方案详情
文
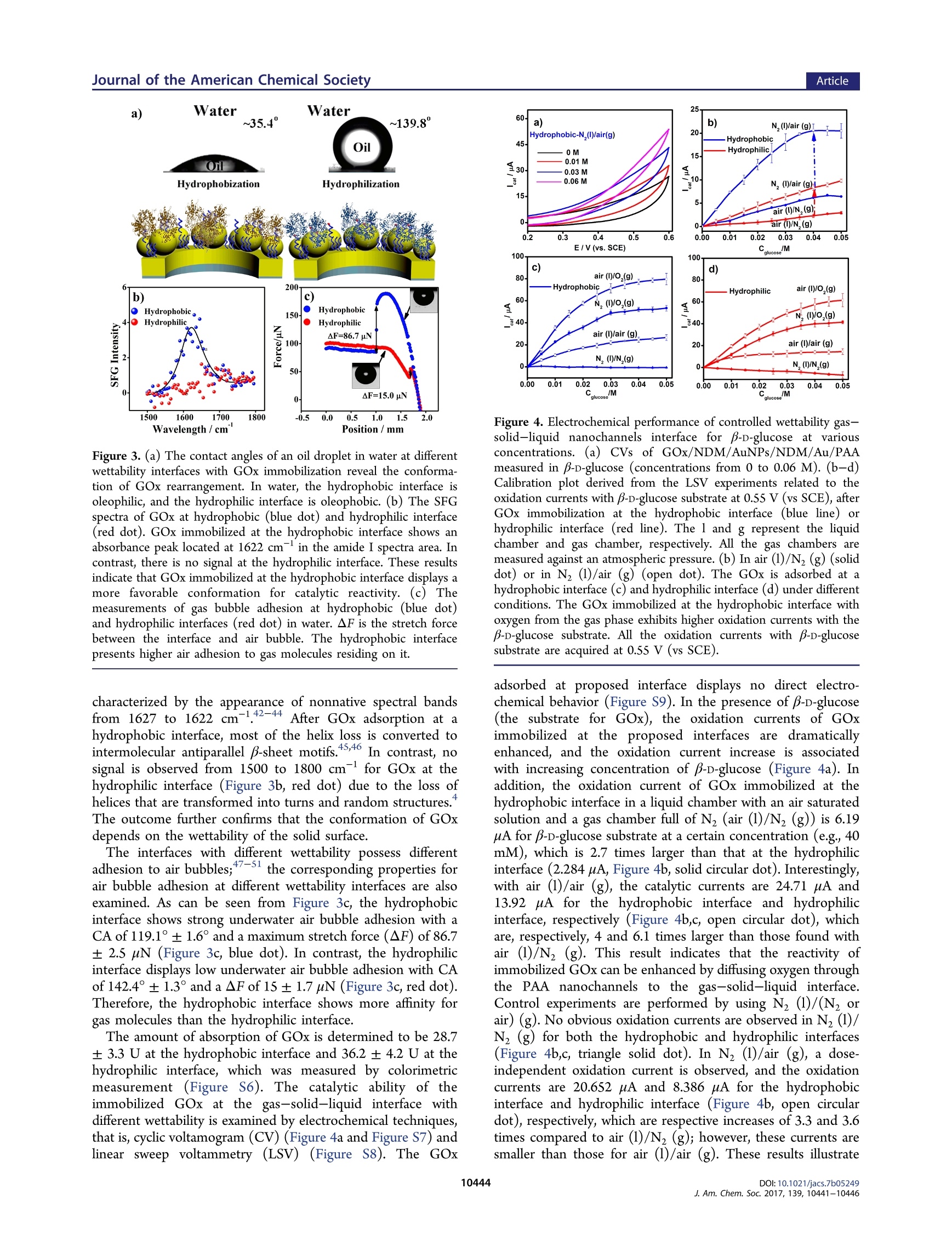
采用立陶宛Ekspla公司的和频光谱测量系统SFG,对纳米通道反应器中联合气固液界面和控制湿润性激励气体反应过程进行了实验测量和理论分析研究。
方案详情

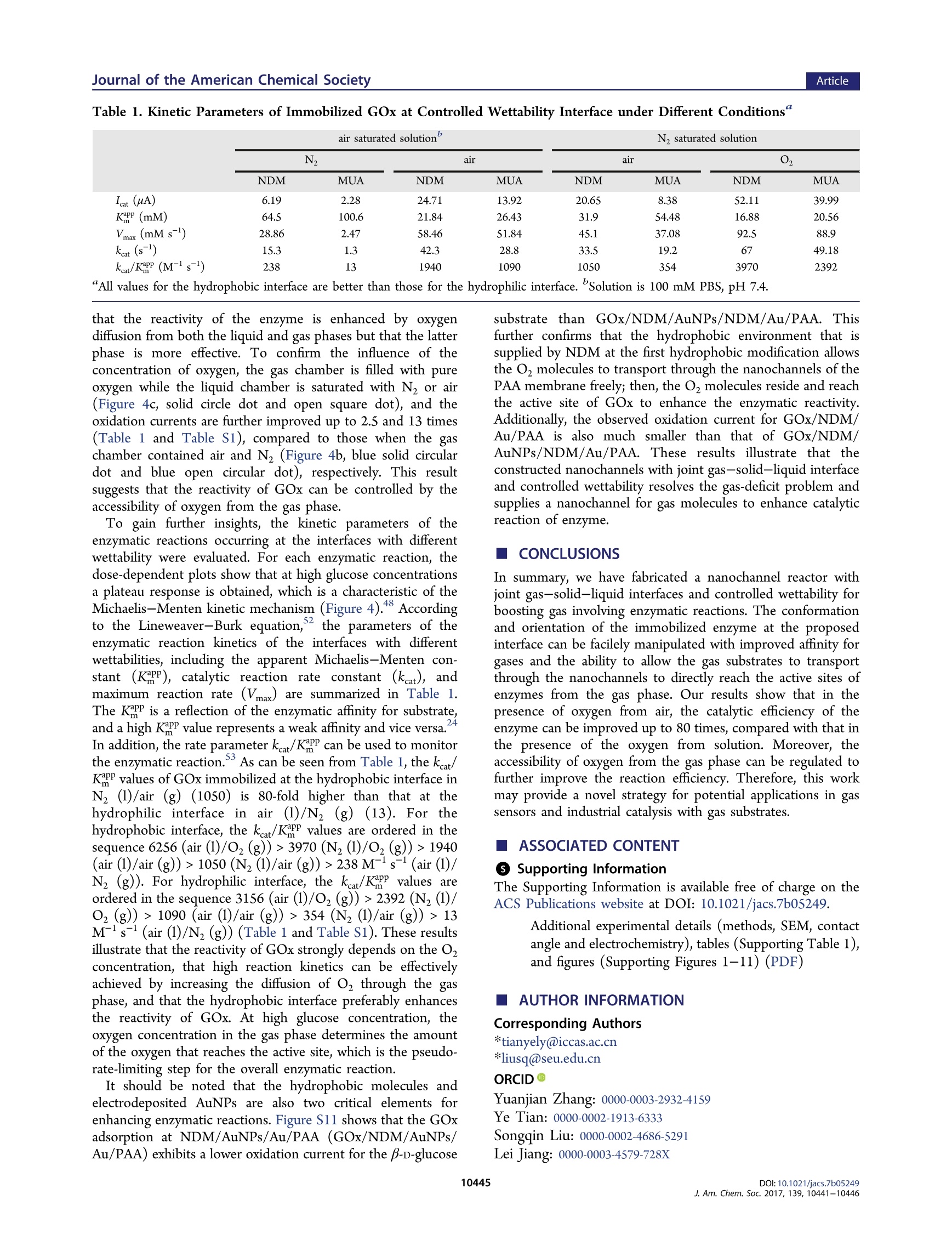
This is an open access article published under an ACS AuthorChoice License,which permitscopying and redistribution of the article or any adaptations for non-commercial purposes. ArticleJournal of the American Chemical Society Boosting Gas Involved Reactions at Nanochannel Reactor with JointGas-Solid-Liquid Interfaces and Controlled Wettability Li Mi, Jiachao Yu, Fei He, Ling Jiang, Yafeng Wu, Lijun Yang, Xiaofeng Han, Ying Li, Anran Liu,Wei Wei,Yuanjian Zhang,.TDD yYe Tian*@Songqin Liu,*o’and Lei JiangD 'Key Laboratory of Environmental Medicine Engineering, Ministry of Education, Jiangsu Engineering Laboratory of SmartCarbon-Rich Materials and Device, School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, ChinaBeijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Green Printing, Institute of Chemistry, ChineseAcademy of Sciences, Beijing, 100190, China Key Laboratory of Bioinspired Smart Interface Sciences, Technical Institute of Physics and Chemistry, Chinese Academy ofSciences, Beijing, 100190, China ABSTRACT: The low solubility of gases in aqueous solution isthe major kinetic limitation of reactions that involve gases. Toaddress this challenge, we report a nanochannel reactor with jointgas-solid-liquid interfaces and controlled wettability. As a proofof concept, a porous anodic alumina (PAA)nanochannelmembrane with different wettability is used for glucose oxidase(GOx) immobilization, which contacts with glucose aqueoussolution on one side, while the other side gets in touch with thegas phase directly. Interestingly, it is observed that the O2 couldparticipate in the enzymatic reaction directly from gas phase INTRODUCTION Oxygen and other gas molecules have an important role in avariety of reactions such as laboratory synthesis, energyutilization, and biochemical processes.-4,5.6Due to theextremely low solubility of gases in aqueous solution, forexample, O2, CO2, and CH4, the efficiency of these reactions isusually significantly suppressed.-10 To address this limitation,numerous approaches have been taken to adapt; Pickeringemulsions,microfluidic devices,or "tube-in-tube"techni-ques have been developed for increasing the interfacial areasor gas adsorption. 4,15 Those methods require extra additives orcomplicated design techniques, and the low concentration ofgas molecules in solution is still a major limitation. In theseregards, a novel method for solving the gas-deficit problem andadjusting the concentration of gases for the specific systems ishighly anticipated. In this work, we report a nanochannel reactor with joint gas-solid-liquid interfaces and controlled wettability for boostinggas involving reactions. A porous anodic alumina (PAA)nanochannel membrane is used for immobilizing an enzyme,which contacts the aqueous solution on one side, while theother side contacts the gas phase directly. Glucose oxidase(GOx), a type of aerobic oxidase, is selected as a model enzyme. Oxygen molecules from the gas phase can transportthrough the modified PAA nanochannels and reach the activesites of the GOx directly, significantly improving theconcentration of oxygen for the reaction. Moreover, it isobserved that the reaction efficiency of the enzyme at thehydrophobic interface is muchi greater than that at thehydrophilic interface in our system. Further mechanisticinvestigation reveals that at the hydrophobic interface theprotein exhibits a favorable conformation and orientation,16-19as well as high adhesive force for oxygen. As a result, in thenanochannel reactor with joint gas-solid-liquid interfaces, thereaction efficiency of enzyme with oxygen diffusing directlyfrom air is enhanced up to 80 times compared with the freestate in traditional aqueous air-saturated electrolyte. Suchproposed strategy is expected not only to access the biocatalyticreaction but also to be of use in industrial catalysis andsynthesis. ( Rece i v e d: May 22, 2017 ) ( P u bl is h ed: June 3 0, 2017 ) ACS Publications MUA/AuNPs/NDM/Au/PAA GOD/MUA/AuNPs/NDM/Au/PAA Figure 1. Schematic illustration for the construction process of a nanochannel with joint gas-solid-liquid interfaces and controlled wettability. First,the PAA membrane is covered with a gold film via sputtering (Au/PAA). Then, the modified PAA membrane is incubated