采用立陶宛Ekspla公司PL2251A型脉冲皮秒Nd:YAG激光器和PG501型光学参量发生器构成的振动和频光谱测量系统(SFG)对花生酸朗缪尔单分子膜与三价离子La3+和Fe3+的相互作用进行了实验研究
方案详情

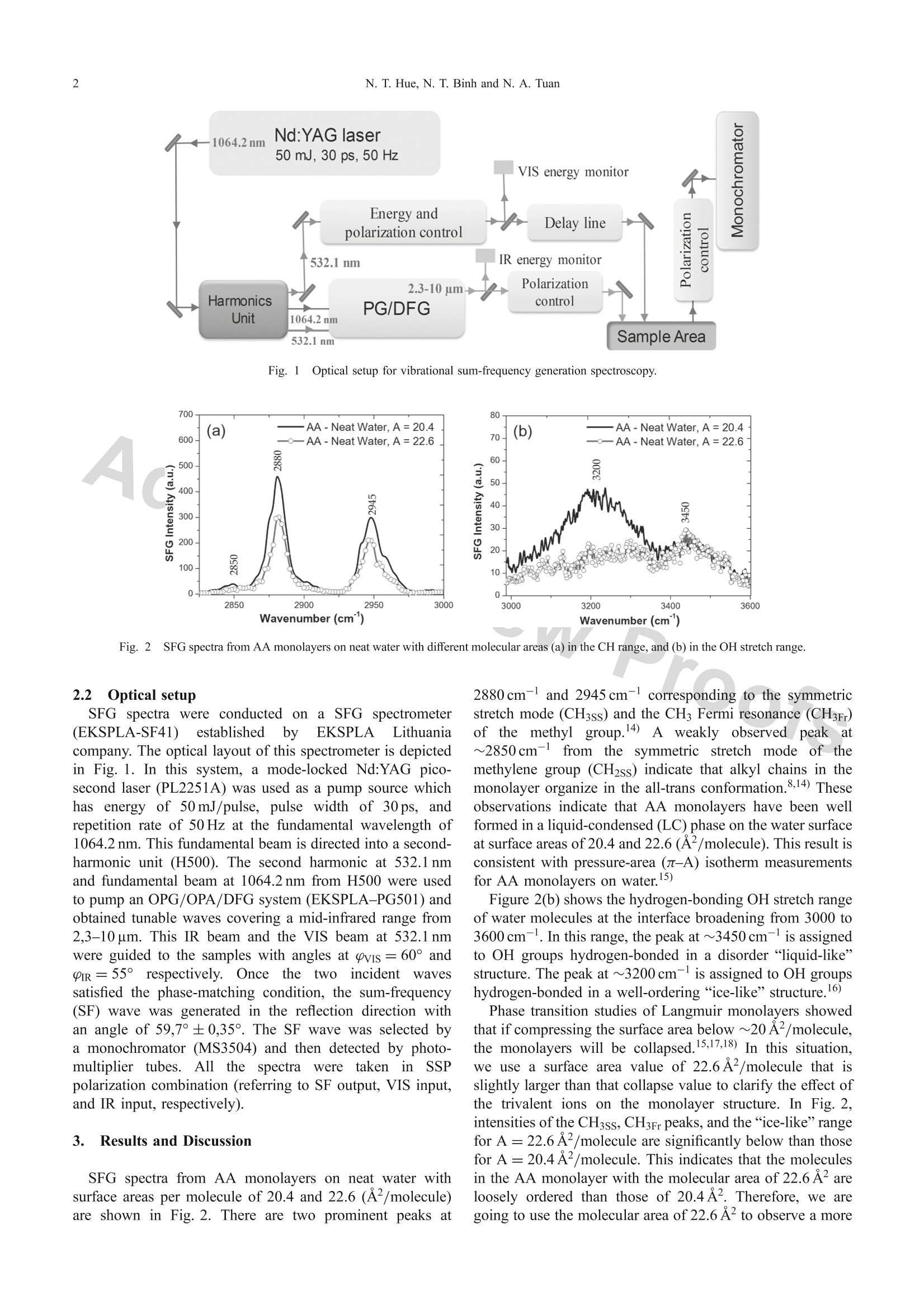
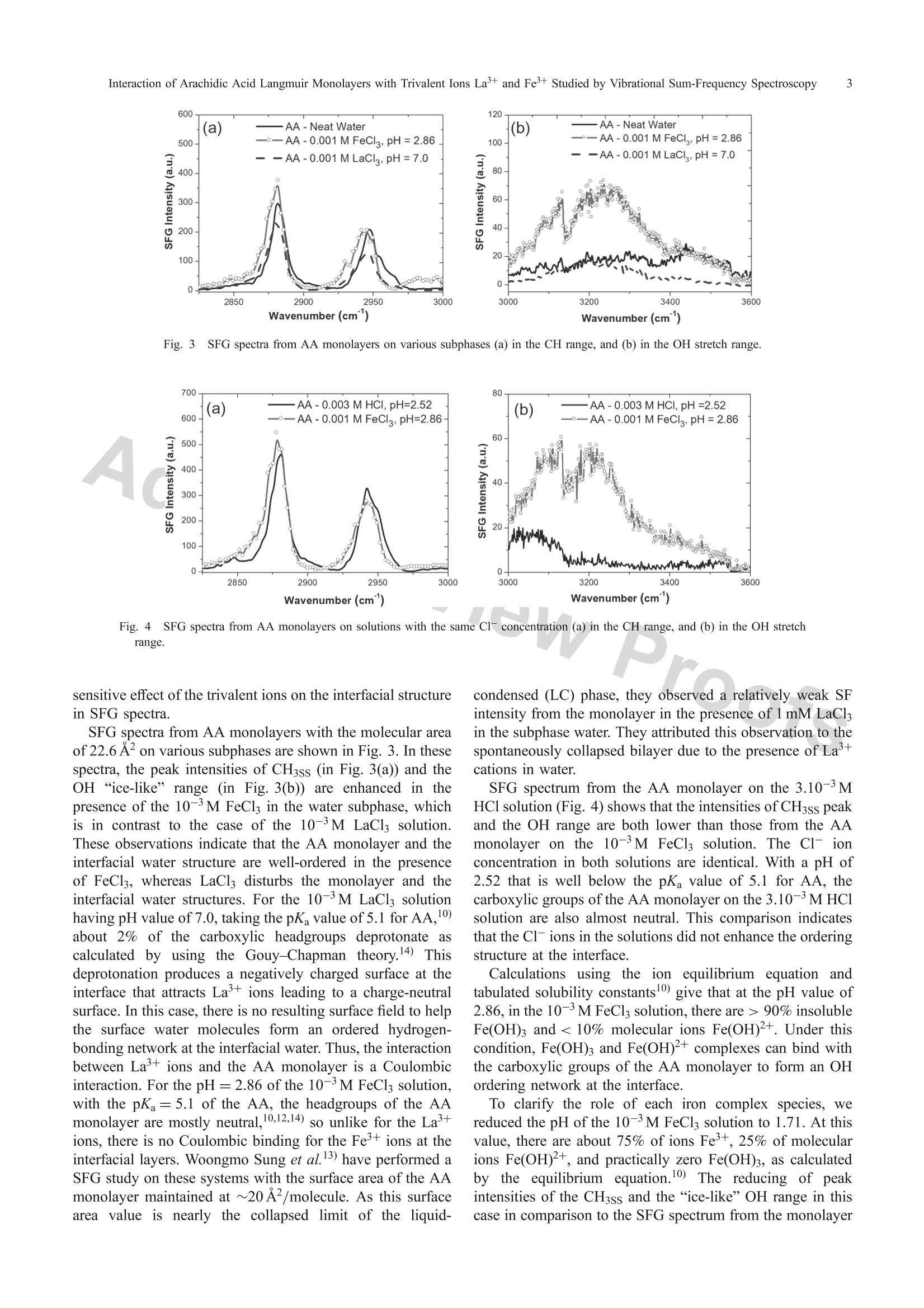
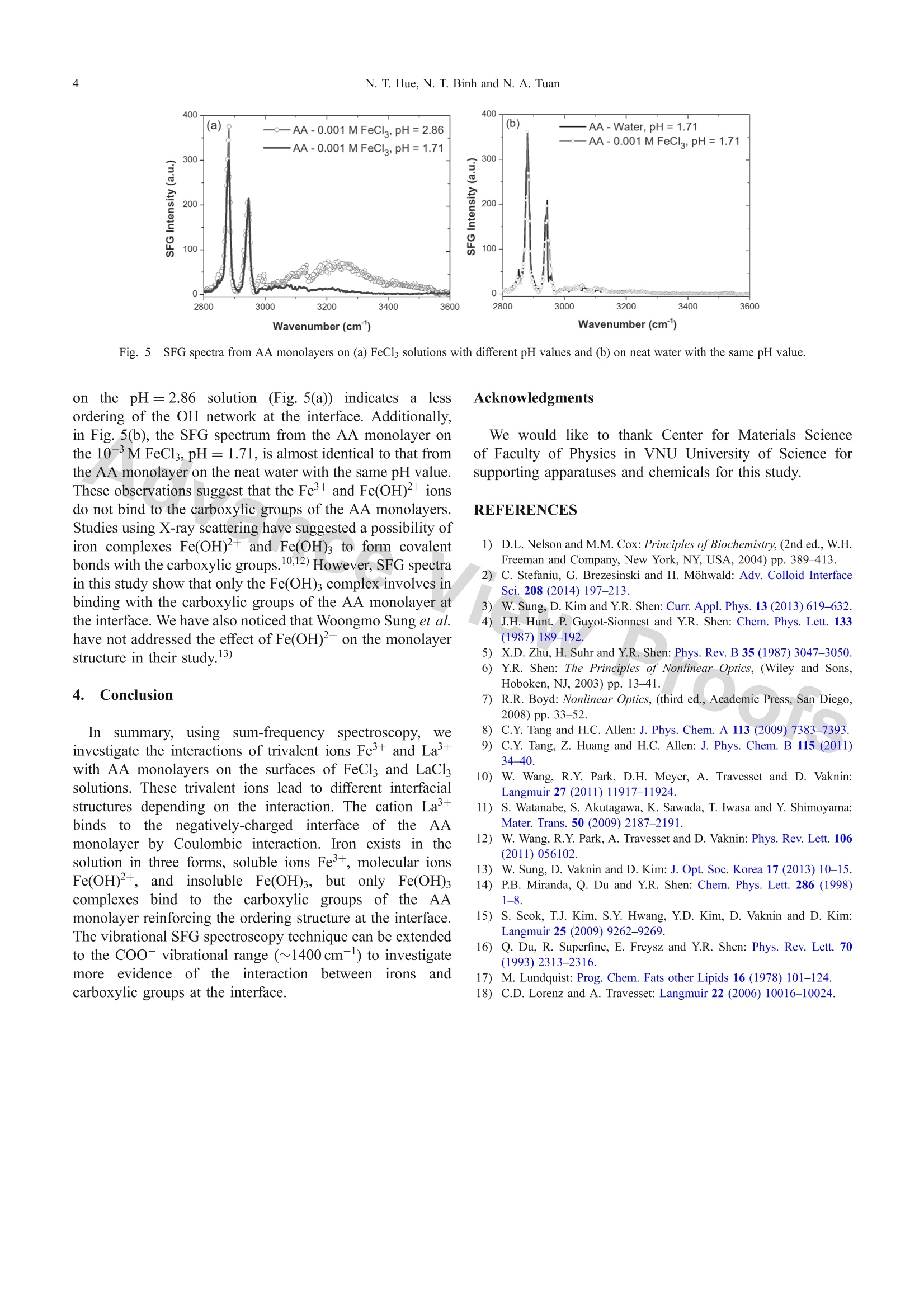
Materials TransactionsSpecial Issue on Nanostructured Functional Materials and Their Applications II◎2018 The Japan Institute of Metals and Materials 2N. T. Hue, N.T. Binh and N. A. Tuan *Corresponding author, E-mail: tuanphysics@vnu.edu.vn Interaction of Arachidic Acid Langmuir Monolayers with Trivalent Ions La+and Fe+Studied by Vibrational Sum-Frequency Spectroscopy Nguyen Thi Huel·2, Nguyen The Binh and Nguyen Anh Tuanl* 'Faculty ofPhysics, VNU University of Science, Hanoi 10000, Vietnam Hung Vuong University, Phu Tho province 290000, Vietnam Interaction of carboxylic acid headgroups of arachidic acid (AA) monolayers with trivalent ions Fe+ and La’+ is studied by usingvibrational sum-frequency generation (VSFG) spectroscopy. Comparing SFG spectra of AA Langmuir monolayers on 10-'M LaCl; solutionsand 10- M FeCl solutions with various pH values, we show that, the cation La+binds to carboxylic groups by Coulombic interaction as theAA monolayers deprotonate at high pH (pH≥). On the other hand, insoluble Fe(OH)3 complexes in the FeCl; solution bind to the carboxylicheadgroups of AA molecules enhances the ordering structure of AA monolayers as well as that of the OH network at the interfacial layers.[doi:10.2320/matertrans.MD201708] (Received November 16, 2017; Accepted February 19, 2018; Published March 30, 2018) Keywords: trivalent ions, iron complex, sum-frequency generation spectroscopy, arachidic acid,Langmuir monolayer 1.IIntroduction Ions in intracellular/extracellular fluids are essential forliving organisms, they can diffuse across the cell membraneand can govern important cell functions. For example,sodium and potassium ions can regulate muscle contractionand determine the transmission of nerve impulses in the brainas well as from the brain to other parts of the body.Langmuir monolayers are widely used as a two-dimensionalmodel to study the interaction mechanism of ionsonbiological membranes.2.3) Since being developed in the early 1980s, sum-frequencygeneration (SFG) spectroscopy has been extensively used asa highly surface-specific technique for studies of surfacesand interfaces.4,5) As a second-order nonlinear process, SFGis