GC-O-MS技术正是一个用于研究食品风味的强大工具,广泛应用于各种食品的香气和风味分析。GC-O-MS技术可以解决食品中的多种风味问题如“气味活性化合物的图谱锁定“,“关键气味活性化合物的鉴定“等。 借此机会,我们向您介绍哲斯泰在食品风味研究领域的优秀解决方案 “热脱附+嗅觉检测口” ,并且结合气味物质提取三大法宝“动态顶空DHS” + “搅拌棒吸附萃取SBSE”+“固相微萃取SPME”。
方案详情

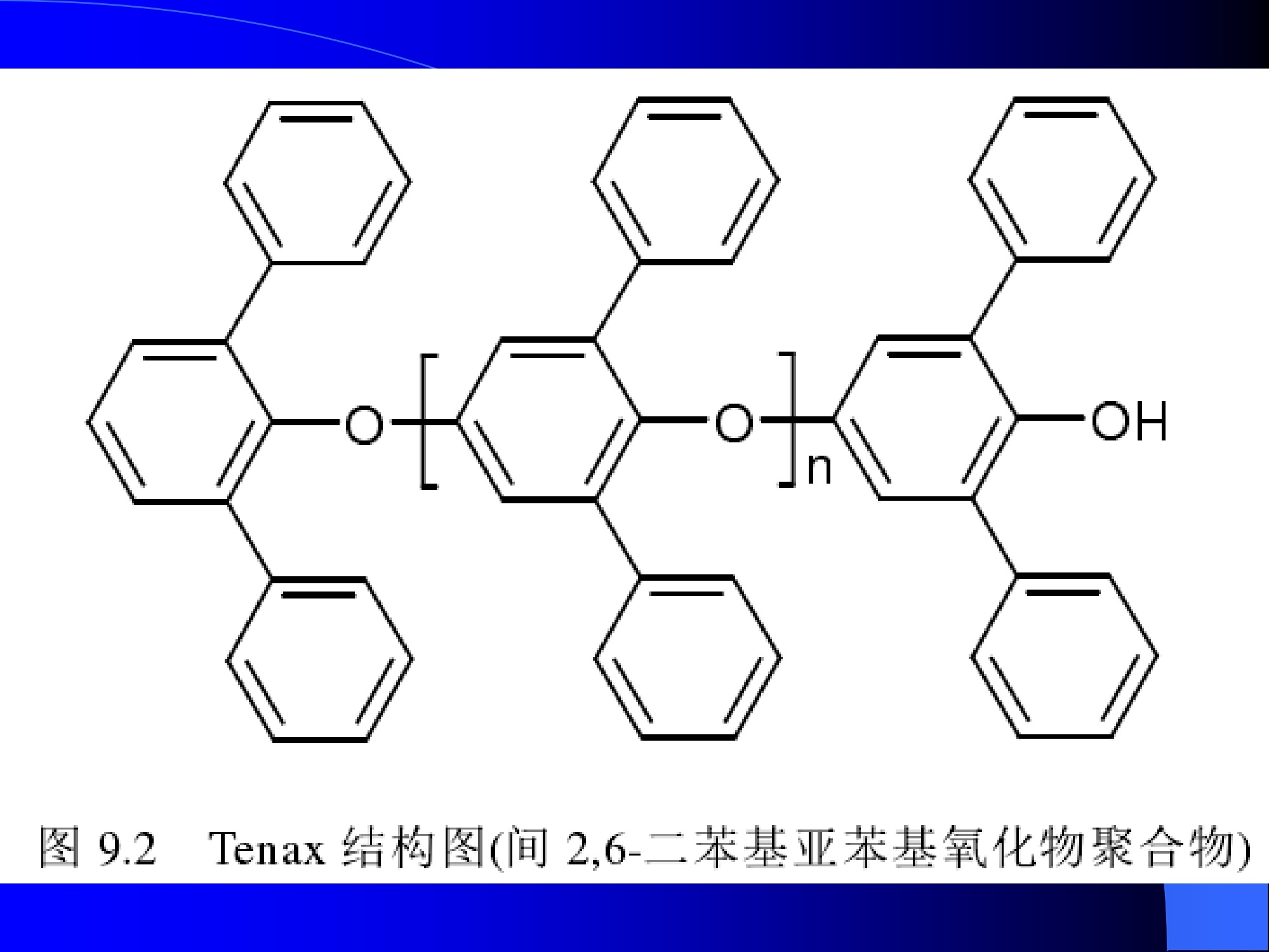
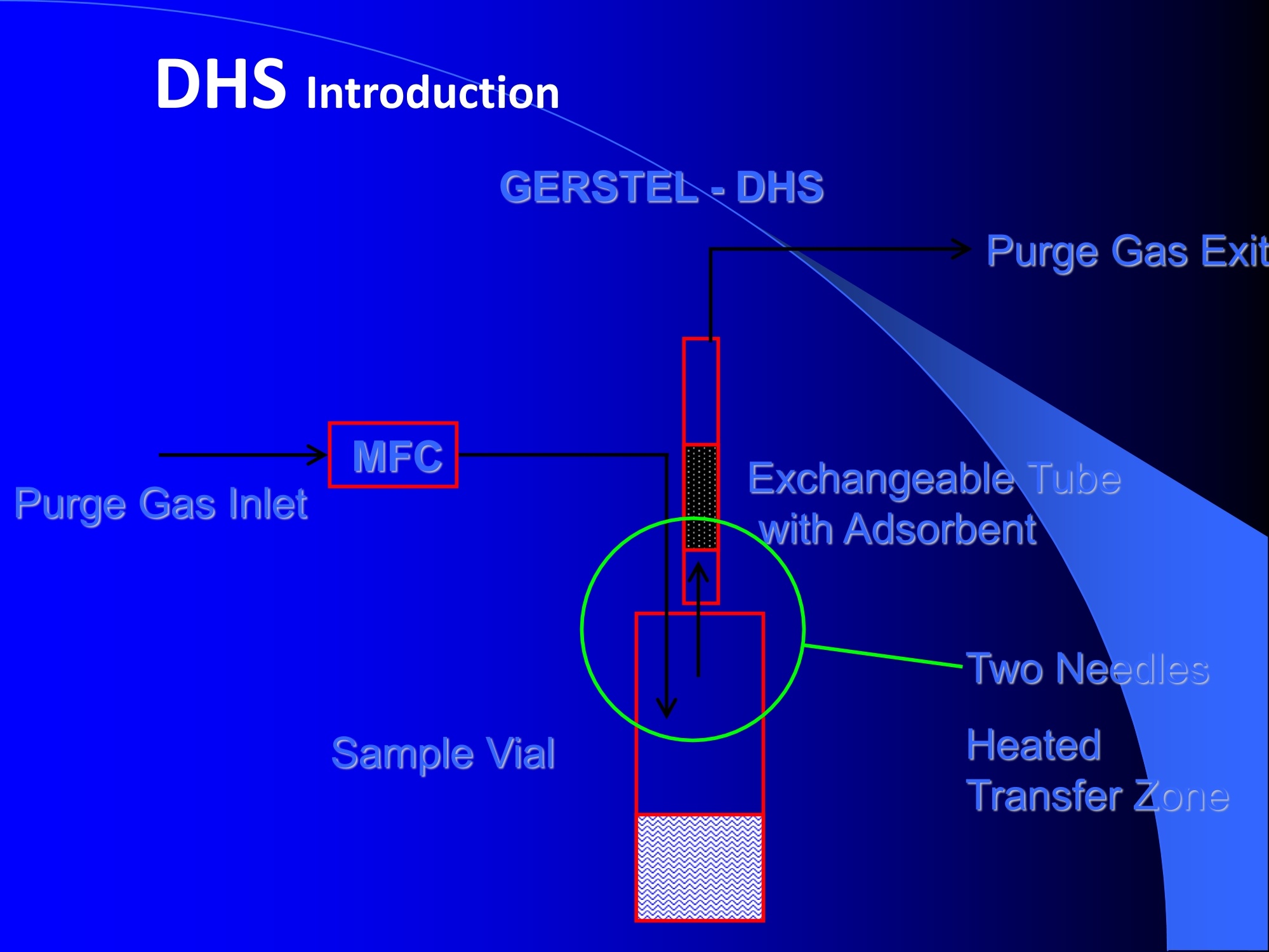
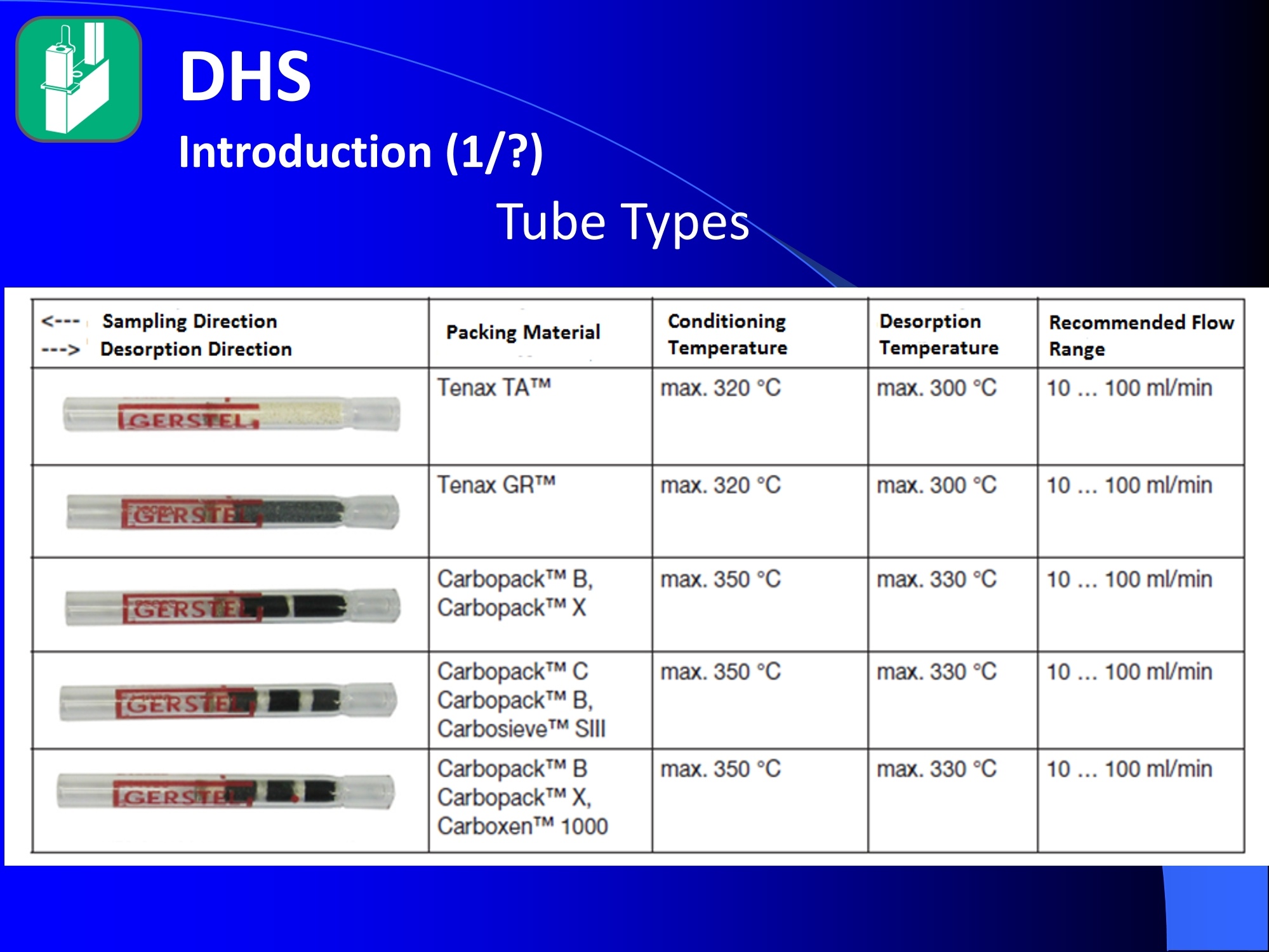
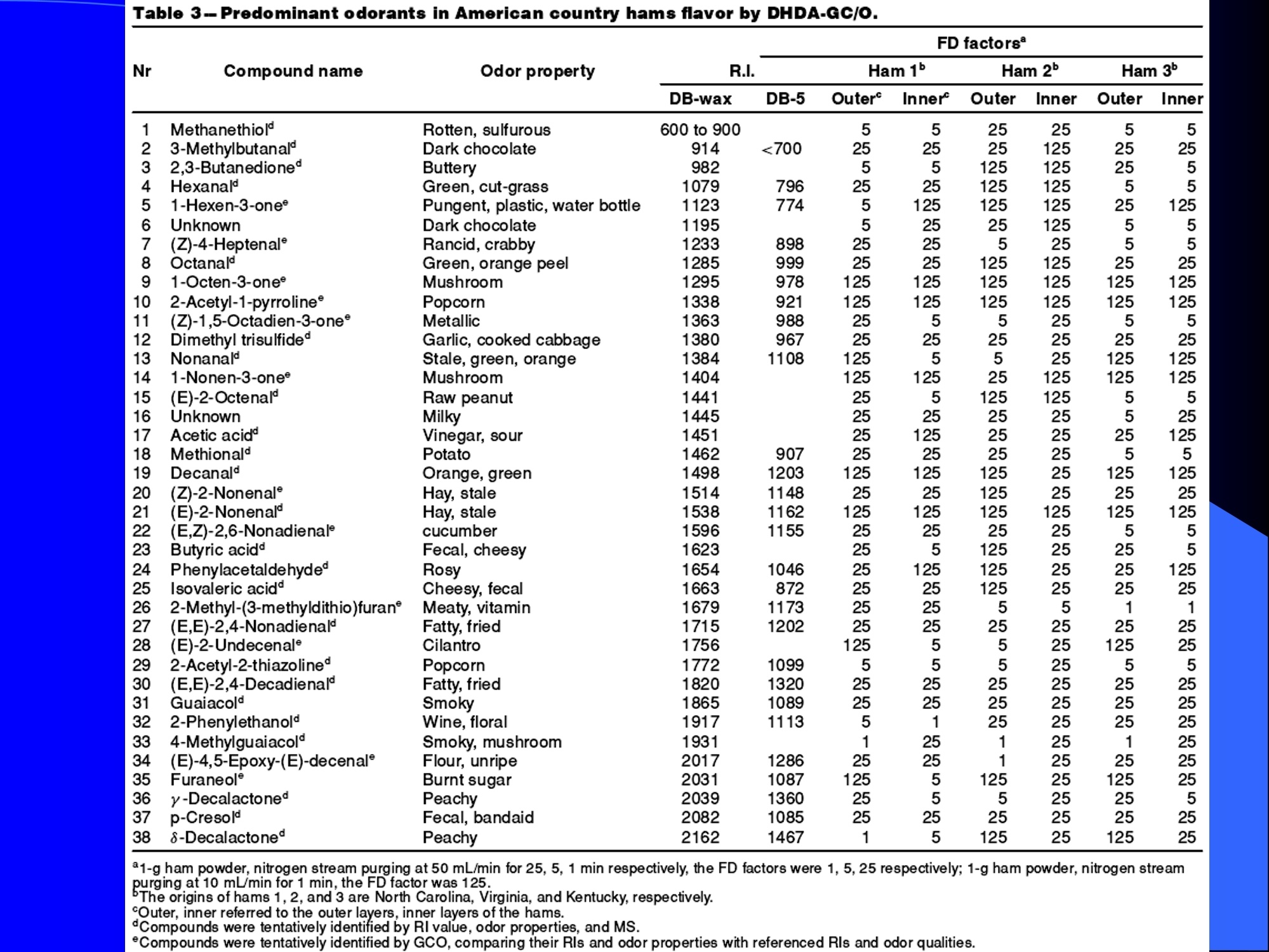
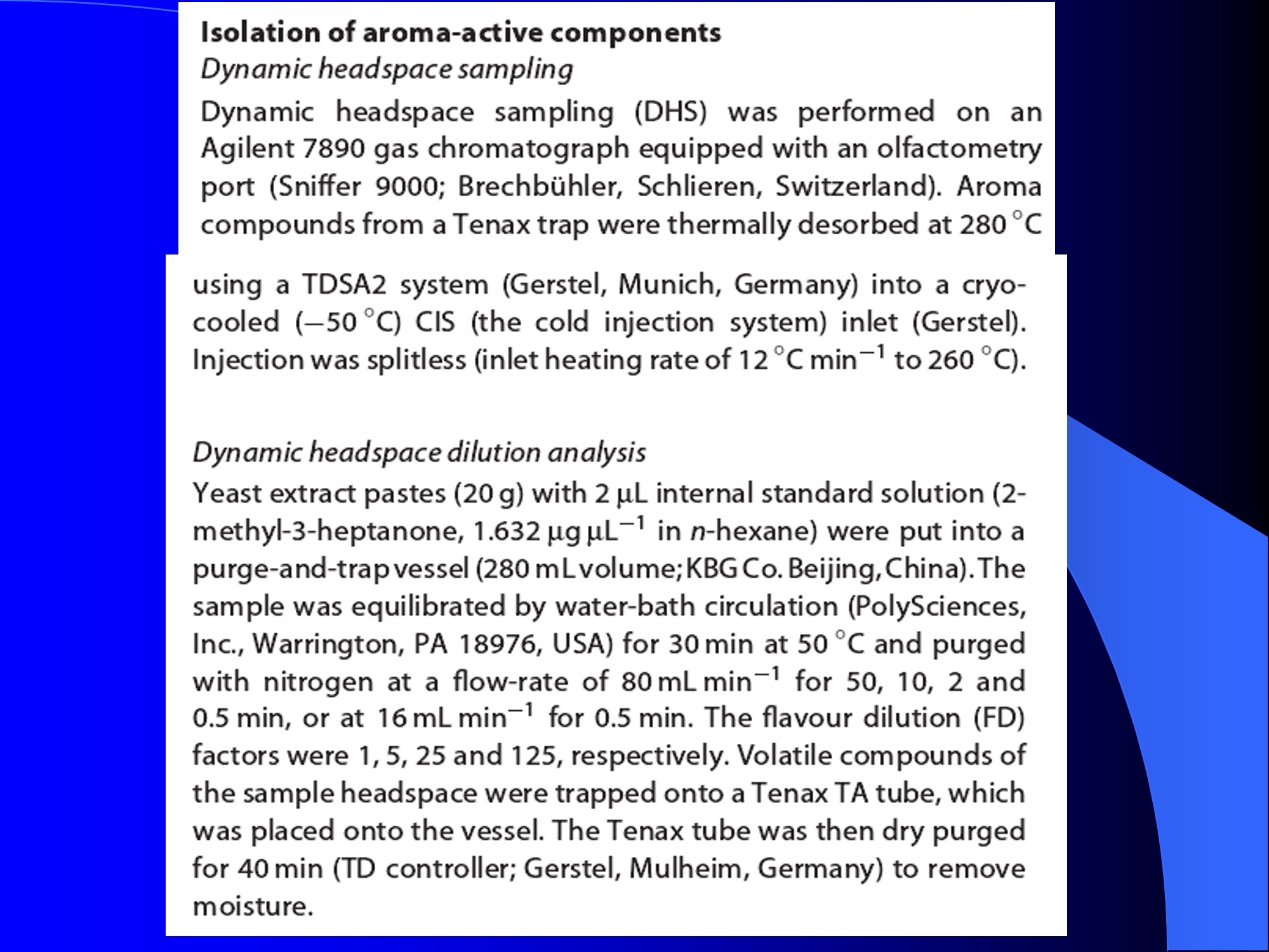
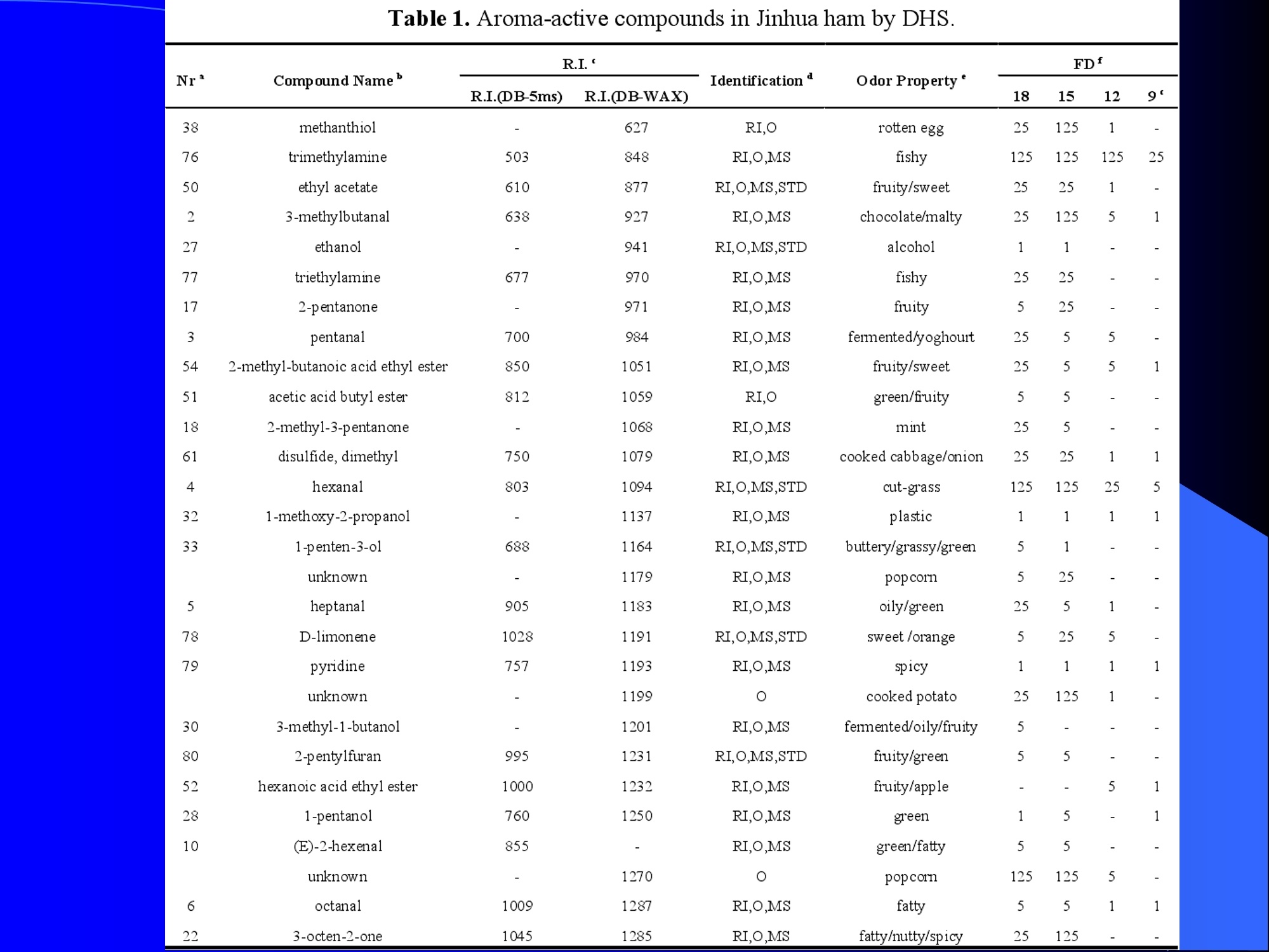
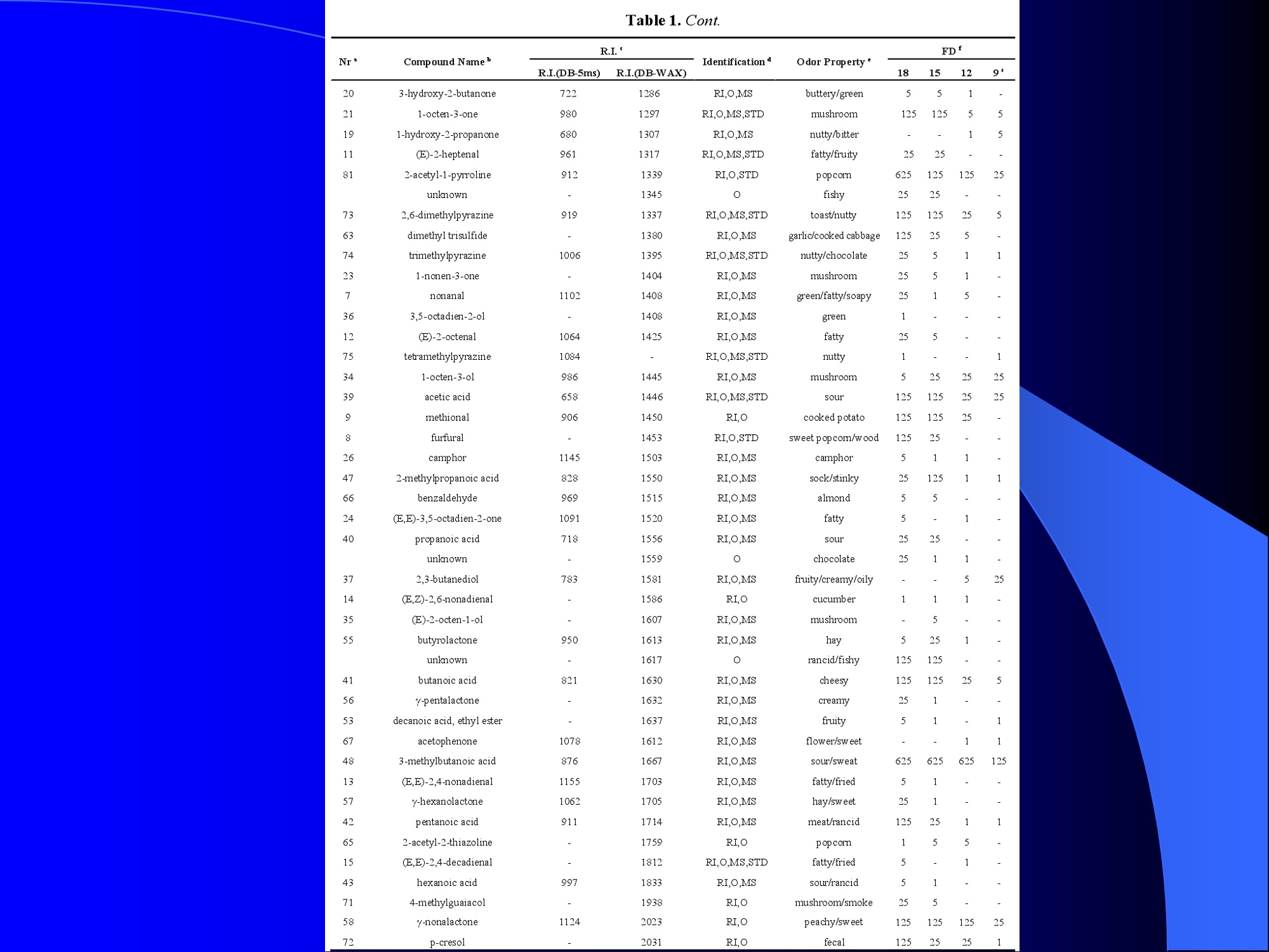
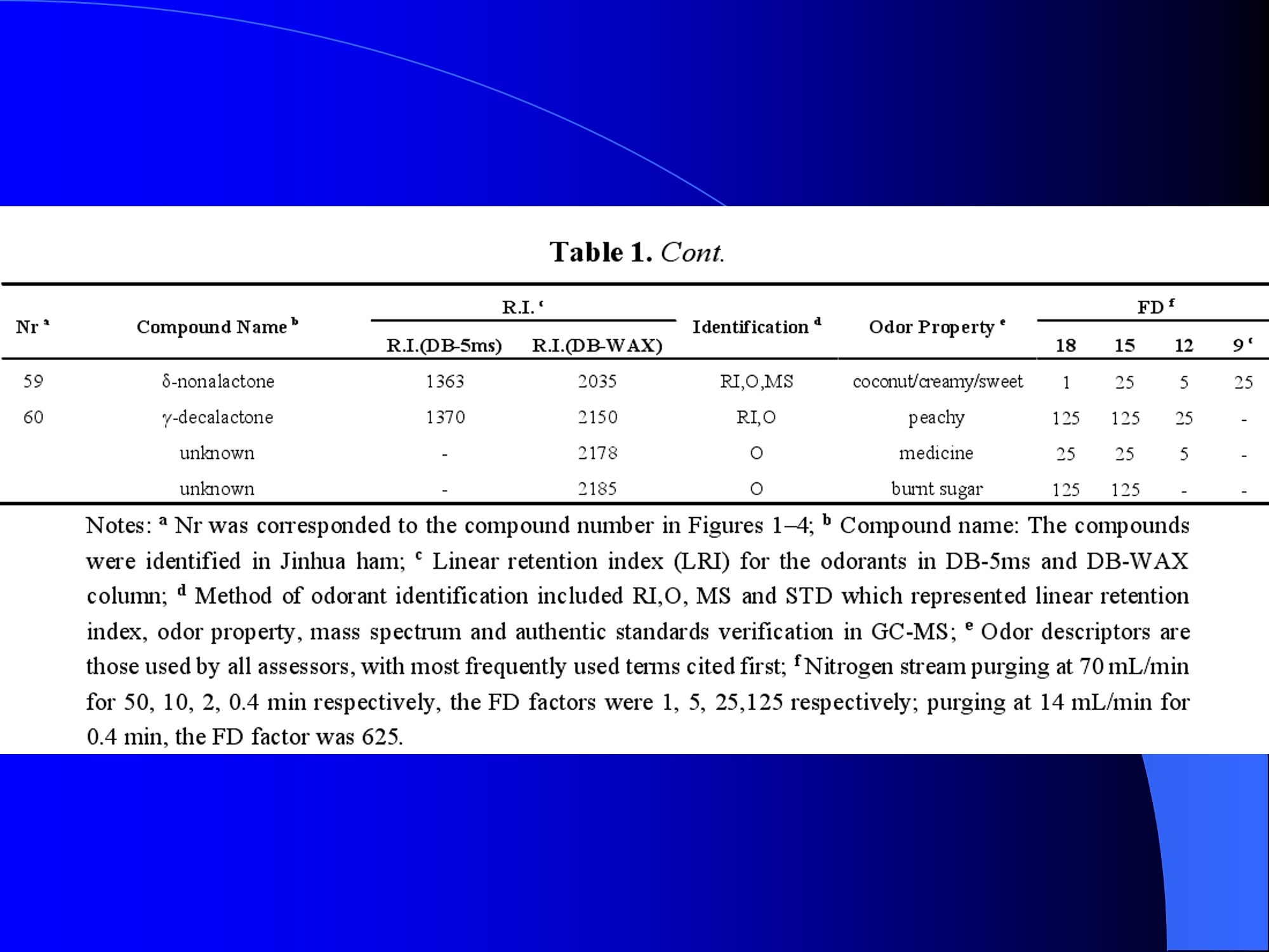
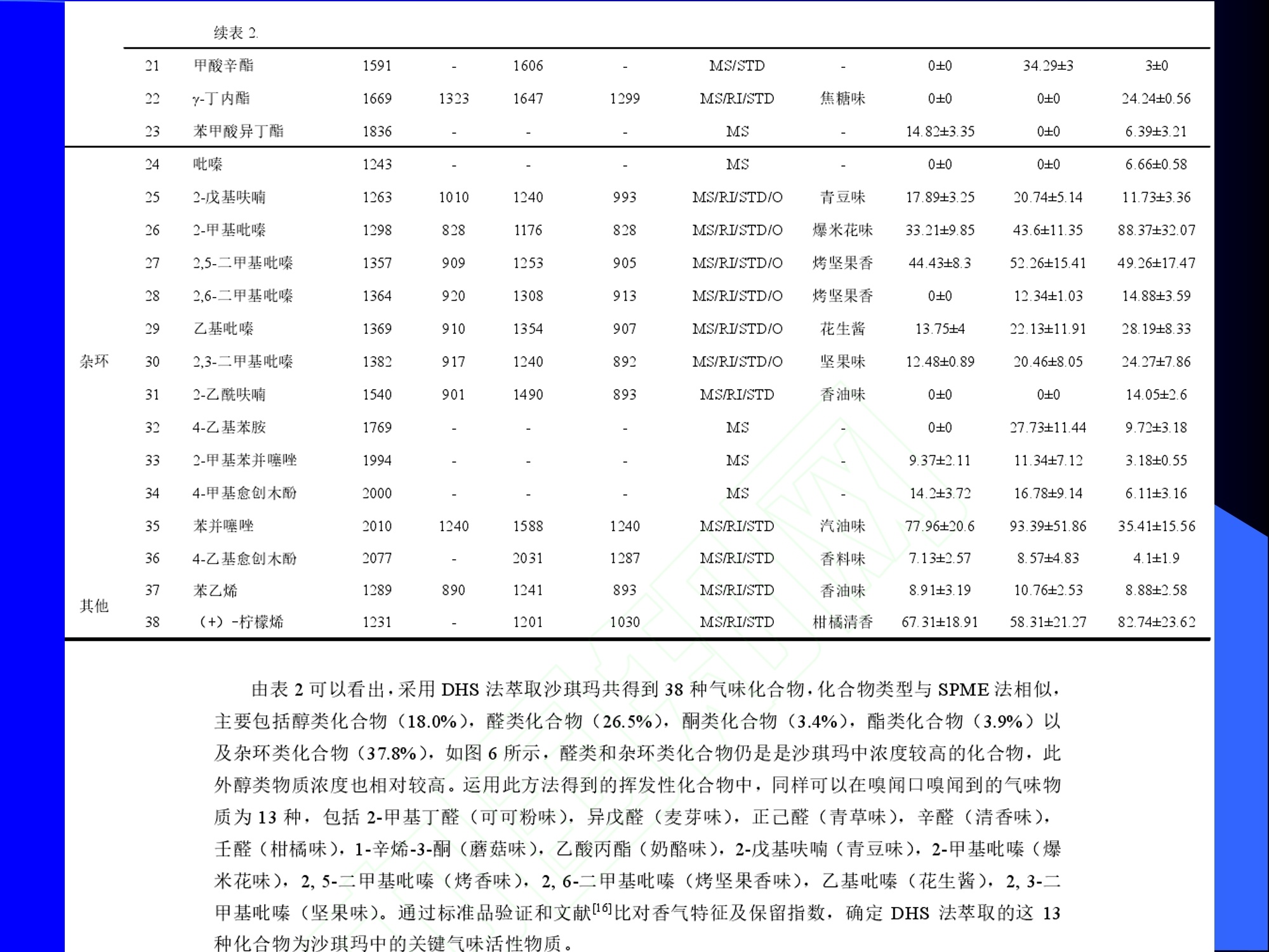
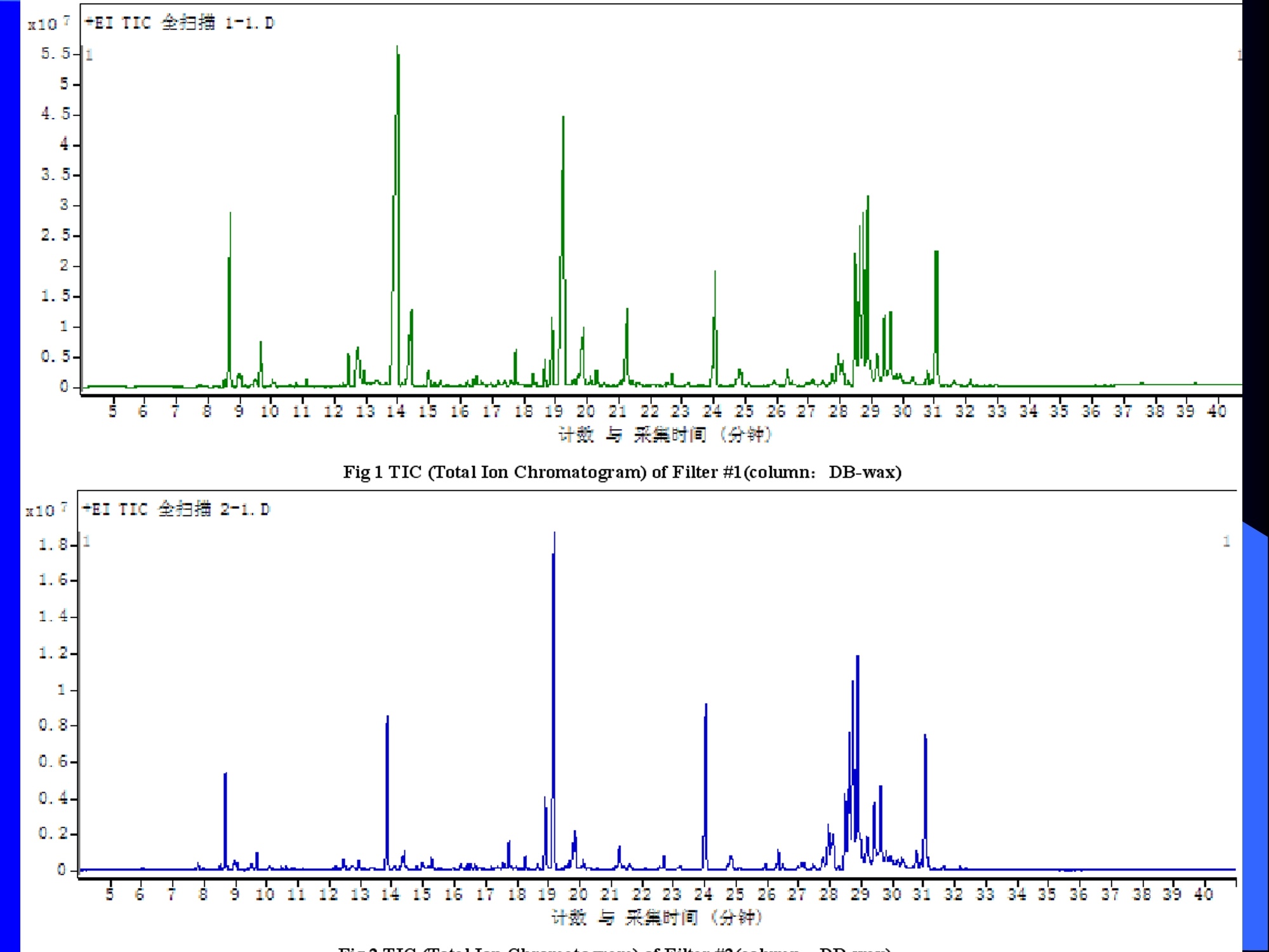
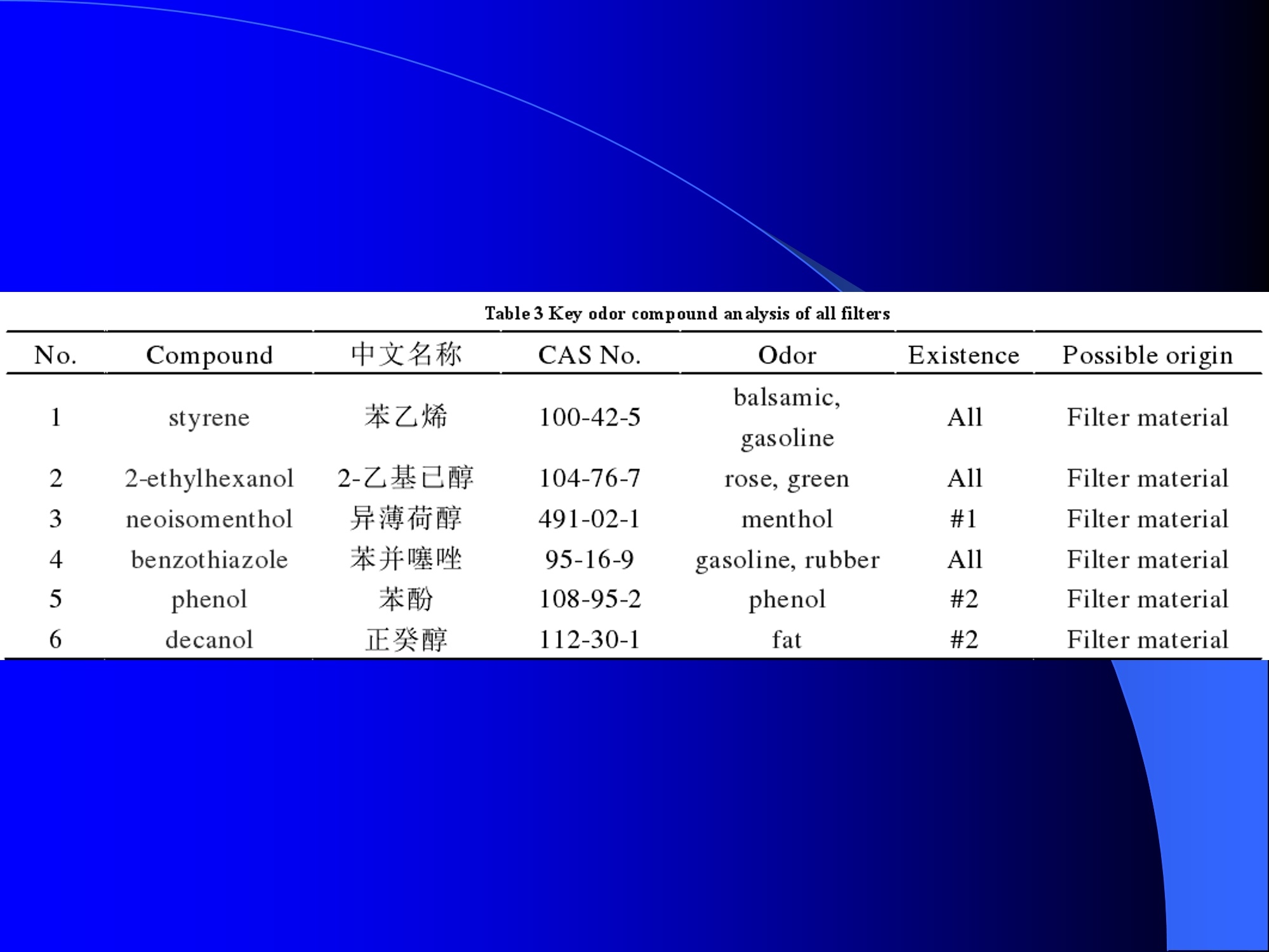
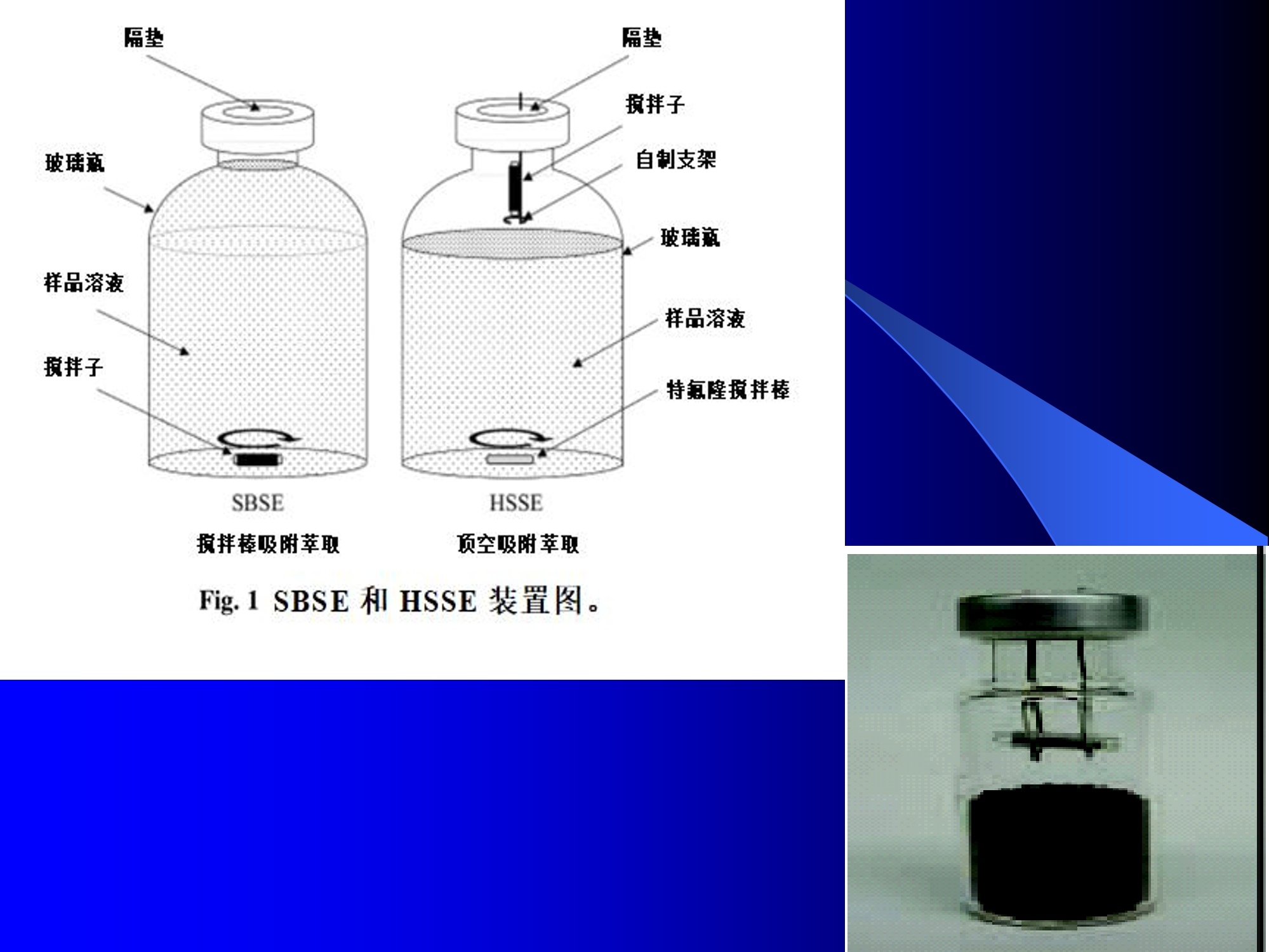
Dynamic Headspace Sampling Introduction (1/?) 动态顶空制样(DHS) 、口无乔气 在食品香气分析中的应用 北京工商大学食品与健康学院 宋焕禄 2019.10.25 DHS的基本原理 动态顶空样品制备 (Dynamicheadspace sampling-DHS) 用惰性气体如氮气对密闭容器中样品表面进行吹扫,那么样品的顶空中的香气成分便会被载(如 气带出来,当混合气体通过一个捕集物Tenax柱)时,就会被吸收。这样经过一段时间Tenax柱上就会聚集一定量的香气化合物。然后将Tenax柱加热让香气化合物挥发出来,进入气相色谱仪或气一质联用进行成分分析。Tenax柱中的填料是一种多聚物吸附剂,称为聚(2,6-二苯基-p-苯烯基氧化物),,能有效地吸附香气挥发物,现已被广泛应用于食品香气、水中挥发性 有机物 (VOC)以及环境检测中。 - —OH 图9.2 Tenax 结构图(间2,6-二苯基亚苯基氧化物聚合物) useful method for samples containing low levelsofvolatile compounds in the headspace. an inert gas (nitrogen or helium) is swept gt over orthrough the thermostatted sample for a period oftime sufficient to “extract”most of the volatilesample constituents. (references 17-22) Purge & Trap DHS C LDRIGOH VW MOSE OUEN tin - E 559 使用动态顶空的两个关键环节包括冷阱捕集和热脱附。,而热解吸的目的是让捕集的气味成分以很窄的形式瞬间进入气相色谱柱进行分析。快速加热解吸的过程,角解吸时间短,样品在更短的时间内进入气相色谱,这样引入到色谱系统的载气、水、二氧化碳的量就会很少,从而更好的提高了气相色谱分析的分离度及检测的灵敏度。 冷阱捕集也称为低温冷冻浓缩,它可以 避免吸附材料在高温热解析时有芳香族 杂质释放出来,对微量分析结果产生干 扰,不在冷冻浓缩时一般不使用吸附剂,而是在冷阱捕集管内填充一些玻璃珠、玻璃棉或其他的惰性材料以增加接触面 积。 为保证香气萃取物以一个“窄带 进入毛细管柱,冷冻浓缩液气化后,可以再经过一次冷聚焦(cryofocusing 从而提高气相色谱分析的分辨率与灵敏度。样品冷冻浓缩一般用-196℃的液氮做冷却剂,因为液氮的冷冻浓缩效果非常好9可以使很多强挥发性易于穿透吸附剂的物质被很好地冷凝。 Thermal Desorption Unit (TDU) TDU Twisteerr- wister PTV PTV-Liner GERSTEL-DHS MFC Purge Gas Inlet Exchangeable Tubewith Adsorbent -Two Needles Sample Vial HeatedTransfer Zone Tube Types <---Sampling Direction ---> Desorption Direction Packing Material ConditioningTemperature DesorptionTemperature Recommended FlowRange GERSSIRTEL Tenax TATM max. 320°℃ max.300℃ 10 ... 100 ml/min CERSTEL Tenax GRTM max. 320°℃ max.300°℃ 10 ...100 ml/min GERSTEl CarbopackTM B,CarbopackTM X max. 350℃ max.330°℃ 10 ... 100 ml/min GERSTE CarbopackTM CCarbopackTM B,CarbosieveTM SII max. 350℃ max.330℃ 10 ... 100 ml/min GERS CarbopackMBCarbopackTM X,CarboxenTM 1000 max.350C max.330℃ ... 100 ml/min DHS TDU-Liner 一 Adsorbent. Trapping 20-70°C OC Thermaldesorption20-350°C Gas Sample. Extraction10-200°C selectable Sample introduction dry purge Optionalstep DHS 二应用 美国 乡村 try ham) Aroma Components of American Country Ham H. SONG AND K.R. CADWALLADER ABSTRACT: The aroma-active compounds of American country ham were investigated by using direct solventextraction-solvent assisted flavor evaporation (DSE-SAFE), dynamic headspace dilution analysis (DHDA), gaschromatography-olfactometry (GCO), aroma extract dilution analysis (AEDA), and gas chromatography-mass spec-trometry (GC-MS). The results indicated the involvement of numerous volatile constituents in the aroma of coun-try ham. For DHDA, 38 compounds were identified as major odorants, among them,1-octen-3-one, 2-acetyl-1-pyrroline, 1-nonen-3-one, decanal, and (E)-2-nonenal were the most predominant, having FD-factors 125 inall 3 hams examined,followed by 3-methylbutanal, 1-hexen-3-one,octanal, acetic acid,phenylacetaldehyde, andFuraneolTM. For the DSE-SAFE method, the neutral/basic fraction was dominated by 1-octen-3-one, methional,guaiacol, (E)-4,5-epoxy-(E)-decenal,p-cresol as well as 3-methylbutanal, hexanal,2-acetyl-1-pyrroline, phenylac-etaldehyde, and y-nonalactone. The acidic fraction contained mainly short-chain volatile acids (3-methylbutanoicacid, butanoic acid, hexanoic acid, and acetic acid) and Maillard reaction products (for example, 4-hydroxy-2,5-dimethyl-3(2H)-furanone). The above compounds identified were derived from lipid oxidation, amino acid degra-dation, and Maillard/Strecker and associated reactions. Both methods revealed the same nature of the aroma com-ponents of American country ham. Keywords: American country ham, aroma-active compound,aroma extractidilutionnanalysis, gaschromatography-olfactometry,solvent assisted flavor extraction Introduction Materials and Methods ry-cured ham is an important proD:duct worldwide. There are3 ham belts in the world, including the southeastern UnitedStates, southern and central Europe, and southern China. Virginia,Tennessee, Kentucky, and North Carolina are major producingareas for American salt-cured country hams. The 9- to 12-mo fer-mentation period gives a final product with a distinctive and pleas-ant flavor-being very different from that of thermally processedmeat products (Voltz and Harvell 1999; Arnold 2004). Samples and chemicals Hams. Hams 1,2, and 3 (raw, unsmoked) were purchased frommanufacturers located in North Carolina,Virginia, and Kentucky,respectively. The lean meat portions of the hams were cut into smallpieces (approximately1 cm), frozen in liquid nitrogen, and groundto fine powder. Chemicals. Diethyl ether (anhydrous, 99.8%), sodium chlo-A considerable amount of research has been conducted on dry-ridee(99%), sodium sulfate (anhydrous), sodium bicarbon-cured hams, mostly on European hams such as Iberian and Parmaate (99.7%), and hydrochloric acid (36.5%) were obtainedhams (Berdague and others 1991; Garcia and others 1991; Barbi-ifrom Fisher Scientific (Pittsburgh, Pa., U.S.A.). 