with NDM solution toobtain a hydrophobic surface (NDM/Au/PAA). Third, Au nanoparticles (AuNPs) are electrodeposited (AuNPs/NDM/Au/PAA). After that, thestructure is incubated with either NDM (NDM/AuNPs/NDM/Au/PAA) or MUA (MUA/AuNPs/NDM/Au/PAA) to form a hydrophobic orhydrophilic surface, respectively. Finally, GOx is immobilized at the hydrophobic or hydrophilic interfaces. The electrochemical characteristics of theas-prepared membrane are obtained with a homemade device that has two chambers: one chamber is equipped with a counter Pt wire electrode(CE), an SCE reference electrode (RE), and 0.1 M, pH 7.4, phosphate solution as an electrolyte, and the other chamber is used for gas storage. TheGOx modified PAA, which is the working electrode(WE), is fixed between the two chambers, with the GOx immobilization side immersed into theelectrolyte and the other side in contact with the gas phase for gas transport through the nanochannels to form a gas-solid-liquid interface. Materials. The porous anodic alumina (PAA) membrane with porediameter of 90 ±10 nm and length of 100 ±20 um is purchased fromPuyuan Nanotechnology (Hefei, China). 11-Mercaptoundecanoic acid(MUA), n-dodecanethiol (NDM), and glucose oxidase (GOx, EC1.1.3.4, from Aspergillus niger, 196.6 kU g-) were received fromSigma-Aaldrich (Shanghai, China). HAuCL4H,O (Au%= 47.8%)was received from Nanjing Reagent Co. (Nanjing, China). Phosphate-buffer solution (PBS, 100 mM, pH7.4) was prepared by mixing stocksolutions of Na,HPO4 and NaH,PO4· Apparatus. The scanning electron micrograph (SEM) images wererecorded with a field emission scanning electron microscope (ULTRAPLUS, ZEISS, Germany), coupled toanenergy dispersivespectrometer (EDS). Measurement of the contact angle (CA) ofwater or oil droplets (dichloroethane) in water was performed with avideo-based optical contact angle measuring instrument (Dataphysics,German). The adhesive force of a bubble in water on the PAAmembrane surface was measured by a high sensitivity micro-electromechanical balance system (Data-Physics DCAT 11, Germany).The electrochemical measurements were performed with a CHI 750electrochemical workstation (Shanghai Chenhua, China). All experi-ments were conducted with this three-electrode system, composed of asaturated calomel electrode (SCE), a Pt electrode, and the PAAmembrane as reference, counter, and working electrodes, respectively.The secondary structure of GOx was analyzed with a sum frequencygeneration spectrometer (SFG, EKSPLA, Lithuania). The right-angleCaFz prisms were distributed in n-trimethoxyoctadecylsilane andsuccine anhydride to obtain the hydrophobic and hydrophilic surfaces,respectively. GOx was then dropped onto the modified prism andincubated for 4 h to reach equilibrium. SFG spectra in the amide Ifrequency region were collected from the immobilized enzyme from1500 to 1800 cm- using both ssp (s-polarized SFG signal, s-polarizedvisible input, and p-polarized input IR) and ppp (p-polarized SFGsignal, p-polarized visible input, and p-polarized input IR) polarizationcombinations,20-24,which can be used to determine the enzymeorientation. The SFG ssp spectra were fitted using the a standardspectral fitting method. Preparation of a Nanochannel Reactor with Joint Gas-Solid-Liquid Interfaces and Controlled Wettability. A thin goldfilm with about 100 nm thickness, determined from SEM images, wasfirst sputtered onto one face of the PAA membrane at 5 mA for 3 min.The resulting gold film coated PAA membrane was then incubatedwith 0.01 mM NDM in ethanol overnight, washed with ethanol 3times, and dried in N. After that, the electrochemical deposition ofgold nanoparticles was performed at -0.2 V for 20 min by dipping theside of the PAA membrane with the gold film into 2.4 mM HAuCl4solution in 100 mM PBS, pH 7.4. The PAA membrane sputtered goldfilm with gold nanoparticle electrodeposition (AuNPs/NDM/Au/ PAA) was further modified with NDM or MUA overnight to obtainsurface hydrophobic NDM/AuNPs/NDM/Au/PAA or surface hydro-philic MUA/AuNPs/NDM/Au/PAA. Finally, both hydrophobic andhydrophilic PAA membranes were incubated with 750 U mLglucoseoxidase solution in 100 mM PBS, pH 7.4, at 4°C for 4 h, followed byrinsing thoroughly with PBS to obtain enzyme modified three-phaseelectrodes (GOx/NDM/AuNPs/NDM/Au/PAA or GOx/MUA/AuNPs/NDM/Au/PAA) and storage at 4 C for further experiments. RESULTS AND DISCUSSION Figure 1 shows the typical approach to construct