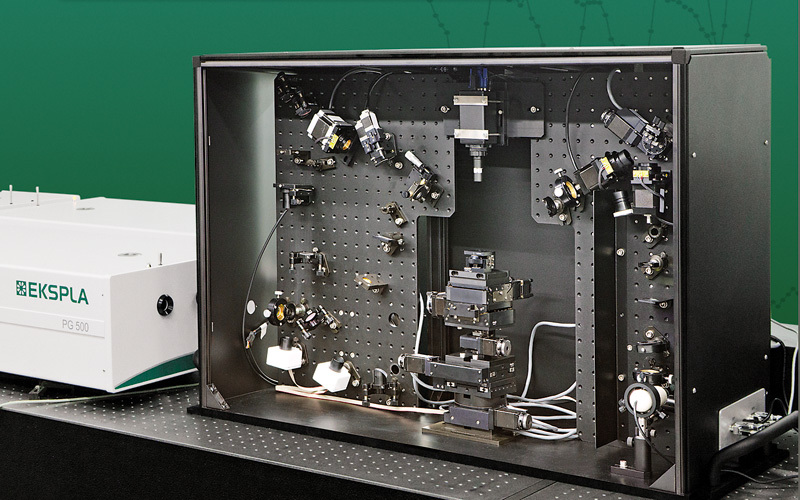
采用立陶宛Ekspla公司PL2251A型皮秒脉冲激光器和PG501型皮秒光学参量发生器构成的振动和频光谱测量系统(SFG)对具有末端苯丙氨酸环和链内酰胺基团的硫醇的自组装单层的形成进行了实验测量研究。
方案详情

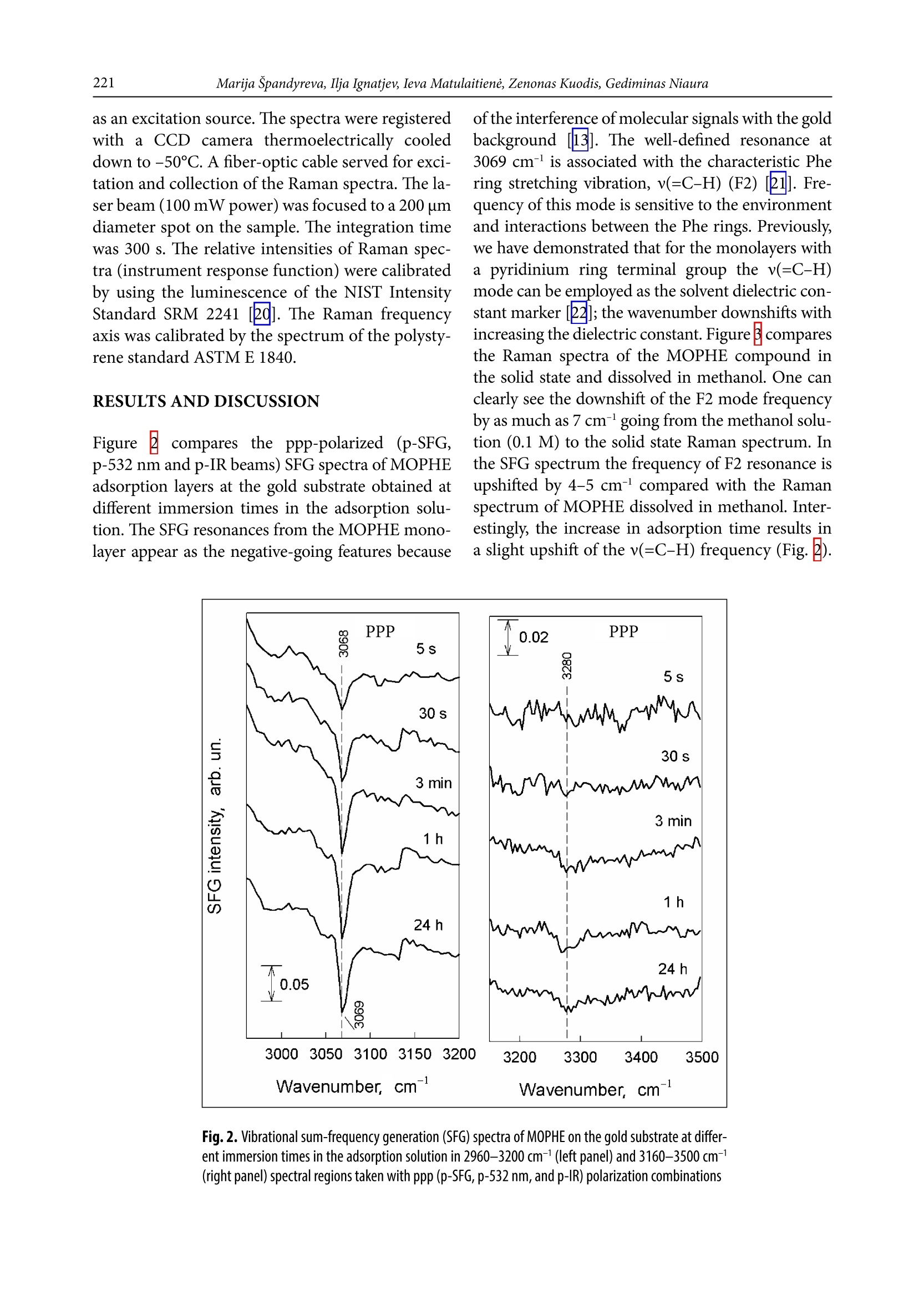
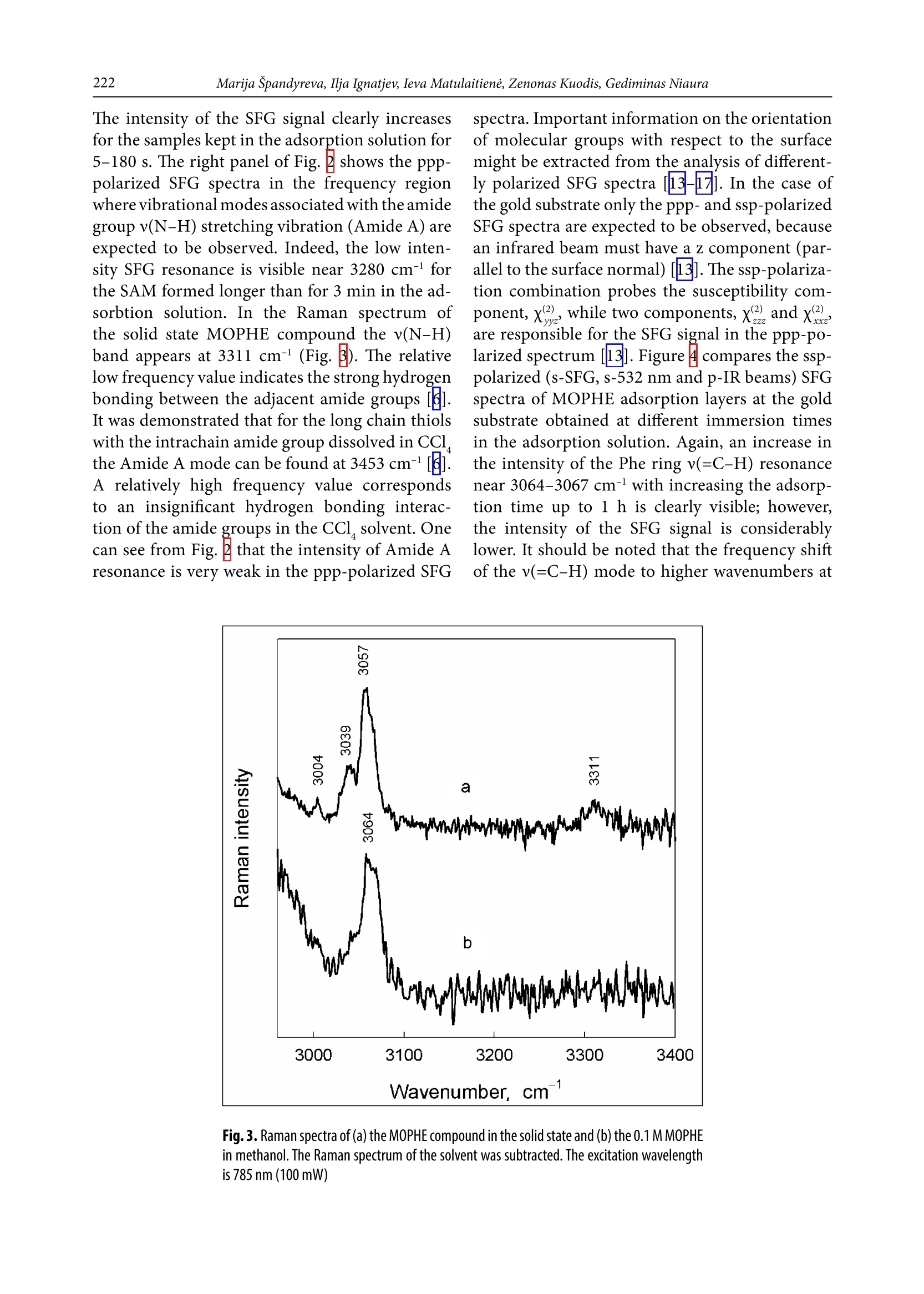
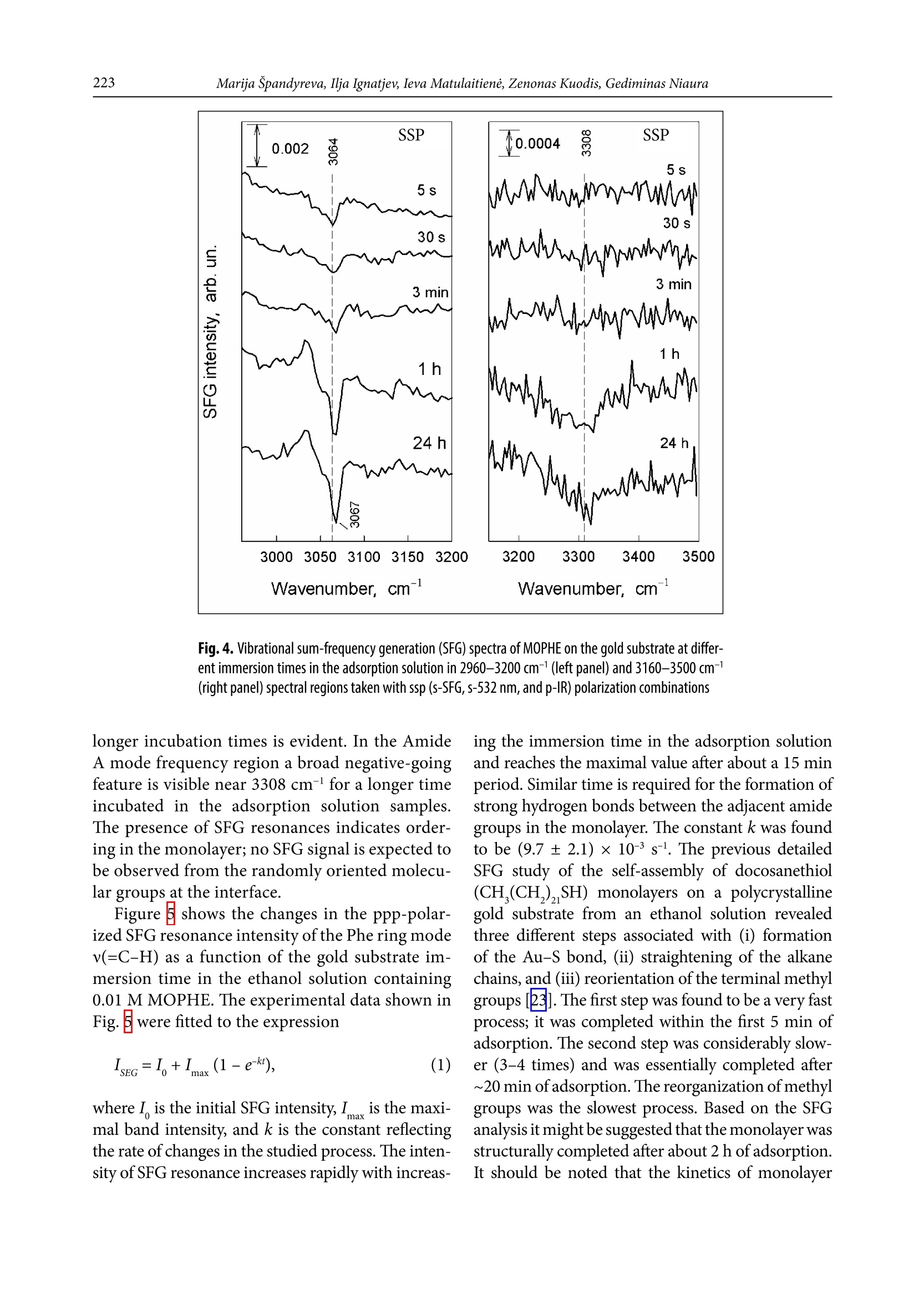
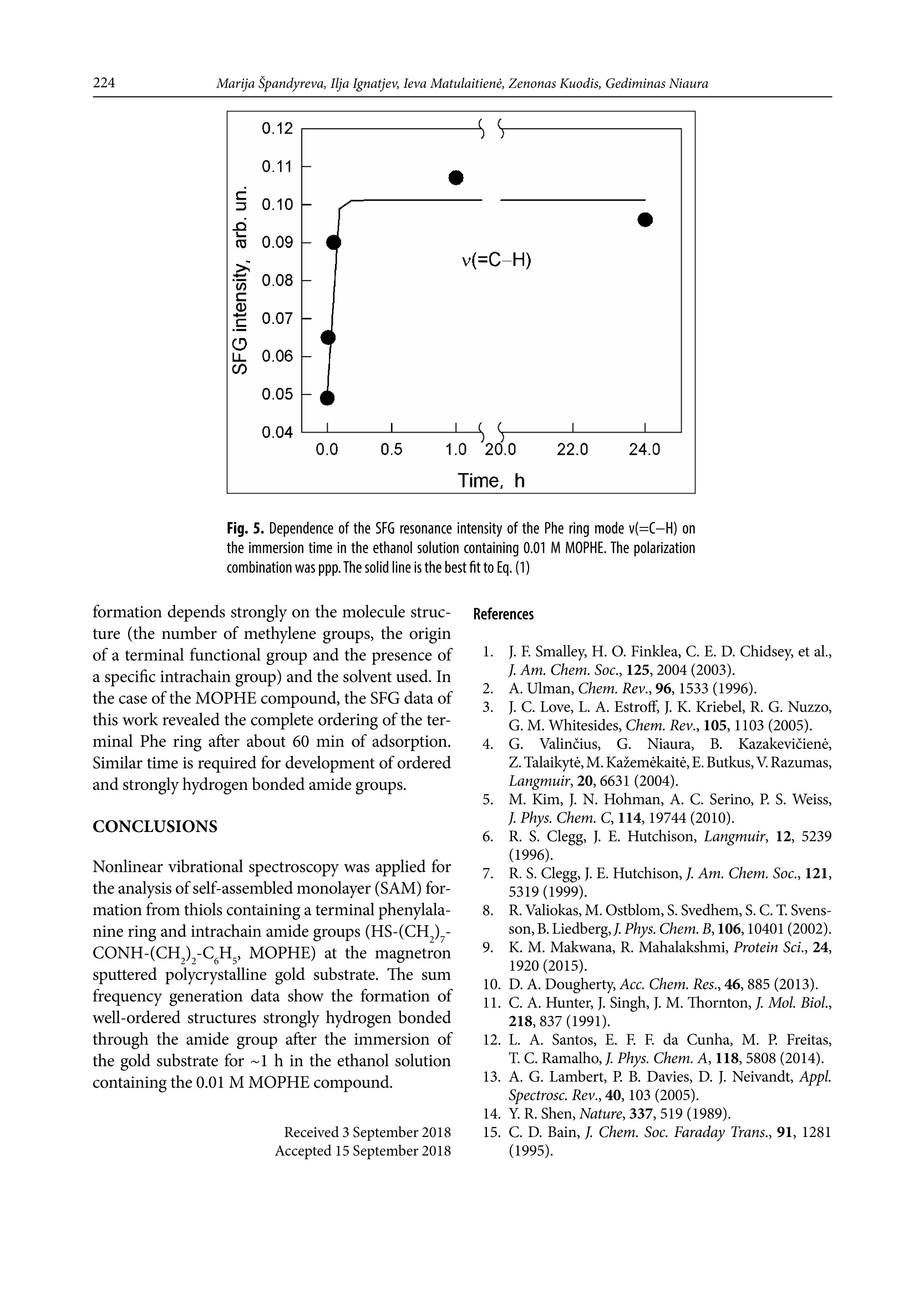
CHEMIJA.2018. Vol. 29. No. 4. P. 219-226@ Lietuvos moksly akademija, 2018 220Marija Spandyreva, Ilja Ignatjev, Ieva Matulaitiene, Zenonas Kuodis, Gediminas Niaura Sum frequency generation spectroscopy probing offormation of self-assembled monolayers from thiolswith terminal phenylalanine ring and intrachain amidegroups Marija Spandyreva, Ilja Ignatjev,Ieva Matulaitiene, Zenonas Kuodis, Gediminas Niaura* Department of Organic Chemistry,Center for Physical Sciences and TechnologySauletekio Ave. 3, 10257, Vilnius, Lithuania Formation of a self-assembled monolayer onto the polycrystalline goldsubstrate from thiols containing a terminal phenylalanine (Phe) ringand intrachain amide groups (HS-(CH,)-CONH-(CH,)-CH,) wascharacterized by vibrational sum frequency generation (SFG) spectros-copy. The temporal evolution of the characteristic Phe ring stretchingvibration v(=C-H) near 3069 cm-and the Amide A mode were moni-tored by nonlinear vibrational spectroscopy. The SFG data revealedthe complete ordering of the terminal Phe rings after about 60 min ofadsorption. Formation of a strong hydrogen bonding between the adjacent chains of adsorbed molecules was evident by appearance ofthe Amide A mode at the relative low frequency (3280-3308 cm-l).The well-ordered and strongly hydrogen bonded SAM with the termi-nal Phe ring functionality is a promising platform for the analysis ofinteractions and the function of aromatic rings in biomolecular pro-cesses. Keywords: SAM, phenylalanine, amide, SFG,Raman INTRODUCTION Self-assembled monolayers (SAMs) of bifunc-tional thiol compounds on noble metal surfacesprovide a useful platform for a detailed analysisof the interaction of functional groups with solu-tion components and adsorbed molecules, stud-ies of electron transfer, construction of (bio)sen-sors, electrocatalytical studies, and developmentof bioelectronics devices [1-4]. The