unclear, more experimental results suggested that the aggregation of thosegroups played a crucial role to induce their strong emissions. This is because the aggregation ofthese non-emissive groups can cause restricted vibro-rotational motions with reduced non-radiative relaxations and induce unanticipated fluorescence emission.38 Therefore, we attributethe fluorescence intensity increase of PHU1 over reaction time at 105℃ to the increase of theconstraint degree of carbamate linkages in the system. As the reaction proceeds at 105℃, moretranscarbamoylation reactions occur, leading to the formation of more molecules with more thantwo carbamates and the aggregation of carbamates (Scheme S1). This can cause more motionrestrictions of the carbamate groups, consequently increase the fluorescence intensity of PHU1. Figure 3. Photographic images, fluorescence spectra and UV absorption spectra of PHU1 afterspecific reaction time at 105°C (Oh, 2h, 4h, 6h, 8h, 10h and 12h), (a) images taken under natural light; (b) images taken under UV irradiation ()= 365 nm); (c) excitation and emission spectrataken at neat liquid state ;(d) UV absorption spectra in the solution of ethanol. Solid Polyhydroxyurethane Preparation and Fluorescence Properties Investigation.Since the primary amine, n-hexylamine (a) is monofunctional, the resultant polyhydroxyurethane(PHU1) is just a white viscous liquid even if the reaction at 105C would be extended to twodays, which limits its use for fabrication of solid luminescent devices. In order to preparepolyhydroxyurethane material with sufficient mechanical properties while maintaining theunique luminoscencent characteristics, we used a polyfunctional amine, tris(2-aminoethyl)amine(b), instead of n-hexylamine (a), to react with bis(6-membered cyclic carbonate) (BCC) andprepared polyhydroxyurethane (PHU2). Because both tris(2-aminoethyl)amine (b) and bis(6-membered cyclic carbonate) (BCC) are polyfunctional, and their polymerizations are based onthe chemical reactions between the cyclic carbonate and primary amine group (Scheme 1), acovalently cross-linked polyhydroxyurethane, PHU2, was successfully prepared, and isolated aswhite transparent solid that is conformed to the mold used (Figure S5). Similar to PHU1, PHU2 obtained at room temperature is not luminescent and thefluorescence emission of PHU2 can only be observed after a further reaction at 90 C. Figures 4ashows the photographic images of polyhydroxyurethane (PHU2) under natural light and UVirradiation (X= 365 nm), respectively. We can clearly found that all polyhydroxyurethane (PHU2)sampled at different times at 90 °C were not luminescent under natural light but exhibit emissionunder UV radiation. The fluorescence intensity increased as the curing time was prolonged. Tensamples with different curing times (Oh, 2h, 4h, 6h, 8h, 10h, 12h, 14h, 16h and 18h) wereselected for detailed investigation. It was found that the florescence intensity gradually increasedand no emission quenching existed when the curing time was increased from Oh to 18h (Figure 4b). A further tensile testing shows that PHU2 has a gradual increase of tensile strength over thecuring time. When the curing time is 18h, the tensile strength of PHU2 is up to 37.35 MPa withstrain-at-break of 4.42% (Figures 4c and S6). This is attributed to that at 90 °C the free hydroxylgroups can nucleophilically attack the carbamates in PHU2,resulting in covalently crosslinkednetworks between different polymer chains. Different from linear siloxane/poly(hydroxyurethane)copolymers synthesized from five-membered cyclic carbonate functionalized reactants,35 thecrosslinked network structure of PHU2 not only leads to highly restricted motions of carbamategroups, inducing the unique luminescence of PHU2, but also offers PHU2 with excellentmechanical properties. We compared the fluorescent intensities of PHU1 and PHU2 with same curing time (12h)under identical conditions and the quantum yields of PHU1 and PHU2 at different reaction times.PHU2 have lower fluorescence intensity and quantum yields than that of PHU1. (Figures S7-S8)This is because several carbamate groups (-NHCOO-) were attached at one small molecule inPHU1 and result in higher restraint of cabamate groups than that in PHU2. Higher restraint ofcabamate groups leads to higher fluorescence activity of PHU1. The excitation wavelength-dependent fluorescence experiment indicated that both PHU1 and PHU2 showed excitation-dependent luminescence properties, where excitation at longer wavelength resulted in a red-shifted emission (Figure S9). We also found that the excitation spectra of PHU1 and PHU2 allappeared red-shifted in comparison1 with their absorption spectra (Figure S10, PHU1:Aabs=273nm and Aex=355nm; PHU2: Aabs=315nm and Aex=365nm). These results indicate theunusual fluorescence of PHUs is attributed to the clustering of carbamtes, and subsequentelectron cloud overlap (delocalization) together with simultaneous conformation restraining,namelyclustering-triggered emission (CTE)i mechanism accordingtothe literature.339 Furthermore, the repeated measures of the luminescence spectra of PHU1 and PHU2 at specifictime intervals show