当前位置:
仪器信息网
>
行业应用
>
畜禽肉及副产品检测方案
>
Rhodamine Salicylaldehyde Hydrazone Dy(III) Complexes: Fluorescence and Magnetism
方案详情
文
calculations illustrate that the disposition of predominant Dy−O phenoxy bonds affects the magnetic anisotropy of the Dy(III) ions
and relaxation processes of complexes 2 and 3.
方案详情

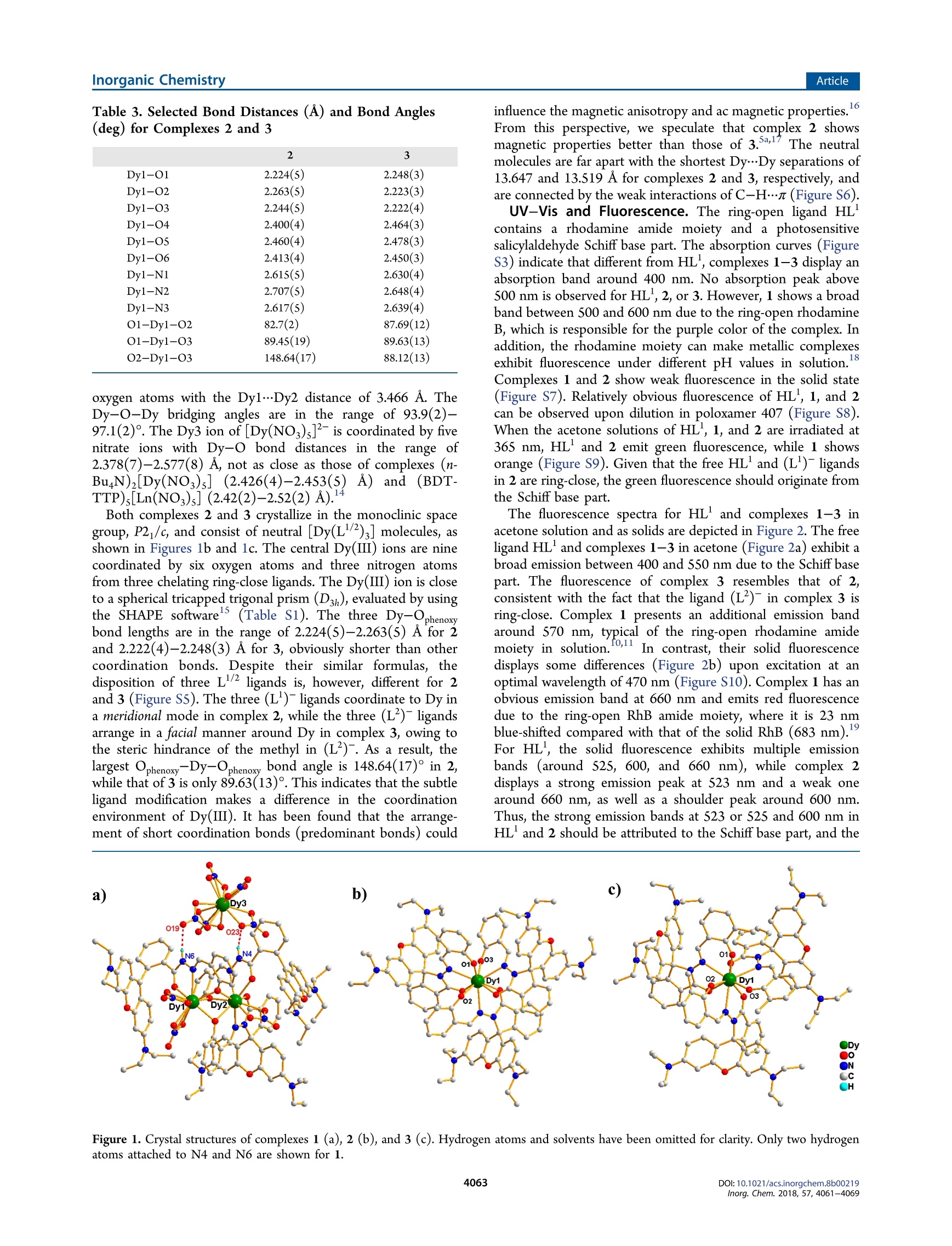
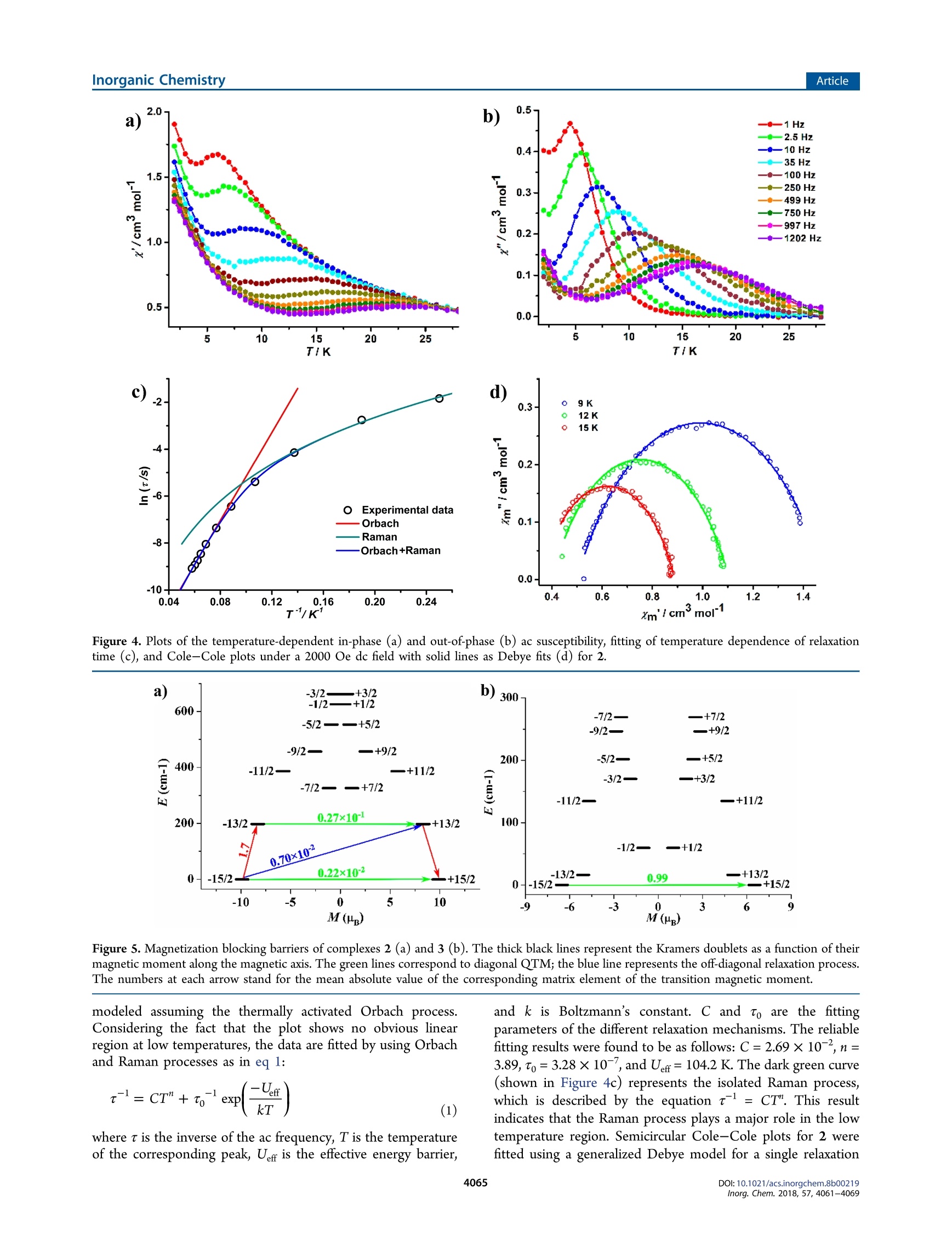
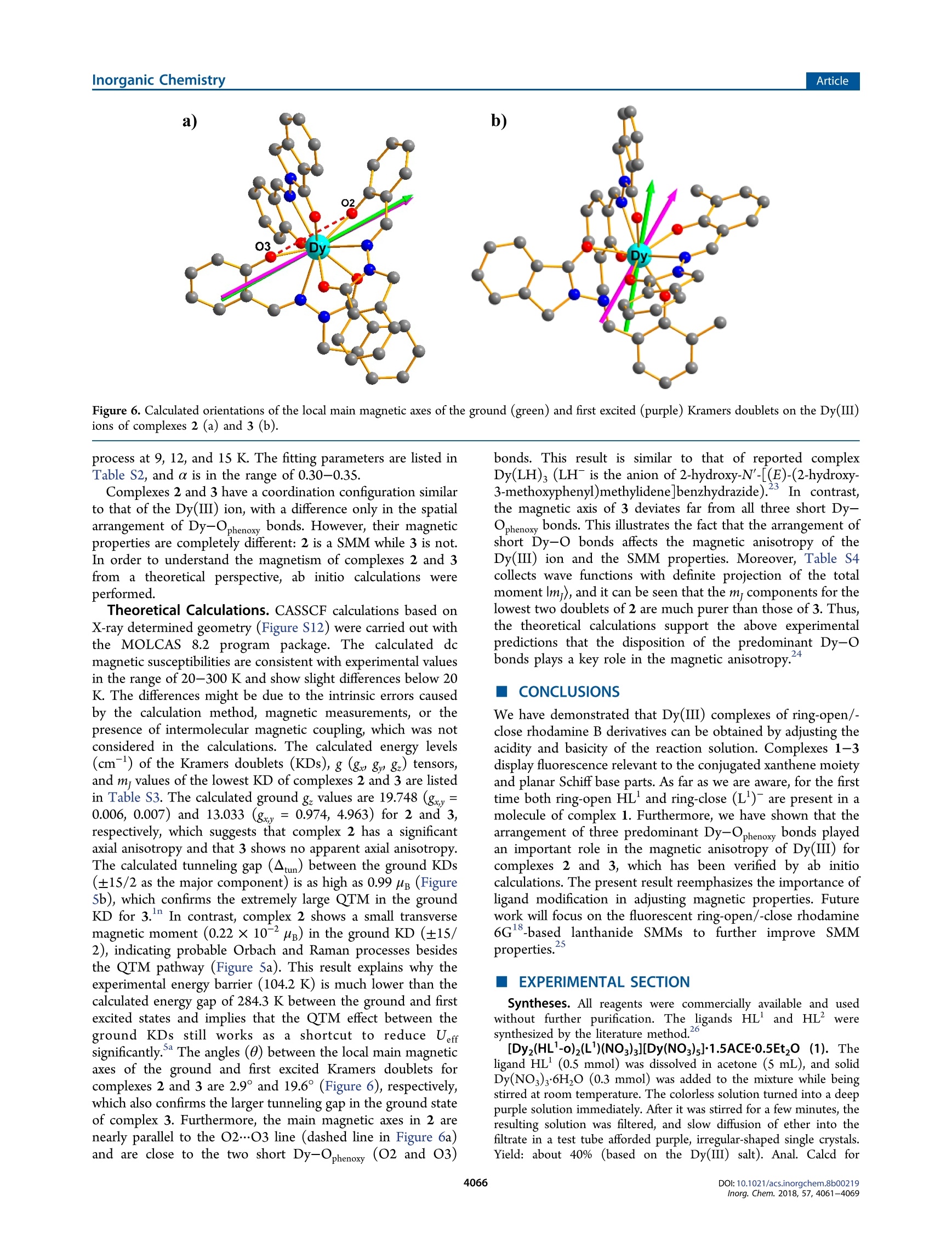
Inorganic Chemistry.Article) Cite This: Inorg. Chem. 2018,57, 4061-4069pubs.acs.org/lC Inorganic ChemistryArticle Rhodamine Salicylaldehyde Hydrazone Dy(III) Complexes:Fluorescence and Magnetism Mei-jJiao Liu, Juan Yuan, Jin Tao, Yi-Quan Zhang* *Dt oCai-Ming Liu,D and Hui-Zhong Kou*c 'Department of Chemistry, Tsinghua University, Beijing 100084, P. R. China Jiangsu Key Laboratory for NSLSCS, School of Physical Science and Technology, Nanjing Normal University, Nanjing 210023, P. R.China Beijing National Laboratory for Molecular Sciences, Center for Molecular Science, Institute of Chemistry, Chinese Academy ofSciences, Beijing 100190, P. R. China Supporting Information ABSTRACT: Three new dysprosium(III) complexes [[Dy(HL-0)(L)-(NO3)3][Dy(NO3)s]1.5ACE-0.5Et,O(1), [Dy(L)3]2.5MeOH·MeCN(2), and[Dy(L)3]·MeOH·MeCN(3)(HL= rhodamine Bsalicylaldehyde hydrazine, HL’= rhodamine B 3-methylsalicylaldehydehydrazine)were synthesized and characterized. Purple complex 1contains two ring-open ligands HL-o and shows fluorescence of therhodamine amide moiety, whereas yellow complexes 2 and 33 arecomprised of ring-close ligands (L2) and display fluorescence of thesalicylaldehyde Schiff base part. For 2 and 3, Dy(III) ions are ninecoordinated by the six oxygen and three nitrogen atoms of three chelate(L2)- ligands, but the arrangements of the three ligands are differentowing to the methyl substituent on HL. There are three shortpredominant Dy-Ophenoxy tbonds in 2 and 3. The largest Ophenoxy--Dy-Ophenoxy angle is 148.64(17)° for 2 and 89.63(13)° for 3. Magnetic studies reveal that complex 2 is a field-induced single-molecule magnet (Uerr= 104.2 K under a dc magnetic field of 2000 Oe), and 3exhibits only a magnetic relaxation behavior owing to the quantum tunneling of magnetization (QTM). Furthermore, ab initiocalculations illustrate that the disposition of predominant Dy-Ophenoxyybonds affects the magnetic anisotropy of the Dy(III) ionsand relaxation processes of complexes 2 and 3. INTRODUCTION Recently, studies on single-molecule magnets (SMMs) havemade great strides in enhancing energy barrier (Uerf) andblocking temperature (TB) properties, as shown, for example,in the dysprosium(III) complex [Dy(Cp")z[B(CFs)4], whichpossesses the largest Uefr of 1837 K and the highest Tg of 60 K.Although it has been accepted that the ideal models for Dy(III)SMMs should have strong axial and weak equatorial ligandfields,the implementation of these models is usually not easy.Nevertheless, it can be possibly realized via the optimization ofthe coordination environment by changing coordinated smallmolecules’ or subtle modification of ligands. What’s more,ligand modification might lead to different molecular structuresand coordinated modes. It is well-known that the ratio ofreactants, temperature, and solvents’ of the reaction systemplay an important role in obtaining SMMs with diversestructures and magnetism. The rhodamine-based ligands canrespond to metallic ions and display a color change andfluorescence.This behavior is related to the ring-open or ring-close form of the rhodamine amide moiety. Thus, theintroduction of rhodamine-based derivatives to SMMs couldyield multifunctional complexes. However, current investiga-tions on rhodamine-based ligands focus mainly on chemo- sensors, photochromic systems, or near-infrared fluorescence.To the best of our knowledge, the rhodamine B-basedlanthanide SMMs are very scarce.Under the circumstances,we envisage modifying rhodamine B-based ligands, synthesizinglanthanide SMMs with ring-open/-close forms, and furtherstudying their fluorescence and magnetism. Here, the reactions of ligand HL or HL’ with dysprosiumnitrate hydrates are studied (Scheme 1). Purple complex[Dyz(HL--o)2(L)(NO3)3[Dy(NO3)s]·1.SACE-0.SEt,O (1)and yellow complex [Dy(L')3]2.5MeOH·MeCN (2)) areobtained in the absence or presence of trimethylamine. Yellowcomplex [Dy(L)s]·MeOH·MeCN (3) was obtained inamanner similar to that of complex 2. Moreover, complexes 1and 2 show interesting fluorescence. Complexes 2 and 3 exhibitsimilar coordination modes but have distinct dynamic magneticproperties. RESULTS AND DISCUSSION Synthesis. The reaction of HL1/2 with Dy(NO3)36H,0inacetone gave a purple solution, indicative of the formation of a Scheme 1. Synthesis of Complexes 1-3 ring-open RhB ligand owing to the acidic condition of thereactant solution. Single crystals of complex 1 suitable forstructural determination were obtained by slow diffusion ofether into the acetone reaction solution at room