方案详情
文
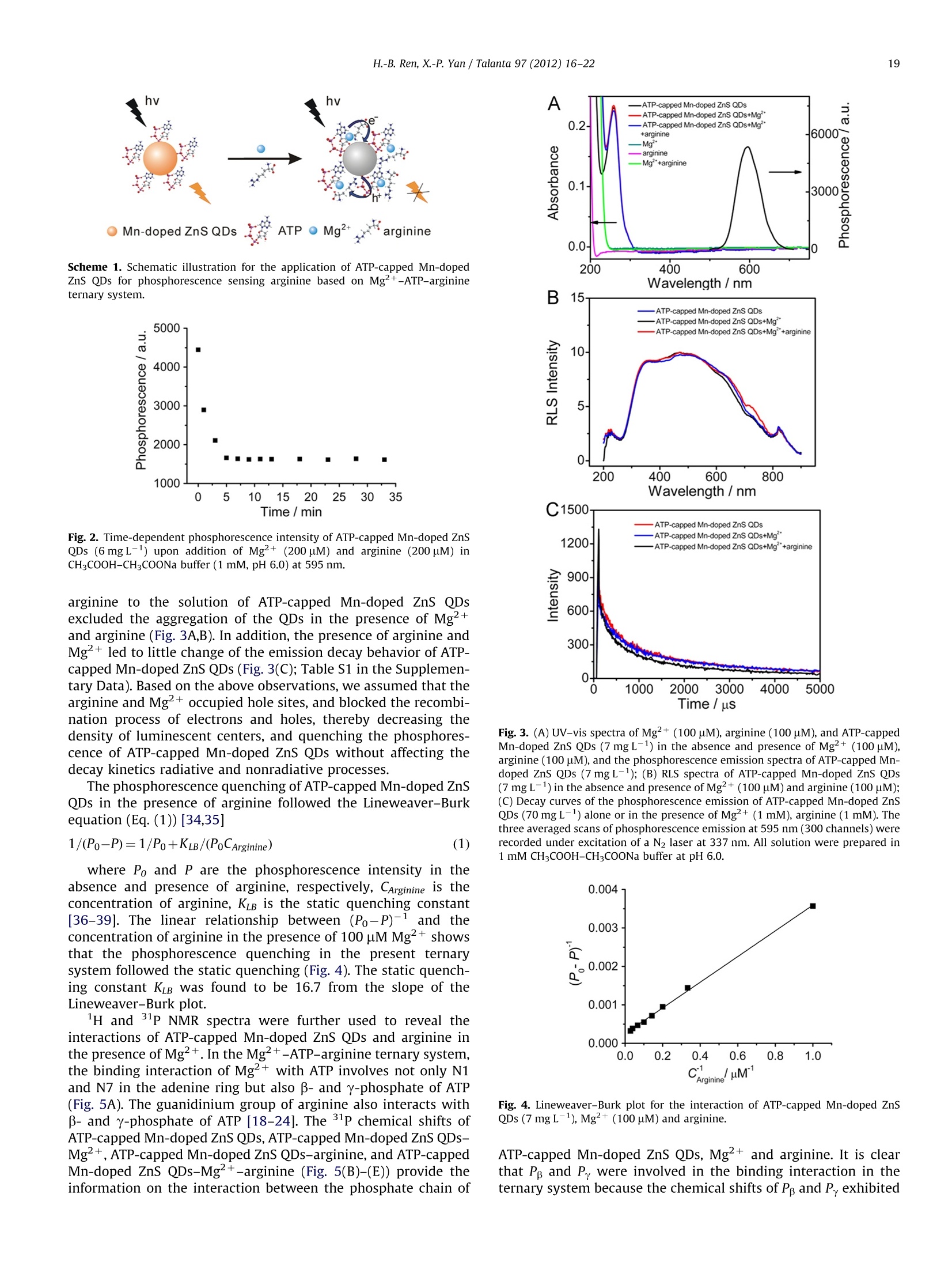
一种超声波辅助合成的ATP封尾的Mn-doped ZnS QDs,用于选择性地检测精氨酸和甲基化精氨酸,其依据是Mg2þ-ATP-精氨酸三元系统的识别性质与Mn-doped ZnS QDs的磷光特性相结合。所开发的探针具有良好的选择性和重现性,并且检测限低。由于有效地消除了散射光和自发荧光的干扰,开发的磷光探针有利于生物应用。
方案详情

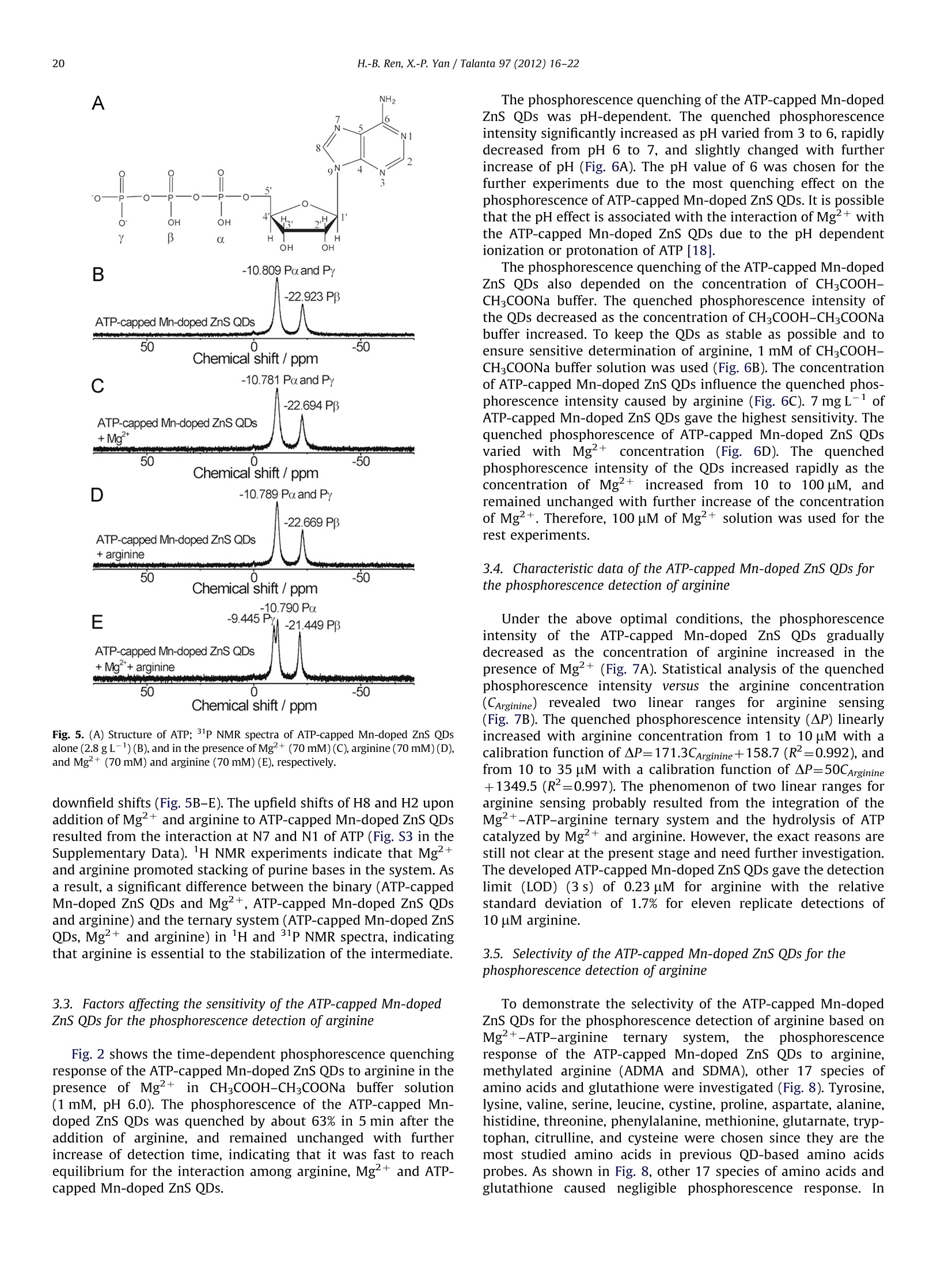
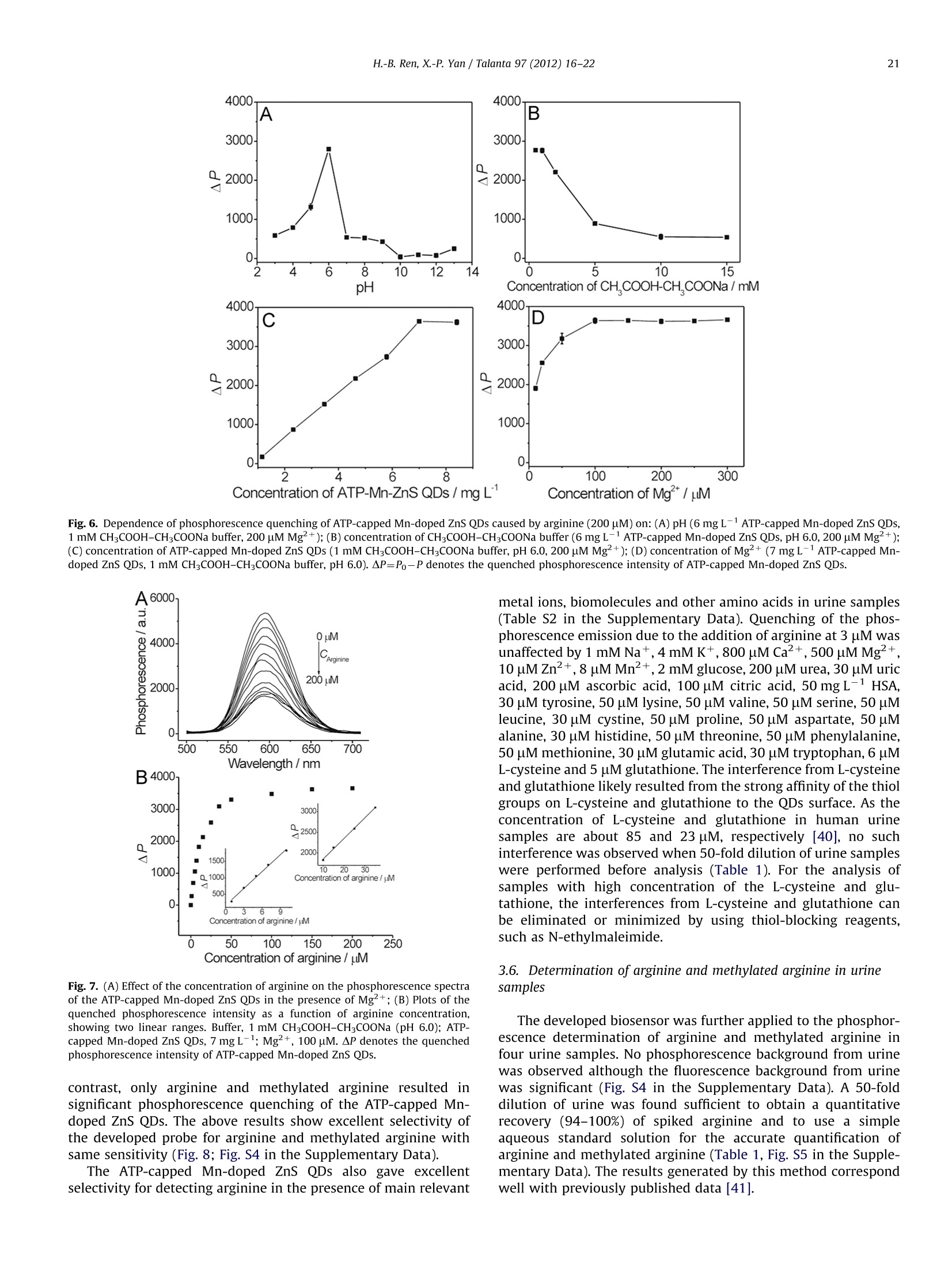
Talanta 97 (2012)16-22Contents lists available at SciVerse ScienceDirect 17H.-B. Ren, X.-P. Yan / Talanta 97 (2012) 16-22 http://dx.doi.org/10.1016/j.talanta.2012.03.055 Talantajournal homepage: www.elsevier.com/locate/talanta Ultrasonic assisted synthesis of adenosine triphosphate cappedmanganese-doped ZnS quantum dots for selective room temperaturephosphorescence detection of arginine and methylated arginine in urinebased on supramoolleeccuullaarr MMgg+-adenosine triphosphate-a nineternary system Hu-Bo Ren, Xiu-Ping Yan* State Key Laboratory of Medicinal Chemical Biology, and Research Center for Analytical Sciences, College of Chemistry, Nankai University, Tianjin 300071, China ARTICLEINFO ABSTRACT Article history: Received 13 February 2012 Received in revised form 21 March 2012 Accepted 21 March 2012 Available online 3 May 2012 An ultrasonic assisted approach was developed for rapid synthesis of highly water soluble phosphor-escent adenosine triphosphate (ATP)-capped Mn-doped ZnS QDs. The prepared ATP-capped Mn-dopedZnS QDs allow selective phosphorescent detection of arginine and methylated arginine based on thespecific recognition nature of supramolecular Mg+-ATP-arginine ternary system in combination withthe phosphorescence property of Mn-doped ZnS QDs. The developed QD based probe gives excellentselectivity and reproducibility (1.7% relative standard deviation for 11 replicate detections of 10 uMarginine) and low detection limit (3 s, 0.23 uM), and favors biological applications due to the effectiveelimination of interference from scattering light and autofluorescence. Keywords:Ultrasonic assisted synthesisPhosphorescenceQuantum dotsAdenosine triphosphateArginine C 2012 Elsevier B.V. All rights reserved. 1. Introduction Arginine is an important component in biological systems,serving as a precursor for the synthesis of not only nitric oxidebut also proteins, urea, proline, polyamines, glutamate, agmatineand creatine [1]. The main products of arginine methylationreactions are NC, NC-dimethylarginine (ADMA) and N, N-dimethylarginine (SDMA) and these methylated arginine areliberated in the course of protein turnover and breakdown [1].With the discovery of novel pathways for arginine catabolism andsynthesis in humans, there is growing recognition that arginine isa conditionally essential amino acid in adult humans, particularlyin cases of trauma or disease [2]. Owing to their biologicalimportance, quite a few methods have been developed for thedetermination of arginine and methylated arginine in biologicalfluids based on high-performance liquid chromatography [3],capillary electrophoresis [4], enzymatic end-point analysiss[5],thin-layer chromatography [6], amino acid analyzers [7], ionexchange paper chromatography [8], high-temperature paperchromatography[9], and voltammetry [10]. Even though, a simple, ( *Corresponding author.Tel./fax : +862223506075. ) ( E-mail a ddresses: xpyan@nankai.edu.cn, xiupingyan@gmail.com (X.-P. Yan). ) ( 0 039-9140/$-see front ma t ter @ 201 2 Elsevier B.V. All ri g hts re s erved. ) highly selective and sensitive, and instantaneous response methodfor the detection of arginine and methylated arginine is among theurgent and emerging challenges. Photoluminescence spectrometry is the technique of choice fordetecting arginine and methylated arginine owing to the apparentadvantages of fluorescent probes over other methods in virtue ofsensitivity and convenience. Miura et al. [11] reported the applica-tion of organic dyes as photoluminescence probe for highly specificdetecting arginine. Quantum dots (QDs) offer unique advantagesover organic dyes such as great photostability, high photolumines-cence efficiency, size-dependent emission wavelengths, broad exci-tation and sharp emission profiles, thus QDs have been widelyexplored for