that of PHU1 and PHU2 all have good repeatability and reproducibility ofthe luminescence properties (Figure S11). According to the change of fluorescence intensity over curing time (Figures 4d), the curingprocess of PHU2 can be divided into two stages. A rapid increase of fluorescence intensity up to8h, is followed by a slow increase to 18h, indicating a fast formation of crosslinking networks ofPHU2 at early stage of curing at 90C. The quantum yields of PHU2 had the similar trend to thefluorescence intensity (Figure 4e). The fluorescence quantum yield of PHU2 has a sharp increasefrom 0.57% to 6.90% in 8 hours, followed by a very slow increase from 6.90% to 9.10% in thenext 10 hours, which is consistent with the tensile testing results of PHU2. Therefore, bycontrolling the curing time of PHU2, we can easily obtain a series of materials with programedfluorescence intensity/quantum yield that can be used for the fabrication of some specificphotoluminescent devices or for anticounterfeiting applications. Cryogenic Long-Persistent Phosphorescence of PHU2. The photophysical properties ofPHU2 were also studied at 77K. We were surprised to find that when the UV light was turnedoff, a long-persistent luminescence lasting for 7 s with changed emission color was clearlyobserved by the naked eyes (Figure 5 and Video S1), which is rarely observed for anonconjugated polymer luminogens.52-56 This is attributed that the rates of internal conversion(IC), intersystem crossing (ISC), and collisional quenching are commonly restricted at lowtemperature due to the rigid state of the frozen sample, which results in phosphorescence withmicrosecond decay times. 1 Figure 4. Photographic images, fluorescence properties and tensile testing results of PHU2 atdifferent reaction time at 90℃, (a) images taken under natural light and UV irradiation (A=365nm), respectively; (b) excitation and emission spectra taken at neat solid state; (c) column chartof tensile strength and strain vs. reaction time (the stress-strain curves see Figure S6); (d) plot offluorescence intensity vs. reaction time; (e) column chart of quantum yield vs. reaction time. Figure 5. Photographic images of PHU2 taken at 77k with one second interval after turning offthe UV light, (a-h) images at 0, 1,2,3,4,5,6, and 7s . Shape Memory and Self-healing properties of PHU2. Besides the unique luminescenceand the good mechanical properties, we also find that PHU2 possesses smart characteristics suchas shape memory and self-healing (Figure 6). The shape memory property of PHU22 wasdemonstrated by using a sheet of PHU2 that was well-curved at 90°℃ for 18h. The sheet was firstheated to 90°℃, then turned into a“coiled circles" shape and cooled to room temperature, whichresulted in a fixed “coiled circles” shape of the PHU2 sheet at room temperature. When thetemperature was increased to 90°℃ again, the “coiled circles” sheet could recover to its originalflat shape very quickly. The recovered flat PHU2 sheet could further be reshaped into anothershape (“helix"), fixed and then recovered by following the same “heating-cooling”procedure(Figure 6a). The good shape memory property of PHU2isattributed to the efficienttranscarbamoylation exchange reactions between hydroxyl groups and carbamates.58,59 Thesereactions are associative in nature and thermally triggered, rendering the covalent bond networkinside PHU2 adjustable at high temperature. Therefore, the deformation of PHU2 can be easilyachieved at 90℃. By decreasing the temperature to 25℃, the external stress for the deformationcan be “locked" inside the network of PHU2 and the deformed shape is fixed. When the temperature is increased again, the PHU2 network tends to return to its thermodynamicequilibrium state and quickly releases the “locked” stress, resulting in a fast recovery of thedeformed shape to its original one. For the self-healing experiment, a blade was first used toscratch the surface of a small sheet of PHU2 and a polarizing microscope was then used tomonitor surface scratch size with time at 160°C. As shown in Figures 6b and 6c, the crack widthon the surface of PHU2 sheet can heal at least 95% in 30 seconds at 160°C, showing good self-healing property of PHU2. This is again due to the efficient transcarbamoylation exchangereactions between hydroxyl groups and carbamates at the crack interface, leading to quickformation of covalent bonds across the crack. The self-healing of the crack with respect to thetemperatureabove 160 'CC iindicated that higher temperature can speedup thetranscarbamoylation exchange reaction between -OH and-NHCOO- groups, resulting in a fasterhealing of the crack (Figures S12-S14). These smart properties of PHU2 mentioned above makePHU2 a kind of smart polymer luminogens with high performance. Figure 6. Smart characteristics demonstration of PHU2, (a) shape memory (photographicimages are taken under natural light and UV light, respectively. the dynamic exhibition of the recovery of a small disc sample of PHU2 see Videos S2 and S3);(b) scratch self-healing at160°C monitored by polarizing microscope; (c) scratch self-healing at 160C monitored by SEM. Applications. Inspired by the unique