2-Methyl-3-eri and others 1992; Ruiz and others 1998; Blank and others 2001;heptanone and 2-ethylbutanoic acid (internal standards forCarrapiso and others 2002a,2002b; Belitz and others 2004). But toneutral/basic fraction and acidic fraction, respectively) wereour knowledge,up to now, little information is available concerningobtained from Aldrich Chemical Co. (St. Louis, Mo., U.S.A.). Au-either the general flavor compounds or the aroma-active compo- thentic reference aroma compounds were also obtained fromnents of dry-cured American country ham. Gas chromatography-7mAldrich.olfactometry (GCO), including aroma extract dilution analysis(AEDA), has served as a very useful tool in flavor research foridentifying and ranking the key odorants in various foods (Grosh1993). The purpose of the present study was to identify and char-acterize the aroma-active compounds of American country hamby using gas chromatography-olfactometry (GCO), including bothdynamic headspace dilution analysis (DHDA) and aroma extractdilution analysis (AEDA) techniques. Isolation of volatiles for instrumental analysis MS 20070593 Submitted 7/28/2007,Accepted10/8/2007. Author Song is withCollege of Chemical & Environmental Engineering, Beijing Technology &Business Uniu, 11 Fucheng Rd., Beijing 100037, China. Author Cadwal-lader is with Dept.ofFood Science and Human Nutrition, Flavor ChemistryLaboratory, Univ. ofIllinois at Urbana-Champaign, 1302 W. Pennsylva-nia Ave., Urbana, IL 61801, U.S.A. Direct inquiries to author Song (E-mail:songhl@th.btbu.edu.cn). Direct solvent extraction-SAFE. Ham powder (100 g) wasplaced in a Teflon bottle and extracted with 100, 75, and 75 mL ofdiethyl ether 3 times, respectively. The solvent-phase extracts werecombined,concentrated using a Vigreux column to approximately100 mL, then applied to a modified SAFE apparatus (ACE Glass-ware, Vineland, N.J., U.S.A.) for solvent extraction. The SAFE ap-paratus was similar to that described by Engel (Engel and others1999), consisting of high vacuum pump, diffusion pump, receiv-ing tube, and waste tube, operating at high vacuum (10- to 10-5Torr) and very low temperature (-196°C) to trap volatile substanceand to avoid the production of artifact. Distillation was carried outfor2 h under vacuum. After distillation, the distillate was concen-trated to about 30 mL by Vigreux column. The concentrated distil-late was then extracted with aqueous NaHCO3 (0.5M,3×30 mL).The solvent phase (upper phase) was concentrated by gentle nitro-gen flow to about 10 mL, then dried over Na2SO4 (anhydrous) and ( ◎ 2 007 Institute of Food Technologists doi: 10.1111/j.1750-3841.2007.00593.x ) Dynamic headspace sampling (DHS). Ham powder (1 g) wasput into a purge-and-trap vessel (280 mL volume, SMS Co.). Af-ter equilibrating 5 min at 50°C (water-bath circulation),the sam-ple was purged with a nitrogen stream at a flow-rate of50 mL/minfor 25,5, or 1 min, or at 10 mL/min forl min, respectively. Volatilecompounds of the sample headspace were trapped onto a Tenax TAtube,which was placed onto the vessel. The Tenax tube was thendry purged for 20 min (TD controller, Gerstel, Mulheim Germany)to remove moistiire. Dynamic headspace dilution analysis (DHDA). DHDA wasperformed on an Agilent 6890 GC equipped with a flame ioniza-tion detector (FID) and an olfactometry port (Gerstel).Aroma com-pounds from Tenax trap were thermally desorbed at 280 °C usinga TDSA2 system (Gerstel into a cryo-cooled (-150"C) CIS inlet(Gerstel).Injection was splitless (inlet heating rate of 12 C/min to260 C). Two different capillary columns were used for identifying Compound name Odor property R.I. Ham 1 Ham 2" Ham 3 DB-Wax DB-5 Outer° Inner Outer Inner Outer 1 inner 1 Methanethiol Rotten, sulfurous 600 to 900 5 5 25 25 5 5 2 3-Methylbutanal Dark chocolate 914 <700 25 25 25 125 25 25 3 2,3-Butanedione° Buttery 982 5 5 125 125 25 5 4 Hexanal Green,cut-grass 1079 796 25 25 125 125 5 5 5 1-Hexen-3-one° Pungent, plastic, water bottle 1123 774 5 125 125 125 25 125 6 Unknown Dark chocolate 1195 5 25 25 125 5 5 7 (Z)-4-Heptenal° Rancid, crabby 1233 898 25 25 5 25 5 5 8 Octanal Green,orange peel 1285 999 25 25 125 125 25 25 9 1-Octen-3-one° Mushroom 1295 