temperature.Interestingly, when triethylamine was added to the abovepurple solution, a light yellow solution formed, from whichcomplex 2 or 3 could be isolated. The color change could beobserved visually when Et N was added in small portions fourtimes (Figure S2 in the Supporting Information). The UV-visabsorption spectra of the solution clearly show that theintensity of the absorption at 560 nm due to the ring-openrhodamine dramatically decreases and that of the shoulder peakat 410 nm increases. The latter is reshuponsible for the yellowcolor of the solution. A comparison of the spectra for HL forcomplexes 1-3 in ethanol (Figure S3) indicates that the newpeak around 400 nm might be due to ligand to metal chargetransfer. Single crystals of complexes 2 and 3 were grown byheating the reaction solution of methanol and acetonitrile in anoven at 60°C. The presence of ring-open ligands in the purplecomplex 1 is proven by the crystal structure data. Complexes 2and 3 are yellow, and their crystal structures show that all the ligands are ring-close. The powder X-ray diffraction (PXRD)patterns and microelemental analyses of CHN for complexes 1and 3 indicate that the solid is pure (Figure S4). Crystal Structures. Crystal data and structural details forcomplexes 1-3 are listed in Table 1, and important bonddistances and angles are collected in Tables 2 and 3. Table 2. Selected Bond Distances (A) and Bond Angles(deg) for Complex 1 Dy1-O1 2.356(6) Dy2-O1 2.292(6) Dy1-O2 2.366(6) Dy2-O2 2.377(6) Dy1-O3 2.317(6) Dy2-O3 2.308(6) Dy1-O4 2.389(6) Dy2-O5 2.419(6) Dy1-O7 2.478(8) Dy2-O6 2.371(6) Dy1-O8 2.409(7) Dy2-O11 2.456(6) Dy1-O9 2.423(7) Dy2-O12 2.473(7) Dy1-O10 2.471(7) Dy2-N2 2.602(7) Dy1-N1 2.571(7) Dy2-N3 2.536(7) Dy1-O1-Dy2 96.5(2) Dy1-O2-Dy2 93.9(2) Dy1-O3-Dy2 97.1(2) Complex 1 crystallizes in the triclinic space group, P1. Themain structure of 1 consists of twoparts, [Dyz(HL-o)(L')(NO3)3]2+ and [[Dy(NO3)s]2- (Figure la). Twomoieties are connected by two hydrogen bonds with N6….019 and N4….023 distances of 2.922 and 2.876 A, respectively.Thee[Dyz(HL'-0)z(L)(NO3)3]2+ cation contains two ring-open ligands HL and one ring-close (L'), chelating twoDy(III) ions. The Dy1 ion is nine coordinated by four oxygenatoms from two nitrate ions, two oxygen atoms and onenitrogen atom from one HL-o ligand, and two phenoxyloxygen atoms from two bridging HL'-o/(L)- ligands. TheDy2 ion is also nine coordinated by two oxygen atoms fromone nitrate ion, four oxygen and two nitrogen atoms from twochelating HL -o/(L')- ligands, and one bridging phenoxyloxygen atom. Dyl and Dy2 ions are bridged by three phenoxyl 2 3 formula C111.5H121Dy3N20O35 C109.5H118DyN13O11.5 C11iH118DyN13010 formula weight 2788.77 1962.66 1956.68 T(K) 173 173 173 crystal system triclinic monoclinic monoclinic space group P1 P2/c P2/c a (A) 17.1579(5) 15.9478(4) 13.5190(4) b (A) 19.1288(5) 20.4636(9) 24.6020(5) c (A) 19.9183(5) 31.9377(7) 30.6886(13) α(deg) 74.850(2) 90 90 β (deg) 83.176(2) 92.527(2) 101.933(3) r (deg) 86.962(2) 90 90 V(A) 6263.8(3) 10412.7(6) 9986.3(6) Z 2 2 Pcale (g cm-) 1.479 1.252 1.301 u(mm-) 10.131 4.355 4.528 F(000) 2816 4096 4084 data/restraints/parameters 23763/1096/1589 21304/52/1255 14358/92/1271 GOF on F 1.108 1.020 1.041 Rint 0.0717 0.0475 0.0395 R, wR,[I>2o(I)] 0.0720,0.1966 0.0836,0.2327 0.0525, 0.1319 R, wR, (all data) 0.1061, 0.2147 0.1277, 0.2714 0.0663, 0.1471 CCDC 1816184 1816185 1816186 Table 3. Selected Bond Distances (A) and Bond Angles(deg) for Complexes 2 and 3 2 3 Dy1-O1 2.224(5) 2.248(3) Dy1-O2 2.263(5) 2.223(3) Dy1-O3 2.244(5) 2.222(4) Dy1-O4 2.400(4) 2.464(3) Dy1-O5 2.460(4) 2.478(3) Dy1-O6 2.413(4) 2.450(3) Dy1-N1 2.615(5) 2.630(4) Dy1-N2 2.707(5) 2.648(4) Dy1-N3 2.617(5) 2.639(4) O1-Dy1-O2 82.7(2) 87.69(12) O1-Dy1-O3 89.4S(19) 89.63(13) O2-Dy1-O3 148.64(17) 88.12(13) oxygen atoms with the Dy1…Dy2 distance of 3.466 A. TheDy-O-Dy bridging angles are in the range of 93.9(2)-97.1(2)°. The Dy3 ion of [Dy(NO3)s]- is coordinated by fivenitrate ions with Dy-O bond distances in the range of2.378(7)-2.577(8) A, not as close as those of complexes (n-BuN)[Dy(NO3)s] (2.426(4)-2.453(5)5))A)and((BDT-TTP)s[Ln(NO3)s](2.42(2)-2.52(2)A).4 Both complexes 2 and 3 crystallize in the monoclinic spacegroup, P2j/c, and consist of neutral [Dy(L)3] molecules, asshown in Figures 1b and 1c. The central Dy(III) ions are ninecoordinated by six oxygen atoms and three nitrogen atomsfrom three chelating ring-close ligands. The Dy(III) ion is closeto a spherical tricapped trigonal prism (D3h), evaluated by usingthe SHAPE softwarels (Table S1). The three Dy-Ophenoxybond lengths are in the range of 2.224(5)-2.263(5) A for 2and 2.222(4)-2.248(3) A for 3, obviously shorter than othercoordination bonds. Despite their similar formulas, thedisposition of three Ll/ ligands is, however, different for 2and 3 (Figure S5). The three (L)-ligands coordinate to Dy ina meridional mode in complex 2, while the three (L’)ligandsarrange in