forbidden in centrosymmetric media under the dipoleapproximation, so that the SFG spectroscopy techniques areselectively sensitive to structures at surfaces and interfaceswhere the inversion symmetry is broken.6,7) To make predictions about surface phenomena related tobiological and atmospheric aerosol interfaces, an in-depthunderstanding of the binding of divalent and trivalent cationsto the carboxylic headgroups of Langmuir monolayers isnecessary. Recently, Cheng Y. Tang et al. have investigatedeffects of alkali ions (Nat and K+) and divalent cations(Mg+ and Ca+) binding to the COOH headgroup of apalmitic acid monolayer by using SFG spectroscopy.8,9)Binding type determination of trivalent cations such asFe+ and La’+ is more complicated than monovalent anddivalent cations because those trivalent cations can exist inwater as ions or complexes. Wenjie Wang et al.10) have useda surface-sensitive X-ray scattering technique to investigateionic specificity of Feand La+ bound to carboxylic groupsof AA monolayers on FeCl and LaCls solutions. Theirresults showed that La+ cations bound to AA monolayersby Coulombic interaction, whereas the Fe+ cations whichhave the same charge with La+ formed covalent bonds byiron complexes of Fe(OH)3 and/or Fe(OH)2+. However, the iron complexes binding to AA monolayers have notbeen studied in detail because X-ray scattering is not able toprobe the subphase water structure. Besides, iron complexesinmagneticcbacteriaahave also drawnnattentionandbeen investigated using FMR (Ferromagnetic Resonance)measurements.1) In this study, we use vibrational sum-frequency spec-troscopy to investigate the interaction of trivalent ions Fe+and La+ with AA Langmuir monolayers on the surfaces of10-3M FeCl; and 10-3M LaCls solutions. The interfacialsurface charge and the composition of Fe in the solutionsare controlled by varying pH levels. We investigate VSFGspectra in the CH stretch range (2800-3000cm-) and theOH stretch range (3000-3600cm-l). The result showsdifferent interactions ofLa+, Fe+, and iron complexes withthe carboxylic groups of AA monolayers at the interface Previous studies10,12,13) on AA monolayers on water oftenused a molecular area value of ~20 A2. In this paper, wewill discuss why using a molecular area of 22.6 A’ leads tomore sensitive effects on the AA monolayer, which helpdistinguish the roles of iron complexes Fe(OH)2++:andFe(OH)3 