978 125 125 125 125 125 125 2-Acetyl-1-pyrroline° Popcorn 1338 921 125 125 125 125 125 125 (Z)-1,5-Octadien-3-one° Metallic 1363 988 25 5 5 25 5 5 Dimethyl trisulfide° Garlic, cooked cabbage 1380 967 25 25 25 25 25 25 Nonanal Stale, green, orange 1384 1108 125 5 5 25 125 125 1-Nonen-3-one° Mushroom 1404 125 125 25 125 125 125 (E)-2-Octenal Raw peanut 1441 25 5 125 125 5 5 Unknown Milky 1445 25 25 25 25 5 25 Acetic acid° Vinegar, sour 1451 25 125 25 25 25 125 Methional· Potato 1462 907 25 25 25 25 5 5 Decanal Orange,green 1498 1203 125 125 125 25 125 125 (Z)-2-Nonenal° Hay, stale 1514 1148 25 25 125 25 25 25 (E)-2-Nonenal° Hay, stale 1538 1162 125 125 125 125 125 125 (E,Z)-2,6-Nonadienale cucumber 1596 1155 25 25 25 25 5 5 Butyric acid Fecal, cheesy 1623 25 5 125 25 25 5 Phenylacetaldehyde° Rosy 1654 1046 25 125 125 25 25 125 Isovaleric acid° Cheesy, fecal a1-g ham powder, nitrogen stream purging at 50 mL/min for 25, 5, 1 min respectively, the FD factors were 1, 5, 25 respectively; 1-g ham powder, nitrogen streampurging at 10 mL/min for 1 min, the FD factor was 125. ( The origins of hams 1,2, and 3 a re No r th Carolina, Vi r ginia, and Kentucky, respectively. ) ( Outer, i n ner referred to the outer layers, inner layers of the hams. ) ( Compounds were tentatively identified by Rl value, od o r pr o perties, and MS. ) Compounds were tentatively identified by GCO, comparing their Rls and odor properties with referenced Rls and odor qualities. Received:25 February 2013 Revised: 19 July 2013 (wileyonlinelibrary.com) DOI 10.1002/jsfa.6330 Aroma-active components of yeast extractpastes with a basic and characteristic meatyflavour Meili Lin,@ Xiaosheng Liu,Qianqian Xu, Huanlu Song,a* Pei Li andJuan Yaob Abstract BACKGROUND:Aroma-active compounds,together with sugars,amino acids, fat and nucleotides, are the main chemical speciesdetermining the characteristic aroma and taste of food. For selecting yeast extract pastes products with a less undesirablearoma, the aroma-active compounds that affect the overall consumeracceptance of yeast extract pastes products were analysedin this work. RESULTS: The aroma-active compounds of yeast extract pastes were extracted by using dynamic headspace extractionor simultaneous distillation extraction, and were detected by gas chromatography-olfactrometry-mass spectrometry inconjunction with dynamic headspace dilution analysis or aroma extract dilution analysis. Sensory results revealed that ameaty, roasted aroma was the dominant of overall aroma. The important aroma-active compounds referred in this workwere mainly aldehydes, acids, ketones, furan derivatives, pyrazines,and sulfur-containing compounds. Of these, six volatilecompounds such as 3-methylbutanal,2,3-butanedione, 2,3,5-trimethyl-pyrazin, acetic acid ethenyl ester, 2-acetyl-1-pyrrolineand (E,E)-2,4-decadienal had never been reported before as key aroma-active compounds of yeast extract pastes. CONCLUSIONS: The key aroma-active compounds were identified in basic and characteristic meaty flavour yeast extract pastes,and their characterisation was determined. G 2013 Society of ChemicalIndustry Keywords: yeast extract pastes; aroma-active compounds; dynamic headspace sampling (DHS); simultaneous distillation extraction(SDE); gas chromatography-olfactrometry-mass spectrometry (GC-O-MS) INTRODUCTION Yeast extract pastes are popular sources of flavour for a rangeof savoury food products, particularly when a meaty aroma isrequired. Ames reported that yeast extracts were a source ofamino acids,peptides,sugars, nucleotides,lipids and B-group vita-mins, all ofwhich could act as meat flavour precursors."Thedomes-tic and international demand for yeast extract has increased signif-icantly in recent years, and it is necessary to produce yeast extractpastes with unique properties that direct the improvement of theprocess. Like any foodseasonings,yeast extract pastes have uniqueorganoleptic properties that couldinfluenceconsumer preference. However, it was indicated that the aroma characteristics ofyeast extracts from different suppliers varied. Additionally, theprocessing conditions, such as nature of the yeast and thepresence of additional components, are likely to lead to flavourdevelopment, and the temperature of concentration or drying,could also have effects on the specific flavour profiles of yeastextract pastes. Munch and others studied several types of yeastextracts from different manufacturing sources and different aromacompounds were identified and characterised.