
方案详情
文
SnTe is a notable member of the IV–VI semiconductors with SnSe and PbTe as two representative
thermoelectric materials, which is considered to be a potentially attractive thermoelectric material due
to its similar rock-salt crystal structure to PbTe. However, the current researches on SnTe are limited
because of the difficult synthesis in aspect of controlling morphology and size, and its thermoelectric
figure of merit is also low due to the high thermal conductivity. In this study, a simple and ultra-fast
microwave hydrothermal method was designed to synthesize the SnTe particles with controlled sizes
from micro-scale to nano-scale. The thermoelectric properties of the corresponding SnTe bulk materials
prepared by spark plasma sintering were investigated in a wide temperature range with a focus on the
size effect. Due to the enhanced phonon scattering caused by the nanometer size effect, a low thermal
conductivity, 0.60 W m1 K1 at 803 K, was obtained in the bulk specimen using 165-nm-sized nanoparticles. The corresponding maximum ZT value at 803 K is enhanced to 0.49, which is about 2.3 times
that of the SnTe bulk samples using mechanically alloyed powders.
& 2016
方案详情

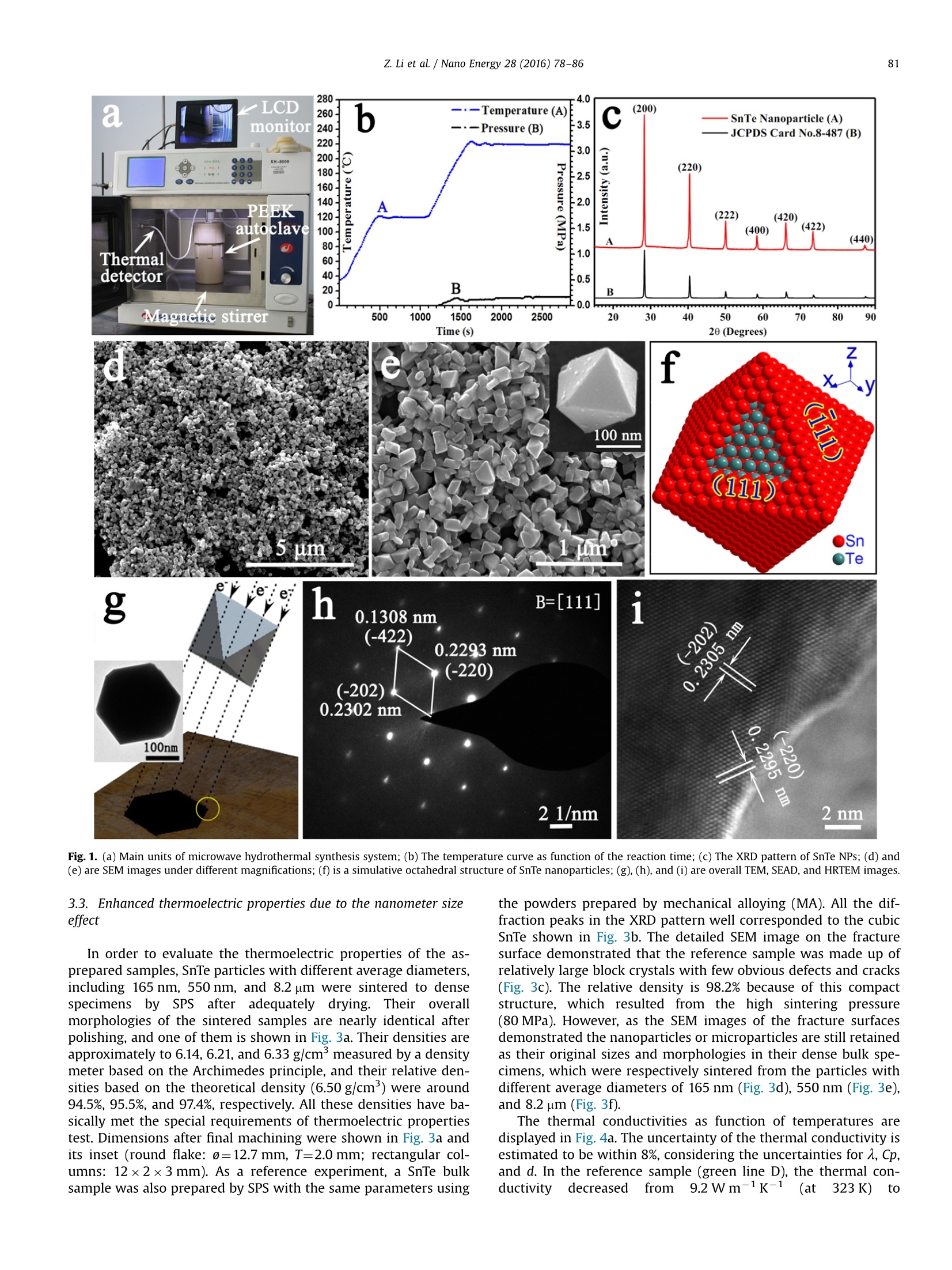
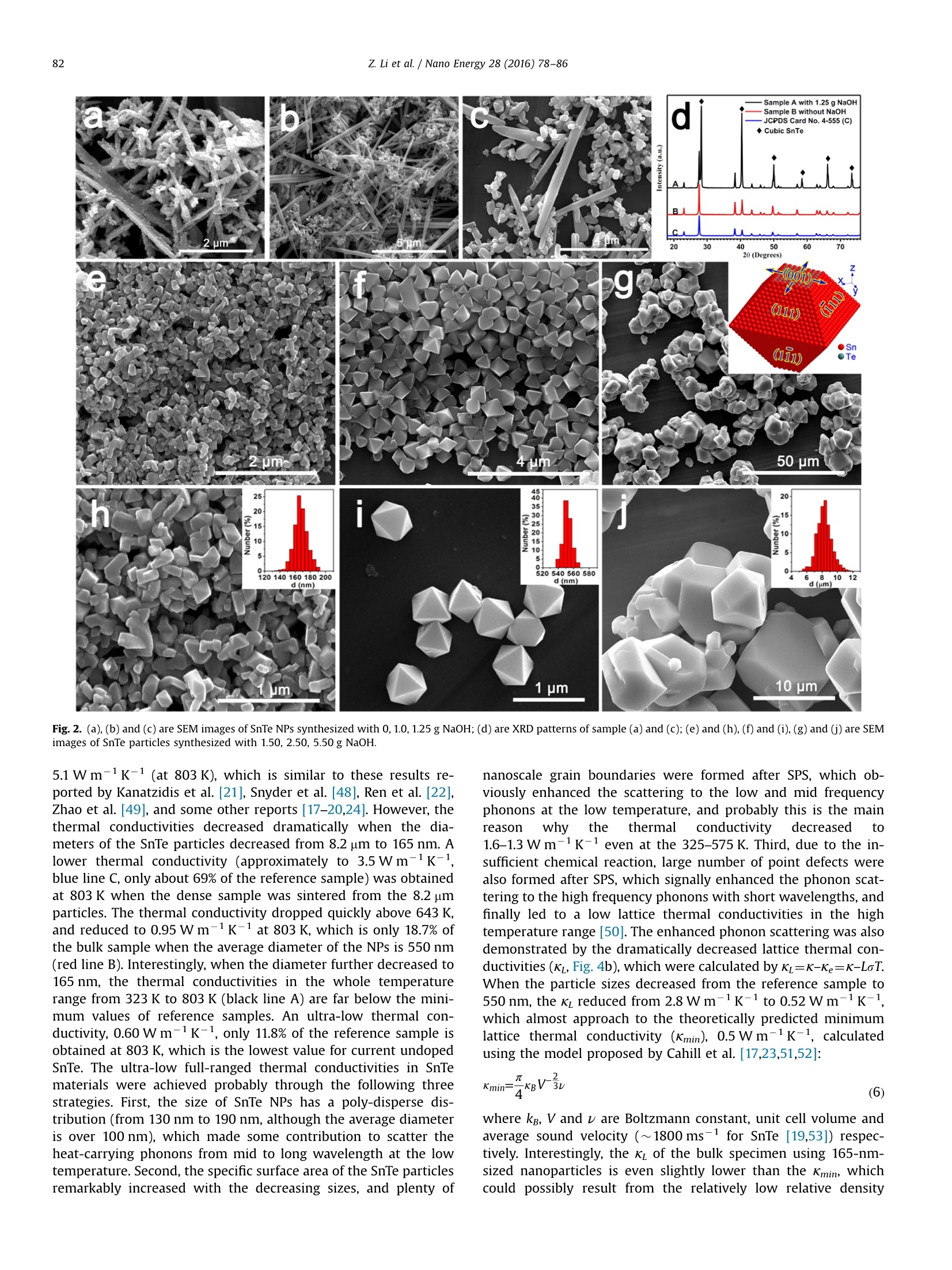
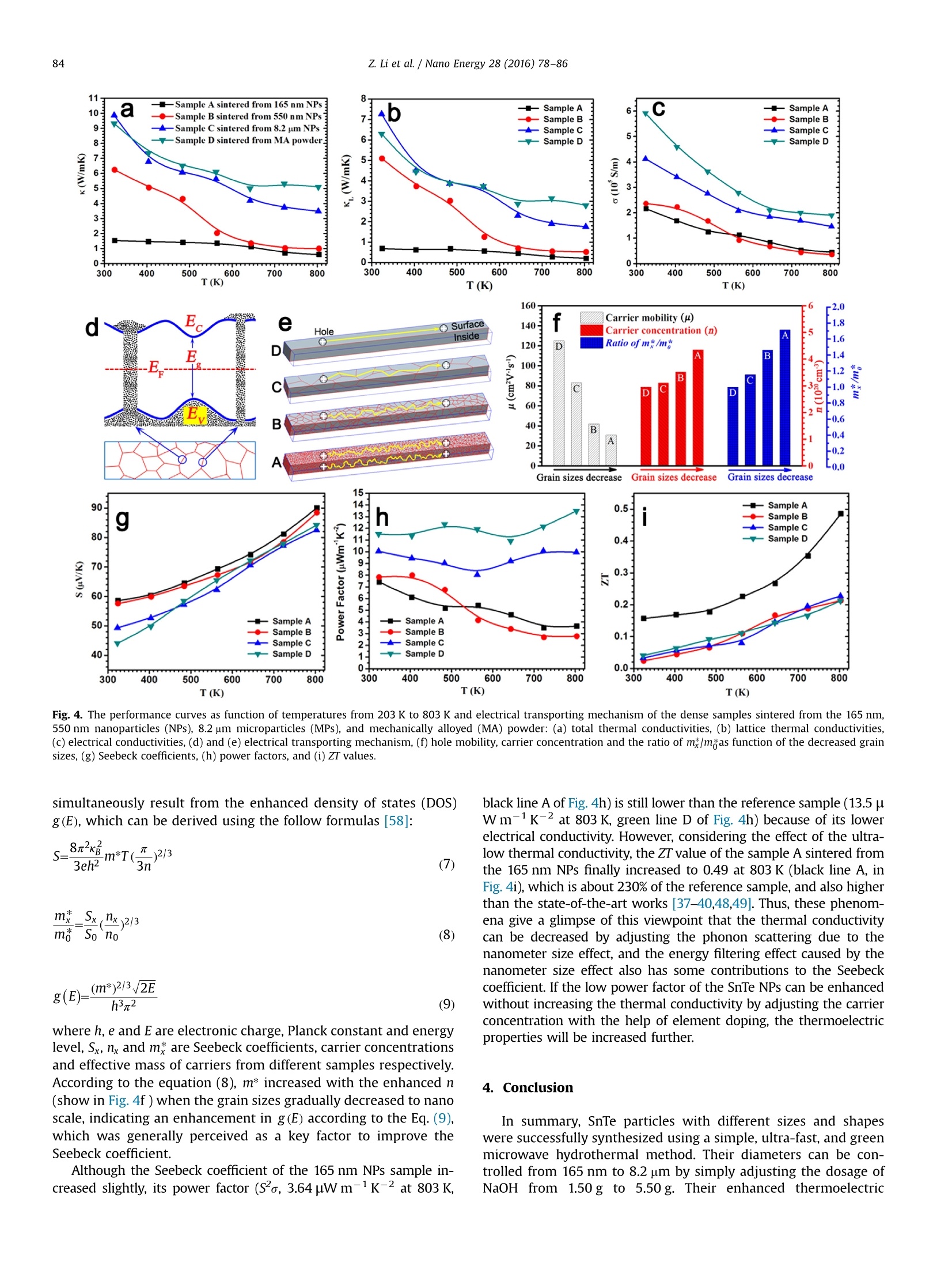
Nano Energy 28 (2016)78-86Contents lists available at ScienceDirectNano Energy 79Z. Li et al./Nano Energy 28 (2016)78-86 http://dx.doi.org/10.1016/j.nanoen.2016.08.008 journal homepage: www.elsevier.com/locate/nanoen Systhesizing SnTe nanocrystals leading to thermoelectric performanceenhancement via an ultra-fast microwave hydrothermal method CrossMark Zhiliang Li, Yudong Chen, Jing-Feng Li, Hong Chen, Lijun Wang, Shuqi Zhenga*,Guiwu Lu d State Key Laboratory of Heavy Oil Processing and Department of Materials Science and Engineering, China University of Petroleum, Beijing 102249,PR China State Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, 100084 Beijing, PR China ARTICLEINF O ABSTRAC T Article history: Received 23 April 2016 Received in revised form 22 July 2016 Accepted 3 August 2016 Available online 11 August 2016 Keywords:Tin tellurideNanoparticleNanometer size effectThermoelectric property Microwave hydrothermal method SnTe is a notable member of the IV-VI semiconductors with SnSe and PbTe as two representativethermoelectric materials, which is considered to be a potentially attractive