方案详情
文
This Application Note presents different online 2D-LC concepts for the high?resolution analysis of complex N-linked glycans in erythropoietin (EPO) using the Agilent 1290 Infinity 2D-LC Solution with fluorescence detection. EPO has a complex glycosylation pattern with differently branched and charged glycans. A combination of hydrophilic interaction chromatography (HILIC) with weak anion exchange chromatography (WAX) enables highly orthogonal separation. Comprehensive 2D-LC analysis with HILIC in the first and WAX in the second dimension facilitates high-resolution 2D chromatography together with simultaneous charge profiling. In addition, multiple heart-cutting 2D-LC analysis, combining WAX and HILIC separation, provides a flexible alternative whereby the user can select multiple peaks to be analyzed in the second dimension and, moreover, run longer gradients.
方案详情

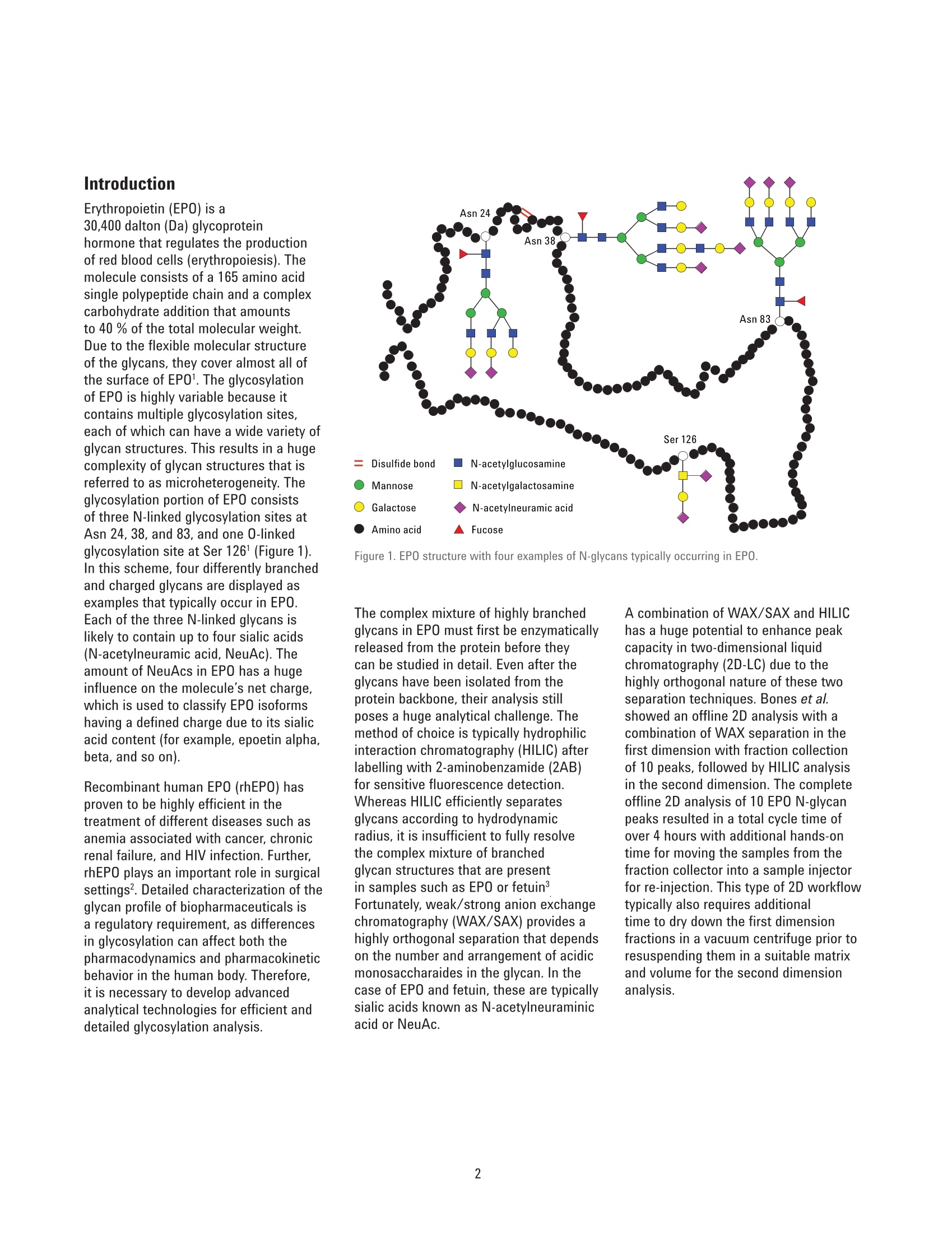
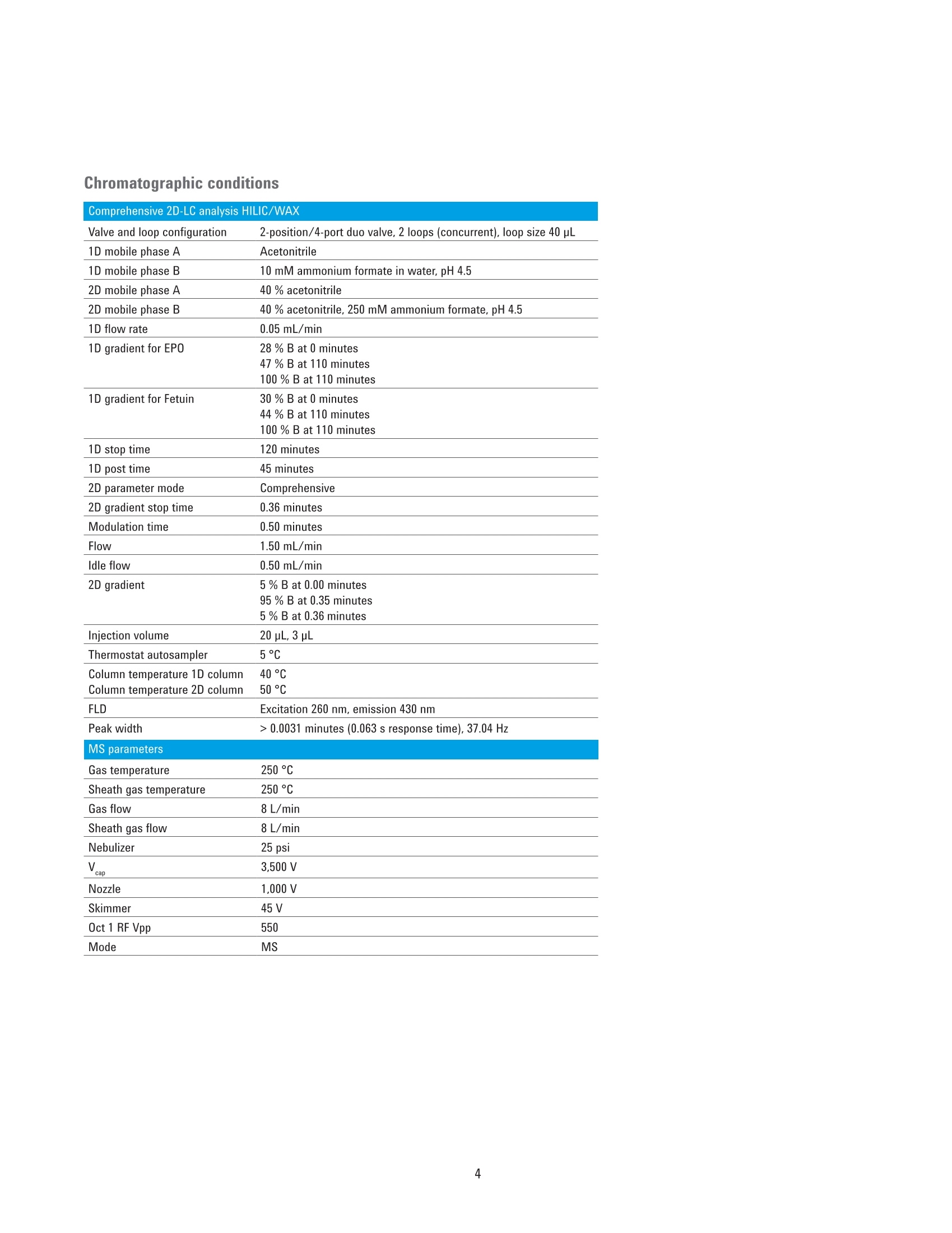
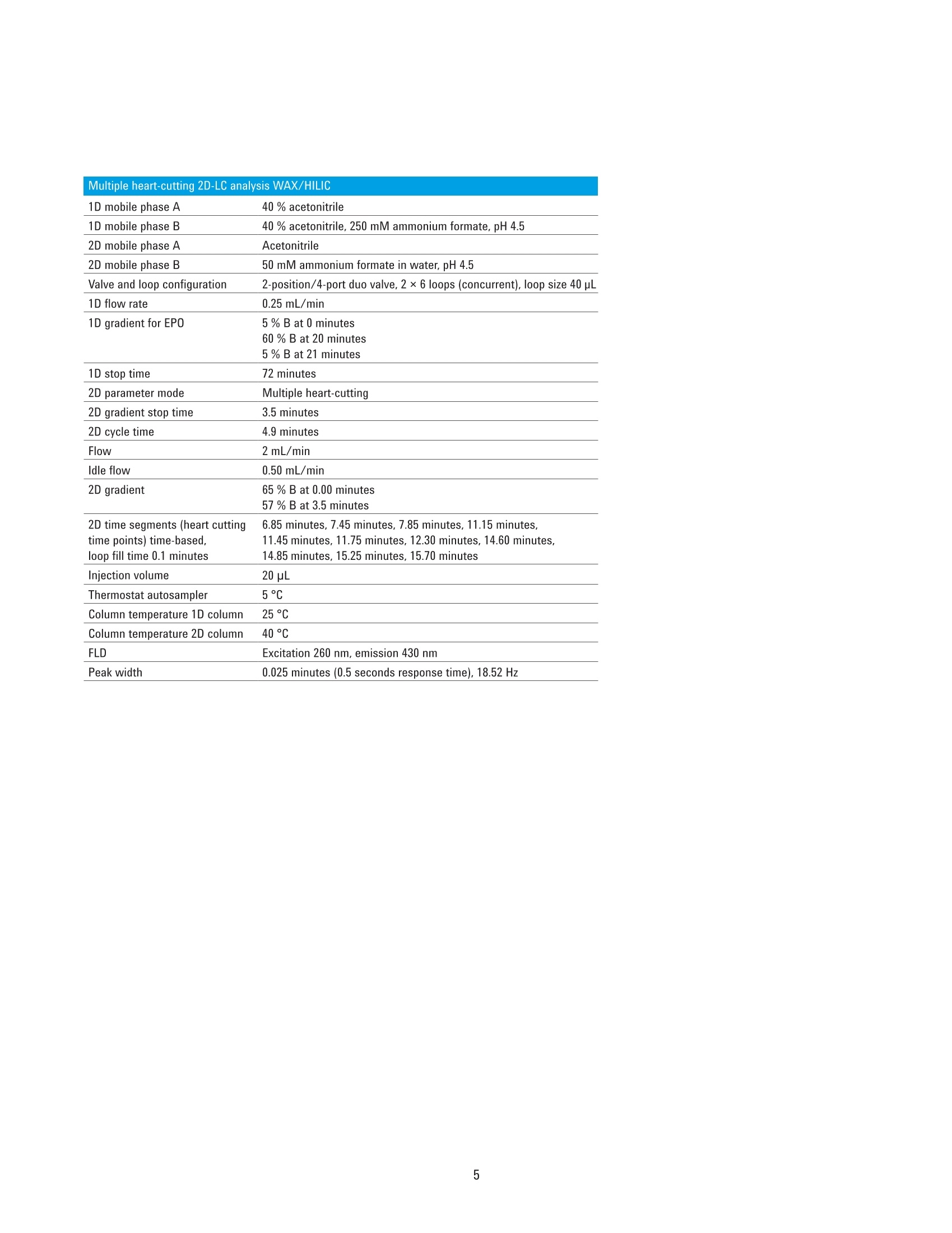
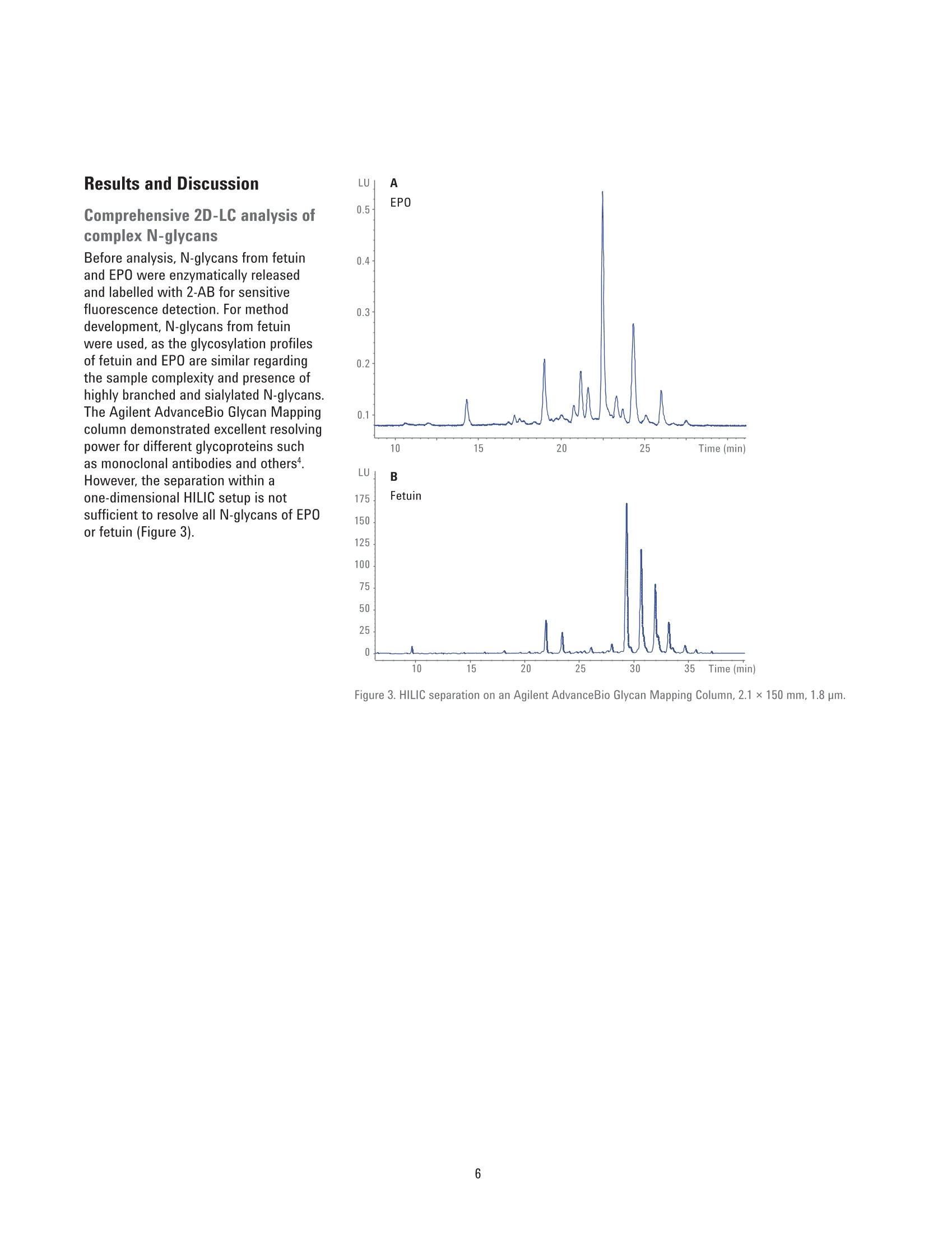
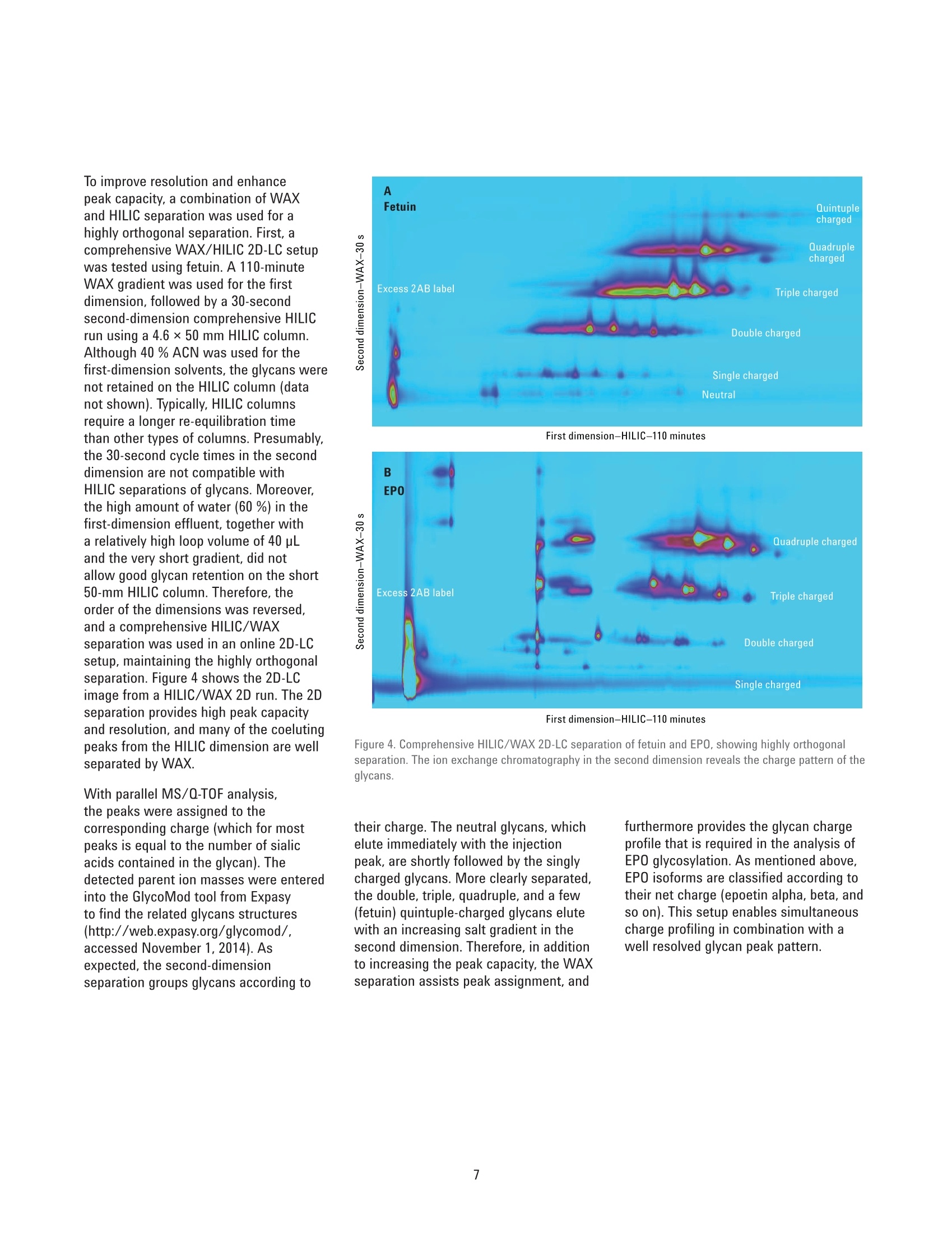
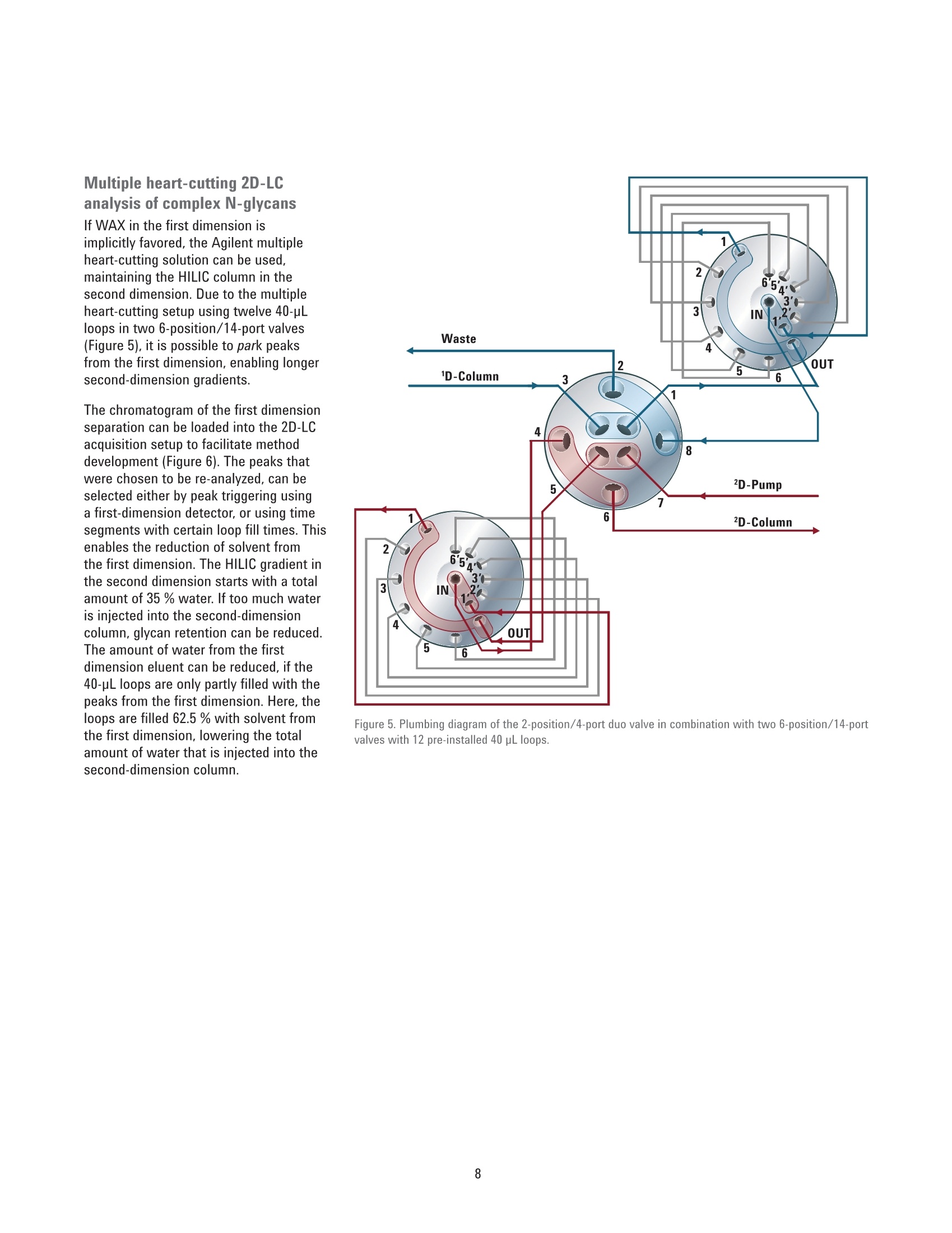
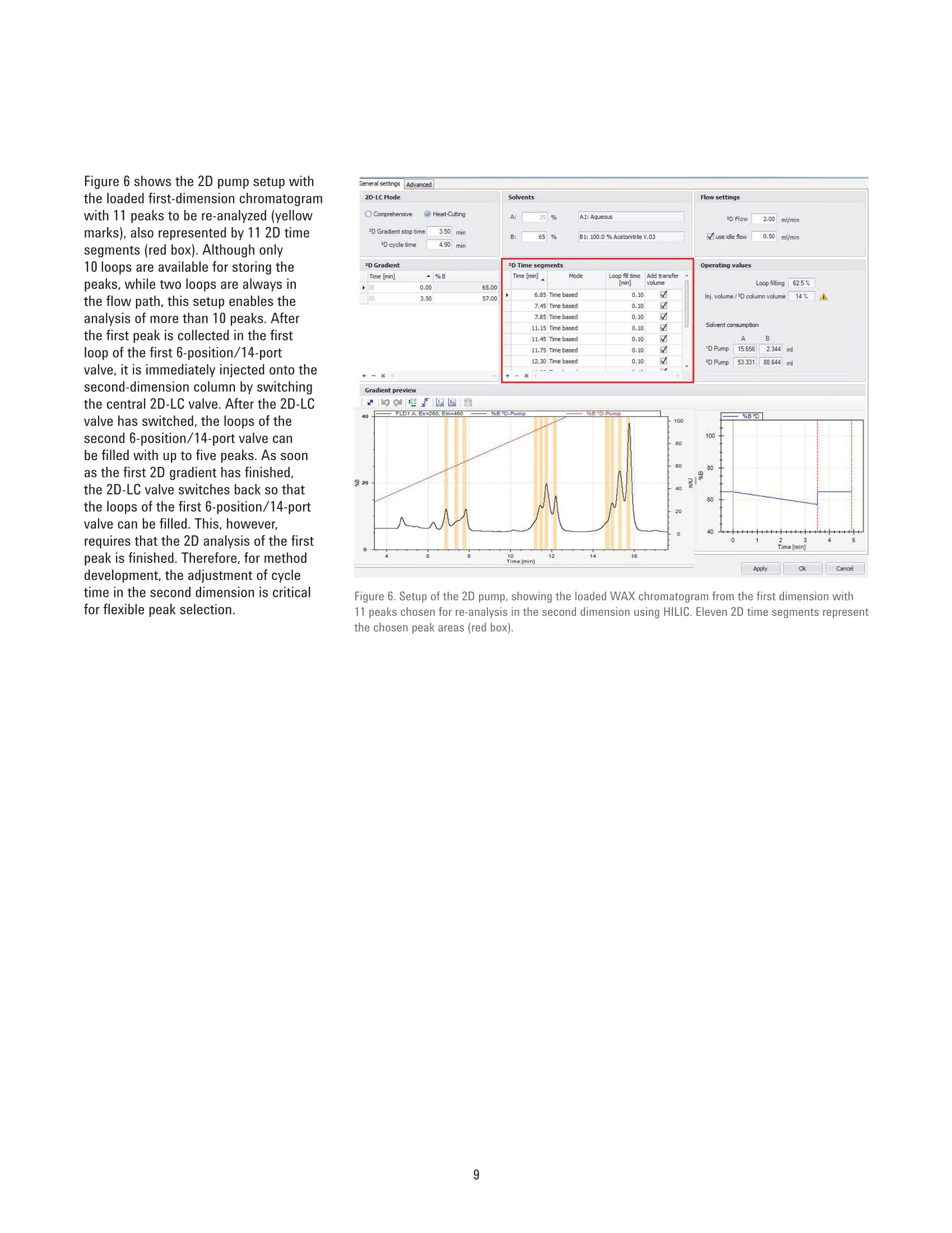
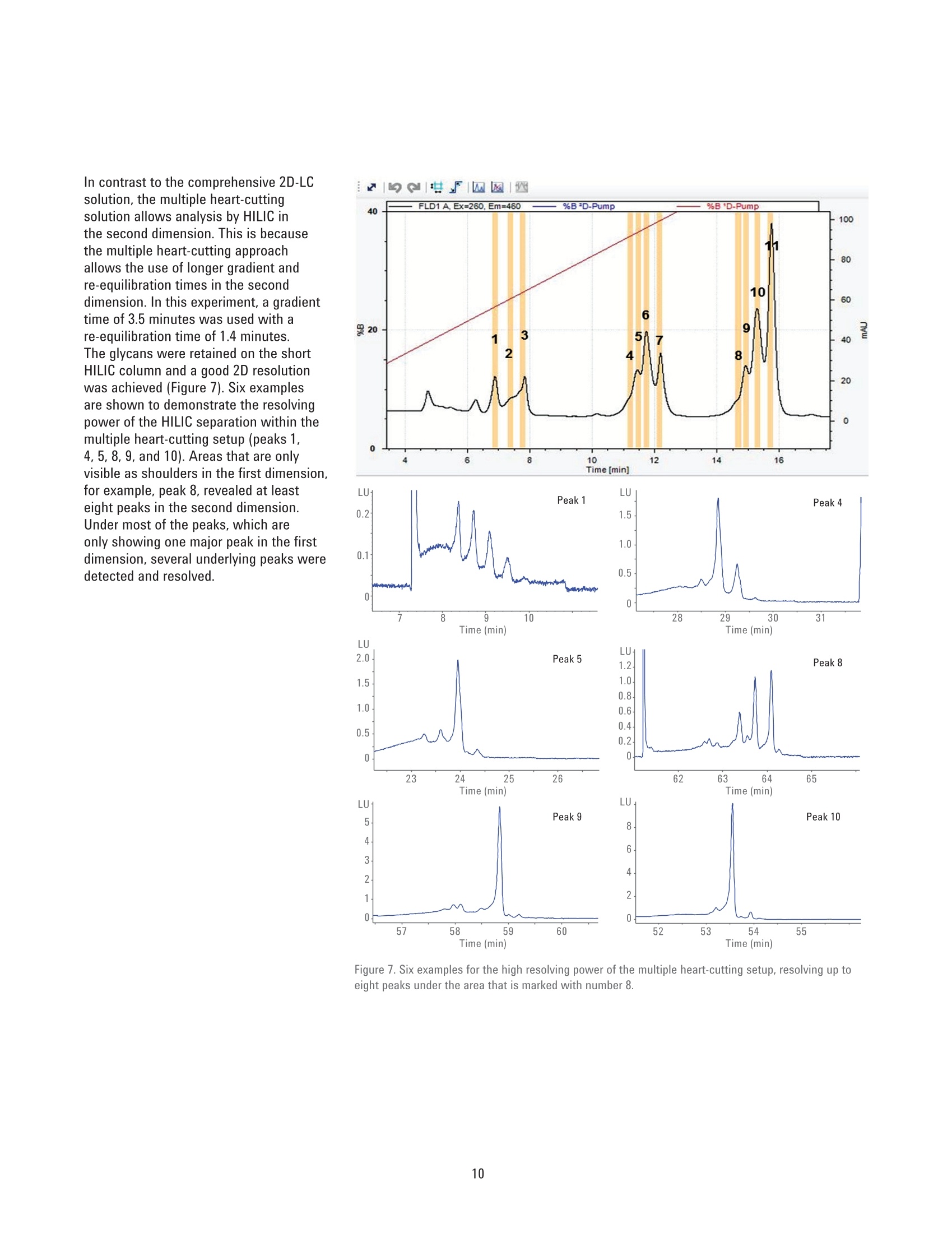
Authors Sonja Schneider, Edgar Naegele, and Sonja KriegerAgilent Technologies, Inc. Waldbronn, Germany Online 2D-LC Analysis of ComplexN-Glycans in BiopharmaceuticalsUsing the Agilent 1290 Infinity 2D-LCSolution Comprehensive and Multiple Heart-Cutting 2D-LCAnalysis for Highest Resolution Application Note Biotherapeutics & Biologics Abstract This Application Note presents different online 2D-LC concepts for thehigh-resolution analysis of complex N-linked glycans in erythropoietin (EPO)using the Agilent 1290 Infinity 2D-LC Solution with fluorescence detection.EPO has a complex glycosylation pattern with differently branched and chargedglycans. A combination of hydrophilic interaction chromatography (HILIC)with weak anion exchange chromatography (WAX) enables highly orthogonalseparation. Comprehensive 2D-LC analysis with HILIC in the first and WAX in thesecond dimension facilitates high-resolution 2D chromatography together withsimultaneous charge profiling. In addition, multiple heart-cutting 2D-LC analysis,combining WAX and HILIC separation, provides a flexible alternative wherebythe user can select multiple peaks to be analyzed in the second dimension and,moreover, run longer gradients. Erythropoietin (EPO) is a 30,400 dalton (Da) glycoproteinhormone that regulates the productionof red blood cells (erythropoiesis). Themolecule consists of a 165 amino acidsingle polypeptide chain and a complexcarbohydrate addition that amountsto 40 % of the