-5 Only a very small amount of research has been conducted onthe volatile compounds of yeast extract pastes. Ames identified atotal of nine sulfur-containing volatiles generated by its thermal treatment. Werkhoff and colleagues also studied the sulfur-containing volatiles compounds from yeast extracts.78Ames andMacLeod reported the volatile compounds of amino-carbonylinteractions in yeast extracts.2 Munch and others comparedkey aroma-active compounds generated by thermal treatment ofthe commercial and self-prepared yeast extracts.In addition,Munch and Schieberle studied the formation of key aroma-active compounds in thermally treated yeast extracts using stableisotope dilution assays.1 The studies by Mahadevan and Farmeron three types of yeast extract pastes from different suppliersidentified the key odours, including 2-methyl-3-furanthiol, 2-methyl-3-methyldithiofuran, methional and 1-octen-3-one." But, to our knowledge, up to now, little information isavailable concerning either the general aroma compounds or ( 爷 C orrespondence to: Hu a nlu Son g , Lab o ratory of Molecular Sensory Scie n ce, B eijing Technology a n d Business Un i versity,11 , F u cheng Road, Beijing 100048,China. E-mail: songhuanlu@yahoo.com.cn ) ( a L aboratory of M olecular S ensory Science, Beijing Technology and BusinessUniversity, 11, Fucheng Road, B eijing 100048, China ) b Yeast Extract Seasoning Division, Hubei Angel Yeast Co.Ltd, Yichang, Hubei,China lsolation of aroma-active componentsDynamic headspace sampling Dynamic headspace sampling (DHS)wassperformeddon anAgilent 7890 gas chromatograph equipped with an olfactometryport (Sniffer 9000;Brechbuhler, Schlieren, Switzerland). Aromacompounds from a Tenax trap were thermally desorbed at 280°℃ using a TDSA2 system (Gerstel, Munich, Germany) into a cryo-cooled (-50°C) CIS (the cold injection system) inlet (Gerstel).lnjection was splitless (inlet heating rate of 12℃min-l to260℃). Dynamic headspace dilution analysisYeast extract pastes (20 g) with 2 u.L internal standard solution (2-methyl-3-heptanone, 1.632 uguL-1in n-hexane) were put into apurge-and-trap vessel (280 mLvolume;KBG Co.Beijing,China).Thesample was equilibrated by water-bath circulation (PolySciences,Inc., Warrington, PA 18976, USA) for 30 min at 50C and purgedwith nitrogen at a flow-rate of 80 mL min-1 for 50, 10, 2 and0.5 min, or at 16mL min-1 for 0.5 min. The flavour dilution (FD)factors were 1, 5, 25 and 125, respectively. Volatile compounds ofthe sample headspace were trapped onto a Tenax TA tube, whichwas placed onto the vessel. The Tenax tube was then dry purgedfor 40 min (TD controller;Gerstel, Mulheim, Germany) to removemoisture. No. Compound name DB-wax DB-5 Odour property Method of identificationd LA LB TB TP TC 1 4-Hydroxy-2-butanone 873 612 Slight sweet MS,LRI,O 1 1 5 1 1 2 2-Methyl-butanal 904 665 Chocolate MS,LRI,O 5 5 5 25 5 3 3-Methyl-butanal 909 656 Dark chocolate MS,LRI,O 25 25 125 ‘ 125 25 4 2,3-Butanedione 981 <600 )Butter,creamy LRI,O 125 25 25 25 125 5 Dimethyl disulfide 1071 745 Meaty,sulfur MS,LRI,O 5 25 25 5 6 Hexanal 1086 801 Green,cut-grass MS,LRI,O 1 1 5 5 7 3-Methyl-1-butanol 1167 736 Malty MS,LRI,O - 5 5 1 8 Methyl-pyrazine 1309 829 Burnt,roasted MS,LRI,O 1 1 5 5 5 9 1-Octen-3-one 1315 916 Mushrooms LRI,O 5 25 25 125 5 10 2,6-Dimethyl-pyrazine 1331 Burnt, fried potato MS,LRI,O 5 1 5 25 25 11 2-Methyl-5-(methio)-furan 1340 1112 Roasted,meaty LRI,O 25 125125 125 125 12 acetic acid,methyl ester 1355 Fruity MS,LRI,O 1 1 5 1 1 13 3-Hydroxy-2-butanone 1363 718 Sweet, buttery MS,LRI,O 1 5 5 1 14 Dimethyl trisulfide 1398 Garlic, cooked cabbage MS,LRI,O — 11 25 5 15 2-Hydroxy-propanoic acid,ethyl ester,(S)- 1394 Fruity MS,LRI,O 1 1 5 16 Nonanal 1412 1103 Hay, stale MS,LRI,O 1 5 1 1 17 2,3,5-Trimethyl-pyrazine 1426 1233 Burnt, bread,musty MS,LRI,O 25 5 125 25 25 18 2-Furan methanethiol 1444 Roasted,sulfur MS,LRI,O 125 25 125 125 125 19 Acetic acid 1471 614 Acid MS,LRI, O 5 5 25 25 5 20 Methional 1478 904 Vegetable,cooked potato LRI, O 125 125125125 125 21 Furaldehyde 1485 Bready, roasted MS,LRI,O 5 5 5 5 5 22 2-Acetylfuran 1516 913 Balsamic,sweet MS,LRI,O 5 5 5 5 5 23 Benzaldehyde 1528 951 Almond MS,LRI,O 5 — 5 5 1 24 Propanoic acid 1538 Acid MS,LRI,O 5 1 1 ( Compound names: compounds were identified in this work. ) ( Linear retention index (LRI) fo r the odours in DB-WAX and DB-5MS columns. ) ‘Odour descriptors are those used by all assessors, with most frequently used terms cited first. Method of identification as compound responsible for odour: MS, LRI, O: mass spectrum,linear retention index, description of odour of appropriateconcentration of authentic compound agree with those detected in yeast extract by GC-O; MS, LRI,and O:mass spectrum, linear retention index,anddescription of odour agree with reference compound in literature,which has been stated. These compounds should be regarded as tentative. eNitrogen stream purging at 80 mLmin- for 50,10, 2, 0.5 min, respectively, the FD factors were 1, 5, 25, 125 respectively; purging at 16 mL min-1 for 0.5 min, the FD factor was 625. ( FD, flavour dilution; L A and LB are two b asic meat aromas; TP, T B a n d TC are three characteristic m e at flavours. ) 金华火 www.mdpi.com/journal/molecules Article Aroma-Active Compounds in Jinhua Ham Produced WithDifferent Fermentation Periods (Jinhuaham) Xiao-Sheng Liu, Jian-Bin Liu, Zheng-Mao Yang, Huan-Lu Song *, Ye Liu and Ting-Ting Zou Laboratory of Molecular Sensory Science, College of Food and Chemical Engineering, Beijing Technologyand Business University, Beijing 100048, China; E-Mails:liuxaosheng@126.com (X.