that the previous studies did not point out. 2. Experimental Procedure 2.1 Sample preparation Arachidic acid (CH(CH2)18COOH) in the solid state(purity >99%, Sigma-Aldrich) was dissolved in chloroformsolvent (purity> 99%, Sigma-Aldrich) to make 10-M AAsolution. Iron(III) chloride and lanthanum(III) chloridesalt solutions with the same concentration of 10-3M wereprepared by dissolving appropriateamountsSof FeCl(purity > 99%, Merck) and LaCl3 (purity > 99%, Merck) inultrapure water (Millipore, Milli-Q,resistivity ~ 18 M cm).The pH values of these salt solutions, just after preparation,were 2.86 for the FeCl solution and 7.0 for the LaCl solution.We then changed those pH values by adding solutionsof HCl or NaOH (purity> 99%,Merck). A Langmuirmonolayer was formed within 10 minutes after spreading. the AA solution on the water surface of the salt solution Fig. l1Optical setup for vibrational sum-frequency generation spectroscopy. Aa Fig. S2FG spectra from AA monolayers on neat water with different molecular areas (a) in the CH range, and (b) in the OH stretch range. 2.2 Optical setup SFG spectra were conducted on a SFG spectrometer(EKSPLA-SF41) establishedbbyyEKSPLA Lithuaniacompany. The optical layout of this spectrometer is depictedin Fig. 1. In this system, a mode-locked Nd:YAG pico-second laser (PL2251A) was used as a pump source whichhas energy of 50 mJ/pulse, pulse width of 30ps, andrepetition rate of 50 Hz at the fundamental wavelength of1064.2 nm. This fundamental beam is directed into a second-harmonic unit (H500). The second harmonic at 532.1 nmand fundamental beam at 1064.2 nm from H500 were usedto pump an OPG/OPA/DFG system (EKSPLA-PG501) andobtained tunable waves covering a mid-infrared range from2,3-10 um. This IR beam and the VIS beam at 532.1 nmwere guided to the samples with angles at pvis = 60° andIR=55° respectively. Once ththe twoincidentt wavessatisfied the phase-matching condition, the sum-frequency(SF) wave was generated in the reflection direction withan angle of 59,7°±0,35°. The SF wave was selected bya monochromator (MS3504) and then detected by photo-multiplier tubes. All thee spectraawwerettaken inSSPpolarization combination (referring to SF output, VIS input,and IR input, respectively). 