采用LaVision公司的ImagerE-lite相机,图像增强器IRO搭建了一套OH平面激光诱导荧光测量系统,并利用该系统对醇类全局和局部湍流预混火焰特性进行了实验研究。
方案详情

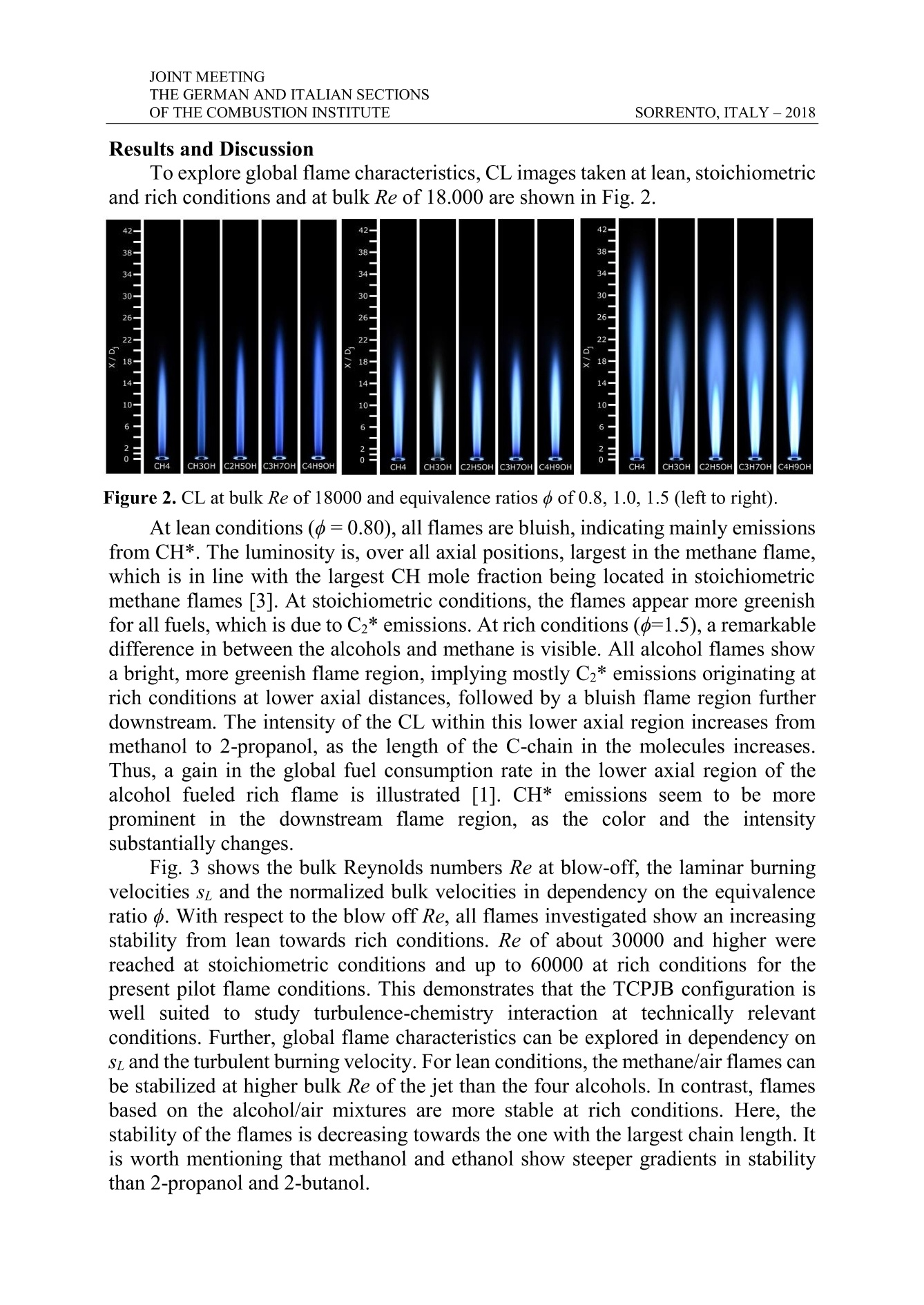
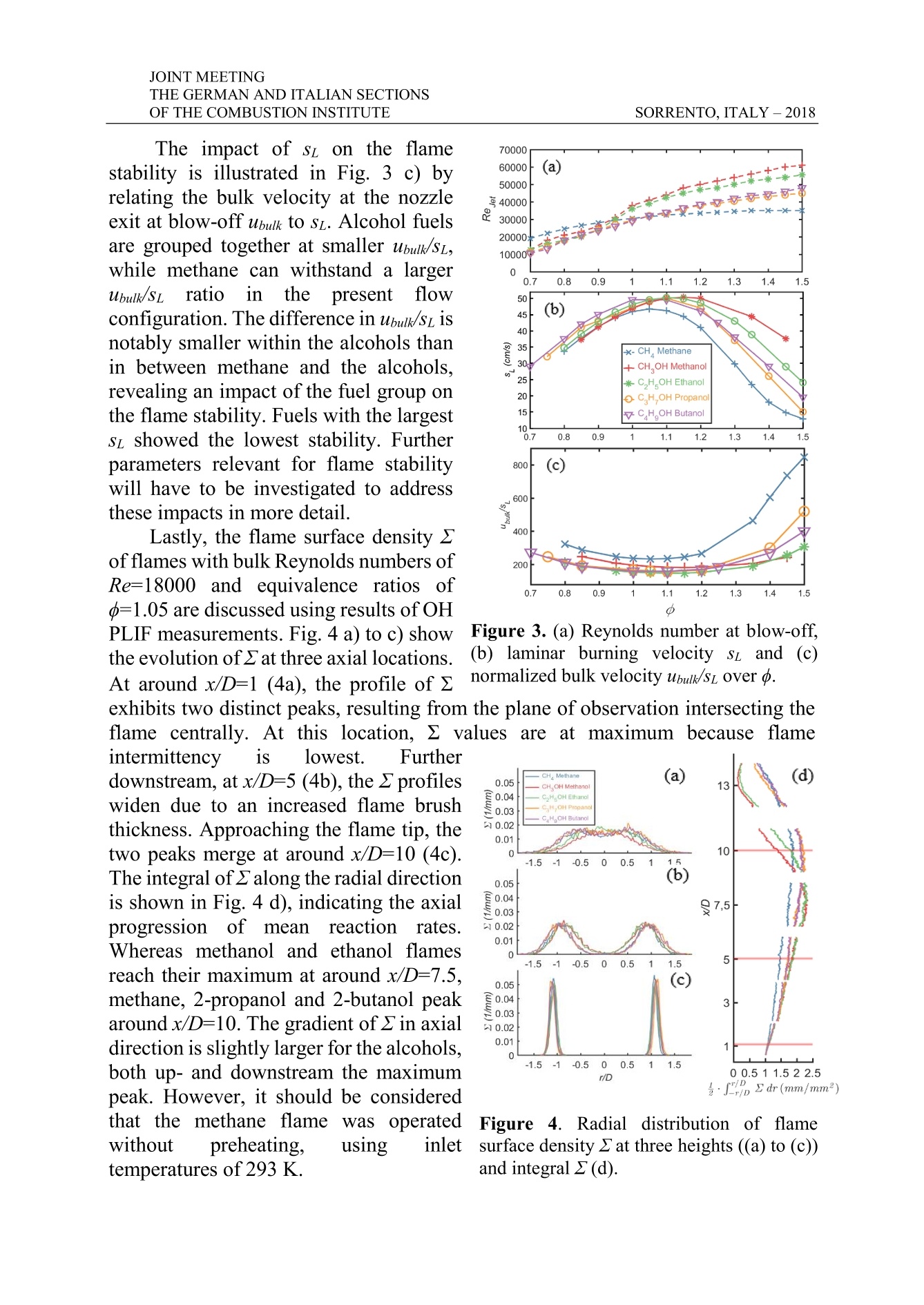
THE GERMAN AND ITALIAN SECTIONSSORRENTO, ITALY-2018OF THE COMBUSTION INSTITUTE V5 JOINT MEETING Experimental Study on Global and LocalTurbulent Premixed Flame Characteristicsof Alcohols J. Trabold*,**,S. Walther*,S. Hartl*,***,A. Dreizler*,D. Geyer* trabold@rsm.tu-darmstadt.de *Thermodynamics, University of Applied Sciences, Darmstadt, Germany **Reactive Flows and Diagnostics (RSM), TU Darmstadt, Germany *** Simulation of reactive Thermo-Fluid Systems (STFS), TU Darmstadt Abstract Turbulent premixed flame characteristics are significant in technically relevantcombustion processes. The here investigated fuels, belonging to the group ofalcohols, are situated among the currently discussed biogenic fuels, which shallcontribute to the emission reduction within the traffic sector. The aim of thisexperimental study is to discuss local and global flame characteristics to identifydifferences of the alcohols methanol,ethanol, 2-propanol and 2-butanol. The well-studied fuel methane is used as reference.All flames are stabilized on the novelTemperature Controlled Piloted Jet Burner (TCPJB), which incorporates an annularpilot flame and an air coflow. First, blow off Reynolds numbers for the differentfuels are compared. Secondly, comparisons of CL images reveal differences in theglobal appearance of the flames. To detect the flame front and subsequently calculatethe flame surface density (2) for varying bulk Reynolds (bulk Re) and equivalenceratios (), Planar Laser Induced Fluorescence (PLIF) of the OH molecule is used.The OH-PLIF images reveal significant differences between the fuels in themagnitude and distribution of 2. Introduction In order to receive predictive modeling approaches for technical applications,turbulent combustion processes have to be assessed in detail, which requirescomprehensive experimental data sets. Additionally, the potential of renewableenergy sources is a central social issue and a topic of current research. In this contest,the turbulence-chemistry interaction and the local and global flame characteristicsfor fuels that are more complex than the widely investigated methane need to beinvestigated. Velocity fields, spatially and temporarily resolved quantitative data of mainspecies concentrations and temperature (Raman/Rayleigh scattering), as well asdetailed information on the flame structure (e.g.OH-PLIF), are required for a moredetailed understanding of flame-chemistry interaction and model validation. Effectsof fuel variations on premixed flame structures were recently investigated by [1] fordifferent C1 -C8 hydrocarbons using flame luminescence. [2] studied the flamestructure of premixed flames of the alkanes methane, ethane and propane, stabilized