photoluminescence of polyhydroxyurethane,wesynthesized PHU2 within the natural cellulose fiber networks and successfully prepared a newclass of transparent polyhydroxyurethane@paper composite material, This was achieved bycarrying out in situ polymerization of bis(6-membered cyclic carbonate) (BCC) and tris(2-aminoethyl)amine (b) in the natural cellulose paper. Since the natural cellulose paper is a kind ofporous materials formed by the random physical interweave of natural cellulose fibers, the twosmall molecular reactants can easily penetrate into the pores of cellulose paper, polymerize andform polyhydroxyurethane directly inside these pores of the paper (Figure S15). By takingadvantage the crosslink-induced luminescence of PHU2 fabricated inside the paper, we facilelymade various fluorescent patterned papers by using a hot stamping method (Figure 7). Thefluorescence intensity can be easily tuned by controlling the stamping time (Figure S16). Asshown in Figure 7, there is no difference for the paper observed at natural light before and afterthe stamping, while the encoded fluorescent pattern“SUST”can be easily observed by using anultraviolet flashlight. Noteworthy, the fluorescent pattern is erasable. By heating the patternedpaper at 100°C for one hour, the fluorescence“SUST” can be thoroughly erased (Figure S17).This feature makes the PHU@paper to be a potential smart package material for temperature-sensitive products. If the temperature of the packaged goods is higher than 100°℃ for a specifictime during its transportation, the fluorescent pattern encoded on the paper will vanish. Bycontrolling the time of hot stamping, we can easily tune fluorescence intensity of the marker andto program the time of pattern disappearance. 1 Paper 31 before patterning Hot Stamping Insulated handle ' Die head Ultraviolet flashlight 21 20 Paper after patterning Figure 7. Anticounterfeiting fluorescent patterns making on paper substrate by using hotstamping (the temperature of the die head is 150℃ and the hot stamping time is 5 seconds), (a)images before stamping; (b) images after stamping (under natural light); (c) images afterstamping (under UV light). Since the PHU2 exhibits an obvious absorption peak at 315nm wavelength in the UVspectrum (Figure S10), we tried to use UV light to induce the crosslink of PHU2 inside the paperto encode fluorescence pattern on paper substrate. As shown in Figure 8, the PHU2@paperprepared at room temperature is first overlapped with a screen stencil used in traditional screenprinting, and then applied to high intensity UV radiation for a period of time. Subsequently, thescreen stencil is removed and a confidential pattern is successfully encoded on the papersubstrate. Under natural light, there was no change of paper after the printing, while a brightfluorescent pattern “SUST”can be observed under UV light. By using this method, more complex patters can also be easily encoded in the paper. The resultant paper can be directly usedfor document printing via commercial laser printer to provide anti-counterfeiting protection ofthe document (Figure S18 and Video S4). Therefore, we successfully developed a facile method,named “light-mediated ink-free screen printing”, to prepare anticounterfeiting materials by usingnatural cellulose paper as substrate. Different from traditional screen printing technology, thenew method used UV-light instead of expensive security ink to encode anticounterfeitingpatterns on the paper substrates. We also determined the stability of fluorescent patterns by submerging the encoded paper invarious wet conditions such as water, oil and harmful organic solvents for 24h (Figure 9). Theresults show that the fluorescence patterns on paper are very stable and no difference is observed,whichh shows promising applications of the resultant PHU2@paper in the fast-growingcounterfeiting of pharmaceuticals, packaging and food industry. The effect of temperature on thefluorescent pattern was studied (Figure S19). The fluorescent pattern on the paper substrate isvery stable and can last at least one month at room temperature. When the environmenttemperature is above 25°C but below 90℃, no obvious changes of the fluorescent patterns wereobserved in at least one day. The stability of the fluorescence patterns to ultraviolet radiation wasalso evaluated. A two-dimensional code (QR Code) was encoded on the PHU2@paper by usingthe“light-mediated ink-free screen printing”method. The fluorescence QR code was monitoredunder high intensity ultraviolet radiation and photos were taken at specific UV radiation time.We can easily found that the fluorescence QR code gradually became obscure over time untilunrecognizable by the QR code recognizer. This is attributed to that the unpatterned part has asharp increase of the fluorescence intensity under the exposure of high intensity UV light, whilethe fluorescence intensity of the patterned QR code already reached to its maximum value in previous printing step. When the unpatterned part reaches its maximum fluorescence intensity asthe QR code, the difference between QR code and unpatterned part would be unrecognizable.The above results indicate that