-S.L.),localcast@163.com (J.-B.L.); yangzhengmao86@163.com (Z.-M.Y.); liuye@th.btbu.edu.cn (Y.L.);zoutingting@th.btbu.edu.cn (T.-T.Z.) Author to whom correspondence should be addressed; E-Mail:songhl@th.btbu.edu.cn;Tel./Fax: +86-10-6898-4025. External Editor: Derek J. McPhee Received: 12 September 2014; in revised form: 21 October 2014/Accepted: 5November2014/Published: 19November 2014 Abstract: The aroma-active compounds in Jinhua ham processed and stored for 9, 12, 15and 18 months were extracted by dynamic headspace sampling (DHS) and solvent-assistedflavor evaporation (SAFE) and analyzed by gas chromatography-olfactometry-massspectrometry (GC-O-MS). In GC-O-MS, volatile compounds were identified based ontheir mass spectrum, linear retention index (LRI), odor properties, or reference compoundcomparisons. The results showed that a total number of 81 aroma-active compoundswere identified by GC-O-MS. Among them, acids (such as acetic acid, butanoic acidand 3-methylbutanoic acid), saturated aldehydes (such as hexanal, heptanal, octanal and3-methylbutanal), benzene derivatives (such as benzeneacetic acid), ester and lactone (suchas y-nonalactone and y-decalactone) were identified as critical compounds in Jinhua hamaroma. The results also indicated that the type and content of the odorants increasedsignificantly with the duration of the fermentation period. Keywords: Jinhua ham; aroma-active compounds; GC-O-MS; DHS; SAFE 3.3. The Extraction of Aroma Compounds by Dynamic Headspace Sampling (DHS) Dynamic headspace sampling (DHS) was done using a dynamic headspace sampling vessel (150 mL,Kimble Glass, Beijing, China). Volatile compounds of the sample headspace were trapped onto a TenaxTA tube, which was placed onto the vessel. The Tenax tube was then dry purged for 20 min (TD controller,Gerstel, Mulheim an der Ruhr, Germany) to remove moisture for aroma analysis. Aroma compoundsfrom Tenax trap were thermally desorbed at 280 °℃ using a TDSA2 system (Gerstel) into a cryo-cooled(-150°C) CIS (the cold injection system) inlet (Gerstel). Injection was splitless (inlet heating rate of12 °C/min to 260 °C). 3.4. Dynamic Headspace Dilution Analysis (DHDA) Small pieces of hams (1 g) and 2-methyl-3-heptanone (500 ng/g) added as the internal standard wereput into the dynamic headspace sampling vessel. After equilibrating 30 min at 50 ℃ (water-bathcirculation), the sample was purged with a nitrogen stream at a flow-rate of 100 mL/min for 60, 12.5, 2.5or 0.5 min, the FD factors were 1, 5,25, 125 respectively. Compounds with higher FD are considered tobe more critical. Nr* R.I. Compound Name " Identification" R.I.(DB-5ms) R.I.(DB-WAX) Odor Property FDf 18 15 12 9 38 methanthiol 627 RI,O rotten egg 25 125 76 trimethylamine 503 848 RI,O,MS fishy 125 125 125 25 50 ethyl acetate 610 877 RI,O,MS,STD fruity/sweet 25 25 2 3-methylbutanal 638 927 RI,O,MS chocolate/malty 25 125 5 27 ethanol 941 RI,O,MS,STD alcohol 1 77 triethylamine 677 970 RI,O,MS fishy 25 25 17 2-pentanone 971 RI,O,MS fruity 5 25 3 pentanal 700 984 RI,O,MS fermented/yoghouit 25 54 2-methy1-butanoic acid ethyl ester 850 1051 RI,O,MS fruity/sweet 25 51 acetic acid butyl ester 812 1059 RI,O green/fruity 5 18 2-methy1-3-pentanone 1068 RI,O,MS mint 25 5 61 disulfide, dimethyl 750 1079 RI,O,MS cooked cabbage/onion 25 25 4 hexanal 803 1094 RI,O,MS,STD cut-grass 125 125 25 5 32 1-methoxy-2-propanol 1137 RI,O,MS plastic 1 1 33 1-penten-3-o1 688 1164 RI,O,MS,STD buttery/grassy/green 5 unknown 1179 RI,O,MS popcorn 5 25 5 heptanal 905 1183 RI,O,MS oily/green 25 5 78 D-limonene 1028 1191 RI,O,MS,STD sweet /orange 5 25 79 pyridine 757 1193 RI,O,MS spicy 1 1 unknown 1199 0 cooked potato 25 125 30 3-methyl-1-butanol 1201 RI,O,MS fermented/oily/fruity 5 80 2-pentylfuran 995 1231 RI,O,MS,STD fruity/green 5 5 52 hexanoic acid ethyl ester 1000 1232 RI,O,MS fruity/apple 5 28 1-pentanol 760 1250 RI,O,MS green 5 1 10 (E)-2-hexenal 855 RI,O,MS green/fatty 5 5 unknown 1270 0 popcorn 125 125 5 6 octanal 1009 1287 RI,O,MS fatty 5 5 22 3-octen-2-one 1045 1285 RI,O,MS fatty/nutty/spicy 25 125 Nr* Compound Name" R.I.‘ Identificationd R.I.(DB-5ms) R.I.