a facial manner around Dy in complex 3, owing tothe steric hindrance of the methyl in (L)-. As a result, thelargest Ophenoxy-Dy-Ophenoxy bond angle is 148.64(17)° in 2,while that of 3 is only 89.63(13)°. This indicates that the subtleligand modification makes a difference in the coordinationenvironment of Dy(III). It has been found that the arrange-ment of short coordination bonds (predominant bonds) could influence the magnetic anisotropy and ac magnetic properties.From this perspective, we speculate that complex 2 showsmagnetic properties better than those of 3.5a17 The neutralmolecules are far apart with the shortest Dy…Dy separations of13.647 and 13.519 A for complexes 2 and 3, respectively, andare connected by the weak interactions of C-H…n(Figure S6). UV-Vis and Fluorescence. The ring-open ligand HLcontains a rhodamine amide moiety and a photosensitivesalicylaldehyde Schiff base part. The absorption curves (FigureS3) indicate that different from HL, complexes 1-3 display anabsorption band around 400 nm. No absorption peak above500 nm is observed for HL, 2, or 3. However, 1 shows a broadband between 500 and 600 nm due to the ring-open rhodamineB, which is responsible for the purple color of the complex. Inaddition, the rhodamine moiety can make metallic complexesexhibit fluores18cence under different pH values in solution.Complexes 1 and 2 show weak fluorescence in the solid state(Figure S7). Relatively obvious fluorescence of HL, 1, and 2can be observed upon dilution in poloxamer 407 (Figure S8).When the acetone solutions of HL , 1, and 2 are irradiated at365 nm, HL and 2 emit green fluorescence, while 1 showsorange (Figure S9). Given that the free HL and (L')- ligandsin 2 are ring-close, the green fluorescence should originate fromthe Schiff base part. The fluorescence spectra for HL and complexes 1-3 inacetone solution and as solids are depicted in Figure 2. The freeligand HL and complexes 1-3 in acetone (Figure 2a) exhibit abroad emission between 400 and 550 nm due to the Schiff basepart. The fluorescence of complex 3 resembles that of 2,consistent with the fact that the ligand (L)- in complex 3 isring-close. Complex 1 presents an additional emission bandaround 570 nm, typical of the ring-open rhodamine amidemoiety in solution.10,11 In contrast, their solid fluorescencedisplays some differences (Figure 2b) upon excitation at anoptimal wavelength of 470 nm (Figure S10). Complex 1 has anobvious emission band at 660 nm and emits red fluorescencedue to the ring-open RhB amide moiety, where it is 23 nmblue-shifted compared with that of the solid RhB (683 nm).For HL, the solid fluorescence exhibits multiple emissionbands (around 525, 600, and 660 nm), while complex 2displays a strong emission peak at 523 nm and a weak onearound 660 nm, as well as a shoulder peak around 600 nm.Thus, the strong emission bands at 523 or 525 and 600 nm inHL and 2 should be attributed to the Schiff base part, and the Figure 1. Crystal structures of complexes 1(a), 2 (b), and 3 (c). Hydrogen atoms and solvents have been omitted for clarity. Only two hydrogenatoms attached to N4 and N6 are shown for 1. Figure 2. Fluorescence spectra for HL' and complexes 1-3 in acetone (a) (Aex=365 nm, [HL']=[1]=[2]=[3]=5×10-6M) and as solids (b)(1=470 nm). relatively weak emission peak at 660 nm in HL’ and 2 is due toa tiny amount of ring-open rhodamine B. Compared with thefluorescence in acetone, the change of emission peaks has beena common phenomenon owing to the aggregation-causedquenching (ACQ) effect of the solid. Magnetic Properties. The variable-temperature magneticsusceptibilities of complexes 1-3 were measured under anexternal magnetic field of 1000 Oe in the temperature range of2-300 K. As shown in Figure 3, therT value of 1 at 300 K is Figure3. Variable-temperature magnetic susceptibilities under a 1000Oe dc field for complexes 1-3. The solid lines represent simulationsfrom ab initio calculations for 2 and 3. Inset:Magnetization curve forcomplexes 1-3 at 2 K. 41.84 cm’K mol-, close to the theoretical value of 42.51 cm3K molof three non-interacting Dy(III) ions (H15/2)g=4/3).When the complex is cooled, the XmT value of 1 decreasesslightly from 300 to 100 K and then decreases rapidly reachingthe minimum of 30.91 cm’K mol- at 4 K. The hightemperature decrease of XmT is probably due to the thermaldepopulation of Stark sublevels for Dy(III) ions. The slightincrease of XmT to 31.64 cm’K mol- from 4 to 2 K suggeststhe presence of a weak intramolecular ferromagnetic interactionbetween two triply Ophenoxy-bridged Dy(III) ions.a-d Themagnetic exchange is related to the Dy-O-Dy bond angle andthe dihedral angle of the Dyz02 core, and a low Dy-O-Dyangle less than 105° and good coplanarity of the Dyz02 corewill lead to Dy.Dy antiferromagnetic exchange.e The Dy-O-Dy angles are very low (93.9(2)-97.1(2)°) in complex 1;however, the Dy2O2 cores are far from a plane, with the Dy-O-Dy-O torsion angles in the range of 