bioanalysis [12-17]. However, to the best of ourknowledge, no work on the utilization of functionalized QDs fordetecting arginine and methylated arginine has been reported so far. The supramolecular interactions of Mg+ and arginine withadenosine-triphosphate (ATP) have been investigated to under-stand the precise function of arginine and Mg2+ in the specificcleavage mechanism of the ATP at the molecular level. ATPhydrolysis is catalyzed by arginine and Mg+, which is involvedin the catalytic mechanism, acting as a binding site for ATP or as acofactor. Selective non-enzymatic catalysis of ATP hydrolysis incomplicated biological system based on Mg+-ATP-arginineternary system have received great attentions [18-24]. Here we show ultrasonic assisted synthesis of ATP-capped Mn-doped ZnS QDs for rapid, selective and sensitive detection ofarginine by taking the advantages of the outstanding opticalproperties of Mn-doped ZnS QDs and the Mg+-ATP-arginineternary system for specific recognition of arginine. The ultrasonicapproach was used for the synthesis of ATP-capped QDs becauseof its rapid, simple, low cost, and efficient merits [25]. Mn-dopedZnS QDs were used as photoluminescent probe due to their lowtoxicity and their long lifetime of phosphorescence which allowedan appropriate delay time to avoid interference from any back-ground fluorescence emission and scattering light [26-28]. Thechoice of ATP as capping ligand in synthesis of functional QDsprovided a powerful means of rationally controlling Mn-dopedZnS QDs properties. ATP could render Mn-doped ZnS QDs stableagainst aggregation and water-soluble, and retain the nucleotidesstructure needed to specially bind to its target [29]. The specificbinding of ATP-capped Mn-doped ZnS QDs to arginine in thepresence of Mg+ led to highly selective quenching of thephosphorescence of the QDs, allowing selective detection ofarginine with a detection limit of 0.23 pM. 2. Experimental section 2.1. Materials and chemicals All chemicals used were at least analytical grade. Arginine,tyrosine, lysine, valine, serine, leucine, cystine, proline, aspartate,alanine, histidine, threonine, phenylalanine, methionine, glutamicacid, tryptophan, L-cysteine, glutathione and ATP disodium salt werepurchased from Newprobe Biotechnology Co. Ltd. (Beijing,China).Mg(NO3)2·6H20, Zn(CHCOO)2·2H20, Mn(CH3COO)2·4H20, andNazS·9H20 were purchased from Tianjing Guangfu Chemical Co.(Tianjing, China). ADMA and SDMA were obtained from Sigma(Steinheim, Germany). Citrulline (CIT) was obtained from ChineseMedicine Shanghai Chemical Reagent Supply Station (Shanghai,China). Ultrapure water (18.2 MQ cm) obtained from a WaterProwater purification system (Labconco Corporation, Kansas City, MO,USA) was used throughout. 2.2. Apparatus The microstructure and morphology of the QDs were character-ized by high-resolution transmission electron microscopy (HRTEM)on a Philips Tecnai G2 F20 microscope (Philips, Holland) operatingat a 200 kV accelerating voltage. The samples for HRTEM wereobtained by drying sample droplets from water dispersion onto a300-mesh Cu grid coated with a lacey carbon film. Fourier trans-form infrared (FT-IR) spectra (4000-400 cm-1) in KBr wererecorded on a Magna-560 spectrometer (Nicolet, Madison, WI).The X-ray diffraction (XRD) spectra were collected on a RigakuD/max-2500 X-ray diffractometer (Rigaku, Japan) with Cu Kradiation. The UV spectra were recorded on a UV-3600 spectro-meter (Shimadzu, Japan). The decayed curves of phosphorescenceemission at 595 nm excited by the N2 laser at 337 nm wererecorded on a PTI QM/TM/NIR system (Birmingham,NJ, USA). Allnuclear magnetic resonance (NMR) experiments were carried outon a Bruker Avance 400 MHz spectrometer operating at 25 ℃. Thechemical shifts of P NMR spectra in ppm were relative to anexternal reference of 85% HPO4. In a typical experiment,20% D2O/H20 was placed in a 5 mm NMR tube. For each sample, the probewas automatically locked, tuned, matched, and shimmed. The ultrasonic assisted synthesis of ATP-capped Mn-DopedZnS QDs was carried out on XiangHu Microwave-ultrasoundreactor (XH-300 A, XiangHu Science and Technology Develop-ment Co. Ltd, Beijing, China). The phosphorescence measurements were performed on an F-4500 spectrofluorometer (Hitachi,Japan)equipped with a plotter unit and a quartz cell (1x1 cm²) in thephosphorescence mode. The photomultiplier tube voltage was setat 950 V. The slit width was 10 nm and 20 nm for excitation andemission, respectively. 