JOINT MEETING on a piloted Bunsen burner employing particle image velocimetry and Miescattering. Still, detailed investigations on turbulent premixed flames of alcohol fuelsin the gaseous phase are sparse and to the knowledge of the authors, a systematicanalysis of global and local flame characteristics on the variation of liquid fuels isyet to be conducted. The novel TCPJB was particularly developed to allow for the stabilization ofgaseous and pre-vaporized liquid fuels over a wide range of equivalence ratios o,mixture temperatures and bulk velocities. Within this work, the CL was captured todiscuss flame appearance and to quantify global flame parameters, such as the flameheight. Further, PLIF experiments were performed to obtain the flame surfacedensity 2. Flames were fueled by the four lowest chain-length alcohols methanol(CH;OH), ethanol (C2H5OH),2-propanol (C3H7OH) and 2-butanol (C4H9OH) wereinvestigated at equivalence ratios o varying from lean (d=0.6) to rich (=1.5)conditions and operated at different bulk Reynolds numbers (bulk Re). Methane(CH4) flames, at the same range of conditions, were employed to explore differencesto a well-known gaseous fuel. The capability to assess and describe the global and local flame structure ofalcohols by means of the bulk blow-off Reynolds number, CL and the flame surfacedensity gives a significant advance for understanding complex combustion processesfor complex and alternative fuels. Experimental Methodology TCPJB System A gear pump and Coriolis type mass flow controllers (MFCs, Bronkhorst, accuracy of0.1%FS) were employed to control the flow rate of the liquid fuels. Theliquids were continuously vaporized over an evaporationmatrix (ADrop, DV4), of which the temperature waslimited to 573 K, to avoid thermal fuel decomposition. 11.34m1m mmFurther, the air flow was metered by a MFC and suppliedto a temperature-controlled gas heater. Subsequently, the ?H 2, C r(1)2H21vaporized fuel and the heated air were mixed in a high CO2, N2 Fuel/airair mixtureshear static mixer. A second gas heater (ADrop NH3)(2was employed for bulk flows exceeding the thermalpower of the first heater. Heated hoses between the gasheater/vaporizer system and the burner prevented partial 3condensation of the gaseous air/fuel mixture at coldspots. For the same reason, all flow conducting partswere temperature controlled by custom designed heatingelements. An illustration of the burner nozzle section isgiven Fig. 1. The TCPJB consists of a central jet (inner f Figure 1. TCPJB jet andpilot area. diameter of D;=11.4 mm), surrounded by a co-annular pilot flame (Di=31.0 mm)and a homogeneous coflow (D;=260 mm) at 0.3 m/s air. The stainless steel tubetemperature was controlled by three nozzle heaters (brown) up to 45 mm upstreamof the burner exit. Thermocouples (type K) incorporated in the heating elements in combination with PID controllers