a nanochannelreactor with joint gas-solid-liquid interfaces with enzyme. ThePAA membrane was coated with a gold film via sputtering (Au/PAA) and then immersed in NDM solution to obtain ahydrophobic surface (NDM/Au/PAA). After that, Au nano-particles (AuNPs) were electrochemically deposited on NDM/Au/PAA to form AuNPs/NDM/Au/PAA. The wettability wasfurther controlled by self-assembly of NDM (NDM/AuNPs/NDM/Au/PAA) or MUA (MUA/AuNPs/NDM/Au/PAA).Finally, GOx was immobilized at the as-prepared interface. Thismodified PAA membrane as working electrode (WE) was fixedbetween two chambers of a homemade electrolytic cell with theGOx side in contact with electrolyte and oxygen diffusingdirectly from the gas phase to the GOx active sites through thenanochannels of the membrane. This device has the merits ofallowing free diffusion of substrates from the liquid-phase andoxygen from the gas-phase to boost the kinetics of reactions. The electrochemical performance and morphology of thenanochannels with gas-solid-liquid interface are demonstratedby electrochemical current response and SEM images,respectively. This interface depends on the pore diameter ofAu/PAA and the electrodeposition time of AuNPs at NDM/Au/PAA. The current increases with the pore diameter andreaches a plateau at 90 nm (Figure 2a). Then, the PAAmembrane with 90 nm pores (channel length of 100±20 um)is verified by scanning electron microscopy (SEM), As shownin Figure 2b, the gold sputtering step forms a covering gold filmwith a thickness of 100 ±5 nm on the PAA surface; no goldnanoparticles are observed in the channels of the PAA. Toprevent liquid outflow from the nanochannels and providehydrophobic nanochannels for gas molecule diffusion, the PAAmembrane is modified to be hydrophobic with NDMdeposition producing a water contact angle (CA) of 133°±4.1°(NDM/Au/PAA)(Figure 2b, inset). In the next step, the Figure 2. Characterization of nanochannels with gas-solid-liquidinterface by electrochemical current response and SEM images. (a)Current increases along with the pore diameter and reaches a plateauat 90 nm; the inset displays the pore diameter of Au/PAA. Scale bar =100 nm. (b) Side-view SEM images of PAA covered with gold film(Au/PAA); the inset shows that the CA of a water droplet on NDM/Au/PAA is 133°±4.1°. Scale bar =100 nm. (c) With an extension ofthe electrodeposition time, the surface density of the gold nano-particles increases and the current response rises. As the depositiontime extends beyond 1200 s, the current decreases sharply. Inset showsthe size of electrodeposited AuNPs. Scale bar = 100 nm. (d) SEMimages of AuNPs/NDM/Au/PAA with insets showing a CA of 23.8°±3.2° for MUA/AuNPs/NDM/Au/PAA (top) and 133.7°±1.5° forNDM/AuNPs/NDM/Au/PAA (bottom). Scale bar =100 nm. (e)Thin GOx coated film at the as-prepared interface. Scale bar=100 nm.(f) Mechanism of GOx reactivity at the as-prepared interface: oxygenfrom the gas phase passes through the nanochannels at this interface toreach the active sites of GOx. current response of NDM/AuNPs/NDM/Au/PAA with differ-ent electrodeposition time of AuNPs is investigated. Theextension of the electrodeposition time causes an increase inthe surface density of the gold nanoparticles (Figure S2) and arise in response current. However, if the deposition time isover-extended, the current decreases sharply (Figure 2c), andthe water CA of electrodes after different electrodepositiontimes of AuNPs are shown in Figure S3. The best depositiontime observed for our system is 1200 s. After electrodeposition,many separated gold nanoparticles could be observed on theNDM/Au/PAA with a size of 100 ± 10 nm (Figure 2d andFigure Slc). The AuNPs/NDM/Au/PAA is further modifiedwith NDM or MUA to either form hydrophobic or hydrophilicsurfaces with water CA of 133.7°±1.5° for NDM/AuNPs/NDM/Au/PAA and 23.8°±3.2°for MUA/AuNPs/NDM/Au/PAA (Figure 2d insets), respectively. Thereafter, the GOxis immobilized at the as-prepared interface to form ahydrophobic interface (GOx/NDM/AuNPs/NDM/Au/PAA)or hydrophilic interface (GOx/MUA/AuNPs/NDM/Au/PAA). As shown in Figure 2e, the SEM images of the GOxcomposite film coated at the as-prepared interface show thatthe composite film is separated from the substrate by theelectrodeposited AuNPs. This architecture facilitates freeoxygen diffusion from air and glucose from the liquid phaseto the gas-solid-liquid nanochannel interface. The water CAafter GOx immobilization