2.3. Ultrasonic assisted synthesis of ATP- capped Mn-doped ZnS QDs The highly luminescent ATP-capped Mn-doped ZnS QDs wereprepared in an aqueous solution at room temperature underultrasonication. Typically, 5 mL of 0.04 M ATP, 5 mL of 0.1 MZn(CH,COO)2 and 0.2 mL of 0.1M Mn(CH;CO0)2 were mixedwith 40 mL ultrapure water in a 100 mL three-necked flask underultrasonication at a power of 1000 Wunder argon for 7 min. 5 mLof 0.1 M Na2S.9H20 was then added and the mixture wassubjected ultrasonification at a power of 1000 W for 30 min toyield highly luminescent ATP-capped Mn-doped ZnS QDs. Theobtained ATP-capped Mn-doped ZnS QDs was precipitated withethanol, and separated by centrifuging at 10,000 rpm for 10 min.The final product was redissolved in ultrapure water, and dilutedto appropriate concentration before detection. Uncapped Mn-doped ZnS QDs were synthesized using a similar procedure butwithout the addition of ATP. 2.4. Urine sample collection and pretreatment Human urine samples were collected from healthy volunteers.The samples were subsequently centrifuged at 3000 rpm for 20 minto remove particulates. All samples were subjected to a 50-folddilution before analysis and no other pretreatments were necessary. 2.5. Measurement procedures To a 10 mL calibrated test tube an appropriate volume of7 mg L-1ATP-capped Mn-doped ZnS QDs solution, 0.1 mL ofCHCOOH-CH,COONa buffer solution (0.1 M, pH 6.0), 100uMMg(NO3)26H20 and a given concentration of arginine/methy-lated arginine standard solution or urine sample solution weresequentially added. The mixture was then diluted to volume withultrapure water, mixed thoroughly, and incubated for 5 min.Finally, the phosphorescence measurements were performed onan F-4500 spectrofluorometer in phosphorescence mode with theexcitation wavelength of 300 nm. 3. Results and discussion 3.1. Preparation and characterization of ATP-capped Mn-doped ZnSQDs The ultrasonic assisted aqueous synthesis of ATP-capped Mn-doped ZnS QDs was carried out on the basis of the reaction of ATP,Zn(CHCOO)2, Mn(CHCOO)2 and NazS at room temperature. Aperiod of 7 min was found sufficient to mix Zn(CHCOO)2,Mn(CH,COO)2 and ATP, and to ensure subsequence synthesis ofhighly luminescent ATP-capped Mn-doped ZnS QDs (Fig. S1 in theSupplementary Data). To show the merits of ultrasonic assistedsynthesis of ATP-capped Mn-doped ZnS QDs, traditional methodfor synthesis of ATP-capped Mn-doped ZnS QDs based on aprevious publication [30] was also used for comparison. A 2-haging of the ATP-capped Mn-doped ZnS QDs was required toimprove the phosphorescence intensity in the traditional method,whereas no such aging was necessary in our present ultrasonicassisted approach (Fig. S2 in the Supplementary Data). Thepresent ultrasonic assisted approach not only provided a muchfaster synthesis (37 min versus 170 min), but also gave 2.3 times higher phosphorescence intensity than the traditional method(Fig. S2 in the Supplementary Data). The prepared ATP-capped Mn-doped ZnS QDs were character-ized by XRD, FT-IR spectroscopy and HRTEM. The XRD pattern ofthe ATP-capped Mn-doped ZnS QDs exhibits a zinc blende withpeaks for (111),(220), and (311) planes (Fig. 1A). The FT-IRspectra of the free ligand (ATP), ATP-capped Mn-doped ZnS QDs,and uncapped Mn-doped ZnS QDs were compared to confirm thecoordination of the ATP on the surface of the QDs (Fig. 1B). ATP-capped Mn-doped ZnS QDs displayed a higher energy shift in the-NH2 feature from 1713 cm-1 in the free ATP to 1650 cm-1,suggesting the amine group on the adenine moiety was coordi-nated to the Mn-doped ZnS QDs surface [31,32]. The featurebands associated with the phosphate group (ca. 800-1300cm-1),the asymmetric stretching vibrations of the POz (ca. 1258 cm),the out of phase symmetrical stretches (ca. 1104cm-), and theP-O stretches of the main chain (ca.971 cm-1) were all broa-dened and slightly shifted in ATP-capped Mn-doped ZnS QDs,confirming the phosphate groups also coordinated to the surfaceof the QDs [33]. However, the bands at 1650, 1250 and 971 cmdisappeared in the FT-IR spectra of uncapped Mn-ZnS QDs. Theseresults indicate the successful capping of ATP on the surface of theQDs, and the -NH2, phosphate groups were involved in theinteractions between ATP and the QDs. The HRTEM images revealspherical shape of ATP-capped Mn-doped ZnS QDs with thediameters of 3-5 nm (Fig. 1C,D). 