方案详情
文
使用热裂解-气质联用鉴定微塑料颗粒的材料成分以及所使用的任何添加剂或已经被颗粒吸收的污染物。对于不易溶解,萃取或水解的聚合物颗粒,热裂解-气质联用是一种非常有用的技术,可根据聚合物在受热分解过程中形成的聚合物单体提供有关大分子聚合物的结构信息。
方案详情

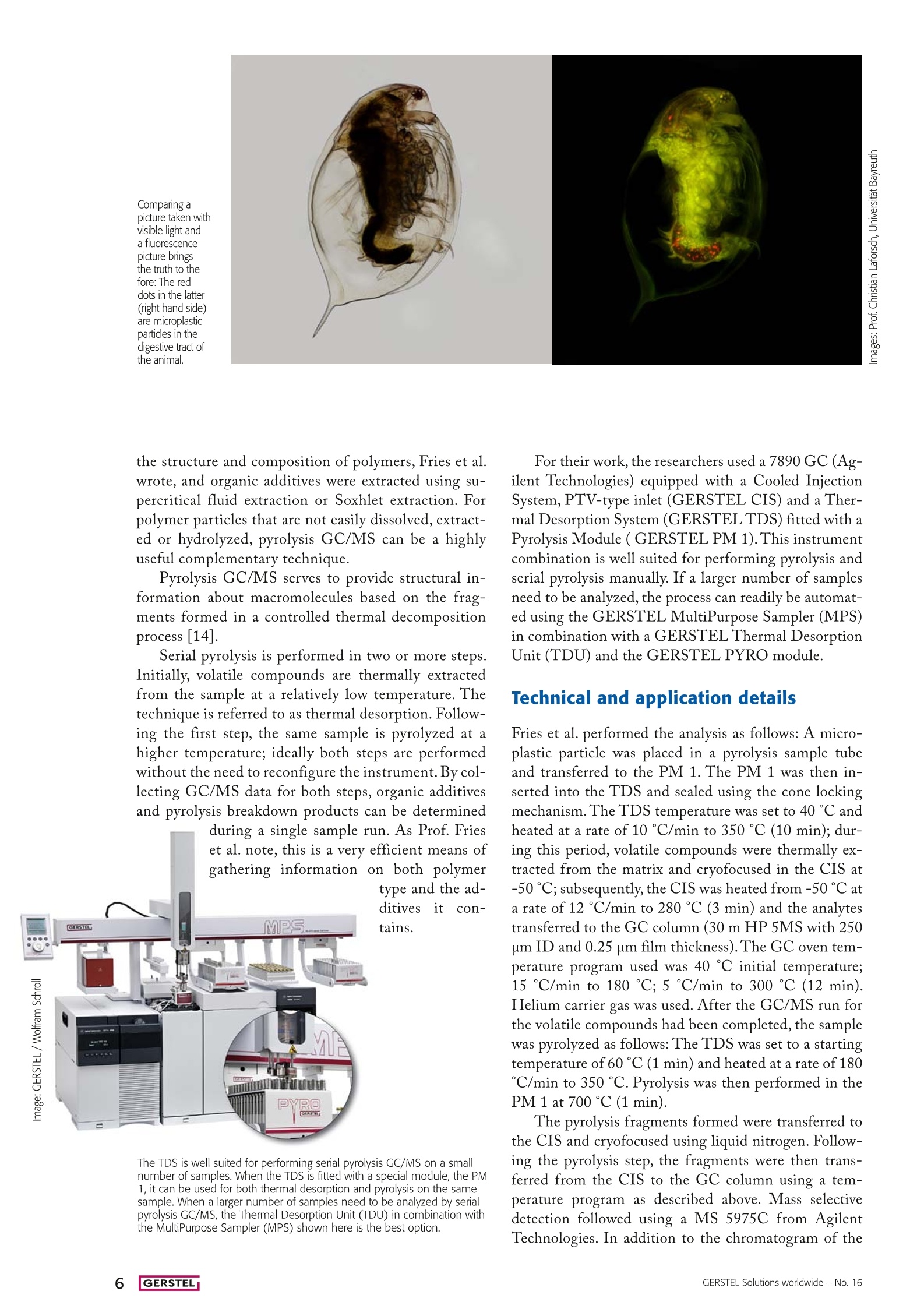
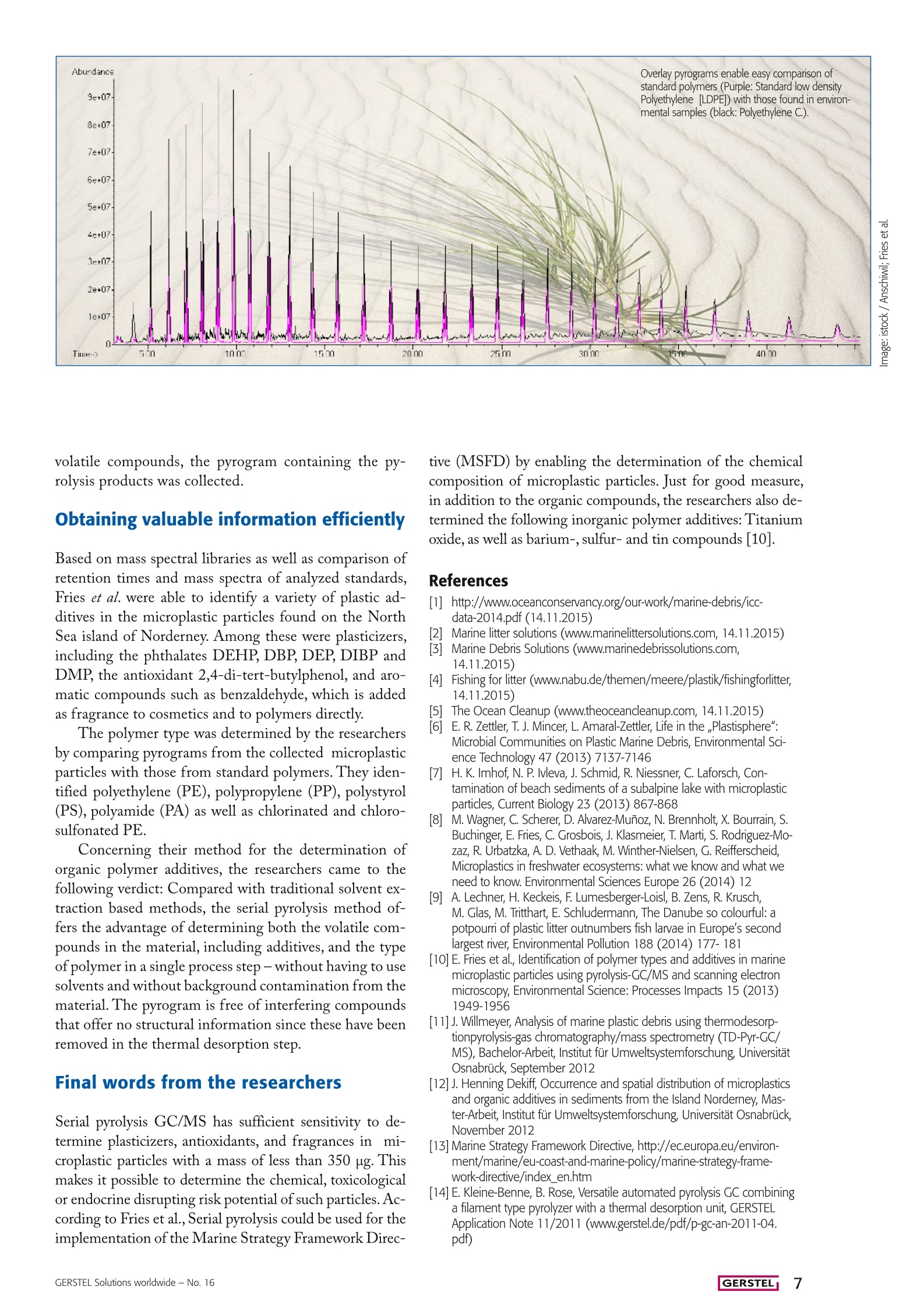
Material and Environmental Analysis A Sea of Polymers Microplastic particles in the marine environment could have abigger impact than previously thought: To get an idea of the extentof the plastic waste pollution in the marine environment, and of howquickly, if at all, it breaks down, science must rise to the challengeand find ways to determine qualitatively and quantitatively what isfloating amongst the aquatic wildlife that makes up a good part ofour food supply. Pyrolysis GC/MS has been found to be a useful toolfor the characterization of microplastics in environmental samples. By Guido Deussing 三 GERSTEL During the International Coastal Cleanup in 2013,648,015 volunteers worked along a stretch of aroundthirteen thousand miles of ocean coastline to removemore than 5,500 tons of waste. The top ten waste itemswere: Cigarette butts (913 tons), paper candy wrapping(794 tons), plastic bottles (426 tons), plastic caps and lids(385 tons), plastic soda straws (252 tons), standard plasticshopping bags (200 tons), glass bottles (179 tons), otherplastic bags large and small (176 tons), paper bags (167tons), and beverage cans (154 tons) [1]. There is no way to correlate the recorded amount ofthese different types of waste to the total amount of wastereleased annually into the oceans, but it does paint a grimpicture if one considers that we are only looking at the tipof the iceberg. Another large source of pollution is plasticmicro beads (less than 5 millimeters in diameter) used inpersonal care products mostly as exfoliating agents. Dur-ing use they enter the waste water stream; however, theypass though waste water treatment plants and end up inour water ways and oceans where fish ingest them at analarming rate. It seems safe to assume that plastics are oneof the major sources of environmental pollution. Not justenvironmental action groups, but also polymer materialproducers see cause for concern and the need for actionas an increasing number of scientific journal papers andreports are referenced in the press documenting the det-rimental effects of microplastics on the environment [2].What then needs to be done - and what can the indi-vidual consumer do to avoid contributing to microplasticspollution or even to help improve the situation? A responsible and sustainable use of polymer materi-als is a