is 105.5°±2.5°for the hydrophobicinterface and 46.7°± 1.9° for the hydrophilic interface (FigureS4). The change in CA after GOx immobilization is due to thedifferent interaction between GOx and a solid surface. Figure 2fis also presented to reveal mechanism of oxygen crossing thenanochannels to the gas-solid-liquid interface and arriving at the active sites of GOx. For the oxygen-mediated enzymaticreaction, the O2 molecules temporarily residing on the proteinsurface is likely the first step for Odiffusing to the active sites.It was previously reported that the hydrophobic residues of aprotein surface are responsible for hosting O, molecules andcarrying O2 molecules inside the protein through severaladditional paths.25 Thus, the wettability environment of aprotein surface is beneficial for encouraging oxygen moleculeresidence, which allows the O2 molecules to easily reach theinterior of the protein and enhance the reactivity of the protein. After immobilization, different conformations and orienta-tions of GOx are displayed at the hydrophobic or hydrophilicinterface. When protein is adsorbed at a solid-liquid interfacein an aqueous environment, protein residues will interact withexposed functionalgroups on the solid surface, and theinteractions between protein and solid surfaces dominate theprotein immobilization and determine the catalytic activities of18,26-.protein. 8.26-34 For a hydrophobic interface, the interactionbetween protein residues and the exposed hydrophobic groupsleads to unfolding or adoption of a non-native conformation ofthe protein to expose the interior hydrophobic residues to theinterface. For a hydrophilic interface, the protein also exposesthe interior hydrophilic residues to the surface to interact withthe hydrophilic functional groups. Because the interfaces withdifferent wettability possess different affinity for oil droplets inwater, the different conformations of GOx at hydrophobicand hydrophilic interfaces can be verified by monitoring thecontact angles of oil droplets in water. The CAs ofoil dropletsin water are 11.3°±1.9° and 143.6°±2.8° for hydrophobicand hydrophilic interfaces without enzyme coating, respectively(Figure S5). After the adsorption of GOx, the CAs of oildroplets in water are 35.4°±2.7°and 139.8°± 3.4° for thehydrophobic and hydrophilic interfaces, respectively (Figure3a). At the hydrophobic interface, the interaction between thehydrophobic amino acids on the GOx surface and the modifiedNDM on the PAA leads to the hydrophobic residues of theimmobilized GOx being exposed, resulting in a CA of the oildroplet in water representative of an oleophilic surface. AfterGOx adsorbs on a hydrophilic surface, the interaction betweenthe immobilized GOx and MUA on the PAA leads to thehydrophilic residues of the immobilized GOx being exposed,resulting in a CA of the oil droplet in water being representativeof an oleophobic surface. These results confirm that the twoCAs of oil droplets in water represent the differentconformations of the GOx at hydrophobic and hydrophilicinterfaces. We reason that the hydrophobic surface made by n-dodecanethiol may promote unfolding of GOx due to theinteraction between hydrophobic groups in the interior of GOxand the alkyl chain of n-dodecanethiol.31-34 Therefore, theadsorption of GOx to hydrophobic surface is due tohydrophobic interactions, which leads to structural changes inthe protein upon adsorption. The nonnative conformation ofGOx at interfaces with different wettability is explored by sumfrequency generation spectroscopy (SFG).36-38 SFG spectrawith ssp (s-polarized SF output, s-polarized visible input, and p-polarized infrared input) polarization are fitted by the standardspectral fitting method.21,22 The GOx immobilization at thehydrophobic interface shows an absorbance peak located at1622 cm- in the amide I spectra area (Figure 3b, bluedot).2324 Previous studies demonstrated that unfolding of anative protein was involved in the conversion of a solubleprotein into a B-sheet-rich structured aggregate,38-41.which is Figure 3. (a) The contact angles of an oil droplet in water at differentwettability interfaces with GOx immobilization reveal the conforma-tion of GOx rearrangement. In water, the hydrophobic interface isoleophilic, and the hydrophilic interface is oleophobic. (b) The SFGspectra of GOx at hydrophobic (blue dot) and hydrophilic interface(red dot). GOx immobilized at the hydrophobic interface shows anabsorbance peak located at 1622 cm in the amide I spectra area. Incontrast, there is no signal at the hydrophilic interface. These resultsindicate that GOx immobilized at the hydrophobic interface displays amore favorable conformation for catalytic reactivity.