the PHU2@paper maybe can also be used as a kind of smart labelfor UV-light sensitive products by recording the exposure time of the products to sunlight duringtheir transporting, since by controlling the mesh size of screen stencil and the exposure timeduring light-mediated screen printing, the fluorescence intensity of the encoded patterns can beeasily tuned to program the appearance time of the patterns under sunlight. Figure 8. Anticounterfeiting fluorescent pattern making on paper substrate by using light-mediated ink-free screen printing (The ultraviolet ray irradiation for printing has a luminousintensity of 2mW/cm² by using a high-pressure discharge mercury lamp. The mesh size of screen stencil used for printing is 200, which means the number of holes in one square centimeter of thescreen stencil is 200.). (a) In wet conditions (b) Exposure to high intensity ultraviolet Figure 9. Stability evaluations of fluorescent patterns on paper substrate (the fluorescent patternswas made via light-mediated ink-free screen printing method),(a) in various wet conditions;(b)exposure to high intensity ultraviolet (the ultraviolet ray irradiation has a luminous intensity of2mW/cm). Recycleanddrreuseeoof paper@PHU2 composite. Noteworthy,when the encodedinformation on paper@PHU2 was erased by heating, the resultant material can be reused as anew kind of fluorescent material that not only possess high mechanical properties but also smartproperties as bulk PHU2 such as shape memory and self-healing (Figure S20 and Video S5). Thetensile testing showed that the resultant fluorescent paper has a tensile strength up to 44.3 MPathat is much higher than blank paper (4.3MPa) (Figure S21). Furthermore, the blank paper can beeasily recycled and the PHU2 inside the paper can be degraded by submerging the resultant 1 fluorescent paper in 1M HCl solution for 24h (Figure S22). The recycled paper can berecombined with the polymerization again to prepare new paper/PHU composites for encodingnew information (Figure 10). Figure 10. Recycling, refabricating and re-encoding of paper@PHU2. MATERIALS AND METHODS Materialsand Chemicals. Di(trimethylopropane) (98%), diphenyl carbonate(98%),diethyl ether (98%) and Whatman medium-rate qualitative filter paper (with a medium filteringspeed and a basis weight of 80±4 gsm) were purchased from J&K and used as received. n-Hexylamine (99%), tris(2-aminoethyl)amine (98%) and dichloromethane (98%) were purchasedfrom Macklin, stirred over CaH2 for 48h and distilled prior to use. Bis(6-membered cyclic carbonate) (BCC) Synthesis. The monomer, bis(6-memberedcyclic carrbboonnaatte)e) (BCC),wassynthesize1d according to literature vwithh mnodifications.60 Di(trimethylopropane) (10 g, 39.9 mmol) and diphenyl carbonate (85.7 g, 400mmol) were mixedtogether and then reacted at 140C. After 48 h, the reaction mixture was cooled to ambienttemperature and washed with diethyl ether several times to remove excess diphenyl carbonate.The resultant crude white solid was further purified by crystallization in tetrahydrofuran (THF)to yield bis(6-membered cyclic carbonate)(BCC) as white crystals (6 g, 60%). Synthesis of PHU1. In a beaker, bis(6-membered cyclic carbonate) (BCC) (4.00 g, 0.0132mol) was dissolved in 12 ml of anhydrous CH2Cl2 and then n-hexylamine (a) (2.68 g, 0.0264 mol)in 4ml anhydrous CHCl2 was added. After 12 h of reaction at room temperature for thenucleophilic ring-opening of BCC by a, the beaker was then heated to 105 ℃ gradually toremove most of the solvent (CH2Cl2) in the reaction system. Subsequently, the reaction mixturewas placed in an oven to allow further reaction at 105°℃ for 12 h. Samples for fluorescence studywere taken every one hour from the system without further purification and stored in quartzweighing bottles. Synthesis of PHU2. In a beaker, bis(6-membered cyclic carbonate) (BCC) (4.00 g, 0.0132mol) was dissolved in 12 ml of anhydrous CH2Cl2, then tris(2-aminoethyl)amine (b) (0.64g,0.0044mol) in 4ml anhydrous CH2Cl2 was added. After 12 h of reaction at room temperature forthe nucleophilic ring-opening of BCC by b, the beaker was gradually heated to 90 ℃ to removemost of the solvent (CH2Cl2) in the reaction system. The resultant sample was then taken out ofthe beaker and placed in an oven to allow further curing reaction at 90°℃ for 12 h, resulting in aquantitative yield of PHU2. The transcarbamoylation exchange reaction can happen at bothtemperatures (90°℃ and 105℃), but a high curing temperature of 105°℃ for PHU2 could result inan unwanted yellow color for the final product. In order to prevent the color from interferingwith the fluorescence test of PHU2, a lower temperature of 90℃ was used for the curing reaction and the PHU2 was obtained in colorless state. As for the fluorescence study of PHU2,samples were taken every one hour from the reaction system during curing without furtherpurification and stored in quartz weighing bottles. Preparation of PHU2@Paper Composite. In a beaker, bis(6-membered cyclic carbonate(BCC)(0.50 g, 1.65mmol) and tris(2-aminoethyl)amine (b) (0.08g, 0.55mmol) were dissolved in1.5)ml and 0.5ml anhydrous CH2Cl2, respectively and quickly mixed together