27.6-45.1°. The deviation from coplanarity for the Dy2O2 cores and the largedihedral angle between the DyO2 plane and the benzene planeshould be responsible for the ferromagnetic exchange in 1.At room temperature, the xmTvalues are 13.84 and 14.06 cm’K molfor 2 and 3, respectively, comparable to the theoreticalvalue of 14.17 cm’K mol- for an isolated Dy(III) ion. As thetemperature is lowered, the XmT values of 2 and 3 remainessentially constant with only a little decrease until 100 K. Withfurther cooling, the XmT value for 2 decreases regularly to aminimum of 11.25 cm’K molat 2 K. In contrast to 2, theXmT value of 3 decreases more rapidly with decreasingtemperature to 9.35 cm’K molat 6 K and then increasesto a maximum of 9.52 cm’K molat 4 K, before decreasingagain to 9.27 cm’K mol-at 2 K. Considering that complexes 2and 3 are mononuclear, the high temperature decrease of xmTshould be due to the thermal depopulation of Stark sublevelsfor the Dy(III) ion and/or intermolecular antiferromagneticinteractions. The field dependence of the magnetization with the appliedmagnetic field in the range of 0-50 kOe was investigated at 2K. As shown in Figure 3 (inset), the magnetization values for1-3 increase rapidly at a low field (0-10 kOe) and then growslowly reaching the maximum of 18.0 Nβ (1), 5.3 Np (2), and6.6 NB (3) at 50 kOe, still less than the theoretical saturatedvalues of 30 NB (1) and 10 NB (2 and 3) expected for isolatedDy(III) ions. This can be attributed to crystal field effectsleading to significant magnetic anisotropy of the Dy(III)ions. To investigate magnetic dynamics for complexes 1-3, thetemperature-dependent ac magnetic susceptibilities werecollected under zero and 2000 Oe dc fields. Complexes 1and 3 show non-zero out-of-phase (x") values under a dc fieldof 2000 Oe, but no peaks are observed (Figure S11). Thissuggests that the quantum tunneling of magnetization (QTM)process is still very strong under a 2000 Oe dc field. Forcomplex 2, under a zero dc field, x" signals show no peaks until2 K due to QTM. When an external dc field of 2000 Oe isapplied, x" displays strong frequency dependence and peaks,typical of a field-induced single-molecule magnet. Below 5 K,the increase of x" indicates that the QTM has not beencompletely suppressed at low temperature, which is a commonphenomenon in lanthanide SMMs. In the high temperatureregion, the Arrhenius law (t=/k7To exp(Ue/kT)) is employed toextract parameters to (4.84 ×10-s) and energy barrier Ue(93.8 K) (red line in Figure 4c). The In t vs 1/T plot is notlinear, indicating that magnetic dynamics cannot be simply Figure 4. Plots of the temperature-dependent in-phase (a) and out-of-phase (b) ac susceptibility, fitting of temperature dependence of relaxationtime (c), and Cole-Cole plots under a 2000 Oe dc field with solid lines as Debye fits (d) for 2. Figure 5. Magnetization blocking barriers of complexes 2 (a) and 3(b). The thick black lines represent the Kramers doublets as a function of theirmagnetic moment along the magnetic axis. The green lines correspond to diagonal QTM; the blue line represents the off-diagonal relaxation process.The numbers at each arrow stand for the mean absolute value of the corresponding matrix element of the transition magnetic moment. modeled assuming the thermally activated Orbach process.Considering the fact that the plot shows no obvious linearregion at low temperatures, the data are fitted by using Orbachand Raman processes as in eq 1: where t is the inverse of the ac frequency, T is the temperatureof the corresponding peak, Uefr is the effective energy barrier, and k is Boltzmann’s constant. C and to are the fittingparameters of the different relaxation mechanisms. The reliablefitting results were found to be as follows: C=2.69×10,n=3.89, to=3.28×10-7, and Ueff= 104.2 K. The dark green curve(shown in Figure 4c) represents the isolated Raman process,which is described by the equation t-= CT". This resultindicates that the Raman process plays a major role in the lowtemperature region. Semicircular Cole-Cole plots for 2 werefitted using a generalized Debye model for a single relaxation Figure 6. Calculated orientations of the local main magnetic axes of the ground (green) and first excited (purple) Kramers doublets on the Dy(III)ions of complexes 2 (a) and 3 (b). process at 9, 12, and 15 K. The fitting parameters are listed inTable S2, and a is in the range of 0.30-0.35. Complexes 2 and 3 have a coordination configuration similarto that of the Dy(III) ion, with a difference only in the spatialarrangement of Dy-Ophenoxy bonds. However, their magneticproperties are completely different: 2 is a SMM while 3 is not.In order to understand the magnetism of complexes 2 and 3from a theoretical perspective, ab initio calculations wereperformed. Theoretical Calculations. CASSCF calculations based onX-ray determined geometry (Figure S12) were carried out withthe MOLCAS 8.2 program package. The calculated