thermoelectric material dueto its similar rock-salt crystal structure to PbTe. However, the current researches on SnTe are limitedbecause of the difficult synthesis in aspect of controlling morphology and size, and its thermoelectricfigure of merit is also low due to the high thermal conductivity. In this study,a simple and ultra-fastmicrowave hydrothermal method was designed to synthesize the SnTe particles with controlled sizesfrom micro-scale to nano-scale. The thermoelectric properties of the corresponding SnTe bulk materialsprepared by spark plasma sintering were investigated in a wide temperature range with a focus on thesize effect. Due to the enhanced phonon scattering caused by the nanometer size effect, a low thermalconductivity, 0.60 Wm-1K-1 at 803 K, was obtained in the bulk specimen using 165-nm-sized nano-particles. The corresponding maximum ZT value at 803 K is enhanced to 0.49, which is about 2.3 timesthat of the SnTe bulk samples using mechanically alloyed powders. 1. Introduction Thermoelectric (TE) materials have attracted substantial at-tention over the past few decades not only due to their excellentcapabilities on generating electricity from the waste heat, but alsobecause of their broad application prospects in solid-state re-frigeration [1-4]. TE properties are characterized by the di-mensionless figure of merit (ZT), which is defined as ZT=SoT/K,where S,o, T, k are the Seebeck coefficient, electrical conductivity,absolute temperature, and thermal conductivity, respectively [5-8]. In medium temperature region, IV-VI semiconductors, such asSnSe and PbTe, are identified as the best commercial and mostefficient TE materials at present because of their superior aniso-tropy on the crystal structure, and intrinsically low lattice thermalconductivity. PbTe based semiconductor compounds have beenapplied in the thermoelectric generators of "Curiosity",“Galileo”Mars detectors, and“Voyager 1"spacecraft since 1980s [9-11]. TheZT value of SnSe single crystal has reached 2.6 as reported by Zhaoet al., which is undoubtedly the best TE properties among all of thecurrent materials [12,13]. SnTe, as another member of the ⅣV-VI ( * Corresponding author. ) ( E-mail address: zhengsq 0 9@16 3 .com (S . Zheng). ) ( 2211-2855/◎ 2016 El s evier Ltd. All r ights r eserved. ) semiconductors, is considered to be one of the most promisingthermoelectric materials not only because it is a lead-free materialbut also