(c) Themeasurements of gas bubble adhesion at hydrophobic (blue dot)and hydrophilic interfaces (red dot) in water. AF is the stretch forcebetween the interface and air bubble. The hydrophobic interfacepresents higher air adhesion to gas molecules residing on it. characterized by the appearance of nonnative spectral bandsfrom 1627 to 1622 cm-.42-44 After GOx adsorption at ahydrophobic interface, most of the helix loss is converted tointermolecular antiparallel p-sheet motifs.45,46 In contrast, nosignal is observed from 1500 to 1800 cm- for GOx at thehydrophilic interface (Figure 3b, red dot) due to the loss ofhelices that are transformed into turns and random structures.The outcome further confirms that the conformation of GOxdepends on the wettability of the solid surface. The interfaces with different wettability possess differentadhesion to air bubbles;47-51 the corresponding properties forair bubble adhesion at different wettability interfaces are alsoexamined. As can be seen from Figure 3c, the hydrophobicinterface shows strong underwater air bubble adhesion with aCA of 119.1°±1.6° and a maximum stretch force (AF) of 86.7± 2.5 pN (Figure 3c, blue dot). In contrast, the hydrophilicinterface displays low underwater air bubble adhesion with CAof 142.4°±1.3° and a AF of 15 ± 1.7 uN (Figure3s reg 3c, red dot).Therefore, the hydrophobic interface shows more affinity forgas molecules than the hydrophilic interface. The amount of absorption of GOx is determined to be 28.7±3.3 U at the hydrophobic interface and 36.2 ± 4.2 U at thehydrophilic interface, which was measured by colorimetricmeasurement (Figure S6). The catalytic ability of theimmobilized GOx at the gas-solid-liquid interface withdifferent wettability is examined by electrochemical techniques,that is, cyclic voltamogram (CV) (Figure 4a and Figure S7) andlinear sweep voltammetry (LSV) (Figure S8). The GOx 25 Figure 4. Electrochemical performance of controlled wettability gas-solid-liquid nanochannels interface for p-p-glucose at variousconcentrations. (a) CVs of GOx/NDM/AuNPs/NDM/Au/PAAmeasured in B-D-glucose (concentrations from 0 to 0.06 M). (b-d)Calibration plot derived from the LSV experiments related to theoxidation currents with B-D-glucose substrate at 0.55 V (vs SCE), afterGOx immobilization at the hydrophobic interface (blue line) orhydrophilic interface (red line). The l and g represent the liquidchamber and gas chamber, respectively. All the gas chambers aremeasured against an atmospheric pressure. (b) In air (1)/Nz (g) (soliddot) or in Nz (1)/air (g) (open dot). The GOx is adsorbed at ahydrophobic interface (c) and hydrophilic interface (d) under differentconditions. The GOx immobilized at the hydrophobic interface withoxygen from the gas phase exhibits higher oxidation currents with theB-D-glucose substrate. All the oxidation currents with B-D-glucosesubstrate are acquired at 0.55 V (vs SCE). adsorbed at proposed interface displays no direct electro-chemical behavior (Figure S9). In the presence of B-D-glucose(the substrate for GOx), the oxidation currents of GOximmobilized at the proposed interfaces are ddramaticallyenhanced, and the oxidation current increase is associatedwith increasing concentration of B-p-glucose (Figure 4a). Inaddition, the oxidation current of GOx immobilized at thehydrophobic interface in a liquid chamber with an air saturatedsolution and a gas chamber full of Nz (air (1)/Nz (g)) is 6.19uA for p-p-glucose substrate at a certain concentration (e.g., 40mM), which is 2.7 times larger than that at the hydrophilicinterface (2.284 pA, Figure 4b, solid circular dot). Interestingly,with air (1)/air (g), the catalytic currents are 24.71 uA and13.92 uA for the hydrophobic interface and hydrophilicinterface, respectively (Figure 4b,c, open circular dot), whichare, respectively, 4 and 6.1 times larger than those found withair (1)/N (g). This result indicates that the reactivity ofimmobilized GOx can be enhanced by diffusing oxygen throughthe PAA nanochannels to the gas-solid-liquid interface.Control experiments are performed by using Nz (1)/(N, orair) (g). No obvious oxidation currents are observed in Nz (1)/N2 (g) for both the hydrophobic and hydrophilic interfaces(Figure 4b,c, triangle solid dot). In N(1)/air (g), a dose-independent oxidation current is observed, and the oxidationcurrents are 20.652 uA and 8.386 uA for the hydrophobicinterface and hydrophilic interface (Figure 4b, open circulardot), respectively,which are respective increases of 3.3 and 3.6times compared to air (1)/N (g); however, these currents aresmaller than those for air (1)/air (g). These results illustrate Table 1. Kinetic Parameters of Immobilized GOx at Controlled Wettability Interface under Different Conditions" N2 air air NDM MUA NDM MUA NDM MUA NDM MUA Icat (uA) 6.19 2.28 24.71 13.92 20.65 8.38 52.11 39.99 KaPp (mM) 64.5 100.6 21.84 26.43 31.9 54.48 16.88 20.56 Vmax (mM s-) 28.86 2.47 58.46 51.84 45.1 37.08 92.5 88.9 kcat (s-) 15.3 1.3 42.3 28.8 33.5 19.2 67 49.18 kat/KPP(M-s-) 238 13 1940 1090 1050 354 3970 2392 that the reactivity of the enzyme is enhanced by oxygendiffusion from both the liquid and gas phases but that the latterphase is more effective. To confirm the influence of theconcentration of oxygen, the gas chamber is filled with pureoxygen while the liquid chamber is saturated with N or air(Figure 4c, solid circle dot and open square dot), and theoxidation currents are further improved up to 2.5 and 13 times(Table 1 and Table S1), compared to those when the gaschamber contained air and N, (Figure 4b, blue solid circulardot and blue open circular dot), respectively. This resultsuggests that the reactivity of GOx can be controlled by theaccessibility of oxygen from the gas phase. To gain further insights, the kinetic parameters of theenzymatic reactions occurring at the interfaces with differentwettability were evaluated. For each enzymatic reaction, thedose-dependent plots show that at high glucose concentrationsa plateau response is obtained, which is a characteristic of theMichaelis-Menten kinetic mechanism (Figure 4).48 Accordingto the Lineweaver-Burk equation, the parameters of theenzymatic reaction kinetics of the interfaces with differentwettabilities, including the apparent Michaelis-Menten con-stant (Kapp), catalytic reaction rate constant(kcat), andmaximum reaction rate (Vmax) are summarized in Table 1.The KPP is a reflection of the enzymatic affinity for substrate,and a high KP value represents a weak affinity and vice versa.:24In addition, the rate parameter k/KaPP can be used to monitorthe enzymatic reaction. As can be seen from Table 1, the kcat/KPP values of GOx immobilized at the hydrophobic interface inN, (1)/air (g) (1050) is 80-fold higher than that at thehydrophilic interface in air ((1)/N (g)(13). For thehydrophobic interface, the kcat/KpP values are ordered in thesequence 6256 (air (1)/O2 (g))>3970 (Nz (1)/Oz (g))>1940(air (1)/air (g))> 1050 (Nz (1)/air (g))>238 M-s-(air (1)/Nz(g)). For hydrophilic interface, the kat/KPP values areordered in the sequence 3156 (air (1)/O2(g))>2392 (Nz (1)/Oz (g))> 1090 (air (1)/air (g))> 354 (N(1)/air (g))> 13M-s(air(1)/Nz(g)) (Table 1 and Table S1). These resultsillustrate that the reactivity of GOx strongly depends on the O,concentration, that high reaction kinetics can be effectivelyachieved by increasing the diffusion of O2 through the gasphase, and that the hydrophobic interface preferably enhancesthe reactivity of GOx. At high glucose concentration, theoxygen concentration in the gas phase determines the amountof the oxygen that reaches the active site, which is the pseudo-rate-limiting step for the overall enzymatic reaction. It should be noted that the hydrophobic molecules andelectrodeposited AuNPs are also two critical elements forenhancing enzymatic reactions. Figure S11 shows that the GOxadsorption at NDM/AuNPs/Au/PAA (GOx/NDM/AuNPs/Au/PAA) exhibits a lower oxidation current for the p-D-glucose substrate than GOx/NDM/AuNPs/NDM/Au/PAA. Thisfurther confirms that the hydrophobic environment that issupplied by NDM at the first hydrophobic modification allowsthe O2 molecules to transport through the nanochannels of thePAA membrane freely; then, the O2 molecules reside and reachthe active site of GOx to enhance the enzymatic reactivity.Additionally, the observed oxidation current for GOx/NDM/Au/PAA is also much smaller than that of GOx/NDM/AuNPs/NDM/Au/PAA. These results illustrate that theconstructed nanochannels with joint gas-solid-liquid interfaceand controlled wettability resolves the gas-deficit problem andsupplies a