(DB-WAX) Odor Property* FD 18 15 12 9‘ 20 3-hydroxy-2-butanone 722 1286 RI,O,MS buttery/green 5 5 1 21 1-octen-3-one 980 1297 RI,O,MS,STD mushroom 125 125 5 5 19 1-hydroxy-2-propanone 680 1307 RI,O,MS nutty/bitter - 1 5 11 (E)-2-heptenal 961 1317 RI,O,MS,STD fatty/fruity 25 25 - - 81 2-acetyl-1-pyrroline 912 1339 RI,O,STD popcon 625 125 125 25 unknown - 1345 0 fishy 25 25 - 73 2,6-dimethylpyrazine 919 1337 RI,O,MS,STD toast/nutty 125 125 25 5 63 dimethyl trisulfide 1380 RI,O,MS garlic/cooked cabbage 125 25 5 74 trimethylpyrazine 1006 1395 RI,O,MS,STD nutty/chocolate 25 5 1 1 23 1-nonen-3-one - 1404 RI,O,MS mushroom 25 5 1 7 nonanal 1102 1408 RI,O,MS green/fatty/soapy 25 1 5 36 3.5-octadien-2-o1 1408 RI,O,MS green 1 12 (E)-2-octenal 1064 1425 RI,O,MS fatty 25 5 - 75 tetramethylpyrazine 1084 - RI,O,MS,STD nutty 1 - - 1 34 1-octen-3-ol 986 1445 RI,O,MS mushroom 5 25 25 25 39 acetic acid 658 1446 RI,O,MS,STD sour 125 125 25 25 9 methional 906 1450 RI,O cooked potato 125 125 25 8 furfural - 1453 RI,O,STD sweet popcomn/wood 125 25 - 26 camphor 1145 1503 RI,O,MS camphor 5 1 1 47 2-methylpropanoic acid 828 1550 RI,O,MS sock/stinky 25 125 1 1 66 benzaldehyde 969 1515 RI,O,MS almond 5 5 - 24 (E,E)-3,5-octadien-2-one 1091 1520 RI,O,MS fatty 5 - 1 40 propanoic acid 718 1556 RI,O,MS sour 25 25 - unknown - 1559 0 chocolate 25 1 1 37 2,3-butanediol 783 1581 RI,O,MS fruity/creamy/oily 5 25 14 (E,Z)-2,6-nonadienal 1586 RI,O cucumber 1 1 1 35 (E)-2-octen-1-o1 1607 RI,O,MS mushroom 5 55 butyrolactone 950 Nr* R.I. Compound Name" Identification" R.I.(DB-5ms) R.I.(DB-WAX) Odor Property" FD' 18 15 12 59 8-nonalactone 1363 2035 RI,O,MS coconut/creamy/sweet 1 25 5 60 v-decalactone 1370 2150 RI,O peachy 125 125 25 unknown - 2178 0 medicine 25 25 5 unknown - 2185 0 burnt sugar 125 125 - 0.4 min, the FD factor was 625. 沙琪玛 三种顶空萃取法比较并鉴定沙琪玛中关键气味活性化合物 气味活 杨平,尤梦晨,刘少敏,郑莹莹,宋焕禄' (北京食品营养与人类健康高精尖创新中心,北京市食品添加剂工程技术研究中心,北京工商大学,北京100048) 性化合 物 摘要:采用吹扫捕集法(Purge & trap,P&T)、固相微萃取法(Solid-phase micro-extraction, SPME) 和动态顶空制样法 (Dynamic headspace extraction, DHS)三种顶空萃取方法,结合气相色谱-嗅闻-质谱联用技术 (Gas chromatography-olfactometry-mass spectrometry, GC-O-MS) 对三种沙琪玛样品进行气味分析,分别得到45种和38种挥发性化合物,主要包括醇类、醛类、酮类、酯类以及杂环类化合物,其中醛类和杂环类化合物浓度较高,对沙琪玛整体香气有重要贡献作用。所有挥发性化合物中,共有17种气味物质能够被嗅闻到,具有气味活性,分别贡献出清香味、可可粉味、麦芽香、青草香、柑橘味、面包味、蘑菇味、奶酪味、青豆味、爆米花味、坚果香、烤坚果香、花生油香。结合感官评价得知,沙琪琪的气味轮廓分为烤香、麦香、油脂、清香、甜香、焦糊和蛋香,与17种气味活性物质所贡献的香气特征一致。结合偏最小二乘法 (Partial Least square, PLS)进行统计学分析比较三种沙琪玛可知,经典鸡蛋味和香酥全蛋味对应的挥发性化合物相对于醇浓鸡蛋味样品而言较少,醇浓样品含有更多吡嗪类物质,经典鸡蛋味和香酥全蛋味含有更多的醛类物质。 关键词:沙琪玛;气味活性化合物;固相微萃取(SPME);动态顶空制样(DHS);气相色谱-嗅闻-质谱(GC-O-MS) Optimization of three headspace extraction methods and identification of aroma-active compoundsin Sachima YANG Ping, YOU Mengchen, LIU Shaomin, ZHENG Yingying, SONG Huanlu* (Beijing Innovation Centre ofFood Nutrition and Human Health,Beijing Engineering and Technology ResearchCenter of Food Additives, Beijing Technology & Business University (BTBU),,100048, Beijing, China.) Abstract: Three headspace extraction methods including purge & trap (P & T), solid-phase micro-extraction(SPME) and dynamic headspace extraction (DHS) were utilized to analyze the volatile compounds in three kindsof Sachimas combined with the technology of gas chromatography-olfactometry-mass spectrometry (GC-O-MS).Forty-five and thirty-five compounds were identified respectively, including alcohols, aldehydes, ketones, estersand heterocyclic compounds. Meanwhile, due to the high concentration andlower threshold, the overall profile ofSachima was contributed by the aldehydes and heterocyclic compounds. What’s more, 17 aroma compounds werecaptured by sniffing that were regarded as the aroma-active compounds in Sachima which present thecharacteristic of green, coca, malty, grass, citrus, bread, mushroom, cheesy, green bean, popcomn, peanut, roastednut, peanut butter. The further analysis combined with sensory evaluation were concluded that the characteristic of17 aroma compounds were consistented with the profile of Sachima including roasted, malty, fat, green, sweet, ( 1 收稿 日 期:2017-11-18 ) ( 作 者 简介:杨平 ( 1993-),女, 硕 士 研 究生,研究 方 向: 食 品风味化学 。 E -ma il: yangp _ yp@163.com ) ( 通讯作 者 :宋宋禄( 1 961-),男,教授,博士,研究方向:分 子 感官科学,美 拉 德 反应 。E-mail: ) ( songhl@th.btbu.edu.cn ) 1.3.3 DHS 法提取沙琪玛中的挥发性成分 准确称取25.0g样品于干净无异味的动态顶空瓶中,加入2L浓度为 0.816 ug/uL的2-甲基-3- 庚酮(溶于正己烷)作为内标参照,之后迅速将顶端瓶盖及两侧出口盖好,连接循环水浴,并将循环 水浴温度设为60℃平衡20 min。然后将左端通入氮气,氮气流速设为100 mL/min,右端插入 Tenax 吸附柱,同样温度下吸附40 min,随后通过 MPS 多功能进样器将吸附柱送入进样口,挥发性化合物 依次经过热脱附系统(TDU)、冷进样系统(CIS)、气相色谱-嗅闻-质谱联用仪进行解析分离。每个 样品重复3遍。 TDU升温程序为:起始温度50℃℃,保持1min, 然后以60℃/min 升到2000℃,保持10 min,燃后再以60℃/min升到300)℃,保持10 min。 CIS 升温程序为:先通过液氮将 CIS温度降到-100℃,待 TDU 以10)℃/min升到240℃,保持1min。 方法。 图2P&T法萃取沙琪玛挥发性化合物总离子流图 Fig.2 Total ion chromatograms (TIC) of volatile compounds in Sachim a extracted by P & T. 图3 SPME 法萃取沙琪玛挥发性化合物总离子流图 Fig.3 Total ion chrom atograms (TIC) of volatile compounds in Sachima extr acted by SPME. 