3. Results and Discussion SFG spectra from AA monolayers on neat water withsurface areas per molecule of 20.4 and 22.6 (A2/molecule)are shown in Fig. 2. There are two prominent peaks at 2880 cm-l and 2945 cm-l corresponding to the symmetricstretch mode (CH3ss) and the CH Fermi resonance (CH3Fr)of the methyll group.4) A weakly observed peakk at~2850cm-ffrom the symmetric stretch modee of themethylene group (CH2ss) indicate that alkyl chains in themonolayer organize in the all-trans conformation.8,14) Theseobservations indicate that AA monolayers have been wellformed in a liquid-condensed (LC) phase on the water surfaceat surface areas of 20.4 and 22.6 (A2/molecule). This result isconsistent with pressure-area (n-A) isotherm measurementsfor AA monolayers on water.15) Figure 2(b) shows the hydrogen-bonding OH stretch rangeof water molecules at the interface broadening from 3000 to3600 cm-. In this range, the peak at ~3450 cm-is assignedto OH groups hydrogen-bonded in a disorder "liquid-like”structure. The peak at ~3200cm-is assigned to OH groupshydrogen-bonded in a well-ordering “ice-like”structure.16) Phase transition studies of Langmuir monolayers showedthat if compressing the surface area below ~20 A2/molecule,the monolayers will be collapsed.15,17,18) In this situation,we use a surface area value of 22.6A2/molecule that isslightly larger than that collapse value to clarify the effect ofthe trivalent ions on the monolayer structure. In Fig. 2,intensities of the CH3ss, CH3Fr peaks, and the“ice-like”rangefor A=22.6A2/molecule are significantly below than thosefor A=20.4 A2/molecule. This indicates that the moleculesin the AA monolayer with the molecular area of 22.6 A2areloosely ordered than those of 20.4A2. Therefore, we aregoing to use the molecular area of 22.6 A’ to observe a more 600 (a) .—AA-Neat Water 500 一一AA -0.001 M FeCl, pH=2.86 一--AA-0.001 M LaCl,pH=7.0c u 400 300 200 100 0- 2850 2900 2950 3000 Wavenumber (cm) Fig.3SFG spectra from AA monolayers on various subphases (a) in the CH range, and (b) in the OH stretch range. Fig. 4SFG spectra from AA monolayers on solutions with the same Cl concentration (a) in the CH range, and (b) in the OH stretchrange. sensitive effect of the trivalent ions on the interfacial structurein SFG spectra. SFG spectra from AA monolayers with the molecular areaof 22.6 A2on various subphases are shown in Fig. 3. In thesespectra, the peak intensities of CH3ss (in Fig. 3(a)) and theOH “ice-like” range (in Fig. 3(b)) are enhanced in thepresence of the 10-M FeCls