total molecular weight.Due to the flexible molecular structureof the glycans, they cover almost all ofthe surface of EPO1. The glycosylationof EPO is highly variable because itcontains multiple glycosylation sites,each of which can have a wide variety ofglycan structures. This results in a hugecomplexity of glycan structures that isreferred to as microheterogeneity. Theglycosylation portion of EPO consistsof three N-linked glycosylation sites atAsn 24, 38, and 83, and one 0-linkedglycosylation site at Ser 1261 (Figure 1).In this scheme, four differently branchedand charged glycans are displayed asexamples that typically occur in EPO.Each of the three N-linked glycans islikely to contain up to four sialic acids(N-acetylneuramic acid, NeuAc). Theamount of NeuAcs in EPO has a hugeinfluence on the molecule's net charge,which is used to classify EPO isoformshaving a defined charge due to its sialicacid content (for example, epoetin alpha,beta, and so on). Recombinant human EPO (rhEPO) hasproven to be highly efficient in thetreatment of different diseases such asanemia associated with cancer,chronicrenal failure, and HIV infection. Further,rhEPO plays an important role in surgicalsettings². Detailed characterization of theglycan profile of biopharmaceuticals isa regulatory requirement, as differencesin glycosylation can affect both thepharmacodynamics and pharmacokineticbehavior in the human body. Therefore,it is necessary to develop advancedanalytical technologies for efficient anddetailed glycosylation analysis. Amino acid Fucose Figure 1. EPO structure with four examples of N-glycans typically occurring in EPO. The complex mixture of highly branchedglycans in EPO must first be enzymaticallyreleased from the protein before theycan be studied in detail. Even after theglycans have been isolated from theprotein backbone, their analysis stillposes a huge analytical challenge. Themethod of choice is typically hydrophilicinteraction chromatography (HILIC) afterlabelling with 2-aminobenzamide (2AB)for sensitive fluorescence detection.Whereas HILIC efficiently separatesglycans according to hydrodynamicradius, it is insufficient to fully resolvethe complex mixture of branchedglycan structures that are presentin samples such as EPO or fetuinFortunately, weak/strong anion exchangechromatography (WAX/SAX) provides ahighly orthogonal separation that dependson the number and arrangement of acidicmonosaccharaides in the glycan. In thecase of EPO and fetuin, these are typicallysialic acids known as N-acetylneuraminicacid or NeuAc. A combination of WAX/SAX and HILIChas a huge potential to enhance peakcapacity in two-dimensional liquidchromatography (2D-LC) due to thehighly orthogonal nature of these twoseparation techniques. Bones et al.showed an offline 2D analysis with acombination of WAX separation in thefirst dimension with fraction collectionof 10 peaks, followed by HILIC analysisin the second dimension. The completeoffline 2D analysis of 10 EPO N-glycanpeaks resulted in a total cycle time ofover 4 hours with additional hands-ontime for moving the samples from thefraction collector into a sample injectorfor re-injection. This type of 2D workflowtypically also requires additionaltime to dry down the first dimensionfractions in a vacuum centrifuge prior toresuspending them in a suitable matrixand volume for the second dimensionanalysis. The Agilent 1290 Infinity 2D-LC solutionenables online 2D-LC workflows for eithercomprehensive or (multiple)heart-cuttinganalysis. Comprehensive 2D-LC analysis,using two sample loops within a2-position/4-port-duo valve, captures allpeaks from the first dimension. If higherresolution in the second dimension isdesired, the Agilent 1290 Infinity multipleheart-cutting 2D-LC solution enablesmore flexibility, for example, longercycle times or columns. This solution iscomprised oftwo external valve driveswith 6-position/-14-port valves, each withsix pre-installed 40-pL loops, resultingin twelve loops. Figure 2 shows anAgilent multiple heart-cutting valve withpre-installed loops. This Application Note shows online2D-LC concepts for N-glycan analysis oftherapeutic EPO using a combination ofHILIC/WAX for comprehensive analysisand WAX/HILIC for detailed multipleheart-cutting analysis. Experimental The Agilent 1290 Infinity 2D-LC solutionSGwas comprised of the following modules: Agilent 1260 Infinity Bio-inertQuaternary Pump (G5611A) for thefirst dimension Agilent 1290 Infinity Binary Pump(G4220A) for the second dimension Agilent 1290 Infinity Autosampler(G4226A) with an Agilent 1290Infinity Thermostat (G1330B) Agilent 1290 Infinity ThermostattedColumn Compartment (G1316C) Agilent 1290 Infinity Valve Drive(G1170A) with 2-position/4-portduo valve (G4236A) equipped witheither two 40-pL loops, or Figure 2. Agilent Multiple Heart Cutting Valve with six pre-installed 40-pL loops. Agilent Multiple Heart CuttingValve Upgrade Kit (G4242A) Agilent 1260 Infinity FluorescenceDetector (G1321B), with standard8-mm flow cell MS system Agilent 6530 Accurate-Mass QTOFLC/MS system Columns Agilent AdvanceBio Glycan Mapping column,2.1×150 mm, 1.8 um(p/n 859700-913) Agilent AdvanceBio GlycanMapping column,4.6×50 mm, 2.7 pm (custom) Agilent Bio WAX column,2.1×250 mm, 5 um(p/n 5190-2491) Software Agilent OpenLAB CDSChemStation Edition for LC &LC/MS Systems Rev. C.01.06[61] Agilent