structure ofthe monolayer controls the stability and functionof the terminal group. The important interactionsresponsible for the architecture of the monolayerare (i) covalent bonding between the sulfur atom Such interactions stabilize the tertiary structure ofproteinsSL[12]. Bifunctional monolayers on metalsurfaces may provide a promising platform to getinsights into various interactions of aromatic moi-eties important for the biological function of pep-tides and proteins. To prepare stable and functional monolayerswith controllable properties, detailed molecularlevel information on the structure and interac-tions between the functional groups in the mono-layer is required. Vibrational spectroscopy is ableto provide such knowledge. In particular, sumfrequency generation (SFG) spectroscopy is botha surface and molecule specific tool able to pro-vide rich atomistic information on bonding, inter-actions, and orientation of particular moleculargroups[13-19]. Within the electric dipole ap-proximation the SFG signal arises from the over-lapped visible and IR beams at the interface wherethe inversion symmetry is broken. The signal gen-eration is forbidden in the centrosymmetric me-dia or in the case of randomly oriented molecules.Because at the interfaces the inversion symmetryis broken, SFG is an intrinsically surface specif-ic technique. Molecular sensitivity comes fromthe capacity to record the vibrational spectrum.To be active in the SFG spectrum the particularvibrational mode must be active in both the Ra-man and infrared spectra. In this work, SFG spectroscopy was appliedto determine structural transformations duringthe formation of a self-assembled monolayer fromthe thiols containing a terminal phenylalaninering and an intrachain amide group. EXPERIMENTAL Millipore-purified water (18 MQ cm) was usedfor the preparation of monolayer formation solu-tions. The monolayer forming compound (N-(2-phenylethyl)-8-sulphanyloctanamide)(MOPHE)(Fig. 1) was synthesized in our laboratory. The syn-thesis details will be published elsewhere. 