nanochannel for gas molecules to enhance catalyticreaction of enzyme. CONCLUSIONS In summary, we have fabricated a nanochannel reactor withjoint gas-solid-liquid interfaces and controlled wettability forboosting gas involving enzymatic reactions. The conformationand orientation of the immobilized enzyme at the proposedinterface can be facilely manipulated with improved affinity forgases and the ability to allow the gas substrates to transportthrough the nanochannels to directly reach the active sites ofenzymes from the gas phase. Our results show that in thepresence of oxygen from air, the catalytic efficiency of theenzyme can be improved up to 80 times, compared with that inthe presence of the oxygen from solution. Moreover, theaccessibility of oxygen from the gas phase can be regulated tofurther improve the reaction efficiency. Therefore, this workmay provide a novel strategy for potential applications in gassensors and industrial catalysis with gas substrates. ASSOCIATED CONTENT ⑤ Supporting Information The Supporting Information is available free of charge on theACS Publications website at DOI: 10.1021/jacs.7b05249. Additional experimental details (methods, SEM, contactangle and electrochemistry), tables (Supporting Table 1),and figures (Supporting Figures 1-11) (PDF) AUTHOR INFORMATION Corresponding Authors tianyely@iccas.ac.cn *liusq@seu.edu.cn ORCID D Yuanjian Zhang: 0000-0003-2932-4159 Ye Tian: 0000-0002-1913-6333 ( Songqin Liu:00 00-0002-4686-5291 ) ( Lei Jiang: 0000-0003-4 5 79-728X ) Notes The authors declare no competing financial interest. ■ACKNOWLEDGMENTS We gratefully appreciate the support from National NaturalScience Foundation of China (Grants 21635004, 21627806,and 21671194). ( REFERENCES ) ( (1) H uang, J. P.; C heng, F . Q.; Binks, B. P .; Yang, H. Q .J . Am. C h em. S oc. 2015, 137, 15015. ) ( (2) Zhang, G . R . ; M u noz, M.; Etzold, B. J.M. Ang e w. Chem , Int. E d. 2016, SS, 2257. ) ( (3) L i u, F . J ; W ang, L. ; Sun, Q. ; Zhu, L. F .; M eng , X. J. ; X i ao , F. S. J. Am. C hem. Soc. 2012, 134, 16948. ) ( (4) S aam, J.; I vanov, I .; Walther, M.; H olzhutter, H. G . ; K u hn, H. P roc. N atl. Acad. S ci. U. S. A. 2007, 1 04, 1 3 319. ) ( (S) Wang, H.; Z h ou, W.; Li u , J. X.; S i,R.; S un, G .; Zhong, M. Q.; S u , H . Y.; Zhao, H. B.; Rodriguez, J. A .; Pennycook, S. J . ; Idrobo,J. C.; Li, W. X.; K ou, Y.; Ma, D . J. Am. C h em. So c . 2013, 13S, 4149. ) ( (6) X u, C . L. ; S o ng, Z. Q. ; Xiang, Q; Jin, J.; Fen g , X. J . N a noscale 2016, 8 , 7 391. ) ( (7) A tencia,J.; Beebe, D. J. N ature 2005, 4 3 7, 6 4 8. ) ( (8) Strong, P. J.; Xie, S . ; C l arke, W. P. Env i ron. Sci. Tec h nol. 2015, 49, 4001. ) ( (9) K ang, P.; Zhang, S.; Meyer, T. J.; Brookhart, M . Angew . Chem, Int. Ed. 2014, 53, 8709. ) ( (10) C h an, S. I . ; L u, Y . J.; Nagababu, P .; Maji, S.; Hung, M. C.; L ee, M. M.; Hsu, I . J.; Minh, P. D .; L ai, J. C. H.; Ng, K. Y.; et al . Angew. C hem., I nt. Ed. 2013, 52, 3731. ) ( (11) Y u, Y . H .; F u, L . M .; Z hang, F . W.; Z h ou, T.; Yang,H. Q. ChemPhysChem 2014, 15, 841. ) ( (12) Gunther, A.; K h an, S. A.; Thalmann, M.; Tra c hsel, F.; Jensen, K. F . Lab C hip 2004, 4, 278. ) ( (13) K obayashi, J. S cience 2004, 3 04, 1305-1308. ) ( (14) Lei, Y . J.; S un, R . Z . ; Z h ang, X. C .; Fen g , X. J.; J i ang,L. Adv. Mater. 2016, 2 8, 1477. ) ( (15) Wang, J.; Lu, F. J.Am. C hem. Soc. 1 998, 120,1048. ) ( (16) A nand, G . ; S h arma, S. ; Dutta, A. K.; Kumar, S. K.; Be l fort, G . Langmuir 2010, 26, 1 0803. ) ( (17) Ciaccafava, A.; I n fossi, P .; I lbert, M.; Guiral, M.; L ecomte, S. ; Giudici-Orticoni, M. T .; Lojou, E. Angew. Chem., Int. Ed . 2012, 51,953. ) ( (18) S ethuraman, A.; B e lfort, G . Biophys.J. 2005, 88, 1 3 22. ) ( (19) S eo, J.; Hoffmann, W.; Warnke, S.; B o wers, M. T. ; Pa g el, K.; von Helden, G . Angew. Ch e m., Int. Ed. 2016, SS , 14173. ) ( (20) Zhuang, X.; Miranda, P. B.; Kim, D.; Shen, Y. R . P h ys . Rev. B: Condens. Matter Mater. Phys. 1999, 59, 12632. ) ( (21) Nguyen, K. T .; Le Cla i r, S. V.; Ye, S. J.; Chen, Z. J. Phys. Chem. B 2009,113,12169. ) ( (22) Wang, J.; Lee, S. H.; Chen, Z. J. Phys . Chem. B 2008, 112, 2281.