allowed for a temperature precision of about ±1 Kup to temperatures of more than 400 K at the nozzle exit. As pilot flame, a co-annularslot, consisting of four rings holding 36 circular evenly distributed holes each, isutilized. Gas mixtures supplied to the pilot consisted of the five gases air, hydrogen,acetylene, carbon dioxide and nitrogen at ambient temperature. The gas compositionwas selected to match the C/H and C/O atom ratio, as well as the adiabatictemperature of the respective fuel/air mixture in the central jet at an equivalence ratioof =0.7. A constant pilot bulk velocity of up= 1.0 m/s was employed throughoutall fuels. Chemiluminescence imaging Temporally averaged CL of the flames were captured using a Nikon D5600 andan 18-35 mm F1.8 Sigma lens at 31 mm with a pixel resolution of approximately335 um/pixel. The three major emitters in hydrocarbon flames within the sensitivityrange of the experimental setup are CH* emitting around 431 nm, the Swan bandsof C2*, emitting between 430 nm and 650 nm, as well as the broadband emittingCO2* [3]. Note that the experimental setup is insensitive in the wavelength regionaround 307 nm, where OH*radiates. OH-PLIF imaging OH-PLIF was used to image flame structures of the jet at six different axialpositions. The laser beam emitted from a pulsed UV laser system at 283 nm and wasformed into a 28 mm high and 0.2 mm thick sheet to excite the Q1(6) transition inthe A-X(1-0) band. The OH fluorescence signal of a 24 x 32 mm²area was imagedonto a CCD camera (Imager E-lite, LaVision) using Intensified Relay Optics (High-speed IRO, LaVision). The pixel resolution of the detection system was 23 um/pixel. In a first step of data post-processing, images were background corrected. Next,the laser sheet intensity distribution was normalized using an in-situ averaged sheetprofile [4]. The flame front was deduced to generate a binary burned/unburned imageby tracking the highest gradient of OH [5]. Splines were fitted to recover the flamefront. To deduce flame surface densities 2 as a measure of the mean reaction rate[6], flame lengths were determined in quadratic control areas. The size of the controlareas was set to 0.2 x 0.2 mm². This was smaller than 20% of the smallest observedflame brush thickness (near the nozzle), but larger than the reaction zone thickness,as recommended by [7]. At fixed axial positions, cross sections of2 are evaluated.Furthermore, 2 was integrated in radial direction to receive an axial distribution of习. Laminar burning velocities An in-house flame solver [8] was used to simulate one-dimensional adiabaticsteady laminar premixed flames. The methane flames are modelled using the GRI30mechanism by [9]. For methanol and ethanol the recently developed ELTEmechanism by [10] is used and for 2-propanol and 2-butanol the POLIMI/CRECKmechanism was chosen [11]. Results and Discussion To