8-Bro-)-mooctanoic acid (97%) and 2-phenylethylamine(99%) were purchased from Sigma-Aldrich Che-mie GmbH (Germany) and were used withoutany additional purification. N-(2-phenylethyl)-Q8-sulphanyloctanamidewas obtained throughthe amidation of 8-bromooctanoic acid with phe-nylethylamine and further by replacing bromine to thiol. The used inorganic salts were of ACS gradeand were purchased from Sigma-Aldrich ChemieGmbH (Germany). Fig. 1. The molecular structure of N-(2-phenylethyl)-8-sulphanyloc-tanamide (MOPHE) Gold substrates were prepared by magnetronsputtering of the Cr sublayer (~3 nm) and sub-sequently the 200 nm layer of Au on the cleanedglass substrates. These substrates were immersedinto a 10 mM MOPHE ethanol solution for vari-ous adsorption times. Subsequently the substrateswere rinsed with ethanol (≥99.8%) and dried un-der N, flow. Sum frequency spectra were collected on anEKSPLA(Vilnius, Lithuania) picosecond SFGspectrometer PG501/DFG+PL2251A + SFG046.The spectrometer is based on a mode-lockedEKSPLA PL2251A Nd:YAG laser generating 30 pspulses at 1064 nm with the 50 Hz repetition rate.The second harmonic radiation from this laser wasused as a visible beam (w). The tunable infraredpulses (w ) in the 1000-3800 cm- frequency rangewere produced in a parametric generator EKSPLAPG501/DFG. The bandwidth of infrared radiationwas <6 cm. To produce SFG spectra, the wandw beams were incident at angles of 55 and 60°,Wvisrespectively, and overlapped at the sample withinan area of 0.04 mm. The sum frequency(w) ra-diation was filtered by a holographic notch filterand monochromator and detected by a photomul-tiplier tube (PMT) and gated registration system.Typically, the signal was averaged over 200 puls-es. The SFG spectra were normalized accordingto the intensity of the infrared beam to take intoaccount the changes in w energy with the wave-length. The frequencies were calibrated by the IRspectrum of the polystyrene film placed in the pathof the infrared beam. Raman spectra were recorded with an EchelleSspectrometer RamanFlex 400 (PerkinElmer, Inc.)by using the 785 nm radiation of the diode laser as an excitation source. The spectra were registeredwith a CCD camera thermoelectrically cooleddown to -50℃. A fiber-optic cable served for exci-tation and collection of the Raman spectra. The la-ser beam (100 mW power) was focused to a 200 umdiameter spot on the sample. The integration timewas 300 s. The relative intensities of Raman spec-tra (instrument response function) were calibratedby using the luminescence of the NIST IntensityStandard SRM 2241 [20]. The Raman frequencyaxis was calibrated by the spectrum of the polysty-rene standard ASTM E 1840. RESULTS AND DISCUSSION Figure ccompares theppp-polarized (p-SFG,p-532 nm and p-IR beams) SFG spectra of MOPHEadsorption layers at the gold substrate obtained atdifferent immersion times in the adsorption solu-tion. The SFG resonances from the MOPHE mono-layer appear as the negative-going features because ofthe interference of molecular signals with the goldbackground [13]. The well-defined resonance at3069 cm- is associated with the characteristic Phering stretching vibration,v(=C-H) (F2) [21]. Fre-quency of this mode is sensitive to the environmentand interactions between the Phe rings. Previously,we have demonstrated that for the monolayers witha pyridinium ring terminal group the v(=C-H)mode can be employed as the solvent dielectric con-stant marker [22]; the wavenumber downshifts withincreasing the dielectric constant. Figure B comparesthe Raman spectra of the MOPHE compound inthe solid state and dissolved in methanol. One canclearly see the downshift of the F2 mode frequencyby as much as 7 cm-going from the methanol solu-tion (0.1 M) to the solid state Raman spectrum. Inthe SFG spectrum the frequency of F2 resonance isupshifted by 4-5 cm-compared with the M01Ramanspectrum of MOPHE dissolved in methanol. Inter-estingly, the increase in adsorption time results ina slight upshift of the v(=C-H) frequency (Fig.2). Fig.2. Vibrational sum-frequency generation (SFG) spectra of MOPHE on the gold substrate at differ-ent immersion times in the adsorption solution in 2960-3200 cm-1(left panel) and 3160-3500 cm-(right panel) spectral regions taken with ppp (p-SFG, p-532 nm, and p-IR) polarization combinations The intensity of the SFG signal clearly increasesfor the samples kept in the adsorption solution for5-180 s. The right panel of Fig. 2 shows the ppp-polarized SFG spectra in the frequency regionwhere vibrational