in the water subphase, whichis in contrast to the case of the 10-3M LaCl solution.These observations indicate that the AA monolayer and theinterfacial water structure are well-ordered in the presenceof FeCl, whereas LaCl disturbs the monolayer and theinterfacial water structures. For the 10-3M LaCl solutionhaving pH value of 7.0, taking the pKa value of 5.1 for AA,about 2% of the carboxylic headgroups deprotonate ascalculated by using the Gouy-Chapman theory.14) Thisdeprotonation produces a negatively charged surface at theinterface that attracts La+ions leading to a charge-neutralsurface. In this case, there is no resulting surface field to helpthe surface water molecules form an ordered hydrogen-bonding network at the interfacial water. Thus, the interactionbetween La’+ions and the AA monolayer is a Coulombicinteraction. For the pH=2.86 of the 10-3M FeCls solution,with the pKa=5.1 of the AA, the headgroups of the AAmonolayer are mostly neutral,10,12,14) so unlike for the La’+ions, there is no Coulombic binding for the Fe+ ions at theinterfacial layers. Woongmo Sung et al.13) have performed aSFG study on these systems with the surface area of the AAmonolayer maintained at ~20A2/molecule. As this surfacearea value is nearly the collapsed limit of the liquid- condensed (LC) phase, they observed a relatively weak SFintensity from the monolayer in the presence of 1 mM LaCl3in the subphase water. They attributed this observation to thespontaneously collapsed bilayer due to the presence of Lacations in water. SFG spectrum from the AA monolayer on the 3.10-3MHCl solution (Fig. 4) shows that the intensities of CH3ss peakand the OH range are both lower than those from the AAmonolayer on the 10-3M FeCl solution. The Cl- ionconcentration in both solutions are identical. With a pH of2.52 that is well below the pKa value of 5.1 for AA, thecarboxylic groups of the AA monolayer on the 3.10-3 M HClsolution are also almost neutral. This comparison indicatesthat the C1- ions in the solutions did not enhance the orderingstructure at the interface. Calculations using the ion equilibrium equation andtabulated solubility constants) give that at the pH value of2.86, in the 10-3M FeCl solution, there are > 90% insolubleFe(OH)3 and < 10% molecular ions Fe(OH)2+. Under thiscondition, Fe(OH)3 and Fe(OH)2+complexes can bind withthe carboxylic groups of the AA monolayer to form an