explore global flame characteristics, CL images taken at lean, stoichiometricand rich conditions and at bulk Re of 18.000 are shown in Fig.2. Figure 2. CL at bulk Re of 18000 and equivalence ratios d of 0.8, 1.0, 1.5 (left to right). At lean conditions (=0.80), all flames are bluish, indicating mainly emissionsfrom CH*. The luminosity is, over all axial positions, largest in the methane flame,which is in line with the largest CH mole fraction being located in stoichiometricmethane flames [3]. At stoichiometric conditions, the flames appear more greenishfor all fuels, which is due to C2* emissions. At rich conditions (o=1.5), a remarkabledifference in between the alcohols and methane is visible. All alcohol flames showa bright, more greenish flame region, implying mostly Cz* emissions originating atrich conditions at lower axial distances, followed by a bluish flame region furtherdownstream. The intensity of the CL within this lower axial region increases frommethanol to 2-propanol, as the length of the C-chain in the molecules increases.Thus, a gain in the global fuel consumption rate in the lower axial region of thealcohol fueled rich flame is illustrated [1]. CH*emissions seem to be moreprominent in the downstream flame region, as the color and the intensitysubstantially changes. Fig. 3 shows the bulk Reynolds numbers Re at blow-off, the laminar burningvelocities sL and the normalized bulk velocities in dependency on the equivalenceratio o. With respect to the blow off Re, all flames investigated show an increasingstability from lean towards rich conditions. Re of about 30000 and higher werereached at stoichiometric conditions and up to 60000 at rich conditions for thepresent pilot flame conditions. This demonstrates that the TCPJB configuration iswell suited to study turbulence-chemistry interaction at technically relevantconditions. Further, global flame characteristics can be explored in dependency onsz and the turbulent burning velocity. For lean conditions, the methane/air flames canbe stabilized at higher bulk Re of the jet than the four alcohols. In contrast, flamesbased on the alcohol/air mixtures are more stable at rich conditions. Here, thestability of the flames is decreasing towards the one with the largest chain length. Itis worth mentioning that methanol and ethanol show steeper gradients in stabilitythan 2-propanol and 2-butanol. The impact of sL on the flamestability is illustrated in Fig. 3 c) byrelating the bulk velocity at the nozzleexit at blow-off ubulk to sz. Alcohol fuelsare grouped together at smaller ubulk/sz,while methane can withstand a largerubulk/Stratio)1inthepresentflowconfiguration. The difference in Ubulk/sz isnotably smaller within the alcohols thanin between methane and the alcohols.revealing an impact of the fuel group onthe flame stability. Fuels with the largestst showed the lowest stability. Furtherparameters