modes associated with the amidegroup v(N-H) stretching vibration (Amide A) areexpected to be observed. Indeed, the low inten-sity SFG resonance is visible near 3280 cm- forthe SAM formed longer than for 3 min in the ad-sorbtion solution. In the Raman spectrum ofthe solid state MOPHE compound the v(N-H)band appears at 3311 cm- (Fig.B). The relativelow frequency value indicates the strong hydrogenbonding between the adjacent amide groupsIt was demonstrated that for the long chain thiolswith the intrachain amide group dissolved in CCl4the Amide A mode can be found at 3453 cm-1A relatively high frequency value correspondsto an insignificant hydrogen bonding interac-tion of the amide groups in the CCl solvent. Onecan see from Fig.that the intensity of Amide Aresonance is very weak in the ppp-polarized SFG spectra. Important information on the orientationof molecular groups with respect to the surfacemight be extracted from the analysis of different-ly polarized SFG spectra [13-17]. In the case ofthe gold substrate only the ppp- and ssp-polarizedSFG spectra are expected to be observed, becausean infrared beam must have a z component (par-allel to the surface normal)[13]. The ssp-polariza-tion combination probes the susceptibility com-ponent,x2), while two components, x(2)and x2),are responsible for the SFG signal in the ppp-po-larized spectrum133]. Figurecompares the ssp-polarized (s-SFG, s-532 nm and p-IR beams) SFGspectra of MOPHE adsorption layers at the goldsubstrate obtained at different immersion timesin the adsorption solution. Again, an increase inthe intensity of the Phe ring v(=C-H) resonancenear 3064-3067 cm- with increasing the adsorp-tion time up to 1 h is clearly visible; however,the intensity of the SFG signal is considerablylower. It should be noted that the frequency shiftof the v(=C-H) mode to higher wavenumbers at Fig. 3. Raman spectra of (a) the MOPHE compound in the solid state and (b) the 0.1 M MOPHEin methanol. The Raman spectrum of the solvent was subtracted. The excitation wavelengthis 785 nm (100mW) Fig. 4. Vibrational sum-frequency generation (SFG) spectra of MOPHE on the gold substrate at differ-ent immersion times in the adsorption solution