3.2. Consideration of supramolecular Mg+-ATP-arginine ternarysystem for QDs based phosphorescence sensing of arginine The supramolecular interactions of Mg+and arginine withATP have been investigated in complicated biological system [18-24]. In the Mg+-ATP-arginine ternary system, the bindinginteraction of Mg+with ATP involves not only N1 and N7 in theadenine ring but also phosphate groups of ATP. The binding forcesare mainly cation (Mg+)-n interaction and electrostatic interac-tion. Arginine is fully protonated under physiological conditions,and enables interaction with phosphate in ATP because of thestrong basic nature of the guanidinium group. The role of arginineis to stabilize Mg+-ATP in the ternary system because the sizeand shape of arginine and ATP are compatible with a creationarrangement. Also, electrostatic interaction and coordinationbonds are possibly involved in the interactions [18]. Such supra-molecular Mg+-ATP-arginine ternary system gives us a hint todevelop selective QDs based phosphorescence sensing of argininein the presence of Mg2+. To take the advantages of the specific recognition of argininebased on the Mg+-ATP-arginine ternary system and the distin-guished photoluminescence intrinsic of Mn-doped ZnS QDs, wedesigned and prepared ATP-capped Mn-doped ZnS QDs for sen-sing arginine in the presence of Mg+(Scheme 1). ATP not onlyrenders Mn-doped ZnS QDs water-soluble and stable in aqueoussolution against aggregation, but also retains the nucleotidesstructure needed to specifically bind to its target [29]. It is worth mentioning that the phosphorescence of ATP-capped Mn-doped ZnS QDs was quenched quickly upon additionof arginine in the presence of Mg+, and reached equilibriumwithin 5 min (Fig. 2). We ruled out the energy transfer from ATP-capped Mn-doped ZnS QDs to arginine in the presence of Mgasth1ee Iphosphorescence quenching mechanism because of no spec-tra overlap between the absorption spectra of arginine (and/orMg) and the phosphorescence emission spectra of ATP-cappedMn-doped ZnS QDs (Fig. 3A). The negligible variation in UV andresonance light scattering (RLS) spectra after adding Mg+ and Scheme 1. Schematic illustration for the application of ATP-capped Mn-dopedZnS QDs for phosphorescence sensing arginine based on Mg2+-ATP-arginineternary system. Fig. 2. Time-dependent phosphorescence intensity of ATP-capped Mn-doped ZnSQDs (6mg L-) upon addition of Mg+(200uM) and arginine (200 pM) inCHCOOH-CHCOONa buffer (1 mM, pH 6.0) at 595 nm. arginine to the solution of ATP-capped Mn-doped ZnS QDsexcluded the aggregation of the QDs in the presence of Mg+and arginine (Fig. 3A,B). In addition, the presence of arginine andMg+led to little change of the emission decay behavior of ATP-capped Mn-doped ZnS QDs (Fig. 3(C); Table S1 in the Supplemen-tary Data). Based on the above observations, we assumed that thearginine and Mg+occupied hole sites, and blocked the recombi-nation process of electrons and holes, thereby decreasing thedensity of luminescent centers, and quenching the phosphores-cence of ATP-capped Mn-doped ZnS QDs without affecting thedecay kinetics radiative and nonradiative processes. The phosphorescence quenching of ATP-capped Mn-doped ZnSQDs in the presence of arginine followed the Lineweaver-Burkequation (Eq.