(23) K e lly, J. W. C u rr. Opin. St r uct. Biol. 1998, 8, 1 01 . ) ( (24) W ang, J.; Even, M. A. ; Ch e n, X. Y .; Sch m aier, A. H.; W aite, J.H.; Chen, Z . J . Am. Chem. Soc.2003, 1 2 5, 9914. ) ( (25) B aron, R.; R iley, C.; Chenprakhon, P . ; Thotsaporn, K.; W i nter, R . T .; A lfieri, A.; Forneris, F .; van Berkel, W. J. H . ; C h aiyen, P.; Fraaije, M. W.; Mattevi, A .; McCammon, J. A. Proc. Na t l. Acad. Sci. U. S. A.2009,106,10603. ) ( (26) Aissaoui, N.; Bergaoui, L . ; Boujday, S.; Lambert, J. F.; Methivier, C.; Landoulsi, J. Langmuir 2014, 30, 4066. ) ( (27) R odrigues, R . C . ; O r tiz, C.; Berenguer-Murcia, A. ; Torres, R. ; Fernandez-Lafuente, R. C hem. Soc. R e v. 2013, 42, 6290. ) ( (28) Guiomar, A. J.; Guthrie, J. T.; E v ans, S. D. Langmuir 1999 , 15, 1198. ) ( (29) Badieyan, S . ; Wang, Q. M.; Zou , X. Q ; Li, Y. X . ; Herron, M.; Abbott, N. L.; Chen, Z.; Marsh, E. N. G. J. Am. Ch e m. Soc. 2017, 139, 2872. ) ( (30) Wu, F.; Su, L.; Yu,P.; Mao, L. Q. J. Am. Chem. Soc. 2017, 139, 1565. ) ( (31) Duinhoven, S.; P oort, R.; Vandervoet, G.; Agterof, W. G. M.;Norde, W . ; Ly k lema, J. J. Co l loid Interface Sc i . 1995, 170, 340 . ) ( ( 32) R o silio, V .; B oissonnade, M. M. ; Zh a ng, J. Y.; Ji a ng, L.; B a szkin, A. Langmuir 1997, 13,4669. ) ( ( 33) Sun, S . C.; Hosi, P. H.; Harrison, D . J . Langmuir 19 9 1, 7, 727.(34) Z hang, J; R o silio, V .; Goldmann, M.; Bo i ssonnade, M. M.; Baszkin, A. Langmuir 2 000, 16, 1 226. ) ( (35) L iu, M . J.; Wang, S. T.; W ei, Z. X.; Song, Y. L.; Jiang, L. Adv. Mater. 2009, 2 1, 665. ) ( (36) Fu, L.; Ma, G.; Yan, E. C. Y. J. Am. Chem. Soc.20 1 0, 132, 5405. ( 37) B o oth, D . R . ; Sunde, M. ; Bellotti, V . ; R o binson, C . V. ; ) Hutchinson, W. L.; Fraser, P. E.; Hawkins, P. N.; Dobson, C. M.; ( Radford, S. E .; B lake, C. C . F.; P epys, M. B. Nature 1997, 385 , 787.(38) D obson, C. M. Tr e nds Biochem. Sci . 19 9 9, 24, 32 9 . ) ( (39)S t efani, M.; Dobson, C . M. J. Mol. Med. 2003,81,678. ( 40) D o ng, A. C. ; Randolph, T. W .; Carp e nter, J. F. J. B i ol. Che m . 2000,275,27689. ) ( (41) Dong, A. C. ; Prestrelski, S. J.; Allison, S. D .; C arpenter, J. F. J . Pharm. Sci. 1995, 84, 415. ) ( (42) Zurdo,J.; Gu i jarro,J. I.; Jimenez, J. L. ; Sa i bil, H. R.; D o bson, C. M. J. Mol. Biol. 2001, 311, 325. ) ( ( 43) S okolowski, F.; Modler, A. J.; Masuch, R.; Zirwer, D.;Baier, M.;Lutsch, G.; Moss, D. A.; G ast, K.; N aumann, D. J. B iol. C h em. 2003, 278,40481. ) ( (44) M i litello, V . ; Casarino, C. ; Em a n uele, A.; G i ostra, A.; Pullara, F.; Leone, M . Biophys. Chem. 2 004, 1 07, 1 75. ) ( (45) C h en, L. ; L y ubimov, A. Y.; Br a mmer, L.; V ri e link, A.; Sampson,N. S . Biochemistry 2008, 47, 5368. ) ( ( 46) Lario, P . I .; Sampson, N.; Vrielink, A . J . Mol. Biol. 2003, 326, 1635. ) ( ( 47) Tian, Y.; S u, B .; Jiang, L. Adv. Mater. 2 014, 26, 6872. ) ( (48) K amin, R. A.; W ilson, G. S. A nal. Chem. 1 980, 52,1198. ) (49) Yen, T. H. Mol. Phys. 2015, 113, 3783. ( ( 50) E rcan, B.; K hang, D . ; C a rpenter, J.; W e bster, T. J. In t . J . Nanomed. 2013, 8, 7 5. ) ( ( 51) L i akopoulos, A.; Sofos, F . ; Ka r akasidis, T. E . Mi c rofluid.Nanofluid. 2016, 20, 24. ) ( (52) L aviron, E. J. E lectroanal. Chem. Interfacial Electrochem. 1979, 101, 19. (53) K linman, J. P. Acc. Chem. Res. 2007, 40, 325. ) OI:jacs.b. Am. Chem. Soc. American Chemical Society OI:jacs.b. Am. Chem. Soc. The low solubility of gases in aqueous solution is the major kinetic limitation of reactions that involve gases. To address this challenge, we report a nanochannel reactor with jointgas−solid−liquid interfaces and controlled wettability. As a proof of concept, a porous anodic alumina (PAA) nanochannel membrane with different wettability is used for glucose oxidase(GOx) immobilization, which contacts with glucose aqueous solution on one side, while the other side gets in touch with the gas phase directly. Interestingly, it is observed that the O2 could participate in the enzymatic reaction directly from gas phase through the proposed nanochannels, and a hydrophobic interface is more favorable for the enzymatic reaction due to the rearrangement of GOx structure as well as the high gas adhesion. As a result, the catalytic efficiency of GOx in the proposed interface is increased up to 80-fold compared with that of the free state in traditional aqueous air-saturated electrolyte. This triphase interface with controlled wettability can be generally applied to immobilize enzymes or catalysts with gas substrates for high efficiency.
确定




还剩4页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《纳米通道反应器中和频光谱检测方案(其它光谱仪)》,该方案主要用于其他中其他检测,参考标准--,《纳米通道反应器中和频光谱检测方案(其它光谱仪)》用到的仪器有Ekspla SFG 表面和频光谱分析系统
推荐专场
相关方案
更多
该厂商其他方案
更多