good start for individual consumers, companiesand organizations alike. On a grander scale, well orga-nized collection and recycling systems must be available.Various projects have been initiated with the aim of re-ducing the problem of waste in the oceans [2-6]. In themeantime, inland waterways and water bodies are alsobecoming the focus of attention since they seem to beimpacted significantly as well [7-8]. Obviously, for any long term strategy to have a chanceof success,it needs to be built on solid and reliable data.At this time, too little is known about transport routes,transformation processes, as well as effects and where-abouts of polymer residues in the environment. The sci-entific community is working to close this knowledge gapwith a special focus on microplastics [7,9-12]. The big problem posed by thetiniest particles Polymer materials are very rugged. Even when exposed toenergy-rich solar radiation, chemicals, mechanical force,or microbial attack, they never really seem to degrade or disappear from the environment. Sooner or later, polymermaterials become brittle and are ground into ever finerparticles until they end up in the microplastics range. Microplastic particles, due to their small size, rangingfrom a few micrometers to a few millimeters,and theirirregular shape and color, are often mistaken for food andare ingested by aquatic organisms and sea birds. Increasingly, substantial amounts of polymer frag-ments are being found in the digestive tracts of deadseabirds. Whether the microplastic particles caused thedeath of the animals is uncertain.Nevertheless, it is notunreasonable to assume that animals which often ingestplastic material could suffer from malnutrition. Scien-tists predict that in 2050 we will find plastic residues inaround 80 percent of all seabirds. Further, microplasticparticles can contain unhealthy or toxic additives as wellas pesticides, heavy metals or other toxins that are con-centrated in the polymer from the surrounding environ-ment. Since humans are at the top of the food chain thiscould eventually become a threat to us as well. International efforts to learn more The European Commission's Marine Strategy Frame-work Directive (MSFD) [13] focuses on protecting theseas and on managing natural marine resources efficient-ly. According to the MSFD, the type and compositionof micro particles, and microplastic particles in particular,need to be characterized. This is exactly what research-ers at the Universities of Osnabruck and Darmstadt, bothlocated in Germany, have set