MassHunter Workstationsoftware, Version B.05.01,Build 4.0.479.0 Glycan structures were createdwith GlycoWorkbench, Version 2.1,stable (146) Solvents and samples All solvents used were LC grade.Fresh ultrapure water was obtainedfrom a Milli-QIntegral system equippedwith a 0.22-pm membrane point-of-usecartridge (Millipak). Ammonium formate,fetuin, and ovalbumin, PNGase F fromElizabethkingia miricola, GlycoProfil2-AB Labeling Kit and GlycoProfil GlycanCleanup Cartridges were purchased fromSigma-Aldrich, St.Louis, USA. B-EPO wasused as example of a therapeutic EPO. Chromatographic conditions Multiple heart-cutting 2D-LC analysis WAX/HILIC 1D mobile phase A 40 % acetonitrile 1D mobile phase B 40 % acetonitrile, 250 mM ammonium formate, pH 4.5 2D mobile phase A Acetonitrile 2D mobile phase B 50 mM ammonium formate in water, pH 4.5 Valve and loop configuration 2-position/4-port duo valve, 2×6 loops (concurrent), loop size 40 pL 1D flow rate 0.25 mL/min 1D gradient for EPO 5 % B at 0 minutes 60 % B at 20 minutes 5% B at 21 minutes 1D stop time 72 minutes 2D parameter mode Multiple heart-cutting 2D gradient stop time 3.5 minutes 2D cycle time 4.9 minutes Flow 2 mL/min Idle flow 0.50 mL/min 2D gradient 65% B at 0.00 minutes 57 % B at 3.5 minutes 2D time segments (heart cutting 6.85 minutes, 7.45 minutes, 7.85 minutes, 11.15 minutes, time points) time-based, 11.45 minutes, 11.75 minutes, 12.30 minutes, 14.60 minutes, loop fill time 0.1 minutes 14.85 minutes, 15.25 minutes, 15.70 minutes Injection volume 20 pL Thermostat autosampler 5°C Column temperature 1D column 25 °C Column temperature 2D column 40°C FLD Excitation 260 nm, emission 430 nm Peak width 0.025 minutes (0.5 seconds response time), 18.52 Hz Results and Discussion Comprehensive 2D-LC analysis of complex N-glycansBefore analysis, N-glycans from fetuinand EPO were enzymatically releasedand labelled with 2-AB for sensitivefluorescence detection.For methoddevelopment, N-glycans from fetuinwere used, as the glycosylation profilesof fetuin and EPO are similar regardingthe sample complexity and presence ofhighly branched and sialylated N-glycans.The Agilent AdvanceBio Glycan Mappingcolumn demonstrated excellent resolvingpower for different glycoproteins suchas monoclonal antibodies and others4.However, the separation within aone-dimensional HILIC setup is notsufficient to resolve all N-glycans of EPOor fetuin (Figure 3). Figure 3. HILIC separation on an Agilent AdvanceBio Glycan Mapping Column, 2.1×150 mm, 1.8 pm. To improve resolution and enhancepeak capacity, a combination of WAXand HILIC separation was used for ahighly orthogonal separation. First, acomprehensive WAX/HILIC 2D-LC setupwas