possesses the similar crystal and band structures to PbTecrystal [14-18]. However, unfortunately, the recent researcheshave revealed that the ZT values of SnTe crystals are still very lowbecause of their inherently high thermal conductivities (usuallyhigher than 2.5 W m-1K-1 at 800 K)[19,20]. Endotaxial CdS orZnS nanoscale precipitates were introduced into the cubic phaseSnTe by Tan et al. [21], and its thermal conductivity was reduced to2.1 W m-K-1 from 3.8 Wm-1K-1 (dropped by 34%), leading toan increase in ZT values to 0.60 from 0.42 (increased by 50%). It istrue that increasing Seebeck coefficients and electrical con-ductivity is effective in raising the ZT values in this crystal, how-ever, in terms of the final value, more than half of the contributionon the enhanced ZT values came from the reduced thermal con-ductivities. Similar phenomenon was also observed on the In-doped SnTe crystal reported by Zhang et al. [22], whose thermalconductivity is reduced by 18%, resulting in 32% increase in the ZTvalue. Thus, the thermal conductivity probably also plays a deci-sive role in improving the thermoelectric properties of SnTecrystals [23,24]. In fact, according to theoretical calculations, the total thermalconductivity (K) is composed of electrical thermal conductivity (Ke) andlattice thermal conductivity (KL), and in many semiconductors K is Thus, in this study, we designed a facile, ultra-fast, green, andhigh-yield microwave hydrothermal method to synthesize SnTenanoparticles (NPs) with controlled sizes from micro-scale tonano-scale. The oriented attachment growth mechanism andmorphology controlling technologies were systematically dis-cussed. In order to prove and understand the nanometer size effectfurther, SnTe reference sample, as a comparison, was also preparedby ball-milling combining with spark plasma sintering (SPS).Comparing with the thermoelectric performance of the pure SnTebulk material,, ultra-lowthermalconductivities(:(from1.5 Wm-K-to 0.60 W m-K-1,323-800 K), relatively higherSeebeck coefficients (58-90 uV K-1, 323-800 K), and much higherZT value (about 0.49 at 803 K), which can be ascribed to the en-hanced phonon scattering and the intensified energy filtering ef-fect, were discovered in the specimen that was sintered from theNPs with an average diameter of 165 nm. 2. Experimental 2.1. Materials and reactors Commercial powders of tellurium dioxide (TeO2, 99.99%), tell-urium (Te, 