(1))[34,35] where Po and P are the phosphorescence intensity in theabsence and presence of arginine, respectively, CArginine is theconcentration of arginine, KuB is the static quenching constant[36-39]. The linear relationship between (Po-P)-1 and theconcentration of arginine in the presence of 100 uM Mg2+ showsthat the phosphorescence quenching in the present ternarysystem followed the static quenching (Fig. 4). The static quench-ing constant KuB was found to be 16.7 from the slope of theLineweaver-Burk plot. lH and 31P NMR spectra were further used to reveal theinteractions of ATP-capped Mn-doped ZnS QDs and arginine inthe presence of Mg2+. In the Mg2+-ATP-arginine ternary system,the binding interaction of Mg+ with ATP involves not only N1and N7 in the adenine ring but also β- and y-phosphate of ATP(Fig.5A). The guanidinium group of arginine also interacts withp- and y-phosphate of ATP [18-24]. The 31P chemical shifts ofATP-capped Mn-doped ZnS QDs, ATP-capped Mn-doped ZnS QDs-Mg2+, ATP-capped Mn-doped ZnS QDs-arginine, and ATP-cappedMn-doped ZnS QDs-Mg+-arginine (Fig. 5(B)-(E)) provide theinformation on the interaction between the phosphate chain of Fig. 3. (A) UV-vis spectra of Mg2+(100 uM), arginine (100 pM), and ATP-cappedMn-doped ZnS QDs (7 mg L) in the absence and presence of Mg+(100 uM),arginine (100 uM), and the phosphorescence emission spectra of ATP-capped Mn-doped ZnS QDs (7mg L-); (B) RLS spectra of ATP-capped Mn-doped ZnS QDs(7 mgL-) in the absence and presence of Mg²+(100 uM) and arginine (100 pM);(C) Decay curves of the phosphorescence emission of ATP-capped Mn-doped ZnSQDs (70 mg L-1) alone or in the presence of Mg2+(1 mM), arginine (1 mM). Thethree averaged scans of phosphorescence emission at 595 nm (300 channels) wererecorded under excitation of a N2 laser at 337 nm. All solution were prepared in1 mM CH3COOH-CH,COONa buffer at pH 6.0. Fig. 4. Lineweaver-Burk plot for the interaction of ATP-capped Mn-doped ZnSQDs (7 mgL-), Mg+(100 pM) and arginine. ATP-capped Mn-doped ZnS QDs, Mgand arginine. It is clearthat Pp and Py were involved in the binding interaction in theternary system because the chemical shifts of Pg and Py, exhibited NH. Fig. 5. (A) Structure of ATP; P NMR spectra of ATP-capped Mn-doped ZnS QDsalone (2.8gL-)(B), and in the presence of Mg+(70mM)(C), arginine (70 mM)(D),and Mg+(70 mM) and arginine (70 mM) (E), respectively. downfield shifts (Fig.5B-E). The upfield shifts of H8 and H2 uponaddition of Mg+and arginine to ATP-capped Mn-doped ZnS QDsresulted from the interaction at N7 and N1 of ATP (Fig. S3 in theSupplementary Data). H NMR experiments indicate that Mg2+and arginine promoted stacking of purine bases in the system. Asa result, a significant difference between the binary (ATP-cappedMn-doped ZnS QDs and Mg2+, ATP-capped Mn-doped ZnS QDsand arginine) and the ternary system (ATP-capped Mn-doped ZnSQDs, Mg2+ and arginine) in lH and 31P NMR spectra, indicatingthat arginine is essential to the stabilization of the intermediate. 3.3. Factors affecting the sensitivity of the ATP-capped Mn-dopedZnS QDs for the phosphorescence detection of arginine Fig. 2 shows the time-dependent phosphorescence quenchingresponse of the ATP-capped Mn-doped ZnS QDs to arginine in thepresence of Mg+ in CHCOOH-CHCOONa buffer solution(1 mM, pH 6.0). The phosphorescence of the ATP-capped Mn-doped ZnS QDs was quenched by about 63% in 5 min after theadditi1oonn of arginine, and remained unchanged with furtherincrease of detection time, indicating that it was fast to reachequilibrium for the interaction among arginine, Mg+ and ATP-capped Mn-doped ZnS QDs. The phosphorescence quenching of the ATP-capped Mn-dopedZnS QDs was pH-dependent. The quenched phosphorescenceintensity significantly increased as pH varied from 3 to 6, rapidlydecreased from pH 6 to 7, and slightly changed with furtherincrease of pH (Fig. 6A). The pH value of 6 was chosen for thefurther experiments due to the most quenching effect on thephosphorescence of ATP-capped Mn-doped ZnS QDs. It is possiblethat the pH effect is associated with the interaction of Mg+withthe ATP-capped Mn-doped ZnS QDs due to the pH dependentionization or protonation of ATP [18]. The phosphorescence quenching of the ATP-capped Mn-dopedZnS QDs also depended on the concentration of CHCOOH-CHCOONa buffer. The quenched phosphorescence intensity ofthe QDs decreased as the concentration of CHCOOH-CHCOONabuffer increased. To keep the QDs as stable as possible and toensure sensitive determination of arginine, 1 mM of CH3COOH-CHCOONa buffer solution was used (Fig. 6B). The concentrationof ATP-capped Mn-doped ZnS QDs influence the quenched phos-phorescence intensity caused by arginine (Fig. 6C). 