at11roomtemperature. A piece of medium-rate qualitative filter paper (around 6cm in diameter and a basisweight of 80±4 gsm) was then put into the beaker. After ultrasonic concussion for ten minutes,the beaker was sealed by using parafilim and left at room temperature for 12h. The beaker wassubsequently gradually heated to 90 °C to remove the solvent (CH2Cl2) in the reaction system,resulting in PHU2@paper composite for hot-stamping or light-mediated printing experiments. Fluorescent Pattern Making by Light-Mediated Ink-Free Screen Printing. The UVlight mediated printing was carried out by using a box type UV reactor (XH-300UL+) equippedwith a high-pressure discharge mercury lamp (250W). The PHU2@paper prepared at roomtemperature was first overlapped with a nylon screen stencil (200 mesh) and then put into thecuring chamber of the instrument. A luminous intensity of 2mW/cm² of ultraviolet ray irradiationform the high-pressure discharge mercury lamp was applied on the surface of the nylon screenstencil by tuning the distance between the mercury lamp and nylon screen stencil. Ultravioletlight intensity tester was used to measure the luminous intensity applied on the surface of nylonscreen stencil. After 15 minutes of UV radiation, the overlapped PHU2@paper and nylon screenstencil were taken out of the curing chamber of the instrument. The screen stencil was removedor reused for next printing and a confidential pattern is successfully encoded on the papersubstrate. No change of paper was observed under natural light after the printing, while a bright fluorescent pattern could be observed under UV light. By using screen stencils with predesignedpatterns, more complex patters can also be easily encoded on the paper. Measurements and Characterizations. The infrared spectra of liquid or solid sampleswere recorded on a Bruker TENSOR II spectrometer by using KBr pellets. 2mg sample wasmixed with 200 mg dried KBr, milled and then pressed into transparent pellets for testing. The insitu FT-IR monitoring of the reaction between bis(6-membered cyclic carbonate) and n-hexylamine in dichloromethane was carried out by using TENSOR II with MCT detector. Adiamond ATR probe was connected with TENSOR Ⅱ via AgX 9.5mm x l1.5m Fiber. Theautomatic sampling interval for the in situ FT-IR monitoring is 10 seconds.1H NMR spectrawere recorded on a Bruker AV500 spectrometer. The UV-vis absorption spectra of liquidsamples were measured in ethanol solution by using a Mettler Toledo UV5 spectrophotometer atambient temperature. For the solid samples, the UV-vis absorption spectra were recorded on anAgilent Cary5000 spectrophotometer at ambient temperature. Fluorescent excitation/emissionspectra of samples were measured on an Edinburgh integrated steady state transient fluorescencespectrometer FS5 with a slit width of 5 nm under xenon discharge lamp excitation. The quantumyields of samples were measured on a steady/transient-state fluorescence spectrometer coupledwith an integrating sphere. The uniaxial tensile testing was performed on an AI-7000-AGDtesting machine with an extension rate of 5 mm/min by following ASTMD1708-18 method. Thereported values are the averages of at least five replicates. The UV light mediated printing wascarried out by using XH-300UL+ instrument (Beijing Xiang-Hu Science and TechnologyDevelopment Reagent Co., Ltd., China). The ultraviolet ray irradiation has a luminous intensityof 2mW/cm²by using a high-pressure discharge mercury lamp. CONCLUSIONS In summary, we report a new kind of nonconventional polymer luminogens based onpolyhydroxyurethane with dynamic covalent cross-linked network for the first time. The newluminogens are readily synthesized from aliphatic polyfunctional cyclic carbonate and amines,avoiding the use of toxic isocyanates. The new luminogens not only exhibit intrinsic strongfluorescent emissions at solid states, but also possess high mechanical properties along with goodshape memory and self-healing properties, showing promising applications for smart solidluminescent device fabrication. By utilizing these unique characteristics of the resultantluminogens, a facile method, named“light assistant ink-free screen printing”was developed toprepare anticounterfeiting materials. The new method used UV-light instead of expensivesecurity ink, to create anticounterfeiting patterns on paper substrates. The anticounterfeitingpatterns on the substrates are easy-reading and stable under various wet conditions. Thedeveloped process concept shows promising applications in the fast-growing counterfeiting ofpharmaceuticals, packaging and food industry. ASSOCIATED CONTENT Supporting Information. The Supporting Information is available free of charge on the ACS Publications website at DOI: FT-IR, and lH NMR spectra of PHU1 taken at different curing time; Stress-strain curves ofPHU2 film with different curing time; Fluorescence pattern making and easing on paper (PDF). Cryogenic long-persistent phosphorescence of PHU2 (Video-S1) (MP4) Recovery demonstration of PHU2 under natural light (Video-S2) (MP4)Recovery demonstration of PHU2 under UV light (Video-S3) (MP4) 1 PHU2@paper used as anticounterfeiting ink-jet printing