relevant for flame stabilitywill have to be investigated to addressthese impacts in more detail. Figure 3. (a) Reynolds number at blow-off,(b) laminar burning velocity si and (c) location, 2 values are at maximum because flame Lastly, the flame surface density 2offlames with bulk Reynolds numbers ofRe=18000 and equivalence ratios of=1.05 are discussed using results ofOHPLIF measurements. Fig. 4 a) toc) showthe evolution of2 at three axial locations.At around x/D=1 (4a), the profile of 2normalized bulk velocity ubuw/sz over d.exhibits two distinct peaks, resulting from the plane of observation intersecting theflame centrally. At thisintermittency is lowest. Furtherdownstream, at x/D=5 (4b), the 2profiles 0 . 05 CHMethane(a) (d)=0., CH.OH Methano 130w4iden due to an increased flame br ush CH OH Ethanal0.03 CHOHPopmn一 CH,OH Butanolthickness. Approaching the flame tip, the 0.020.01two peaks merge at around x/D=10 (4c). 0 10-1.5 -0.5 0 0.5The integral of2 along the radial direction 0.05is shown in Fig. 4 d), indicating the axial 0.040.03 977,.55Fprogression of meanreaction1rrates. 0.020.01Whereas methanol and ethanol flames 0-1.5 -1 -0.5 0.5 1 1.55reach their maximum at around x/D=7.5. 0.05 (c)methane, 2-propanol and 2-butanol peak 0.04 3+0.03around x/D=10. The gradient of2 in axial W0.02direction is slightly larger for the alcohols, 0.0110-1.5b-1oth up- and downstr eam the maximum -0.5 0 0.5 1 1.5r/D0 r/01.51 1.5 2 2.5.2 IdDr(mm/mm)peak. However, it should be consideredthat the methane flame was operatedwithout preheating, using inlettemperatures of 293 K. Figure 4. Radial cdistribution of flamesurface density 2 at three heights ((a) to (c))and integral2(d). JOINT MEETING This reduces sL (36.3 m/s instead of 46.7 m/s at 343 K) and reactivity isaccordingly lower. Conclusion This experimental study discusses local and global flame characteristics of thealcohols methanol, ethanol,2-propanol and 2-butanol. Significant differencesbetween the fuels were found in the blow off Reynolds number, chemiluminescenceimages and the flame surface density. This serves as a indicator that flame stability,size and turbulence of the TCPJB flames are strongly dependent on the fuel type. Innear future, experimental data will be analyzed with regards to influences of thermo-diffusive and hydrodynamic instabilities. Further, combined Raman- and Rayleighmeasurements shall yield even more detail on the turbulence-chemistry interactionof alcohol flames, particularly of Ethanol. Acknowledgements We gratefully acknowledge financial support bythe DeutscheForschungsgemeinschaft (DFG)1through GE2523/2-1 andDR374/17-1.A. Dreizler was financially supported by the Gottfried Wilhelm Leibniz-Preis(DFG). D. Geyer was financially supported by the ZFE of the Darmstadt Universityof Applied Sciences. References [1] Carbone, F., et al., “Comparative behavior of piloted turbulent