7 mg L-1 ofATP-capped Mn-doped ZnS QDs gave the highest sensitivity. Thequenched phosphorescence of ATP-capped Mn-doped ZnS QDsvaried with Mg+ concentration (Fig. 6D). The quenchedphosphorescence intensity of the QDs increased rapidly as theconcentration of Mg+ increased from 10 to 100 uM, andremained unchanged with further increase of the concentrationof Mg+. Therefore, 100 uM of Mg+ solution was used for therest experiments. 3.4. Characteristic data of the ATP-capped Mn-doped ZnS QDs forthe phosphorescence detection of arginine Under the above optimal conditions, the phosphorescenceintensity of the ATP-capped Mn-doped ZnS QDs graduallydecreased as the concentration of arginine increased in thepresence of Mg2+(Fig. 7A). Statistical analysis of the quenchedphosphorescence intensity versus the arginine concentration(CArginine))1revealed two linear rannggeess for arginine sensing(Fig. 7B). The quenched phosphorescence intensity (AP) linearlyincreased with arginine concentration from 1 to 10 uM with acalibration function of ^P=171.3CArginine+158.7 (R’=0.992), andfrom 10 to 35 uM with a calibration function of AP=50CArginine+1349.5 (R=0.997). The phenomenon of two linear ranges forarginine sensing probably resulted from the integration of theMg+-ATP-arginine ternary system and the hydrolysis of ATPcatalyzed by Mg+and arginine. However, the exact reasons arestill not clear at the present stage and need further investigation.The developed ATP-capped Mn-doped ZnS QDs gave the detectionlimit (LOD) (3s) of 0.23 uM for arginine with the relativestandard deviation of 1.7% for eleven replicate detections of10 uM arginine. 3.5. Selectivity of the ATP-capped Mn-doped ZnS QDs for thephosphorescence detection ofarginine To demonstrate the selectivity of the ATP-capped Mn-dopedZnS QDs for the phosphorescence detection of arginine based onMg+-ATP-arginineternary system, tthephosphorescenceresponse of the ATP-capped Mn-doped ZnS QDs to arginine,methylated arginine (ADMA and SDMA), other 17 species ofamino acids and glutathione were investigated (Fig.8). Tyrosine,lysine, valine, serine, leucine, cystine, proline, aspartate, alanine,histidine, threonine, phenylalanine,methionine, glutarnate, tryp-tophan, citrulline, and cysteine were chosen since they are themost studied amino acids in previous QD-based amino acidsprobes. As shown in Fig. 8, other 17 species of amino acids andglutathione caused negligible phosphorescence response. In 14 4000- C 3000- 2000- 1000- 0 2 4 6 8 Concentration of ATP-Mn-ZnS QDs /mg L Fig. 6. Dependence of phosphorescence quenching of ATP-capped Mn-doped ZnS QDs caused by arginine (200 uM) on:(A)pH (6 mg L-1 ATP-capped Mn-doped ZnS QDs,1 mM CHCOOH-CHCOONa buffer, 200 pMMg+);(B) concentration of CHCOOH-CHCOONa buffer (6 mg L-1ATP-capped Mn-doped ZnS QDs, pH 6.0, 200 pM Mg+);(C) concentration of ATP-capped Mn-doped ZnS QDs (1 mM CH,COOH-CH;COONa buffer, pH 6.0, 200 uM Mg2+); (D) concentration of Mg2+(7 mg L-1ATP-capped Mn-doped ZnS QDs, 1 mM CH;COOH-CH,COONa buffer, pH 6.0). AP=Po-P denotes the quenched phosphorescence intensity of ATP-capped Mn-doped ZnS QDs. Fig. 7. (A) Effect of the concentration of arginine on the phosphorescence spectraof the ATP-capped Mn-doped ZnS QDs in the presence of Mg+; (B) Plots of thequenched phosphorescence intensity as a function of arginine concentration,showing two linear ranges. Buffer, 1 mM CHCOOH-CH,COONa (pH 6.0); ATP-capped Mn-doped ZnS QDs, 7 mg L-1; Mg?