99.99%), tin (Sn, 99.99%) and polyvinyl pyrrolidone(PVP, K-30, Mw=40,000) were used as starting materials. The tindichloride dehydrate (SnClz·2H20), sodium hydroxide (NaOH),ethylene glycol (EG), and absolute ethyl alcohol were analytical-grade and were not purified further before using. The syntheticprocesses of SnTe NPs were carried out in a special microwavehydrothermal synthesis system (MHSS, XH-8000, Beijing XiangHuScience and Technology Development Co., Ltd, China). The reactiontemperatures can be accurately regulated by the power programcontrol (from 300 W to 1500 W). A magnetic stirrer was equippedinto the MHSS to obtain homogeneous solution. 2.2. Preparations Controlled synthesis of SnTe NPs: in typical synthesis, 250 mLEG, 10.0 g PVP, 1.330 g TeO2, 1.881 g SnCl2·2H20 and 1.5 g NaOHwere orderly added to a 500 mL special teflon autoclave with highstrength shell (polyetheretherkrtone, PEEK). Then the mixture washeat to 120℃ within 8 min in the MHSS with vigorous and continuous magnetic stirring. Subsequently, the temperature wasincreased to 220 ℃ at a heating rate of 15C/min, and last for20 min with a power of 550 W. The final products were naturallycooled to room temperature and washed several times with dis-tilled water and absolute ethanol. SnTe NPs with a mean diameterof 165 nm were obtained, and they were adequately dried topowder in a vacuum oven at 60℃ for 8 h. In the similar reactionconditions, SnTe particles with different sizes and morphologieswere obtained by only adjusting the NaOH dosage from 0 g to 0.50 g. The dense specimens were prepared in a graphite die by sparkplasma sintering (SPS-211Lx, Fuji Electronic Industrial Co., Ltd, Ja-pan) at 450℃ for 8 min under an axial pressure of 80 MPa and avacuum of 3.0 Pa. And then, the circular samples were accuratelypolished to 2.0 mm in thickness, and 12.7 mm in diameter toevaluate their thermal conductivities. Finally, the dense specimenswere cut into rectangular columns with the size of 12×2×3 mm tomeasure the Seebeck coefficient and the electrical resistivity. Preparation of SnTe reference materials: as a comparison spe-cimen, SnTe bulk samples were synthesized by the followingprocesses. 