dcmagnetic susceptibilities are consistent with experimental valuesin the range of 20-300 K and show slight differences below 20K. The differences might be due to the intrinsic errors causedby the calculation method, magnetic measurements, or thepresence of intermolecular magnetic coupling, which was notconsidered in the calculations. The calculated energy levels(cm-) of the Kramers doublets (KDs), g (gw 8w &) tensors,and my values of the lowest KD of complexes 2 and 3 are listedin Table S3. The calculated ground ge values are 19.748 (gxy=0.006, 0.007) and 13.033 (gxy= 0.974, 4.963) for 2 and 3,respectively, which suggests that complex 2 has a significantaxial anisotropy and that 3 shows no apparent axial anisotropy.The calculated tunneling gap (Atun) between the ground KDs(±15/2 as the major component) is as high as 0.99 uB (FigureSb), which confirms the extremely large QTM in the groundKD for 3." In contrast, complex 2 shows a small transversemagnetic moment (0.22×10-2 fB) in the ground KD (±15/2), indicating probable Orbach and Raman processes besidesthe QTM pathway (Figure 5a). This result explains why theexperimental energy barrier (104.2K) is much lower than thecalculated energy gap of 284.3 K between the ground and firstexcited states and implies that the QTM effect between theground KDs still works as a shortcut to reduce Ueffsignificantly. The angles (0) between the local main magneticaxes of the ground and first excited Kramers doublets forcomplexes 2 and 3 are 2.9° and 19.6° (Figure 6), respectively,which also confirms the larger tunneling gap in the ground stateof complex 3. Furthermore, the main magnetic axes in 2 arenearly parallel to the O2…..03 line (dashed line in Figure 6a)and are close to the two short Dy-Ophenoxy (O2 and O3) bonds. This result is similar to that of reported complexDy(LH)3 (LH"is the anion of 2-hydroxy-N'-[(E)-(2-hydroxy-3-methoxyphenyl)methylidene]benzhydrazide).23 In contrast,the magnetic axis of 3 deviates far from all three short Dy-Ophenoxy bonds. This illustrates the fact that the arrangement ofshort Dy-o bonds affects the magnetic anisotropy of theDy(III) ion and the SMM properties. Moreover, Table S4collects wave functions with definite projection of the totalmoment lm), and it can be seen that the m components for thelowest two doublets of 2 are much purer than those of 3. Thus,the theoretical calculations support the above experimentalpredictions that the disposition of the predominant Dy-Obonds plays a key role in the magnetic anisotropy. CONCLUSIONS We have demonstrated that Dy(III) complexes of ring-open/-close rhodamine B derivatives can be obtained by adjusting theacidity and basicity of the reaction solution. Complexes 1-3display fluorescence relevant to the conjugated xanthene moietyand planar Schiff base parts. As far as we are aware, for the firsttime both ring-open HL and ring-close (L)are present in amolecule of complex 1. Furthermore, we have shown that thearrangement of three predominant Dy-Ophenoxy bonds playedan important role in the magnetic anisotropy of Dy(III) forcomplexes 2 and 3, which has been verified by ab initiocalculations. The present result reemphasizes the importance ofligand modification in adjusting magnetic properties. Futurework will focus on the fluorescent ring-open/-close rhodamine6G-based lanthanide SMMsto further improve SMMproperties. EXPERIMENTAL SECTION Syntheses. All reagents were commercially available and usedwithout further purification. The ligands HL and HL2' wweresynthesized by the literature method. [Dy2(HL'-o)2(L')(NO3)3][Dy(NO3)s]1.5ACE·0.5Etz0 (1). Theligand HL (0.5 mmol) was dissolved in acetone (5 mL), and solidDy(NO3)36H,O (0.3 mmol) was added to the mixture while beingstirred at room temperature. The colorless solution turned into a deeppurple solution immediately. After it was stirred for a few minutes, theresulting solution was filtered, and slow diffusion of ether into thefiltrate in a test tube afforded purple, irregular-shaped single crystals.Yield: about 40% (based on the Dy(III) salt). Anal. Calcd for C111.sH121Dy3N20O3s (2788.77): C, 48.02; H, 4.37; N, 10.05. Found:C, 47.9; H, 4.3; N, 10.0. [Dy(L')]2.5MeOH·MeCN (2). The ligand HL (0.3 mmol) wassuspended in methanol (18 mL) and acetonitrile (9 mL), and solidDy(NO3)36H,O (0.1 mmol) was added to the mixture while beingstirred at room temperature to afford a deep purple solution.Triethylamine (0.6 mmol, 84 uL) was added to the above reactionsolution, and the color of the solution became yellow. After it wasstirred for a few minutes, the solution was filtered, and the filtrate wasplaced in an oven at 60°C for 24 h. Small yellow block-shaped crystalssuitable for X-ray single-crystal diffraction were obtained. The crystalswere efflorescent in the air. Yield: about 60% (based on the Dy(III)salt). [Dy(L)g]MeOH·MeCN (3). Yellow block-shaped crystals ofcomplex 3 suitable for X-ray single-crystal diffraction were obtainedin