paper (Video-S4) (MP4)Recovery demonstration of PHU2@paper under natural light (Video-S5)(MP4) AUTHOR INFORMATION Corresponding Author * E-mail: zhwgah1028@126.com(W. Z.) ORCID Wei Zhao: 0000-0001-7333-0420 ( Yonghao Ni: 0000-0001-6107-6672 ) ( Author Contributions ) The manuscript was written through contributions of all authors. All authors have given approvalto the final version of the manuscript. Notes The authors declare no competing financial interest. ACKNOWLEDGMENT This work is supported by grants from National Natural Science Foundation of China (No.21774071) and Natural Science Foundation of Shaanxi University of Science and Technology(No. 2017QNBJ-07). W. Z. thanks the support from the Youth Hundred-Talent Program ofShaanxi Province(No.SXBR9227),theNationalHigh-LevelForeignExpertFProject(No.GDT20186100425) and Biomass Chemistry and Materials Acadamician WorkstationProject in SUST (No.134090002). 1 REFERENCES ( (1) Burroughes, J. H.; Bradley,D. D.; Brown, A. R .; Marks, R. N .; Mackay,K. ; Friend, R . H . ;Burns P. L.; Holmes, A . B. L ight-Emitting Diodes Based on Conjugated Polymers. Nature 199 0 , 348,352-352. ) ( (2) Grimsdale, A. C.; Leok Chan, K.; Martin, R. E .; Jokisz, P. G.; Holmes, A. B. Synthesis ofLight-Emitting Conjugated Polymers for A pplications in Electroluminescent Devices. Chem.Rev. 2009,109,897-1091. ) ( (3) Samuel, I. D. W.; Turnbull, G. A . O rganic Semiconductor La s ers. Chem. Rev.2007 , 107, 1272-1295. ) ( (4) McGehee, M. D . ; H e eger, A. J . S emiconducting (Conjugated) P olymers as Materials forSolid-State Lasers.Adv. Mater. 2000, 12 , 1655-1668. ) (5) Feng, X.;Liu, L.; Wang, S.; Zhu, D. Water-Soluble Fluorescent Conjugated Polymers andtheir Interactions with Biomacromolecules for Sensitive Biosensors. Chem. Soc. Rev. 2010.39,2411-2419. (6) Basabe-Desmonts, L.; Reinhoudt, D. N.; Crego-Calama, M. Design of Fluorescent Materialsfor Chemical Sensing. Chem. Soc. Rev. 2007, 36, 993-1017. (7) Thomas, S. W.; Joly, G. D.; Swager, T. M. Chemical Sensors Based on AmplifyingFluorescent Conjugated Polymers. Chem. Rev. 2007,107,1339-1386. (8) Adhikari, B.; Majumdar, S. Polymers in Sensor Applications. Prog. Polym. Sci. 2004, 29,699-766. (9) Mei, J.; Leung, N. L.; Kwok, R. T.; Lam, J.W.; Tang, B. Z. Aggregation-Induced Emission:Together We Shine, United We Soar!. Chem. Rev. 2015,115,11718-11940. (10) Hu, R.; Leung, N. L.; Tang, B. Z. AIE Macromolecules: Syntheses, Structures and 1 Functionalities. Chem. Soc. Rev.2014,43,4494-4562. (11) Gong, Y.; Zhao, L.; Peng, Q.; Fan, D.; Yuan, W. Z.; Zhang, Y.; Tang, B. Z. Crystallization-Induced Dual Emission from Metal- and Heavy Atom-Free Aromatic Acids and Esters. Chem.Sci. 2015,6,4438-4444. (12) Kawamura, Y.; Brooks, J.; Brown, J.J.; Sasabe, H.; Adachi, C. Intermolecular Interactionand a Concentration-Quenching Mechanism of Phosphorescent Ir (III) Complexes in a SolidFilm.Phys. Rev. Lett. 2006,96,017404. (13) Kubota, Y.; Uehara, J.; Funabiki, K.; Ebihara, M.; Matsui, M. Strategy for the Increasingthe Solid-State Fluorescence Intensity of Pyrromethene-BF2 Complexes. Tetrahedron Lett. 2010,51,6195-6198. (14) Luo, J.; Xie, Z.; Lam, J. W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Zhu, D.;Tang, B. Z. Aggregation-Induced Emission of 1-Methyl-1, 2, 3, 4, 5-Pentaphenylsilole. Chem.Commun.2001,(18),1740-1741. (15) Tang, B. Z.; Zhan, X.; Yu, G.; Lee, P. P. S.; Liu, Y.; Zhu, D. Efficient Blue Emission fromSiloles. J. Mater. Chem.2001, 11,2974-2978. (16) Lamere, J. F.; Saffon, N.; Dos Santos, I.; Fery-Forgues, S. Aggregation-Induced EmissionEnhancement in Organic Ion Pairs. Langmuir2010,26,10210-10217. (17) Kokado, K.; Nagai, A.; Chujo, Y. Poly (y-glutamic acid) Hydrogels with Water-SensitiveLuminescence Derived from Aggregation-Induced Emission of o-Carborane. Macromolecules2010,43,6463-6468. (18) Qian, L.; Tong, B.; Shen, J.; Shi, J.; Zhi, J.; Dong, Y.; Lam, J. W.; Liu, Y.; Tang, B. Z.Crystallization-Induced Emission Enhancementina Phosphorus-Containing HeterocyclicLuminogen.J. Phys. Chem. B 2009, 113,9098-9103. 1 (19) Zheng, Y. S.; Hu, Y. J. Chiral Recognition Based on Enantioselectively Aggregation-Induced Emission. J. Org. Chem. 2009, 74,5660-5663. ( (20) An, P .; Shi, Z. F.; Dou, W.; Cao, X. P . ; Zhang, H . L. Synthesis of 1, 4 - Bis[2, 2- b is(4-alkoxyphenyl)vinyl]benzenes and S ide C hain Modulation o f their Solid-State Emission. Org. ) Lett.2010, 12,4364-4367. ( (21) Chen, J.; Law, C. C.; Lam, J. W.; Dong, Y.; Lo, S. M.; Williams, I.D.; Zhu, D.; Tang, B. Z.Synthesis, L ight E mission, N a noaggregation, and Res t ricted Int r amolecular Rotation of 1 , 1-Substituted 2,3, 4, 5-Tetraphenylsiloles. Chem. Mater. 