out to do. As part of theirresearch [10, 11],Professor Elke Fries and her colleaguesinvestigated marine microplastic particles, which theyhad extracted from sand samples collected on the NorthSea Island of Norderney, Germany. In order to deter-mine the material composition of the microplastic par-ticles collected as well as any additives used or pollutantsthat had been absorbed by the particles, Prof. Fries andher colleagues turned to pyrolysis-GC/MS as the first scientists ever to do so for this type of sample the structure and composition of polymers, Fries et al.wrote, and organic additives were extracted using su-percritical fluid extraction or Soxhlet extraction. Forpolymer particles that are not easily dissolved, extract-ed or hydrolyzed, pyrolysis GC/MS can be a highlyuseful complementary technique. Pyrolysis GC/MS serves to provide structural in-formation about macromolecules based on the frag-ments formed in a controlled thermal decompositionprocess [14]. Serial pyrolysis is performed in two or more steps.Initially, volatile compounds are thermally extractedfrom the sample at a relatively low temperature. Thetechnique is referred to as thermal desorption. Follow-ing the first step, the same sample is pyrolyzed at ahigher temperature; ideally both steps are performedwithout the need to reconfigure the instrument. By col-lecting GC/MS data for both steps, organic additivesand pyrolysis breakdown products can be determined during a single sample run. As Prof. Frieset al. note, this is a very efficient means ofgathering information on both polymer The TDS is well suited for performing serial pyrolysis GC/MS on a smallnumber of samples. When the TDS is fitted with a special module, the PM1, it can be used for both thermal desorption and pyrolysis on the samesample. When a larger number of samples need to be analyzed by serialpyrolysis GC/MS, the Thermal Desorption Unit (TDU) in combination withthe MultiPurpose Sampler (MPS) shown here is the best option. For their work, the researchers used a 7890 GC (Ag-ilent Technologies) equipped with a Cooled InjectionSystem, PTV-type inlet (GERSTEL CIS) and a Ther-mal Desorption System (GERSTEL TDS) fitted with aPyrolysis Module ( GERSTEL PM 1). This instrumentcombination is well suited for performing pyrolysis andserial pyrolysis manually. If a larger number of samplesneed to be analyzed, the process can readily be automat-ed using the GERSTEL MultiPurpose Sampler (MPS)in combination with a GERSTEL Thermal DesorptionUnit (TDU) and the GERSTEL PYRO module. Technical