a manner similar to that for complex 2 except that HL’ was usedinstead of HL. The obtained crystals were stable in the air. Yield:about 46% (based on the Dy(III) salt). Anal. Calcd forC11H118DyN1301o(1956.68): C, 68.13; H, 6.08; N, 9.31. Found: C,67.6; H, 6.1; N, 9.0. Physical Measurements. PXRD measurements were recorded ona Bruker D8 ADVANCE X-ray diffractometer. The IR spectra with aKBr tablet were obtained on WQF-510A FTIR equipment. Elementalanalyses (C, H, and N) were performed on an Elementar Vario CarioErballo analyzer. The UV-vis absorption spectra were measured witha TU-1901 spectrophotometer. Single-crystal X-ray data were collectedon a Rigaku SuperNova, Dual, Cu at zero, AtlasS2. The structure wassolved by direct methods (SHELXS-2013) and refined by a full matrixleast-squares method based on F using the SHELXL-2013 orSHELXL-2014/7 program. Hydrogen atoms were added geometricallyand refined using a riding model.The fluorescence spectra wererecorded on an F98 fluorospectrophotometer. Temperature- and field-dependent magnetic susceptibility measurements were carried out on aQuantum Design MPMS-XLS SQUID magnetometer. The exper-imental susceptibilities were corrected for diamagnetism of Pascal'sconstants. Ab Initio Computations. Complete active space self-consistentfield (CASSCF) calculations on complexes 2 and 3 (see Figure S6 forthe calculated model structures of complexes 2 and 3) based on single-crystal X-ray determined geometry have been carried out with theMOLCAS 8.2 program package." The basis sets for all atoms useatomic natural orbitals from the MOLCAS ANO-RCC library: ANO-RCC-VTZP for the Dy(III) ion, VTZ for close O and N, and VDZ fordistant atoms. The calculations employed the second-order Douglas-Kroll-Hess Hamiltonian, where scalar relativistic contractions weretaken into account in the basis set and the spin-orbit couplings werehandled separately in the restricted active space state interaction(RASSI-SO) procedure. For the fragment of an individual Dy(III) ion,active electrons in the 7 active spaces include all f electrons (CAS(9 in7)) in the CASSCF calculation. To exclude all doubts, we calculated allthe roots in the active space. We have mixed the maximum number ofspin-free states that was possible with our hardware (all from 21sextets, 128 from 224 quadruplets, and 130 from 490 doublets). ASSOCIATED CONTENT ⑤Supporting Information The Supporting Information is available free of charge on theACS PublicationswebsiteeatDOI::10.1021/acs.inorg-chem.8b00219. Additional crystal structures and data, FT-IR spectros-copy and powder X-ray diffraction analyses, dc and acmagnetic susceptibility data, spin density distributions,photoluminescence spectra and lifetimes, and TD-DFTcomputational results (PDF) Accession Codes CCDC 1816184-1816186 contain the supplementary crystal-lographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by email-ing data_request@ccdc.cam.ac.uk, or by contacting TheCambridge Crystallographic Data Centre, 12 Union Road,Cambridge CB2 1EZ, UK; fax: +44 1223 336033. AUTHOR INFORMATION Corresponding Authors *E-mail: kouhz@mail.tsinghua.edu.cn. *E-mail: zhangyiquan@njnu.edu.cn. ORCID O Yi-Quan Zhang: 0000-0003-1818-0612 Cai-Ming Liu: 0000-0001-7184-6693 Hui-Zhong Kou: 0000-0002-7510-052X Notes The authors declare no competing financial interest. ACKNOWLEDGMENTS ThisworkwasSsupportedby thee 9733program(2013CB933403), the National Natural Science Foundationof China (21571113, 21771115, and 11774178), and theNatural Science Foundation of the Jiangsu Higher EducationInstitutions of China (16KJB430020). REFERENCES (1) (a) Liu,J.; Chen, Y.-C.; Liu, J.-L.; Vieru, V.; Ungur, L.; Jia, J.-H.;Chibotaru, L. F.; Lan, Y.; Wernsdorfer, W.; Gao, S.; Chen, X.-M.;Tong, M.L. A Stable Pentagonal Bipyramidal Dy(III) Single-IonMagnet with a Record Magnetization Reversal Barrier over 1000 K. J.Am. Chem. Soc. 2016, 138, S441-5450. (b) Xiong, J.; Ding, H.-Y.;Meng, Y.-S.; Gao, C.; Zhang, X.J.; Meng,Z.-S.; Zhang, Y.-Q.; Shi, W.;Wang, B.-W.; Gao, S. Hydroxide-bridged five-coordinate Dysingle-molecule magnet exhibiting the record thermal relaxation barrier ofmagnetization among lanthanide-only dimers. Chem. Sci. 2017, 8,1288-1294.(c) Blagg, R. J.; Ungur, L.; Tuna, F.; Speak, J.; Comar, P.;Collison, D.; Wernsdorfer, W.; McInnes, E. J. L.; Chibotaru, L. F.;Winpenny, R. E. P. Magnetic relaxation pathways in lanthanide single-molecule magnets. Nat. Chem. 2013, S,673-678.(d) Pugh, T.; Vieru,V.; Chibotaru, L. F.; Layfield, R. A. Magneto-structural correlations inarsenic- and selenium-ligated dysprosium single-molecule magnets.Chem. Sci. 2016, 7, 2128-2137.(e) Lin, P.-H.; Burchell, T. J.; Ungur,L.; Chibotaru, L. F.; Wernsdorfer, W.; Murugesu, M. A PolynuclearLanthanide Single-Molecule Magnet with a Record AnisotropicBarrier. Angew. Chem., Int. Ed. 2009, 48, 9489-9492. (f) Milios, C.J.; Vinslava, A.; Wernsdorfer, W.; Moggach, S.; Parsons, S.; Perlepes, S.P.; Christou, G.; Brechin, E. K. A Record Anisotropy Barrier for aSingle-Molecule Magnet. J. Am. Chem. Soc.2007,129, 2754-2755.