图 4 DHS法萃取沙琪玛挥发性化合物总离子流图 Fig.4 Total ion chromatograms (TIC) of volatile compounds in Sachima extracted by DHS Table 2 The volatile compoun ds and their relative concentration in Sachima extracted by DHS 分类 序号 气味化合物 RI值 参考RI值 鉴别方法 DB-WAX DB-5 DB-WAX DB-5 气味描述 相对浓度(ng/g) 经典鸡蛋味 香酥全蛋味 醇浓鸡蛋味 醇 1 正戊醇 1279 <800 1255 726 MS/RI/STD 香油味 0±0 7.07±1.7 10.86±0.31 2 1-辛烯-3-醇 1481 981 1394 982 MS/RI/STD 蘑菇味 22.3±1.41 38.55±15.65 26.49±0.71 3 2-乙基己醇 1522 1487 1032 MS/PI 玫瑰清香 33.18±5.57 35.97±10.27 30.39±8.37 4 2-呋喃甲醇 1693 856 1199 851 MS/RI/STD 焦香 47.18±11.9 60.31±19.24 95.06±26.37 5 2-甲基丁醛 956 <800 MS/RI/STD/O 可可粉味 3.14±1.24 4.36±1.48 4.12±0.8 6 异戊醛 959 <800 910 650 MS/RI/STD/O 麦芽味 5.14±1.28 4.04±1.68 3.7±1.23 醛 7 正己醛 1114 810 1084 801 MS/RI/STD/O 青草味 41.63±17.59 32.98±7.86 45.36±33.34 8 庚醛 1216 901 1174 903 MS/RI/STD 脂肪味 9.58±1.71 11.81±3.15 0±0 9 辛醛 1321 1006 1280 1010 MS/RI/STD/O 清香味 11.63±2.8 15.28±4.59 9.32±5.77 10 壬醛 1427 1009 1385 1104 MS/RI/STD/O 柑橘味 59.22±6.66 112.18±55.09 45.37±21.88 11 反-13辛醛 1487 - - - MS - 0±0 35.8±0.73 0.73±0 12 糠醛 1495 830 1455 829 MS/RI/STD 面包味 0±0 0±0 14.86±6.84 13 苯甲醛 1562 958 1495 960 MS/RI/STD 杏仁味 34.45±6.17 46.63±11.43 28.49±8.21 14 (反,反)-2,4-癸二烯 1855 1320 1710 1317 MS/RI/STD 油脂味 0±0 21.29±6.85 0±0 醛 酮 15 2-庚酮 1213 895 1170 895 MS/RI/STD 清香味 5.3±1.5 4.94±1.19 4.75±2.35 16 1-羟基-2-丙酮 1330 - - - MS - 0±0 0±0 24.99±6.15 17 1-辛烯-3-酮 1313 976 1313 976 PI/STD/O 蘑菇味 0±0 0±0 0±0 18 2(5H)- -呋喃酮 1796 - - - MS - 0±0 23.75±2.93 12.13±8.24 酯 19 乙酸丙酯 972 720 PI/STD/O 奶酪味 0±0 0±0 0±0 20 乙酸丁酯 1106 1075 816 MS/RI/STD 梨香 1.79±1.36 2.83±1.5 3.25±1.97 续表2. 21 甲酸辛酯 1591 一 1606 - MS/STD - 0±0 34.29±3 3±0 22 y-丁内酯 1669 1323 1647 1299 MS/RI/STD 焦糖味 0±0 0±0 24.24±0.56 23 苯甲酸异丁酯 1836 - - 一- MS - 14.82±3.35 0±0 6.39±3.21 24 吡嗪 1243 - - MS - 0±0 0±0 6.66±0.58 25 2-戊基呋喃 1263 1010 1240 993 MS/RI/STD/O 青豆味 17.89±3.25 20.74±5.14 11.73±3.36 26 2-甲基吡嗪 1298 828 1176 828 MS/RI/STD/O 爆米花味 33.21±9.85 43.6±11.35 88.37±32.07 27 2,5-二甲基吡嗪 1357 909 1253 905 MS/RI/STD/O 烤坚果香 44.43±8.3 52.26±15.41 49.26±17.47 28 2,6-二甲基吡嗪 1364 920 1308 913 MS/RI/STD/O 烤坚果香 0±0 12.34±1.03 14.88±3.59 29 乙基吡嗪 1369 910 1354 907 MS/RISTD/O 花生酱 13.75±4 22.13±11.91 28.19±8.33 30 2,3-二甲基吡嗪 1382 917 1240 892 MS/RI/STD/O 坚果味 12.48±0.89 20.46±8.05 24.27±7.86 31 2-乙酰呋喃 1540 901 1490 893 MS/RI/STD 香油味 0±0 0±0 14.05±2.6 32 4-乙基苯胺 1769 - - MS 0±0 27.73±11.44 9.72±3.18 33 2-甲基苯并噻唑 1994 - MS 9.37±2.11 11.34±7.12 3.18±0.55 34 4-甲基愈创木酚 2000 - - MS 14.2±3.72 16.78±9.14 6.11±3.16 35 苯并噻唑 2010 1240 1588 1240 MS/RI/STD 汽油味 77.96±20.6 93.39±51.86 35.41±15.56 36 4-乙基愈创木酚 2077 - 2031 1287 MS/RI/STD 香料味 7.13±2.57 8.57±4.83 4.1±1.9 37 苯乙烯 1289 890 1241 893 MS/RI/STD 香油味 8.91±3.19 10.76±2.53 8.88±2.58 38 (+)-柠檬烯 1231 1201 1030 MS/RI/STD 柑橘清香 67.31±18.91 58.31±21.27 82.74±23.62 由表2可以看出,采用 DHS 法萃取沙琪玛共得到38种气味化合物,化合物类型与 SPME法相似,主要包括醇类化合物(18.0%),醛类化合物(26.5%),酮类化合物(3.4%),酯类化合物(3.9%)以及杂环类化合物(37.8%),如图6所示,醛类和杂环类化合物仍是是沙琪玛中浓度较高的化合物,此外醇类物质浓度也相对较高。运用此方法得到的挥发性化合物中,同样可以在嗅闻口嗅闻到的气味物质为13种,包括2-甲基丁醛(可可粉味),异戊醛(麦芽味),正己醛(青草味),辛醛(清香味),壬醛(柑橘味),1-辛烯-3-酮(蘑菇味),乙酸丙酯(奶酪味),2-戊基呋喃(青豆味),2-甲基吡嗪(爆米花味),2,5-二甲基吡嗪(烤香味),2,6-二甲基吡嗪(烤坚果香味),乙基吡嗪(花生酱),2,3-二甲基吡嗪(坚果味)。通过标准品验证和文献文6]比对香气特征及保留指数, 确定 DHS 法萃取的这13种化合物为沙琪玛中的关键气味活性物质。 比较两种方法可以看出,虽然萃取出的总气味化合物数量 SPME 法多于 DHS法,但是得到的关键香气活性物质数量基本一致,并且种类相同。不同的是正庚醇、正辛醇、糠醛、:2,3-二甲基吡嗪和2-乙酰基吡啶由 SPME/GC-O-MS 法鉴定出,2-甲基丁醛、乙酸丙酯、2,6-二甲基吡嗪、乙基吡嗪由DHS/GC-O-MS 法鉴定出。从图3、图4中可以看出,两种方法得到的出峰数量及峰高差异不大,但DHS 法萃取得到的总离子流图分离效果较好,在整个升温程序中,出峰位置分布均匀,而SPME 法萃取得到的总离子流图出峰较为集中并靠前。从图5、图6中可以看出,对于沙琪玛样品来说, SPME法得到的醛类物质更多,而DHS 法得到的杂环类物质更多,不同的萃取结果说明挥发性化合物的分析不能仅采用一种萃取方法,而是需要多种方法进行相互补充。两种方法得到了不同的定性结果,并且从总浓度来看,DHS 法萃取的总浓度普遍高于 SPME 法的萃取结果,推测这是由于 Tenax柱的填料涂层与 SPME 萃取针相比,更长更厚,因此可以吸附更多的气味成分使浓度更高。此外 DHS 法通过冷肼作用也可以最大程度保留挥发性较强的化合物。通过深入比较分析得知,两种萃取方法各有优劣,可以根据不同需要选择最理想的萃取方法,如果想以快速高效的方式对样品中的挥发性化合物进行直接鉴定则可以选用 SPME 法萃取气味物质,而如果是对样品进行痕量分析,或是对样品中中发性成分进行快速比较,利用 DHS 法将更加有效。 中央空调空气滤网(air filter)异味 Volatile Odor Compound Analysis of Philips Extra-capacity FormaldehydeRemoval Filter Samples (September 2th,2017) 1. Materials 1.1The two samples are packed in vacuum bags and self-contained bags. When first opened, the two samples smelled no bigdifference and no strange smell. In addition, they look cleaner than previous samples. 1.2 Both ofthe two samples were stored in a refrigerator at-80℃ for further analysis. 2. Instruments Gas chromatography-Olfactrometry-Mass Spectrometry: Agilent-Gerstel,7890A-7000B-Sniffer9000; Capillary column: DB-WAX; Tenax absorption tube; DHS self-made bottle: Flow meter: Scissor: Laboratory Electronic Balance; Water bath: N2: Liquid N2: Ultra Low Temperature Upright Freezer. 3. Method Sample:10g±1g,cut into pieces and put them into the DHS self-made bottle to make volatile odor compounds fully trapped Connect the DHS bottle with purging N2 (Flow: 90ml/min) on the one side and fix the Tenax tube on the other side andtrap VOCs for 50min (Bath Temperature remains at 50℃)。 107 +EI TIC 全扫描1-1.D 10111213 141516171181920212223242522627.2829303132计数与采集时间(分钟)Fig1 TIC (Total Ion Chromatogram) of Filter#1(column: DB-wax) 3107+EITIC 全扫描2-1.D 1.8-1 1 1.6- 1.4- 1.2- 1- 0.8- 0.6- 0.4- 0.2- 0- 01 1011121314151617181920212223242526:27282930313233 3435336373839 40 计数与采集时间(分钟) Table 3 Key odor compound an alysis of all filters No. Compound 中文名称 CAS No. Odor Existence Possible origin 1 styrene 苯乙烯 100-42-5 balsamic. All Filter material gasoline 2 2-ethylhexanol 2-乙基已醇 104-76-7 rose, green All Filter material 3 neoisomenthol 异薄荷醇 491-02-1 menthol #1 Filter material 4 benzothiazole 苯并噻唑 95-16-9 gasoline, rubber All Filter material 5 phenol 苯酚 108-95-2 phenol #2 Filter material 6 decanol 正癸醇 112-30-1 fat #2 Filter material 搅拌棒 搅拌棒吸附萃取 顶空吸附萃取 Fig. 1 SBSE 和 HSSE 装置图。 