tested using fetuin. A 110-minuteWAX gradient was used for the firstdimension, followed by a 30-secondsecond-dimension comprehensive HILICrun using a 4.6×50 mm HILIC column.Although 40% ACN was used for thefirst-dimension solvents, the glycans werenot retained on the HILIC column (datanot shown). Typically, HILIC columnsrequire a longer re-equilibration timethan other types of columns. Presumably,the 30-second cycle times in the seconddimension are not compatible withHILIC separations of glycans. Moreover,the high amount of water (60 %) in thefirst-dimension effluent, together witha relatively high loop volume of 40 pLand the very short gradient, did notallow good glycan retention on the short50-mm HILIC column. Therefore, theorder of the dimensions was reversed,and a comprehensive HILIC/WAXseparation was used in an online 2D-LCsetup, maintaining the highly orthogonalseparation. Figure 4 shows the 2D-LCimage from a HILIC/WAX 2D run. The 2Dseparation provides high peak capacityand resolution, and many of the coelutingpeaks from the HILIC dimension are wellseparated by WAX. With parallel MS/Q-TOF analysis,the peaks were assigned to thecorresponding charge (which for mostpeaks is equal to the number of sialicacids contained in the glycan). Thedetected parent ion masses were enteredinto the GlycoMod tool from Expasyto find the related glycans structures(http://web.expasy.org/glycomod/,accessed November 1, 2014). Asexpected, the second-dimensionseparation groups glycans according to First dimension-HILIC-110 minutes First dimension-HILIC-110 minutes Figure 4. Comprehensive HILIC/WAX 2D-LC separation of fetuin and EPO, showing highly orthogonalseparation. The ion exchange chromatography in the second dimension reveals the charge pattern of theglycans. their charge. The neutral glycans, whichelute immediately with the injectionpeak, are shortly followed by the singlycharged glycans.More clearly separated,the double, triple, quadruple, and a few(fetuin) quintuple-charged glycans elutewith an increasing salt gradient in thesecond dimension.Therefore, in additionto increasing the peak capacity, the WAXseparation assists peak assignment, and furthermore provides the glycan chargeprofile that is required in the analysis ofEPO glycosylation. As mentioned above,EPO isoforms are classified according totheir net charge (epoetin alpha, beta, andso on). This setup enables simultaneouscharge profiling in combination with awell resolved glycan peak pattern. Multiple heart-cutting 2D-LCanalysis of complex N-glycans If WAX in the first dimension isimplicitly favored, the Agilent multipleheart-cutting solution can be used,maintaining the HILIC column in