and application details Fries et al. performed the analysis as follows: A micro-plastic particle was placed in a pyrolysis sample tubeand transferred to the PM 1. The PM 1 was then in-serted into the TDS and sealed using the cone lockingmechanism.The TDS temperature was set to 40 ℃ andheated at a rate of 10 °℃/min to 350℃ (10 min); dur-ing this period, volatile compounds were thermally ex-tracted from the matrix and cryofocused in the CIS at-50℃; subsequently, the CIS was heated from -50℃ ata rate of 12 °C/min to 280 ℃ (3 min) and the analytestransferred to the GC column (30 m HP 5MS with 250um ID and 0.25 um film thickness). The GC oven tem-perature program used was 40℃ initial temperature;15C/min to 180 ℃;5C/min to 300 C (12 min).Helium carrier gas was used. After the GC/MS run forthe volatile compounds had been completed, the samplewas pyrolyzed as follows: The TDS was set to a startingtemperature of60℃(1 min)and heated at a rate of 180℃/min to 350 ℃. Pyrolysis was then performed in thePM 1 at 700℃ (1 min). The pyrolysis fragments formed were transferred tothe CIS and cryofocused using liquid nitrogen. Follow-ing the pyrolysis step, the fragments were then trans-ferred from the CIS to the GC column using a tem-perature program as described above. Mass selectivedetection followed using a MS 5975C from AgilentTechnologies. In addition to the chromatogram of the volatile compounds, the pyrogram containing the py-rolysis products was collected. Obtaining valuable information efficiently Based on mass spectral libraries as well as comparison ofretention times and mass spectra of analyzed standards,Fries et al. were able to identify a variety of plastic ad-ditives in the microplastic particles found on the NorthSea island of Norderney. Among these were plasticizers,including the phthalates DEHP,DBP, DEP, DIBP andDMP, the antioxidant 2,4-di-tert-butylphenol, and aro-matic compounds such as benzaldehyde, which is addedas fragrance to cosmetics and to polymers directly. The polymer type was determined by the researchersby comparing pyrograms from the collected microplasticparticles with those from standard polymers. They iden-tified polyethylene (PE), polypropylene (PP), polystyrol(PS), polyamide (PA) as well as chlorinated and chloro-sulfonated PE. Concerning their method for the determination oforganic polymer additives, the researchers came to thefollowing verdict: Compared with traditional solvent ex-traction based methods, the serial pyrolysis method of-fers the advantage of determining both the volatile