(g) Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-Y.; Kaizu, Y.Lanthanide Double-Decker Complexes Functioning as Magnets at theSingle-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694-8695.(h) Guo, Y.-N.; Xu, G.-F.; Guo, Y.; Tang, J. Relaxation dynamics ofdysprosium(III) single molecule magnets. Dalton Trans. 2011, 40,9953-9963. (i) Ungur, L.; Lin, S.-Y.; Tang,J.; Chibotaru, L. F. Single-molecule toroics in Ising-type lanthanide molecular clusters. Chem. Soc.Rev. 2014,43,6894-6905. (j) Zhang, P.; Guo, Y.-N.; Tang, J. Recentadvances in dysprosium-based single molecule magnets: Structuraloverview and synthetic strategies. Coord. Chem. Rev. 2013, 2S7, 1728-1763. (k) Zhang, P.; Zhang, L.; Tang, J. Lanthanide single moleculemagnets: progress and perspective. Dalton Trans. 2015, 44, 3923-3929.(1) Lu,J.; Guo, M.; Tang, J. Recent Developments in LanthanideSingle-Molecule Magnets. Chem. - Asian J. 2017, 12, 2772-2779.(m) Zhu, Z.; Guo, M.; Li, X.-L.; Tang, J. Molecular magnetism oflanthanide: Advances and perspectives. Coord. Chem. Rev. 2017,DOI: 10.1016/j.ccr.2017.10.030. (n) Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev.2018, DOI: 10.1039/C7CS00266A. (2) (a) Guo, F.-S.; Day, B. M.; Chen, Y.-C.; Tong, M.-L.;Mansikkamaki, A.; Layfield, R. A. A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit. Angew. Chem., Int.Ed. 2017, 56, 11445. (b) Goodwin, C. A. P.; Ortu, F.; Reta, D.;Chilton, N. F.; Mills, D. P. Molecular magnetic hysteresis at 60 K indysprosocenium. Nature 2017, S48, 439-442. (3) (a) Gupta, S. K.; Rajeshkumar, T.; Rajaraman, G.; Murugavel, R.An air-stable Dy(III) single-ion magnet with high anisotropy barrierand blocking temperature. Chem. Sci. 2016, 7, 5181-5191. (b) Chen,Y.-C.; Liu, J.-L.; Lan, Y. H.; Zhong, Z.-Q.; Mansikkamaki, A.; Ungur,L.; Li, Q.-W.; Jia, J.-H.; Chibotaru, L. F.; Han, J.-B.; Wernsdorfer, S.;Chen,X.-M.; Tong, M.-L. Dynamic Magnetic and Optical Insight intoa High Performance Pentagonal Bipyramidal Dy Single-Ion Magnet.Chem.- Eur. J. 2017, 23, 5708-5715.(c) Liu,J.-L.; Wu, J.-Y.; Chen, Y.-C.; Mereacre, V.; Powell, A. K;Ungur, L.; Chibotaru, L. F.; Chen, X.-M.; Tong, M.-L. A Heterometallic Fe"-Dy" Single-Molecule Magnetwith a Record Anisotropy Barrier. Angew. Chem., Int. Ed. 2014, S3,12966-12970. (d) Chen, Y.-C.; Liu, J.-L.; Ungur, L.; Liu, J.; Li, Q.-W.;Wang, L.-F.; Ni, Z.-P.; Chibotaru, L. F.; Chen, X.-M.; Tong, M.-L.Symmetry-Supported Magnetic Blocking at 20 K in PentagonalBipyramidal Dy(III) Single-Ion Magnets. J. Am. Chem. Soc. 2016,138,2829-2837. (e) Ding, Y.-S.; Chilton, N. F.; Winpenny, R. E. P.;Zheng, Y.-Z. On Approaching the Limit of Molecular MagneticAnisotropy: A Near-Perfect Pentagonal Bipyramidal Dysprosium(III)Single-Molecule Magnet. Angew. Chem., Int. Ed. 2016, 55, 16071-16074. (4) (a) Blagg, R. J.; Muryn, C. A.; McInnes, E. J. L.; Tuna, F.;Winpenny, R. E. P. Single Pyramid Magnets: Dys Pyramids with SlowMagnetic Relaxation to 40 K. Angew. Chem, Int. Ed. 2011, SO, 6530-6533. (b) Li, J.; Yuan, C.; Yang, L.; Kong, M.; Zhang, J.; Ge, J.-Y.;Zhang, Y.-Q.; Song, Y. Magnetic Anisotropy along a Series ofLanthanide Polyoxometalates with Pentagonal Bipyramidal Symmetry.Inorg. Chem. 2017, S6, 7835-7841. ( (S) (a) S u n, L . ; Z h ang, S. ; Jiang, Z . ; Yang, Q; Che n , S.; Zhang, Y.;Wang, W.; W e i, Q . ; X i e, G . I n terchange b e tween co o rdinated an d l attice solvents g enerates t he h i ghest e n ergy bar r ier wi t hin nin e - c oordinated D y" si n gle m o lecule ma g nets. Da l ton Tra n s. 201 7 , 46,11159-11165.(b) Bis w as, S.; Bej o ymohandas, K. S. ; D a s, S.; Kali t a,P.; Reddy, M . L . P.; O yarzabal, I ; C olacio, E.; Chandrasekhar, V . Mononuclear Lanthanide Complexes: E nergy-Barrier Enhancement by Ligand Substitution in Field-Induced Dy SIMs. Inorg. Chem. 2017,56, 7985-7997. (c) Feng, X. ; Hw a ng, S. J .; Li u , J.-L.; C hen, Y . -C.;Tong, M.-L.; Nocera, D . G . S low Magnetic Relaxation in IntermediateSpin S=3/2 Mononuclear Fe(III) Complexes. J. Am. Chem. Soc. 2017, 139,16474-16477. ) (6) (a) Ding, Z.-Y.; Meng, Y.-S.; Xiao, Y.; Zhang, Y.-Q.; Zhu, Y.-Y.;Gao,S. Probing the influence of molecular symmetry on the magneticanisotropy of octahedral cobalt(II) complexes. Inorg. Chem. Front.2017,4,1909-1916.(b) Chen, G.J.; Guo, Y.-N.; Tian,J.-L.; Tang,J;Gu, W.; Liu, X.; Yan, S.-P.; Cheng, P.; Liao, D.-Z. EnhancingAnisotropy Barriers of Dysprosium(III) Single-Ion Magnets. Chem.-Eur. J. 2012, 18,2484-2487. (7) Lyu, D.-P.; Zheng, J.-Y.; Li, Q.-W.;Liu, J.-L.; Chen, Y.-C.; Jia, J.-H.; Tong, M.-L. Construction of lanthanide single-molecule magnetswith the “magnetic motif[Dy(MQ)4]. Inorg. Chem. Front.2017, 4,1776-1782. (8) Ma, F.; Chen, Q.; Xiong,J.; Sun, H.-L.; Zhang, Y.-Q.; Gao, S.Modulating Slow Magnetic Relaxation of Dysprosium Compoundsthrough the Position of Coordinating Nitrate Group. Inorg. Chem.2017, S6, 13430-13436. ( (9) Huang, W.; Sh e n, F.