以家 7770 射光/0时光模型双单色器, 设定放 19-11002 4 应长准性 036m 液长重复精度 ±0.1 放长扫速度 长移动速度:约3200ut/mix 分类库 0Lm 月 散光 0000%以下(220omNal 10g/L ) 00003%以¥cMmm.UV-39光) 9 浏光方式 双光東光方式(伍反直理比例方式) 10 光类型 吸光度(Abs),透射率(%),反率,量() 1 洲光范围 吸光度: -4-5Ah 透射率0.09999% 1118 光准确度 ±fXZAbs(0~05Abs) t0.001Abu0.5-10Abi) 1.1.19 重复测光精度 t0.001Ahs/0-0.5Aba) ±0.002Abs/05-10Ab) 主要用途: 测定物质的吸光度,对样品成分进行定性定量分析。 MPS2XL HP 3 Agilent Techaolagiei 7890A oC Syn STATUS- Not Ready aiting for preen Host systea not Front inlet purging 北京江南大学/ Agilent Technologies 7000GC/MS Triple Quas E + MS Parameters 回68 白 EE 气相色谱一嗅闻一三重四级杆质谱仪 描速理 7 分辨率 8 杂散光 厕光方式 双光来光方式一 测光类型 哑光度(Abs) 测光范围 吸光度:-4-5Abs 透射率0.0999.9% 1.1.18 测光准瘫度 ±0.002Ahst0-0.5Abs ±0.004Abs/0.5一10Ah 1.1.19 重复测光精度 ±0001Abs.(0~0.SAba ±0002Abs0.5-10Abs) 主要用途: 测定物质的吸光度,对样品成分 Mumip MPS2XL-mister GERSTEL ERSTEL Twister uTDu Agilent Technologie7890As YT GcaSyHaTer hf lode BACK INLETPres SSi ure (PTV) Veentpressure 20. Solven71t DHSlarge utp GERSTEL-1 Rt 请提宝贵意见 谢谢! 划 001山99 号 Vol. Nr. ,JOURNAL OF FOOD SCIENCE(Curther reproduction without permission is prohibited 今天推荐给大家一篇由北京工商大学食品学院的宋焕禄教授主笔的“GC-O-MS的技术及其在食品研究中的应用”。并且向您介绍哲斯泰在食品风味研究领域的优秀解决方案 “热脱附+嗅觉检测口” 。研究食品风味的意义重大,可以帮助产品改良及新产品研发找到异味物质或化学污染物帮助品控或产品分级助于基础研究等GC-O-MS技术正是一个用于研究食品风味的强大工具,广泛应用于各种食品的香气和风味分析。GC-O-MS技术可以解决食品中的多种风味问题如“气味活性化合物的图谱锁定“,“关键气味活性化合物的鉴定“等。 借此机会,我们向您介绍哲斯泰在食品风味研究领域的优秀解决方案 “热脱附+嗅觉检测口” ,并且结合气味物质提取三大法宝“动态顶空DHS” + “搅拌棒吸附萃取SBSE”+“固相微萃取SPME”。GC-O-MS技术及其在食品研究中的应用文章叙述了GC-O-MS技术的操作、优点及应用。阐明了结合仪器分析和人类感官评价来定性和定量地分析食品中的风味物质的重要性和可行性。 具体的介绍了今年来备受欢迎的香气物质的提取技术, 以及活性气味化合物的鉴定方法。全文分为7大部分: 介绍气相色谱-闻嗅技术 GC-O气相色谱-闻嗅-质谱 GC-O-MS 技术及其优势对香气化合物的定性分析对香气化合物的定量分析GC-O-MS的应用案例6.1 气味活性化合物快速谱图锁定 6.2 关键气味活性化合物的鉴定 6.3 食品包材迁移到食品中的异味总结文章的第二和第三部分,分别介绍GC-O,及GC-O-MS技术及其在食品风味研究中的优势。 GC-O是一种结合了气相色谱的分离功能和人类嗅觉识别能力的技术。将挥发性的混合物通过GC分离,然后分别通过嗅觉仪引入人的鼻子来检测分析物的气味。但是嗅觉仪无法有效地对化合物进行分子鉴定。GC-O-MS技术将两种设备的特性合并到一个集成仪器中。嗅觉检测器与气相色谱-质谱法可以特别有效的用于从许多挥发性成分中进行化合物鉴定同时锁定气味活性化合物。哲斯泰GC-O-MS示意图文章的第六部分是整篇文章最精彩的部分,介绍了今年来最广泛应用的风味化合物提取手段,结合GC-O-MS 技术,在对食品气味化合物的定性和定量分析研究领域的典型应用。 6.1部分首先介绍了使用GC-O-MS 技术来实现快速定性分析,以达到快速锁定食品中的气味活性化合物的目的。锁定气味化合物谱图非常重要,因为可以通过了解食品中所有气味性化合物的种类来确定对整个气味特征起到决定性作用的目标气味化合物。在使用GC-O-MS 技术前,还需要把这些挥发性的气味化合物首先从不挥发的基质中萃取出来,文章 介绍了四种重要的风味化合物提取手段:同时蒸馏萃取 SDE/GC-O-MS溶剂辅助风味萃取 SAFE/GC-O-MS固相微萃取 SPME/GC-O-MS动态顶空 DHS/GC-O-MS在最经典的”同时蒸馏萃取”和”溶剂辅助风味萃取”法之外,宋教授还介绍了两种相对简单,无需溶剂,快速的萃取法:固相微萃取和动态顶空。这里我们着重为大家介绍一下动态顶空(DHS)动态顶空DHS:用惰性气体如氮气对密闭容器中样品表面进行吹扫,那么样品的顶空中的香气成分便会被载气带出来,当混合气体通过一个捕集物(如Tenax 柱)时,就会被吸收。这样经过一段时间Tenax 柱上就会聚集一定量的香气化合物。然后将 Tenax 柱加热让香气化合物挥发出来,进入气相色谱仪或气-质联用进行成分分析。Tenax柱中的填料是一种多聚物吸附剂,称为聚 (2,6-二苯基-p-苯烯基氧化物 ),能有效地吸附香气挥发物,现已被广泛应用于食品香气、水中挥发性有机物( VOC )以及环境检测中。“使用动态顶空的两个关键环节包括冷阱捕集和热脱附。而热解吸的目的是让捕集的气味成分以很窄的形式瞬间进入气相色谱柱进行分析。快速加热解吸的过程,解吸时间短,样品在更短的时间内进入气相色谱,这样引入到色谱系统的载气、水、二氧化碳的量就会很少,从而更好的提高了气相色谱分析的分离度及检测的灵敏度。为保证香气萃取物以一个“窄带”进入毛细管柱,冷冻浓缩液气化后,可以再经过一次冷聚焦(cryofocusing),从而提高气相色谱分析的分辨率与灵敏度。样品冷冻浓缩一般用液氮做冷却剂,因为液氮的冷冻浓缩效果非常好,可以使很多强挥发性易于穿透吸附剂的物质被很好地冷凝。” -- 宋教授语录。通常来说,一些关键性的气味化合物的浓度往往非常低,在ppb甚至ppt级别,使用动态顶空可以有效地捕集和富集此类化合物。因此DHS的灵敏度高,适合对样品进行痕量分析,或是快速的进行挥发性物质的成分比较。哲斯泰的DHS动态顶空模块,可以自动化动态顶空采样的过程,样品加热温度可达200°C,并带有震荡功能,如有需要可以冷却样品至10度,以此来模拟食品在加工或食用时的情况,也可以加快整个萃取过程。载气吹扫速率5-100 mL/min,每次采样使用单独的捕集管如Tenax管,Tenax填充容量可达220mg,并且对捕集管进行精确控温20-70°C, 保证结果的可重复性,灵活控制不同挥发性的目标化合物的捕集效果。哲斯泰的DHS动态顶空采样流程+热脱附“使用动态顶空的两个关键环节包括冷阱捕集和热脱附”,哲斯泰的冷阱捕集“冷进样系统CIS”和“热脱附系统/单元 TDS /TDU' 是 ”无冷点,无阀,无传输线“的设计,可以真正做到”无歧视,无残留“的传输效果。无论是使用哪一种萃取手段,都离不开好的热脱附把所得的化合物100%的传输到气相色谱中去,否则只能是事倍功半。 6.2 在定性分析的基础上更近一步,来寻找关键气味化合物。关键风味化合物决定了食品的主要风味特点,它们虽然属于“少数派”,但却“举足轻重”。找到了关键风味化合物,可以对接下进一步的风味分析,无论是定量分析,比较分析还是相关性分析,起到“风向标,指路牌”的作用,达到事半功倍的效果。 文章中介绍了今年来在食品风味研究领域最常用的几个方法:香气萃取物稀释分析 AEDA动态顶空稀释分析 DHDA气味活力值分析 OAV香气重组 Aroma Recombination宋教授在使用嗅觉检测口ODP,仪器安装了热脱附系统TDS北京工商大学食品学院的实验室,安装了热脱附单元 TDU2, 动态顶空DHS,以及1L的大型动态顶空罐 DHS Large哲斯泰食品气味研究的解决方案哲斯泰 “热脱附+嗅觉检测口” 结合独有的样品前处理三大法宝“动态顶空DHS及大型样品罐” + “搅拌棒吸附萃取SBSE”+“固相微萃取SPME”。宋教授认为:“感官评价与仪器分析结合是今后的发展方向”,我们希望可以助广大食品风味研究者们一臂之力。为您提供最灵活的食品风味分析手段,最优质的热脱附表现,和最满意的闻嗅结果。 宋焕禄 北京工商大学 食品学院教授宋焕禄,男,1961年6月出生,汉族,山东烟台市人,工学博士、教授,博士研究生导师,享受国务院特殊津贴专家,美国伊利诺伊大学访问学者。主要讲授 “食品分析”及“食品风味化学与分析”课程。食品科学学科学术带头人,主要研究领域为食品风味化学、食品添加剂、现代食品分析技术等。现为中国食品科学技术学会高级会员。主持或参加完成的科研项目有20余项,其中包括国家“十五”科技攻关项目子课题、国家“十一五”科技支撑项目子课题、国家“十二五”863计划项目子任务、国家自然科学基金、北京市自然科学基金等项目。现主持国家自然科学基金及校企合作项目等多项课题。荣获2014年中国商业联合会科学技术一等奖1项、2000年国家科技进步二等奖1项、教育部1999年度科技进步一等奖1项。发表论文百余篇,其中SCI论文30余篇;主编专著3部;获得授权国家发明专利3项。在本在线课程中,主要负责讲解气味的神经生理学、挥发性香气化合物的提取、分析和鉴定方法。
确定































还剩42页未读,是否继续阅读?
GERSTEL(哲斯泰)为您提供《乳制品,奶酪,牛奶中风味物质,异味物质检测方案(其它色谱仪及分离设备)》,该方案主要用于液体乳中营养成分检测,参考标准--,《乳制品,奶酪,牛奶中风味物质,异味物质检测方案(其它色谱仪及分离设备)》用到的仪器有嗅觉检测口ODP4 (闻香仪,嗅闻仪,嗅辨器)、GERSTEL 搅拌棒Twister (萃取、固相微萃取)、GERSTEL热脱附单元TDU2 (热解吸,热解析)、GERSTEL动态顶空DHS (浓缩捕集)
相关方案
更多
该厂商其他方案
更多