2003,15,1535-1546. ) ( (22) Lo, S. C.; Burn, P. L. Development o f Dendrimers: Macromolecules for Use in OrganicLight-Emitting Diodes and Solar Cells. Chem. Rev. 2007,107,1097-1116. ) ( (23) Yan, J. J.; Wang, Z. K.; Lin, X. S.; Hong, C. Y.; Liang, H. J.; Pan, C. Y; You, Y. Z .Polymerizing Nonfluorescent M o nomers without Incorporating Any Fluorescent Agent ProducesStrong Fluorescent Polymers. Adv. Mater.2012,24,5617-5624. ) (24) Yan, J.; Zheng, B.; Pan, D.; Yang, R.; Xu, Y.; Wang, L.; Yang, M. UnexpectedFluorescence from Polymers Containing Dithio/Amino-Succinimides. Polym. Chem. 2015, 6,6133-6139. (25) Li, W.; Wu, X.; Zhao, Z.; Qin, A.; Hu, R.; Tang, B. Z. Catalyst-Free, Atom-Economic,Multicomponent Polymerizations of Aromatic Diynes, Elemental Sulfur, and Aliphatic Diaminestoward Luminescent Polythioamides. Macromolecules 2015,48,7747-7754. (26) Mohamed, M. G.; Hsu, K. C.; Hong, J. L.; Kuo, S. W. Unexpected Fluorescence fromMaleimide-Containing Polyhedral Oligomeric Silsesquioxanes: Nanoparticle and SquenctDistribution Analyses of Polystyrene-Based Alternating Copolymers. Polym. Chem. 2016, 7,135-145. 1 (27) Zhu, S.; Song, Y.; Shao, J.; Zhao, X.; Yang, B. Non-Conjugated Polymer Dots withCrosslink-Enhanced Emission in the Absence of Fluorophore Units. Angew. Chem., Int. Ed.2015,54,14626-14637. (28) Zhu, S.; Wang, L.; Zhou, N.; Zhao, X.; Song, Y.; Maharjan, S.;Zhang, J.; Lu, L.; Wang, H.;Yang, B. The Crosslink Enhanced Emission (CEE) in Non-Conjugated Polymer Dots: from thePhotoluminescence Mechanism to the Cellular Uptake Mechanism and Internalization. Chem.Commun.2014,50,13845-13848. ( (29) Zhu, S.; Zhang, J.; Wang, L.; Song, Y.; Zhang, G.; Wang, H.; Yang, B. A G e neral Route toMake Non-Conjugated Linear Polymers Luminescent. Chem. Commun. 2012, 48,10 8 89-10891.(30) Sun, M.; H ong, C. Y.; Pan, C. Y. A Unique Aliphatic Tertiary Amine Chromophore:Fluorescence, Polymer Structure, and Application i n Cell Imaging. J. Am. Chem. Soc. 2012, 134,20581-20584. ) (31) Restani, R. B.; Morgado, P. I.; Ribeiro, M. P.; Correia, I. J.; Aguiar-Ricardo, A.; Bonifacio,V. D. Biocompatible Polyurea Dendrimers with pH-Dependent Fluorescence. Angew. Chem., Int.Ed.2012,51,5162-5165. (32) Gao, C.; Lii, S.; Liu, M.; Wu, C.; Xiong,Y. CO2-Switchable Fluorescence of a DendriticPolymer and its Applications. Nanoscale 2016,8,1140-1146. (33) Saha, B.; Choudhury, N.; Seal, S.; Ruidas, B.; De, P. Aromatic Nitrogen Mustard-BasedAutofluorescent Amphiphilic Brush Copolymer as pH-Responsive Drug Delivery Vehicle.Biomacromolecules 2018, 20, 546-557. (34) Saha, B.; Ruidas, B.; Mete, S.; Mukhopadhyay, C. D.; Bauri, K.; De, P. AIE-Active Non-conjugated PFoly(N-vinylcaprolactam) as a FluorescentThermometer forIntracellularTemperature Imaging. Chem. Sci. 2020, 11, 141-147. 1 ( (35) Liu, B.; Wang, Y. L .; Bai, W.; Xu, J. T.; Xu, Z. K. ; Yang, K.; Yang, Y. Z.; Zhang H. X.;Du, B . Y . F luorescent L inear CO2-Derived Po l y (h y droxyurethane) fo r Co o l White LED . J. Mater. Chem. C2017,5,4892-4898. ) ( (36) Saha, B.; Bauri, K.; Bag, A.; Ghorai, P. K.; De, P. Conventional Fluorophore-Free Dual pH- and Thermo-Responsive L u minescent A l ternating; Copolymer. Polym. Chem. 2016. 7. 6895-6900. ) ( (37) Cortez-Lemus, N . A.; L icea-Claverie, A. Poly (N-vinylcaprolactam) , a ComprehensiveReview on a Thermoresponsive Polymer Becoming Popular. Prog. Polym. Sci. 2016, 53,1-51. ) ( (38) Yuan, W. Z.; Zhang, Y. Nonconventional Macromolecular Luminogens w ith Aggregation-Induced Emission Characteristics. Journal of Polymer Science Part A: Polymer Chemistry,2016,55,560-574. ) (39) Zhang, H.; Zhao, Z.; McGonigal, P. R.; Ye, R.; Liu, S.; Lam, J. W. Y.; Kwok, R. T. K.;Yuan, W. Z.; Xie, J.; Rogach, A. L.; Tang, B. Z. Clusterization-Triggered Emission: UncommonLuminescence from Common Materials. Materials Today, 2019.doi:10.1016/j.mattod.2019.08.010. (40) Engels, H.-W.; Pirkl, H.-G.; Albers, R.; Albach, R. W.; Krause, J.; Hoffmann, A.;Casselmann, H.; Dormish, J. Polyurethanes: Versatile Materials and Sustainable Problem Solversfor Today’s Challenges. Angew. Chem., Int. Ed. 2013,52,9422-9441. (41) Nohra, B.; Candy, L.; Blanco, J.-F.; Guerin, C.; Raoul, Y.; Mouloungui, Z. FromPetrochemical Polyurethanes to Biobased Polyhydroxyurethanes. Macromolecules 2013, 46,3771-3792. (42) Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the Versatility ofUrethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-Isocyanate Polyurethane. 1 Chem. Rev. 2013,113, 80-118. ( (43) Hu, S.; Chen, X.; Torkelson, J. M. Biobased Reprocessable Polyhydroxyurethane Networks:Full Recovery of C rosslink D ensity with T' hree C oncurrent D ynamic C hemistries. ACSSustainable Chem. Eng. 2019, 7, 1 0025-10034. ) (44) de Aguiar, K. M. R.; Alves, V. S.; Noeske, P. L. M.; Rischka, K.; Portela, M. B.; Ferreira-Pereira, A.; Rodrigues-Filho, U. P. Hybrid Films Based on Nonisocyanate Polyurethanes withAntimicrobial Activity. Inspired Mater. Biomed. Eng. 2019. 77-116. DOI:https://doi.org/10.1016/B978-0-12-818435-6.00004-9. (45) Wang, Y. L.; Liu, B.; Yang,J. L.; Cao, X. H.; Yang, Y. Z.; Yang, Q.; Greiner, A.; Xu, J.T.;Zhang, X. H. Hyperbranched Fractal Nanocarbons for Bright Photoluminescence in Solid State.Adv. Opt. Mater. 2019,7,1900659. (46) Schimpf, V.; Asmacher, A.; Fuchs, A.; Bruchmann, B.; Mulhaupt, R. PolyfunctionalAcrylic Non-isocyanate HydroxyurethanesasPhotocurable Thermosetsfor 3D Printing.Macromolecules 2019.52.3288-3297. (47) Yadav, N.; Seidi, F.; Crespy, D.; D'Elia, V. Polymers Based on Cyclic Carbonates as Traitd'Union between Polymer Chemistry and Sustainable CO2 Utilization. ChemSusChem 2019, 12,724-754. (48) Niu, S.; Yan, H.; Chen, Z.; Li, S.; Xu, P.; Zhi, X. Unanticipated Bright Blue FluorescenceProduced from Novel Hyperbranched Polysiloxanes Carrying Unconjugated Carbon-CarbonDouble Bonds and Hydroxyl Groups. Poly. Chem. 2016, 7, 3747-3755. (49) Badoux, M.; Kilbinger, A. F. M. Synthesis of High Molecular-Weight Poly(p-benzamide)s.Macromolecules 2017,50,4188-4197. 