com-pounds in the material, including additives, and the typeof polymer in a single process step-without having to usesolvents and without background contamination from thematerial. The pyrogram is free of interfering compoundsthat offer no structural information since these have beenremoved in the thermal desorption step. Final words from the researchers Serial pyrolysis GC/MS has sufficient sensitivity to de-termine plasticizers, antioxidants, and fragrances in mi-croplastic particles with a mass of less than 350 rg. Thismakes it possible to determine the chemical, toxicologicalor endocrine disrupting risk potential of such particles. Ac-cording to Fries et al., Serial pyrolysis could be used for theimplementation of the Marine Strategy Framework Direc- tive (MSFD) by enabling the determination of the chemicalcomposition of microplastic particles. Just for good measure,in addition to the organic compounds, the researchers also de-termined the following inorganic polymer additives: Titaniumoxide, as well as barium-, sulfur- and tin compounds [10]. References ( [1] http://www.oceanconservancy.org/our-work/marine-debris/icc- data-2014.pdf(14.11.2015) ) ( Marine litter solutions (www.marinelittersolutions.com, 1 4.11.2015)NMarine Debris Solutions (www.marinedebrissolu t ions.com, ) 14.11.2015) ( 4] ] F F ishing for litter (www.nabu.de/themen/meere/plastik/fishingforlitter, 14.11.2015) ) ( 5 The Ocean Cleanup ( www.theoceancleanup.com, 14.11.2015) ) ( E . R . Z ettler , T . J. Mincer, L . Amaral-Zettler, L i fe i n t h e,Plastisphere":Microbial Communities on Plastic Marine Debris, E nvironmental S c i- ence T echnology 47 (2013)7137-7146 ) ( [7] H. F K. Imhof, N. P. lvleva, J . Schmid, R. Niessner, C. Laforsch,Con-tamination of beach s ediments of a subalpine lake with m icroplastic particles, Current Biology 23 (2013) 867-868 ) ( 8] M . Wagner, C. Scherer, D. Alvarez-Munoz , N. B rennholt, X. Bourrain, S. B uchinger, E . F r ies, C . G rosbois, J. Klasmeier, T . Marti, S . Rodriguez-Mo-zaz, R. Urbatzka, A. D. Vethaak , M. Winther-Nielsen, G.Reifferscheid,Microplastics in freshwate r ecosystems: what we know and what weneed to know. Environmental Sciences Europe 26 (2014) 12 ) ( 9 A. Lechner, H. Keckeis, F. Lumesberger-Loisl, B. Zens, R. Krusch,M. Glas, M . Tritthart, E . Schludermann, T he Danube so colourful: apotpourri of plastic litter outnumbers fish larvae in Europe's secondlargest river, Environmental Polluti o n 188 (2014) 177- 1 81 ) ( [10] E. F ries et al., Identification of polymer types and