thesecond dimension. Due to the multipleheart-cutting setup using twelve 40-pLloops in two 6-position/14-port valves(Figure 5), it is possible to park peaksfrom the first dimension, enabling longersecond-dimension gradients. The chromatogram of the first dimensionseparation can be loaded into the 2D-LCacquisition setup to facilitate methoddevelopment (Figure 6). The peaks thatwere chosen to be re-analyzed, can beselected either by peak triggering usinga first-dimension detector, or using timesegments with certain loop fill times. Thisenables the reduction of solvent fromthe first dimension. The HILIC gradient inthe second dimension starts with a totalamount of 35 % water. If too much wateris injected into the second-dimensioncolumn, glycan retention can be reduced.The amount of water from the firstdimension eluent can be reduced, if the40-pL loops are only partly filled with thepeaks from the first dimension. Here, theloops are filled 62.5 % with solvent fromthe first dimension, lowering the totalamount of water that is injected into thesecond-dimension column. Figure 5. Plumbing diagram of the 2-position/4-port duo valve in combination with two 6-position/14-portvalves with 12 pre-installed 40 pL loops. Figure 6 shows the 2D pump setup withthe loaded first-dimension chromatogramwith 11 peaks to be re-analyzed (yellowmarks), also represented by 11 2D timesegments (red box). Although only10 loops are available for storing thepeaks, while two loops are always inthe flow path, this setup enables theanalysis of more than 10 peaks. Afterthe first peak is collected in the firstloop of the first 6-position/14-portvalve, it is immediately injected onto thesecond-dimension column by switchingthe central 2D-LC valve. After the 2D-LCvalve has switched, the loops of thesecond 6-position/14-port valve canbe filled with up to five peaks. As soonas the first 2D gradient has finished,the 2D-LC valve switches back so thatthe loops of the first 6-position/14-portvalve can be filled. This, however,requires that the 2D analysis of the firstpeak is finished. Therefore, for methoddevelopment, the adjustment of cycletime in the second dimension is criticalfor flexible peak selection. Figure 6. Setup of the 2D pump, showing the loaded WAX chromatogram from the first dimension with11 peaks chosen for re-analysis in the second dimension using HILIC. Eleven 2D time segments representthe chosen peak areas (red box). In contrast to the comprehensive 2D-LCsolution, the multiple heart-cuttingsolution allows analysis by HILIC inthe second dimension. This is becausethe multiple heart-cutting approachallows the use of longer gradient andre-equilibration times in the