in 2960-3200 cm-1(left panel) and 3160-3500 cm-1(right panel) spectral regions taken with ssp (S-SFG, s-532 nm, and p-IR) polarization combinations longer incubation times is evident. In the AmideA mode frequency region a broad negative-goingfeature is visible near 3308 cm-l for a longer timeincubated in the adsorption solution samples.The presence of SFG resonances indicates order-一ing in the monolayer; no SFG signal is expected tobe observed from the randomly oriented molecu-lar groups at the interface. Figure shows the changes in the ppp-polar-ized SFG resonance intensity of the Phe ring modev(=C-H) as a function of the gold substrate im-mersion time in the ethanol solution containing0.01 M MOPHE. The experimental data shown inFig.5were fitted to the expression where I is the initial SFG intensity, I is the maxi-mal band intensity, and k is the constant reflectingthe rate of changes in the studied process. The inten-sity of SFG resonance increases rapidly with increas- Fig. 5. Dependence of the SFG resonance intensity of the Phe ring mode v(=C-H) onthe immersion time in the ethanol solution containing 0.01 M MOPHE. The polarizationcombination was ppp. The solid line is the best fit to Eq. (1) formation depends strongly on the molecule struc-ture (the number of methylene groups, the originof a terminal functional group and the presence ofa specific intrachain group) and the solvent used. Inthe case of the MOPHE compound, the SFG data ofthis work revealed the complete ordering of the ter-minal Phe ring after about 60 min of adsorption.Similar time is required for development of orderedand strongly hydrogen bonded amide groups. CONCLUSIONS Nonlinear vibrational spectroscopy was applied forthe analysis of self-assembled monolayer (SAM) for-mation from thiols containing a terminal phenylala-nine ring and intrachain amide groups (HS-(CH,),-CONH-(CH,)-C,H, MOPHE) at the magnetronsputtered polycrystalline gold substrate. The sumfrequency generation data show the formation ofwell-ordered structures strongly hydrogen bondedthrough the amide group after the immersion ofthe gold substrate for ~1 h in the ethanol solutioncontaining the 0.01 M MOPHE compound. Received 3 September 2018Accepted 15 September 2018 1.J. F. Smalley, H. O. Finklea, C. E. D. Chidsey, et al.,J. Am. Chem. Soc.,125,2004(2003). 