additives in marine m icroplastic p articles using pyrolysis-GC/MS a nd scanning electron microscopy, Environmental Science: Processes Impacts 15 (2013) 1949-1956 ) ( [11]J. Willmeyer, Analysis of marine plastic debris using thermodesorp-tionpyrolysis-gas chromatography/mass spectrometry (TD-Pyr-GC/MS), Bachelor-Arbeit, I nstitut fur Umweltsystemforschung, Universitat Osnabruck, September 2012 ) ( [12] J. Henning Dekiff, Occurrence and spatial distribution of microplastics a nd o r ganic a d ditives i n sediments fr o m the Island Norderney, Mas- t er-Arbeit, Institu t fur Umweltsystemforschung, Universitat Osnabruck, November 2012 ) ( [13] Marine S t rategy F r amework Directive, http://ec.europa.eu/environ- m ent/marine/eu-coast-and-marine-poli c y/marine-strategy-frame-work-directive/index_ e n.htm ) ( [14]E.K l eine-Benne, B. R o se, Versatile a utomated py r olysis GC combining a f ilament type pyrolyzer with a thermal d e sorption unit, GERSTELApplication Note 11/2011 (www.gerstel.de/pdf/p-gc-an-2011-04.pdf) ) GERSTEL Solutions worldwide -No. 自从2018年10月,科学家在人类粪便样品中发现了微塑料以来,人类的身体正在受到塑料的污染这个话题就一直被关注着。人体中微塑料的来源被猜测是因为食用了海鲜类或是饮用了塑料瓶装水。塑料对环境造成的污染,也因为事关人类的健康而被整个社会再次重视起来。目前,人们对微塑料的形成,转化过程以及环境中聚合物残留物的所造成的影响和它们最终的下落还知之甚少。科学界正在努力弥合这一知识差距。图中红色部分为海洋微生物消化道中的微塑料颗粒位于德国的奥斯纳布吕克大学和达姆施塔特大学的研究人员已经着手研究和表征海洋生物体内微粒的类型和组成。Elke Fries教授及其同事研究了海洋微塑料颗粒,这些颗粒是从德国北海岛诺德奈采集的沙子样品中提取的。为了确定所收集的微塑料颗粒的材料成分以及所使用的任何添加剂或已经被颗粒吸收的污染物,Fries教授和她的同事们选择使用热裂解-GC / MS 【1】。一般确定聚合物的结构和组成主要使用光谱法,而对有机添加剂的检测一般使用超临界流体萃取或索氏提取法提取。对于不易溶解,萃取或水解的聚合物颗粒,热裂解-GC / MS是一种非常有用的技术,可根据聚合物在受热分解过程中形成的聚合物单体提供有关大分子聚合物的结构信息。哲斯泰热裂解PYRO模块基于质谱库以及保留时间和分析标准品质谱的比较,Fries等人能够识别北海诺德奈岛上的微塑料颗粒中的各种塑料添加剂。其中包括邻苯二甲酸酯DEHP,DBP,DEP,DIBP和DMP,抗氧化剂2,4-二叔丁基苯酚和芳香族化合物如苯甲醛,它们作为香料直接加入到化妆品和聚合物中。研究人员通过比较收集的微塑料颗粒的热裂解图和标准聚合物的热裂解图来确定聚合物的类型。他们确定了聚乙烯(PE),聚丙烯(PP),聚苯乙烯(PS),聚酰胺(PA)以及氯化和氯磺化的聚乙烯。根据他们测定有机聚合物添加剂的方法,研究人员得出以下结论:与传统的基于溶剂萃取的方法相比,先把样品在低温热时热脱附,再紧接着使用高温热裂解的连续热裂解法提供了确定材料中挥发性化合物(包括添加剂)和类型的可能性。热解图中也没有干扰化合物,因为这些化合物已经在热脱附步骤中被除去。【1】而这种连续热裂解法,目前只有GERSTEL配置了热裂解PYRO模块的热脱附单元TDU2可以实现。叠加色谱图可以轻松比较标准聚合物(紫色:标准低密度聚乙烯[LDPE])与环境样品中的聚合物(黑色:聚乙烯C.)Fries等人指出热裂解-GC / MS具有足够的灵敏度,可用于测定质量小于350μg的微塑料颗粒中的增塑剂,抗氧化剂和香料。确定这些颗粒的化学,毒理学或内分泌干扰潜在风险。根据Fries等人的说法,通过确定微塑料颗粒的化学成分,可以将热裂解用于实施海洋战略框架指令(MSFD)中。除了有机化合物之外,研究人员还确定了以下无机聚合物添加剂:氧化钛,以及钡,硫和锡化合物。【1】【1】E. Fries et al., Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy, Environmental Science: Processes Impacts 15 (2013) 1949-1956
确定


还剩2页未读,是否继续阅读?
GERSTEL(哲斯泰)为您提供《环境水中微塑料检测方案(热裂解器)》,该方案主要用于环境水(除海水)中微塑料检测,参考标准--,《环境水中微塑料检测方案(热裂解器)》用到的仪器有GERSTEL热裂解PYRO
推荐专场
相关方案
更多
该厂商其他方案
更多