seconddimension. In this experiment, a gradienttime of 3.5 minutes was used with are-equilibration time of 1.4 minutes.The glycans were retained on the shortHILIC column and a good 2D resolutionwas achieved (Figure 7). Six examplesare shown to demonstrate the resolvingpower of the HILIC separation within themultiple heart-cutting setup (peaks 1,4, 5, 8, 9, and 10). Areas that are onlyvisible as shoulders in the first dimension,for example, peak 8, revealed at leasteight peaks in the second dimension.Under most of the peaks, which areonly showing one major peak in the firstdimension, several underlying peaks weredetected and resolved. 40 =FLD1 A. Ex=260.Em=460 %B*D-Pump 9%B 'D-Pump 0 0 57 58 59 60 52 53 54 55 Time (min) Time (min) Figure 7. Six examples for the high resolving power of the multiple heart-cutting setup, resolving up toeight peaks under the area that is marked with number 8. Conclusion This Application Note shows differentconcepts for online 2D-LC analysis ofcomplex N-glycans found in therapeuticEPO using the Agilent 1290 Infinity 2D-LCSolution with fluorescence detection.Differently branched and charged glycansare attached to EPO resulting in a highlycomplex and heterogeneous glycanprofile, in which especially the net chargeof EPO is used for the classification oftherapeutic EPO. The complexity of theEPO glycans requires modern analysistechniques that deliver high resolution. Although current HILIC columns havea high resolving power for glycans, themixture of glycans on therapeutic EPOis so complicated that full resolutioncannot be achieved by a one-dimensionalHILIC separation. Online comprehensive2D-LC with a combination of HILIC in thefirst dimension and a highly orthogonalWAX separation in the second dimensionprovides high resolution and peakcapacity. Complete automation of the2D analysis enables a run time of only110 minutes when compared to a muchlonger run time for offline analysisFurthermore, the resulting data areeasy to interpret because the glycansare grouped according to their chargein the second dimension, satisfying therequirement of charge profiling for EPOglycans. The multiple heart-cutting solution offersgreater flexibility and facilitates the moretechnically demanding combination ofWAX in the first, and HILIC in the seconddimension. With this 2D separationmethod, the user is able to select severalpeaks from the WAX chromatogramfor additional separation in the seconddimension. In addition, because thepeaks are parked in the 40-uL loops ofthe two 6-position/14-port valves, theirsecond-dimension separation is no longerlimited to super short gradients. Instead,the cycle time for the second dimensioncan be