1 (50) Noel, A.; Borguet, Y. P.; Raymond, J. E.; Wooley, K. L. Poly(ferulic acid-co-tyrosine):Effect of the Regiochemistry on the Photophysical and Physical Properties en Route toBiomedical Applications.Macromolecules 2014, 47,7109-7111. ( (51) Pucci, A.; Rausa, R.; Ciardelli F . Aggregation-Induced Luminescence o f P olyisobuteneSuccinic Anhydrides and Imides. Macromol Chem Phys 2008, 209, 900-906. ) ( (52) Zhang, G.; Chen, J.; Payne,S. J . ; Kooi, S. E . ; D e mas, J. N.; Fraser, C. L. Multi-EmissiveDifluoroboron D ibenzoylmethane Po l ylactide E xh ibiting Intense Fluorescence and Oxygen-Sensitive Room Temperature Phosphorescence. J. Am. Chem. Soc. 2007,129,8942-8943. ) ( (53) Zhang, G.; Palmer, G. M.; Dewhirst, M. W.; Fraser, C. L . A Dualemissive-Materials DesignConcept Enables Tumour Hypoxia Imaging. Nat. Mater. 2009, 8,747-751. ) ( (54) Zhang,X.; X i e, T.; Cui, M.; Yang,L. ; Sun, X.; Jiang, J. ; Zhang, G. General De s ign Strategyfor Aromatic K etone-Based S ingle C omponent D u al-Emissive Materials. ACS Appl. M a ter.Interfaces 2014, 6, 2279-2284. ) (55) Zhou, C.; Xie, T.; Zhou, R.; Trindle, C. O.; Tikman, Y.; Zhang, X.; Zhang, G. WaterbornePolyurethanes with Tunable Fluorescence and Room Temperature Phosphorescence. ACS Appl.Mater. Interfaces 2015, 7,17209-17216. (56) Jiang,N.; Li, G. F.; Zhang, B. H.; Zhu, D. X.; Su, Z.M.; Bryce, M. R. Aggregation-InducedLong-LivedPhosphorescenceinNonconjugatedPolyurethaneDerivatives at 77 K.Macromolecules 2018,51,4178-4184. (57) Menning, S.;Kramer, M.; Coombs, B. A.; Rominger, F.; Beeby, A.; Dreuw, A.; Bunz, U.H.Twisted Tethered Tolanes: Unanticipated Long-Lived Phosphorescence at 77 K. J. Am. Chem.Soc.2013,135,2160-2163. (58) Fortman, D. J.; Brutman,J. P.; Cramer, C. J.; Hillmyer,M. A.; Dichtel, W. R. Mechanically Activated, Catalyst-Free Polyhydroxyurethane Vitrimers. J. Am. Chem. Soc. 2015, 137, 14019-14022. (59) Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: fromOld Chemistry to Modern Day Innovations. Advanced Materials 2017, 29,1606100. (60) Matsukizono, H.; Endo, T. Reworkable Polyhydroxyurethane Films with Reversible AcetalNetworks Obtained from Multifunctional Six-Membered Cyclic Carbonates. J. Am. Chem. Soc.2018,140,884-887. Aliphatic Polyhydroxyurethane Based Luminogens and its Application in Anticounterfeiting247x133mm (150x 150 DPI) ACS Paragon Plus Environment 一种基于脂肪族聚氨酯的新型非常规发光剂及其在无墨防伪印刷中的潜在应用关键词:聚氨酯,发光,无墨,防伪,印刷摘要:有机发光体因其独特的光物理特性而具有广泛的应用。近年来,非共轭有机发光体,与传统的结合的发光体,由于其方便的制备、环境的影响,已经获得了很多关注。在这项研究中,一种新的基于非传统发光体的首次报道了在动态共价交联的聚氨酯上出现的16种新的化学反应。该新发光体不仅在固体状态下表现出固有的强荧光发射,而且还拥有高的机械性能以及良好的形状记忆和自修复性能。特别是,新的发光体是由脂肪族多官能环状碳酸酯合成的方法更为直接,避免了使用有毒的异氰酸酯。 调查表明,所产生的发光体的内在发光是由诱导的通过聚合物链的交联,并可以通过控制聚合物链的程度来很好地调整。交联。通过利用所产生的聚合物的独特特性。我们进一步开发了一种简便的方法,命名为 "光介导的无墨屏幕打印",用于防伪纸的制作。与传统的油墨印刷技术不同,新方法使用紫外光而不是昂贵的安全墨水来编码。天然纤维素纸的防伪信息。防伪信息是在各种潮湿条件下都是稳定的,显示出在快速增长的药品、包装和食品行业的假冒。简介: 有机发光体由于其独特的光物理特性,已经吸引了越来越多的关注有机发光二极管、有机激光器中的广泛应用。生物/化学传感器、光导纤维等。常规有机发光剂通常在其分子结构中含有稠合的芳香环,形成显著的π-电子共轭系统,确保强发射。许多传统的有机发光剂在稀溶液中有很强的辐照性,但表现出弱的发光或在其浓溶液或固态中完全不发光,导致浓度猝灭和聚集导致猝灭(ACQ)问题。这是传统有机发光剂的缺点,限制了其作为发光材料的使用固态器件材料。2001年,Tang及其同事发现含有六苯基硅烷的共轭电子该结构在溶液状态下不发光,但在聚集态或固态。这一发现与观察到的聚集引起的猝灭效应形成对比传统的发光体。“聚集诱导发射(AIE)”一词是后来为这一独特现象创造了新的理论,证明了案例也可以对排放发光材料。从那时起,这项开创性的工作启发了许多人的探索具有AIE效应的有机石灰原。目前报道的大多数AIE发光体都很小具有共轭电子结构的分子。对于实际应用,昂贵的处理采用真空升华和气相沉积等技术制备这些材料转化为固体薄膜,限制了这些材料在大面积平板上的应用设备。幸运的是,通过合成AIE聚合物可以缓解这一缺点。AIE聚合物可以很容易地通过简单的工艺制备成大面积的固体薄膜和器件处理技术,如旋涂、静态铸造和喷墨打印。最近,观察到各种非常规发光体的本征发光,没有任何众所周知的发色团类型,和(杂环)芳香族片段。其中27个新兴的发光体、基于聚合物的非常规发光体引起了人们极大的兴趣由于其链的灵活性、结构的可维持性、制备的方便性、环境友好性和生物相容性,显示出重要的基础重要性和有前途的技术应用程序。28–37迄今为止,大多数非常规聚合物发光体都具有线性或超支化结构,显示了固态制造的局限性要求高机械性能的设备。38,39聚氨酯(PUs)是一类具有多种用途的重要聚合物,如粘合剂、涂料、泡沫和密封剂。传统的PUs合成涉及使用剧毒异氰酸酯对人体健康有害。40与传统PUs、聚羟基聚氨酯(PHUs)相比 通过环碳酸酯与伯胺反应制备的碳酸酯吸引了大量近年来,由于其无异氰酸酯的合成路线,增强了性能(与PU)和有前途的应用。41-47在此,我们报告了一种新的非常规首次基于脂肪族聚羟基聚氨酯的发光剂。新的发光体是由多功能脂肪族环状碳酸酯和胺通过简单方法制备,避免使用有毒异氰酸酯。与非常规聚合物发光体相比到目前为止,新的发光体具有动态共价交联网络和展示高机械性能以及智能性能,如形状记忆和自我修复。新发光体的本征发光是由交联引起的并且可以通过聚合物的交联密度轻松调节,提供可微调发光强度的简易工艺概念。利用这一独特的特点,我们进一步开发了一种新的丝网印刷方法,称为“光介油墨”-“免费丝网印刷”,依靠紫外光,而不是昂贵的防伪油墨进行编码基板上的防伪信息。编码的反盗版信息是可编程且在各种条件下稳定,在现代防伪领域。回收、重新制作和重新编码paper@PHU2祥鹄仪器使用过程通过光介导无油墨丝网印刷制作荧光图案。紫外线使用配备有XH-300UL+的箱式UV反应器进行光介导印刷配有高压放电灯(250W)。这个PHU2@paper在房间准备温度首先与尼龙筛网模板(200目)重叠,然后放入仪器的固化室。紫外线照射的发光强度为2mW/cm2在尼龙网表面使用高压放电灯通过调整水银灯和尼龙丝网模具之间的距离来制作模具。紫外线用光强测试仪测量尼龙表面的发光强度屏幕模板。紫外线照射15分钟后,重叠的PHU2@paper和尼龙网模具从仪器的固化室中取出。已删除屏幕模板或再次用于下一次打印,并且在纸上成功地对机密图案进行编码基底印刷后,在自然光下未观察到纸张变化,而在紫外光下可以观察到荧光图案。使用预先设计的屏幕模板图案,更复杂的图案也可以很容易地编码在纸上。结论:总之,我们报道了一种基于首次采用动态共价交联网络的聚氨酯。新的发光剂很容易由脂肪族多功能环碳酸酯和胺合成,避免使用有毒异氰酸酯。新型发光体不仅表现出内在的强固态荧光发射,还具有高机械性能和良好的形状记忆和自愈特性,显示出智能固体的良好应用前景发光器件制造。通过利用结果的这些独特特性发光剂是一种简便的方法,被称为“光助无墨丝网印刷”准备防伪材料。新方法使用紫外光而不是昂贵的防伪油墨,用于在纸张基材上形成防伪图案。反盗版基板上的图案易于读取,在各种潮湿条件下稳定。这个开发的工艺概念在快速增长的假冒中显示出良好的应用前景制药、包装和食品工业。
确定


























还剩34页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《脂肪族多官能环状聚氨酯中催化合成工艺检测方案(微波合成仪)》,该方案主要用于其他中其他检测,参考标准--,《脂肪族多官能环状聚氨酯中催化合成工艺检测方案(微波合成仪)》用到的仪器有电脑微波超声波紫外光组合催化合成仪 XH-300UL+
推荐专场
相关方案
更多
该厂商其他方案
更多
