OHordering network at the interface. To clarify the role of each iron complex species, wereduced the pH of the 10-3M FeCl; solution to 1.71. At thisvalue, there are about 75% of ions Fe+, 25% of molecularions Fe(OH)2+,and practically zero Fe(OH)3, as calculatedby the equilibrium equation.10) The reducing of peakintensities of the CH3ss and the “ice-like”OH range in thiscase in comparison to the SFG spectrum from the monolayer Fig. 5SFG spectra from AA monolayers on (a) FeCl solutions with different pH values and (b) on neat water with the same pH value. o)rn the pH=2.86 solution (Fig. 5(a)) indicates a lessordering of the OH network at the interface. Additionally,in Fig. 5(b), the SFG spectrum from the AA monolayer onthe 10-3M FeCl3, pH=1.71, is almost identical to that fromthe AA monolayer on the neat water with the same pH value.These observations suggest that the Fe+ and Fe(OH)+ ionsdo not bind to the carboxylic groups of the AA monolayers.Studies using X-ray scattering have suggested a possibility ofiron complexes Fe(OH)2+and Fe(OH)3 to form covalentbonds with the carboxylic groups.10,12) However, SFG spectrain this study show that only the Fe(OH)3 complex involves inbinding with the carboxylic groups of the AA monolayer atthe interface. We have also noticed that Woongmo Sung et al.have not addressed the effect of Fe(OH)2+ on the monolayerstructure in their study.13) 4. .Conclusion Insummary, using sum-frequency spectroscopy, weinvestigate the interactions of trivalent ions Fe+and La+with AA monolayers on the surfaces of FeCl and LaClssolutions. These trivalent ions lead to different interfacialstructures depending on the interaction. The cation La’bindssto the negatively-charged interface of the AAmonolayer by Coulombic interaction. Iron exists in thesolution in three forms, soluble ions Fe+, molecular ionsFe(OH)2+, andinsoluble Fe(OH)3, but only Fe(OH)3complexes bind to the carboxylic groups of the AAmonolayer reinforcing the ordering structure at the interface.The vibrational SFG spectroscopy technique can be extendedto the COO-vibrational range (~1400cm-) to investigatemore evidence of the interactionnbetweenirons andcarboxylic groups at the interface. Acknowledgments We would like to thank Center for Materials Scienceof Faculty of Physics in VNU University of Science forsupporting apparatuses and chemicals for this study. REFERENCES 1) D.L. Nelson