adjusted as needed, dependingon the distribution of the peaks withinthe first-dimension chromatogram.In comparison to offline 2D-LC usingWAX/HILIC analysis with fractioncollection (Bones et al.), a time savingsof about 70 % was possible, reducingthe total analysis time from over 4 hoursdown to about 70 minutes. The 1290 Infinity 2D-LC Solutiontogether with the Agilent 1260Infinity Fluorescence Detector andthe combination of HILIC and WAXseparation media enable excellentresolving power for highly complexglycans from therapeutic glycoproteins. References 1. Egrie, J. C; et al. Characterizationand biological effects of recombinanthuman erythropoietin. Immunobiology1986, 172, pp 213-224. 2. Egrie, J. C; Browne,J. K. Developmentand characterization of novelerythropoiesis stimulating protein(NESP). British Journal of Cancer2001, 84 (Supplement 1), pp 3-10. 3. Bones, J; et al. 2D-LC Analysis of BRP3 Erythropoietin N-Glycosylation using. Anion Exchange Fractionation andHydrophilic Interaction UPLC RevealsLong Poly-N-Acetyl LactosamineExtensions. Anal Chem. 2011, 83(11),pp 4154-4162. 4. Schneider, S; Potter, O. N-GlycanAnalysis of mAbs and OtherGlycoproteins with UHPLC andFluorescence Detection, AgilentTechnologies Application Note,publication number 5991-5253EN,2014. www.agilent.com/chem For Research Use Only. Not for use in diagnostic procedures.This information is subject to change without notice. C Agilent Technologies, Inc., 2015, 2017 Published in the USA, November 2,2017 5991-5349EN Agilent Technologies Agilent Technologies This Application Note presents different online 2D-LC concepts for the high?resolution analysis of complex N-linked glycans in erythropoietin (EPO) using the Agilent 1290 Infinity 2D-LC Solution with fluorescence detection. EPO has a complex glycosylation pattern with differently branched and charged glycans. A combination of hydrophilic interaction chromatography (HILIC) with weak anion exchange chromatography (WAX) enables highly orthogonal separation. Comprehensive 2D-LC analysis with HILIC in the first and WAX in the second dimension facilitates high-resolution 2D chromatography together with simultaneous charge profiling. In addition, multiple heart-cutting 2D-LC analysis, combining WAX and HILIC separation, provides a flexible alternative whereby the user can select multiple peaks to be analyzed in the second dimension and, moreover, run longer gradients.
确定




还剩10页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《生物药物中多聚糖检测方案(液相色谱仪)》,该方案主要用于生物药品药物研发中多聚糖检测,参考标准--,《生物药物中多聚糖检测方案(液相色谱仪)》用到的仪器有Agilent 1290 Infinity II 二维液相色谱解决方案、Agilent 1290 Infinity II Multisampler
推荐专场
相关方案
更多
该厂商其他方案
更多

