6.380 g Te powder and 5.936 g Sn powder were put intoa zirconia jar, and mixed by a high-energy planetary ball mill(BM4, Beijing Grinder Instrument Co., Ltd) at 310 rpm for 5 h. Then3.0 g mixture was sintered in to bulk by SPS at 450C for 8 minunder an axial pressure of 80 MPa and a vacuum of 3.0 Pa. 2.3. Characterization methods The crystal structures of the samples were investigated using aBruker AXS XRD-D8 Focus X-ray diffractometer (XRD) equippedwith graphite monochromatized CuKo radiation ()=0.15406 nm).A field emission scanning electron microscope (FESEM, FEI Quanta200F) was used to characterize the morphologies of the as-pre-pared products. High-resolution transmission electron microscopy(HRTEM) image and selected area electron diffraction (SAED)pattern were recorded by an FEI Tecnai G2 F20 field emissiontransmission electron microscope with an acceleration voltage of200 kV. The Seebeck coefficient and resistivity were measuredfrom 323 K to 803 K using a ZEM-3 (M8) Seebeck coefficient/electric resistance measuring system (ULVAC-RIKO, Inc.) whoseuncertainty is 5%. The thermal conductivity can be calculated bythe equation K=aC,d, where A, Cp, d are thermal diffusion coeffi-cient, specific heat capacity and density, respectively. The thermaldiffusion coefficient of the dense specimens was evaluated by alaser flash apparatus (LFA457, Netzsch). The specific heat capacitymeasurements were carried out using a NETZSCH DSC 404C. AnXS105 density meter was used to measure the densities of theSnTe samples after SPS. The particle size distributions were per-formed in a laser particle analyzer (Zetasizer Nano ZS). The carrierconcentration and mobility were measured on the square chipswith the thickness under 0.6 mm using the Hall measurementsystem with 2.0 T magnetic field (ResiTest 8400, Toyo,Japan). 3. Results and discussion 3.1. Characterization of the SnTe NPs Different from the traditional hydrothermal apparatuses, wherestirring cannot be performed due to the sealed and ferric reactor,magnetic stirring was applied to the microwave hydrothermalsynthesis system (MHSS) because the autoclaves used in this re-actor were made up of polymer instead of the stainless steel. Thesolution temperature in the MHSS were accurately probed by athermal detector which was deeply inserted into the solution. Theoverall appearance and main units of the MHSS are shown inFig. 