2A. Ulman, Chem. Rev., 96, 1533 (1996).3 J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo,G. M. Whitesides, Chem. Rev., 105, 1103 (2005). 4. G.Valincius,S,G. Niaura,B. Kazakeviciene,Z. Talaikyte,M. Kazemekaite, E. Butkus, V.Razumas,Langmuir, 20, 6631(2004). 5. M. Kim, J. N. Hohman, A. C. Serino, P. S. Weiss,J. Phys. Chem. C, 114, 19744 (2010). 6.R. S. Clegg, J. E. Hutchison,Langmuir, 12, 5239(1996). 7. R. S. Clegg, J. E. Hutchison, J. Am. Chem. Soc., 121,5319(1999). 8. R. Valiokas,M. Ostblom,S. Svedhem, S. C.T. Svens-son, B. Liedberg,J. Phys. Chem. B,106, 10401 (2002). ( 9. K. M . M akwana, R. Mahalakshmi, Protein Sci., 24, 1920 (2015). ) ( 10. D. A. Dougherty, Acc. Chem. Res., 46,885 (2013). ) ( 11. C. A. Hunter, J. Singh, J. M. Thornton,J. Mo l . Biol., 218,837 (1991). ) ( 12. L. A. Santos, E. F. F. da Cunha, M. P . F r eitas, T. C. Ramalho,J. Phys. Chem. A, 1 1 8,5808 (2014). ) 13. A. G. Lambert, P. B. Davies, D. J. Neivandt, Appl.Spectrosc. Rev., 40, 103 (2005). 14. Y.R. Shen, Nature, 337, 519 (1989). 15. C. D. Bain, J. Chem. Soc. Faraday Trans., 91, 1281(1995). 16. M. R. Watry, M. G. Brown, G. L. Richmond, Appl.Spectrosc.,55,321A (2001). 17. P. B. Miranda, Y. R. Shen, J. Phys. Chem. B, 103,3292(1999). 18. G. L. Richmond, Chem. Rev., 102, 2693 (2002). 19. G.Niaura,Z. Kuprionis, I. Ignatjev, M. Kazemekaite,G. Valincius, Z. Talaikyte, V. Razumas, A. Svendsen,J. Phys. Chem. B, 112, 4094 (2008). 20. E. S. Etz, S. J. Choquette, W. S. Hurst, Microchim.Acta,149, 175 (2005). 21. E. Proniewicz, D. Skoluba, I. Ignatjev, G. Niaura,D. Sobolewski, A. Prahl,L. M. Proniewicz, J. RamanSpectrosc., 44, 655(2013). 22. I. Matulaitiene, Z. Kuodis, A. Matijoska,O. Eicher-Lorka, G. Niaura, J. Phys. Chem. C, 119, 26481(2015). 