premixed jetflames of C1-C8 hydrocarbons“, Combustion and Flame,180:88-101 (2017). [21 Tamadonfar, P. and O. L. Gilder "Experimental investigation of the innerstructure of premixed turbulent methane/air flames in the thin reaction zonesregime",Comb. and Flame 162(1):115-128(2015). [31 Kathrotia,T., et al. "Experimental and numerical study of chemiluminescentspecies in low-pressure flames", Applied Physics B, 107(3):571-584 (2012). ( [41 Peterson, B. et al.,“Early flame propagation in a SI engine measured w ithquasi 4D-diagnostics”, Proc. Comb. Inst., 35:3829-3837 (2015). ) [51 Nauert, A., ,,Laseroptische Untersuchungen ant verdralltenVormisch-flammen“, Technische Universitat Darmstadt (2008). 61 Bray, K. N. C., “Studies of the Turbulent Burning Velocity”, Proceedings ofthe Royal Society A, 431(1882):315-335(1990). [71 Donbar, J. and Driscol, J., “Reaction Zone Structure in TurbulentNonpremixed Jet Flames - From CH-OH PLIF Images”, Comb. and Flame,122:1-19(2000). ( [8] Zschutschke, A., et al.:“Universa l Laminar FlameSolver (ULF)”,https://figshare.com/articles/ULF_code _ pdf/5119855/2 ( 2017). ) [9] Smith, G., P., et al., GRI30, in http://www.me.berkeley.edu/gri_mech/ [101 Olm, C., et al., ELTE,“Development of an Ethanol Combustion MechanismBased on a Hierarchical Optimization Approach”, International Journal ofChemical Kinetics, 48:423-441(2016). [111Frassoldati, R., et al., CRECK,Comb. and Flame, 159:2295-2311(2012). Turbulent premixed flame characteristics are significant in technically relevant combustion processes. The here investigated fuels, belonging to the group of alcohols, are situated among the currently discussed biogenic fuels, which shall contribute to the emission reduction within the traffic sector. The aim of this experimental study is to discuss local and global flame characteristics to identify differences of the alcohols methanol, ethanol, 2-propanol and 2-butanol. The well-studied fuel methane is used as reference. All flames are stabilized on the novel Temperature Controlled Piloted Jet Burner (TCPJB), which incorporates an annular pilot flame and an air coflow. First, blow off Reynolds numbers for the different fuels are compared. Secondly, comparisons of CL images reveal differences in the global appearance of the flames. To detect the flame front and subsequently calculate the flame surface density (Σ) for varying bulk Reynolds (bulk Re) and equivalence ratios (ϕ), Planar Laser Induced Fluorescence (PLIF) of the OH molecule is used. The OH-PLIF images reveal significant differences between the fuels in the magnitude and distribution of Σ.
确定




还剩4页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《湍流预混火焰中OH平面激光诱导荧光(OH-PLIF)检测方案(CCD相机)》,该方案主要用于煤炭中OH平面激光诱导荧光(OH-PLIF)检测,参考标准--,《湍流预混火焰中OH平面激光诱导荧光(OH-PLIF)检测方案(CCD相机)》用到的仪器有德国LaVision PIV/PLIF粒子成像测速场仪、LaVision DaVis 智能成像软件平台
推荐专场
相关方案
更多
该厂商其他方案
更多
