1a. Moreover, microwave is electromagnetic wave generated from magnetrons. The strong electric and magnetic fields with anultra-high frequency can force the polar molecules (like EG orH20) to realign and vibrate acutely. Energy with high power isgenerated from the inner of the solution as a result of the violentcollision and strong friction among the polar molecules. Thus,reagents in the reaction solutions are heated uniformly with anultra-fast heating rate, and therefore, microwave-assisted synth-esis can operate at a high reaction rate and short reaction time[41-44]. As the temperature recorder displayed, the temperaturewas accurately controlled at 220 C as well as the program set, andthe main reaction processes were finished only in 20 min (Fig.1b).The maximum vapor pressure was 0.17 MPa, which is far lowerthan the ultimate pressure (4.0MPa) of PEEK autoclave. The highlypolar and temperate vapor pressure also demonstrated that the EGis an ideal solvent for microwave hydrothermal method at 220℃.The XRD pattern with diffraction angles from 15° to 90° was per-formed after the SnTe NPs were adequately dried. All the Braggdiffraction peaks in the pattern of the as-obtained sample (red lineA in Fig. 1c) are completely indexed to the crystal planes of thestand JCPDS card no. 8-487, including the (200), (220),(420)planes and so on (black line B in Fig. 1c). It is also proved that theas-prepared sample is cubic phase SnTe with a space group ofFm3m (No.225), and the lattice constants are a=b=c=0.6303 nm.The sharp peaks and the small half-peak width suggest that thesample consist of highly crystallized crystals with small diameter.This characteristic is also attested by the overall morphology(Fig. 1d) and the moderate-magnification (Fig. 1e) SEM images.SnTe NPs with various structures are dispersed uniformly, andtheir diameters ranged from 130 nm to 190 nm. Among these NPs,many regular octahedral structures with the side length approxi-mately to 180 nm can be found, and one of their detailed mor-phology is clearly shown in the inset of Fig.1e. As the simulativestructure shows (Fig. 1f), which was established using the pro-fessional structure simulation software based on the Te and Snatom position, the six top atoms of the octahedron are centralatoms of the plane on the face-centered-cubic (FCC) cell. Thus, theeight surface planes of the octahedron belong to {111} plane fa-mily. And interestingly, due to the slow-rate and multistep of Tereductive reaction (from TeO2 to TeO3-, Te and Te-) as well asthe high ionization of SnCl2, we speculate that the most superficialatoms of the octahedron are Sn atoms (Fig. 1f, the red balls), whichis consistent with a simulation. In order to confirm the structureinformation, we