23. M. Himmelhaus, F. Eisert, M. Buck M. Grunze,J. Phys. Chem. B, 104, 576 (2000). Marija Spandyreva, Ilja Ignatjev, Ieva Matulaitiene,Zenonas Kuodis, Gediminas Niaura SAVITVARKIU MONOSLUOKSNIUFORMAVIMOSI IS TIOLIU SU GALINEFENILALANINO ZIEDO FUNKCINE IR AMIDOGRUPEMIS GRANDINEJE TYRIMAS SUMINIODAZNIO GENERACIJOS SPEKTROSKOPIJOS METODU Santrauka Savitvarkiy monosluoksniy formavimasisant poli-kristalinio aukso pavirsiaus i’ tioliy, turinciy galinefenilalanino (Phe) ziedo ir amido grupes grandineje(HS-(CH,),-CONH-(CH,),-C H), buvo tiriamas virpe-sines suminio daznio generacijos (SFG) spektroskopijosmetodu. Charakteringo Phe ziedo valentinio virpesiov(=C-H) ties 3069 cmir amido A virpesio parametrykitimas begant laikui buvo atliekamas panaudojant ne-tiesine virpesine spektroskopija. SFG duomenys parode,kad galiniai Phe ziedai susiformuoja i tvarkinga struktū-ra islaikius aukso plokstele adsorbciniame tirpale maz-daug 60 min. Amido A juosta SFG spektruose buvo ste-bima ties santykinai zemais dazniais (3280-3308 cm-).Tai rodo, kad susiformuoja stiprus vandenilinis rysystarp salia esanciy adsorbuoty molekuliy grandiniy.Tvarkingos struktūros, vandeniliniais rySiais susietassavitvarkis monosluoksnis su galine Phe ziedo funkcinegrupe gali buti naudojamas kaip perspektyvi modelinesistema tiriant aromatiniy amino rūgěiy saveikas irfunkcionaluma biomolekuliniuose procesuose. Formation of a self-assembled monolayer onto the polycrystalline gold substrate from thiols containing a terminal phenylalanine (Phe) ring and intrachain amide groups (HS-(CH2)7-CONH-(CH2)2-C6H5) was characterized by vibrational sum frequency generation (SFG) spectroscopy. The temporal evolution of the characteristic Phe ring stretching vibration ν(=C–H) near 3069 cm–1 and the Amide A mode were monitored by nonlinear vibrational spectroscopy. The SFG data revealed the complete ordering of the terminal Phe rings after about 60 min of adsorption. Formation of a strong hydrogen bonding between the adjacent chains of adsorbed molecules was evident by appearance of the Amide A mode at the relative low frequency (3280–3308 cm–1). The well-ordered and strongly hydrogen bonded SAM with the terminal Phe ring functionality is a promising platform for the analysis of interactions and the function of aromatic rings in biomolecular processes.
确定




还剩6页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《硫醇中和频光谱检测方案(其它光谱仪)》,该方案主要用于醇中理化分析检测,参考标准--,《硫醇中和频光谱检测方案(其它光谱仪)》用到的仪器有Ekspla SFG 表面和频光谱分析系统
推荐专场
相关方案
更多
该厂商其他方案
更多