+, 100 pM. AP denotes the quenchedphosphorescence intensity of ATP-capped Mn-doped ZnS QDs. contrast, only arginine and methylated arginine resulted insignificant phosphorescence quenching of the ATP-capped Mn-doped ZnS QDs. The above results show excellent selectivity ofthe developed probe for arginine and methylated arginine withsame sensitivity (Fig. 8; Fig. S4 in the Supplementary Data). The ATP-capped Mn-doped ZnS QDs also gave excellentselectivity for detecting arginine in the presence of main relevant metal ions, biomolecules and other amino acids in urine samples(Table S2 in the Supplementary Data). Quenching of the phos-phorescence emission due to the addition of arginine at 3 pM wasunaffected by 1 mM Na+, 4 mM K+, 800 uM Ca2+,500 pM Mg+,10 uM Zn2+,8 uM Mn2+,2 mM glucose, 200 pM urea, 30 uM uricacid, 200 pM ascorbic acid, 100 pM citric acid, 50 mg L- HSA,30 pM tyrosine, 50 pM lysine, 50 uM valine, 50 pM serine, 50 uMleucine, 30 uM cystine, 50 pM proline, 50 uM aspartate, 50 pMalanine, 30 pM histidine, 50 pM threonine, 50 pM phenylalanine,50 uM methionine, 30 uM glutamic acid, 30 uM tryptophan, 6 uML-cysteine and 5 uM glutathione. The interference from L-cysteineand glutathione likely resulted from the strong affinity of the thiolgroups on L-cysteine and glutathione to the QDs surface. As theconcentration of L-cysteine and glutathione in human urinesamples are about 85 and 23 uM, respectively [40], no suchinterference was observed when 50-fold dilution of urine sampleswere performed before analysis (Table 1). For the analysis ofsamples with high concentration of the L-cysteine and glu-tathione, the interferences from L-cysteine and glutathione canbe eliminated or minimized by using thiol-blocking reagents,such as N-ethylmaleimide. 3.6. Determination of arginine and methylated arginine in urinesamples Table 1Analytical results for arginine and methylated arginine in human urine samples. Sample Concentration found (mean±s, n=3)(uM) Recovery(%) Urine 1 134±6 94.4+3 Urine 2 148±17 97.8+4 Urine 3 107±9 95.8±3 Urine 4 144±28 96.1±5 The found concentrations were already adjusted for the dilution factor,thereby representing the total concentration of arginine and methylated argininein the original urine samples. " For spiking 150 uM arginine in original urine samples. 4. Conclusions In summary, we have reported an ultrasonic assisted synthesisof ATP-capped Mn-doped ZnS QDs for selective detection ofarginine and methylated arginine based on the specific recogni-tion nature of Mg+-ATP-arginine ternary system in combinationwith the phosphorescence property of Mn-doped ZnS QDs. Thedeveloped probe gives excellent selectivity and reproducibility,and low detection limit. The developed phosphorescence probefavors biological applications since the interference from scatter-ing light and autofluorescence is effectively eliminated. Acknowledgments This work was supported by the National Basic ResearchProgram of China (No. 2011CB707703), the National NaturalScience Foundation of China (No. 20935001), and the TianjinNatural Science Foundation (No. 10JCZDJC16300). Appendix A. Supporting information Supplementary data associated with this article can be found inthe online version at http://dx.doi.org/10.1016/j.talanta.2012.03.055. References ( [1] G. Wu, S.M. Morris, Biochem. J. 336(1998) 1-17. ) ( [2] A. Barbul,J. Parenter. Enteral. Nutr. 10 (1986)227-238. ) [3] V. Gopalakrishnan, P.J. Burton, T.F. Blaschke, Anal. Chem. 68 (1996) 3520-3523. [4] D.L. Olson, M.E. Lacey, A.G. Webb, J.V. Sweedler, Anal. Chem. 71 (1999)3070-3076. ( [5] R.M. de Ordufa, J. Agric. Foo d Chem. 49 (2001) 549-552. ) ( [6] S. Bahl, S. Naqvi, T.A. Venkitasubramanian,J. Agric. Fo o d Chem. 2 4 (1976) 56-59. ) ( [7] A. Bastone, L. Diomede, R . Parini, F. Carnevale, M. Salmona, Anal. Biochem. 191 (1990)384-389. ) [8] H.R. Roberts, M.G. Kolor, Anal. Chem. 31 (1959)565-566. ( [9] : S .M. S ibalic, N.V . Radej, Anal. Chem . 33 (1961 ) 1223-1224. ) ( [10] F.G. Bánica, D. G uziejewski, S . Skrzypek, W. Ciesielski, D . K a zmierczak, Electroanalysis 2 1 (2009 ) 1711-1718. ) ( [11] T. Miura, M. Kashiwamura, M. Kimura, Anal. Biochem. 139 (1984) 432-437. ) ( [12] M.J. Ruedas- R ama, E.A.H. Hall, Anal. Chem. 80 (2008)8 2 60-8268. ) ( [13] W.C.W. Chan, S. Nie, Science 28 1 (1998) 2016-2018. ) ( [14] M . Bruchez, M . Moronne, P. Gin, S. We i ss, A.P. Alivisatos, Science 281 (199 8 )2013-2016. ) [15] W.C.W. Chan, D.J. Maxwell, X. Gao, R.E. Bailey, M. Han, S. Nie, Anal.Biotechnol. 