took a TEM image in a single octahedral NPs, anda similar hexagon shadow was obtained (inset of Fig.1g) when theelectron beams vertically pass through the (111) plane. Similarhexagon shadow was also obtained in the 3D simulation structureunder the same conditions (Fig.1g). The SAED pattern was per-formed on the thin area of the octahedron (Fig. 1h). The zone axisis [111], and the interplanar spacings from the three diffractionspots to the center transmission spot are 0.2293 nm, 0.2302 nmand 0.1308 nm, which respectively correspond well to the (-220),(-202),(-422) planes of the cubic SnTe. All these planes com-bined with the transmission spot can form a regular parallelogram,and meet the principle of vector superposition as well. Fig. 1i is aHRTEM image, the smallest interplanar spacings in different di-rections are 0.2295 nm and 0.2305 nm, matching well to the(-220) and(-202) planes (both of their interplanar spacings are0.2229 nm, which can be calculated from the interplanar spacingformula or found in JCPDS card no. 8-487). All these phenomenademonstrate that the NPs are cubic SnTe once again. 3.2. Formation mechanisms and size control from micro-scale tonano-scale In order to obtain the SnTe particles with different sizes frommicro-scale to nano-scale, we tried to control the reaction rates by adjusting the dosage of NaOH that act as an accelerant of reducingagent in this reaction. A relatively slow oxidation-reduction reac-tion rate between the TeO2 and EG occurred when there is noNaOH added into the solution. The Te4+ ions were reduced to Teatoms, then nucleated, and grew into Te nanorods along its in-trinsic growth orientation [001], which has been discussed in ourprevious works [45-47]. However, Te atoms was not reduced toTe2-ions further because of the absence of OH-ions, and also didnot react with Sn²+ions to form SnTe crystals. Therefore, the re-actions without NaOH were run incompletely, and only Te na-norods adhered lots of non-reacted reactants on their surface weregenerated. Their overall morphologies are displayed in Fig. 2a, andtheir XRD pattern (red line B in Fig.2d) corresponds well to thehexagonal Te crystal (JCPDS card no. 4-555, blue line C in Fig.2d).Te nanorods were transformed into nanowires when some butinsufficient NaOH granules were added to the reaction solution,and a few of SnTe NPs appeared in the final products. The reactionmechanism can be understood by the following