and M.M. Cox: Principles of Biochemistry, (2nd ed., W.H.Freeman and Company, New York, NY, USA, 2004) pp. 389-413. ( 2) C . Stefaniu, G. Brezesinski and H. Mohwald: A d v. C ollo id I nter f ace Sci. 208 (2014 ) 197-213. ) )X.D. Zhu, H. Suhr and Y.R. Shen: Phys. Rev. B 35 (1987) 3047-3050. ( Y.R. S hen: T h e Pr i nciples of Nonlinear Optics, (W i ley and Son s , H oboken, NJ, 2003) pp. 1 3-41. ) ( 7) R.R . B o yd: Nonlinear Optics, (t h ird e d ., Academic Pr e ss, San Di e go,2008)pp. 33-52. ) ( 8) C ( .Y. T ang and H.C. Allen: J . Ph ys . Chem. A 1 1 3 ( 2 00 9 ) 7 3 8 3-73 93 . ) ( 9) C.Y. T ang, Z. H uang and H. C . Al l en: J. P h ys. Che m . B 1 15 (2011) 3 4-40. ) 10)\W. Wang, R.Y. Park, D.H. Meyer, A. Travesset and D. Vaknin:Langmuir 27 (2011) 11917-11924. ( 11)SS. Watanabe, S. Akutagawa, K. S a wada, T. I w asa a n d Y . S h imoyama: Mater. T rans. 50 (2009) 2187- 2 191. ) ( 12) W. Wang, R. Y. Park, A. T ravesset a nd D. V aknin: P hys. R e v. Lett. 106(201 1 )056102 ) ( 13)W. Sung, D. Vaknin and D . K i m: J . Opt. Soc. K orea 17 (2013 ) 1 0- 15 . ) 14) PF.B. Miranda, Q. Du and Y.R. Shen: Chem. Phys. Lett. 286 (1998)1-8. 15)S$. Seok, T.J. Kim, S.Y. Hwang, Y.D. Kim, D. Vaknin and D. Kim:Langmuir 25 (2009) 9262-9269. 16)(Q. Du, R. Superfine, E. Freysz and Y.R. Shen: Phys. Rev. Lett. 70(1993)2313-2316. 17) MN. Lundquist: Prog. Chem. Fats other Lipids 16 (1978) 101-124. ( 18) C. D . Lo r enz a n d A. T r avesset: L ang m ui r 2 2 (2006) 10 0 16-10 0 24. ) Interaction of carboxylic acid headgroups of arachidic acid (AA) monolayers with trivalent ions Fe3+ and La3+ is studied by using vibrational sum-frequency generation (VSFG) spectroscopy. Comparing SFG spectra of AA Langmuir monolayers on 10¹3M LaCl3 solutionsand 10¹3M FeCl3 solutions with various pH values, we show that, the cation La3+ binds to carboxylic groups by Coulombic interaction as the AA monolayers deprotonate at high pH (pH ² 7). On the other hand, insoluble Fe(OH)3 complexes in the FeCl3 solution bind to the carboxylic headgroups of AA molecules enhances the ordering structure of AA monolayers as well as that of the OH network at the interfacial layers.
确定

还剩2页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《花生酸朗缪尔单分子膜中振动和频光谱(VS-SFG)检测方案(其它光谱仪)》,该方案主要用于其他中其他检测,参考标准--,《花生酸朗缪尔单分子膜中振动和频光谱(VS-SFG)检测方案(其它光谱仪)》用到的仪器有Ekspla SFG 表面和频光谱分析系统、PL2250 闪光灯泵浦皮秒Nd:YAG激光器
推荐专场
相关方案
更多
该厂商其他方案
更多