13(2002) 40-46. ( [16]X . M i chalet, F. F . Pinaud, L.A. Bentolila, J.M. Tsay, S. Doose, J.J.Li, G. Sundare- san, A.M. Wu, S.S. Gambhir,S. W eiss, S cience 3 07 ( 2005) 5 38-544. ) [17] ]I.L. Medintz, H.T.Uyeda, E. Goldman, H. Mattoussi, Nat. Mater. 4 (2005)435-446. ( [18] Y.Q. Ma, G.X. Lu, Dalton Trans. (2008)1081-1086. ) ( [19] M . F okkens, T. Schrader, F.G. Klarner, J. Am. Chem. Soc. 127 (2005) 14415-14421. [20] M .F. Bush, J.T. O 'Brien, J.S. Prell, R.J. Saykally, E.R. Williams, J. Am. Chem. Soc. 129 (2007) 1612-1622. ) ( [2 1 ] A.S. Woods, S. Ferre, J. Proteome Res. 4(2005 ) 1397-1402. ) ( [22] A.S. Woods, J. Proteome R e s. 3 ( 2004) 478-484. ) ( [23] M.J. Rashkin, R.M. Hughes, N.T. Calloway, M . L. Waters, J. Am. Chem. Soc. 12 6 (2004)13320-13325. ) ( [24] R .M. Hughes, M.L. Waters, J. Am. Chem. Soc. 128 (2006)13586-13591. ) ( [251 J .L. Luche, C. Bianchi , Plenum Press, New York, 1998, pp. 91. ) ( [26] H .F. Wang, Y. H e, T.R. Ji , X.P. Ya n , Ana l . Chem . 81 (2009) 1615-1621. ) ( [27] Y . H e, H.-F. Wang, X.-P. Yan, Anal . Chem. 80 (2008) 3832-3837. ) ( [28] H .-B. Ren, B.-Y. Wu, J.-T . Chen , X.-P. Yan, Ana l . Chem. 83 (2011)8239-8244. ) [29]J].H. Choi, K.H. Chen, M.S. Strano, J. Am. Chem. Soc. 128 (2006)15584-15585. ( [30]J . Q. Zhuang, X.D. Zhang, G. Wang, D.M. Li, W .S. Yang, T.J. Li, J. Mater. Chem. 13 (2003)1853-1857. ) ( [31] M. Green , D . S myth-Boyle, J. H arries, R. Taylor, Chem. C ommun. (2 0 05)4830-4832. ) ( [32] H. Sigel, Inorg. Chi m . Acta 198-200(1992) 1-11. ) ( [33] M . Green, R. Taylor, G. Wakefield, J. Mater. Chem. 1 3 ( 2003)1859-1861. ) [34] K. Sauer, H. Scheer, P. Sauer, Photochem. Photobiol. 46 (1987) 427-440. ( [35] J.R . Lakowicz , G. Weber , Biochemistry 12 (1973) 4161-4170. ) ( [36] R.M. Jones, T.S. Bergstedt , D.W. McBranch, D .G. W hitten, J. Am. C hem. S o c. 123 (2001)6726-6727. ) ( [37] C.B. Murphy, Y . Z h ang, T. T r oxler, V . F e rry, JJ. M artin, W . E. J o nes, J . Phys. Chem. B 108 ( 2004) 1537-1543. ) ( [38] M.S. Baptista, G.L. Indig, J. Phys. Chem. B 10 2 (1998)4678-4688. ) ( [39] J.R. Lakowicz, Principle of Fluorescence Spectroscopy, 2nd ed., Plenum Pr e ss, N ew York, 1999, pp. 237-259. ) ( [40] Y . Zhang, Y. L i, X . -P. Yan, Anal. Chem. 81 ( 2009) 5001-5007. ) ( [41 ] J.M. Lobenhoffer, S.M. Bode-Boger, J.Chromatogr. B 7 98 (2003) 231-239. ) 基于超分子Mg2þ-三磷酸腺苷-精氨酸三元体系的超声辅助合成三磷酸腺苷封端的锰掺杂ZnS量子点用于选择性室温磷光检测尿液中的精氨酸和甲基化的精氨酸 简介 精氨酸是生物系统中的一个重要组成部分,不仅是一氧化氮的前体,也是蛋白质、尿素、脯氨酸、多胺、谷氨酸、琼脂的前体。和肌氨酸[1]。精氨酸甲基化反应的主要产物是NG,NG-二甲基精氨酸(ADMA)和NG,NG0-二甲基精氨酸(SDMA),这些甲基化的精氨酸在蛋白质周转和分解过程中被释放出来[1]。随着人类发现新的精氨酸分解和合成途径,人们越来越认识到,精氨酸是成年人类的一种条件性必需氨基酸,特别是在创伤或疾病的情况下[2]。由于精氨酸在生物学上的重要性,人们开发了相当多的方法来测定生物液体中的精氨酸和甲基化精氨酸,这些方法基于高效液相色谱法[3]、毛细管电泳法[4]、酶学终点分析法[5]、薄层色谱法[6]、氨基酸分析仪[7]、离子交换纸色谱法[8]、高温纸色谱法[9]和伏安法[10]。即使如此,一种简单、高选择性、高灵敏度、瞬时反应的检测精氨酸和甲基化精氨酸的方法仍是迫切的、新出现的挑战。由于荧光探针在灵敏度和便利性方面比其他方法有明显的优势,因此光致发光光谱法是检测精氨酸和甲基化精氨酸的首选技术。Miura等人[11]报道了有机染料作为光致发光探针的应用,用于高度精确地检测精氨酸。量子点(QDs)比有机染料具有独特的优势,如巨大的光稳定性、高的光致发光效率、随尺寸变化的发射波长、宽广的辐射和清晰的发射图,因此QDs已被广泛用于生物分析[12-17]。然而,据我们所知,到目前为止,还没有关于利用功能化QDs检测精氨酸和甲基化精氨酸的工作报告。我们研究了Mg2þ和精氨酸与三磷酸腺苷(ATP)的超分子相互作用,以了解精氨酸和Mg2þ在分子水平上对ATP裂解机制的确切功能。ATP的水解是由精氨酸和Mg2 þ催化的,Mg2 þ参与催化机制,作为ATP的结合点或作为辅助因子。在复杂的生物系统中,基于Mg2 þ-ATP-精氨酸的ATP水解的选择性非酶催化作用三元系统的ATP水解的非酶催化作用得到了极大的关注[18-24]。在此,我们展示了超声波辅助合成的ATP封尾的Mn-掺杂ZnS QDs,通过利用Mn-掺杂ZnS QDs出色的光学特性和Mg2 þ -ATP-精氨酸三元体系对精氨酸的精确识别,快速、选择性和灵敏地检测精氨酸。超声波方法被用于合成ATP封端的QDs,因为它具有快速、简单、低成本和高效的优点[25]。掺锰的ZnS QDs被用作光致发光探针,因为其毒性低,磷光寿命长,可以有适当的延迟时间来避免任何背地荧光发射和散射光的干扰[26-28]。在合成功能性QDs时选择ATP作为封盖配体,为合理控制Mn掺杂的ZnS QDs特性提供了强有力的手段。ATP可以使Mn掺杂的ZnS QDs稳定地防止聚集和水溶性,并保留所需的核苷酸结构以专门结合到其目标[29]。在有Mg2þ存在的情况下,ATP封顶的Mn-doped ZnS QDs与精氨酸的特定结合导致QDs的磷光的高度选择性淬灭,允许选择性地检测精氨酸,检测极限为0.23 mM。 结论 综上所述,我们报告了一种超声波辅助合成的ATP封尾的Mn-doped ZnS QDs,用于选择性地检测精氨酸和甲基化精氨酸,其依据是Mg2þ-ATP-精氨酸三元系统的识别性质与Mn-doped ZnS QDs的磷光特性相结合。所开发的探针具有良好的选择性和重现性,并且检测限低。由于有效地消除了散射光和自发荧光的干扰,开发的磷光探针有利于生物应用。
确定




还剩5页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《尿液中精氨酸和甲基化精氨酸检测方案(微波合成仪)》,该方案主要用于尿液中生化检验检测,参考标准--,《尿液中精氨酸和甲基化精氨酸检测方案(微波合成仪)》用到的仪器有电脑微波超声波组合催化合成萃取仪、电脑微波超声波组合合成萃取仪 Soniwave XH-300A+型
推荐专场

