phase chemicalreaction equations: The ratio of Te nanowires to SnTe NPs was highly governed bythe dosage of NaOH. Fig. 2b and c are the SEM images of theproducts with 1.0 g or 1.25 g NaOH. The proportion of the Te na-nowires decreases, and the amount of SnTe NPs increases gradu-ally because of the increasing OH- concentrations, which madethe Te crystals generated from Eqs. (2) and (3) re-dissolve andtransform to Te-ions according to the Eq.(4). Their XRD patterns(black line A in Fig. 2d) demonstrated that both the hexagonal Teand the cubic SnTe crystals coexisted in these samples. The reac-tion rate of Eq.(4) enhanced sharply when NaOH increased to1.50 g. All the Te nanowires were involved into the reactions,andonly pure SnTe NPs with octahedral structure or irregular shapesare found in Fig. 2e and h. Their average diameters ranged from130 to 190 nm,which were measured by the laser particle analy-zer, and the sharp peak appears at 165 nm (inset of Fig. 2h). Whenthe dosage of NaOH increased to 2.50 g, the reaction of Eq. (4)further enhanced because of the increasing OH- concentration,and also result in a relatively high Te2- concentration, whichmade the Eq. (5) occur fluently. Probably, in this case, some re-action equilibrium was formed between the generated rate andconsumed rate of Te-ions, which led to the SnTe crystals grownalong one of intrinsic crystal morphology, and ultimately formedthe regular octahedrons shown in Fig. 2f and i. Their averagediameter also increased from 165 nm to 550 nm (inset of Fig. 2i)due to the high yield Te-ions. However, excess OH- ions and tooviolent reaction may make some lattice planes, like (00l, l/0)planes (inset of Fig. 2g), that once relatively stabilized in the lowOH-concentration solution involve into the reaction dramaticallv.At the same time, more Te032-ions were formed (Eqs.(1) and(4))and first transformed to Te crystals (Eqs. (2) and (3)), then Te-ions (Eq. (4)), and finally SnTe NPs (Eq. (5)), which result in a highSnTe yield. So the SnTe particles become irregular, and theiraverage diameters increased to 5-12 um when the NaOH was ad-ded up to 5.50 g (Fig. 2g and j). Thus, the grain sizes of SnTecrystals can be controlled by simply adjusting the dosage of NaOHin this microwave hydrothermal method.
确定






还剩7页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《纳米晶体中提高热电性能检测方案(微波合成仪)》,该方案主要用于其他中提高热电性能检测,参考标准--,《纳米晶体中提高热电性能检测方案(微波合成仪)》用到的仪器有
相关方案
更多