-X.; Wu , S.- Q .; Liu , L.; Wu, D.; Zheng, Z.; X u,J.; Zhang, M.; Hu a ng, X.-C.; Jia n g,J; Pan, F.-F.; Li, Y.; Zhu , K.;Sato, O. M etallogrid S ingle-Molecule M agnet: S olvent-InducedNuclearity T ransformation a n d Magnetic Hy s teresis at 16 K. Inorg.Chem. 2016, S5, 5476-5484. ) ( (10) ( a) D ujols, V .; F ord, F .; Czarnik, A. W. A L o ng-Wavelength Fluorescent Chemodosimeter Selective for Cu(II) Ion in Water. J. Am.Chem. S oc. 1997, 119, 7386-7387. ( b) Xiang, Y.; Tong, A. A New ) Rhodamine-Based Chemosensor Exhibiting Selective Fe-AmplifiedFluorescence. Org. Lett. 2006, 8, 1549-1552. (11) (a) Zhao, Y.; Zhang, X.-B.; Han, Z.-X.; Qiao, L.; Li, C.-Y.; Jian,L.-X.; Shen, G.-L.; Yu, R.-Q. Highly Sensitive and SelectiveColorimetric and Off-On Fluorescent Chemosensor for Cu+ inAqueous Solution and Living Cells. Anal. Chem.2009, 81,7022-7030.(b) Huang, W.; Zhu, X.; Wua, D.; He, C.; Hu, X.; Duan, C. Structuralmodification of rhodamine-based sensors toward highly selectivemercury detection in mixed organic/aqueous media. Dalton Trans.2009, 10457-10465. (c) Li, K.; Xiang, Y.; Wang, X.; Li, J.; Hu, R.;Tong, A.; Tang, B. Z. Reversible Photochromic System Based onRhodamine B Salicylaldehyde Hydrazone Metal Complex. J. Am.Chem. Soc. 2014, 136, 1643-1649.(d) Liu, C.; Jiao, X.; Wang, Q;Huang, K.; He, S.; Zhao, L.; Zeng, X. A unique rectilinearly n-extendedrhodamine dye with large Stokes shift and near-infrared fluorescencefor bioimaging, A unique rectilinearly n-extended rhodamine dye withlarge Stokes shift and near-infrared fluorescence for bioimaging. Chem.Commun. 2017, 53, 10727-10730. (e) Huang, W.; Wu, D.; Guo, D.;Zhu, X.; He, C.; Meng, Q.; Duan, C. Efficient near-infrared emission ofa Ytterbium(III) compound with a green light rhodamine donor.Dalton Trans. 2009, 2081-2084. (12) Huang, W.; Xu, J.; Wu, D.; Huang, X.; Jiang, J. Rhodamine-based field-induced single molecule magnets in Yb(III) and Dy(III)series. New J. Chem. 2015, 39, 8650-8657. ( (13) M orimoto, M .; M iyasaka, H.; Y amashita, M.; Irie, M.Coordination A ssemblies o f [Mn4] Single-Molecule Magnets L i nkedby Photochromic L igands: P hotochemical C ontrol o f the Magnetic P roperties. J . Am. Chem. Soc. 2009, 131,9823-9835. ) ( ( 14) ( a) C hen, L .; Zhou, J.J; Y uan, A.; Song, Y. Slow magneticrelaxation in luminescent mononuclear dysprosium(III) a n d erbium-(III) pentanitrat e complexes with the same LnO1o coordinationgeometry. D alton Trans. 2017, 46 , 15812-15818.(b) Cui, H.; Otsuka,T.; Kobayashi, A .; Takeda, N .; I shikawa, M.; M isaki, Y.; Kobayashi, H.Structural, Electrical, and Magnetic Properties of a Series of Mo l ecularConductors Based on BDT-TTP a n d L a nthanoid Nitrate ComplexAnions ( BDT-TTP= 2, S -B i s(1,3-dithiol-2-ylidene)-1,3,4,6-tetrathia-pentalene. Inorg. Chem. 2003, 42, 6 114-6122. ) ( (1 5 ) Llunell, M . ; Casanova, D.; Cirera, J.; Ale m any, P.; Alvar e z, S. SHAPE, v e rsion 2 . 1; University of B arcelona: Barcelona, Spain, 2013. (16) Wu, J.; Cador, O.; Li, X . -L.; Z hao, L . ; Le G u ennic, B. ; Ta n g,JAxial Ligand Field in D4a C o ordination Symmetry: Ma g netic Relaxation of Dy SMMs Perturbed b y C o unteranions. I n org. C hem. 2 017, S6, 11211-1121 9 .(b) Li, M .; Wu, H.; Yang, Q.;Ke, H.; Yin, B .; Shi, Q; W ang, W . ; W e i, Q ; Xi e , G. ; Ch e n, S. Ex p erimental an d Theoretical Interpretation on the Magnetic B ehavior i n a S eries ofPentagonal-Bipyramidal Dy" Single-Ion Magnets. Chem.-Eur. J. 2017, 23, 1 7775-17787. ) ( (17) Liu, S.-S.; Meng, Y.-S.; Zhang, Y.-Q.; Meng, Z.-S.; Lang, K; Z hu,Z.-L . ; Shang, C.-F.; Wang, B .-W.; G ao, S . A Six-CoordinateDysprosium S i ngle-Ion Magnet wi t h Tr i gonal-Prismatic Ge o metry. Inorg. Chem. 2017, S6, 7320-7323. ) ( ( 18) W u, J.-S.; Hwang, I .-C. ; Kim, K. S.; Kim, J. S . Rhodamine-Based Hgt-Selective C hemodosimeter in Aqueous Sol u tion: Fl u orescent OFF-ON. Org. Lett.2007, 9,907-910. ) ( ( 19) Wang, C.-F.;Li, R.-F.; Chen, X.-Y.; Wei, R.J.;Zheng, L.-S.; Tao,J. Synergetic Spin Crossover a nd Fluorescence i n O n e-Dimensional H ybrid C omplexes. Angew. C hem., Int. Ed . 2015 , S4, 1574-1577. ) ( (20) Birks, J. B . Photophysics of Aromatic M olecules; Wiley- Interscience: London, 1 970. ) ( (21) (a) G uo, Y . -N.; Chen, X . -H.; X u e, S . ; Tang, J. Modulating Magnetic D ynamics o f Three D yz Complexes t h rough K e to-Enol Tautomerism of th e o- V anillin Pi c olinoylhydrazone Lig a nd. Inorg.Chem. 2011, 50, 9705-9713. (b) Z o u, L.; Zhao, L.; Chen, P.; Guo, Y .-N.; Guo, Y .; L i, Y.-H.; Tang, J. Phenoxido a nd a lkoxido-bridgeddinuclear dysprosium c omplexes s h owing sin g le-molecule ma g netbehaviour. D alton T rans. 2012, 4 1, 2 966-2971. (c) Moreno Pin e da,E.; Chilton , N. F .; Marx, R.; D orfel, M .; Sells, D. O.; Neugebauer, P.;Jiang, S.-D. ; Collison, D.; van S lageren, J; M cInnes, E . J. L.;Winpenny, R . E . P . Direct measurement of d ys p rosium(III)….. ) dysprosium(III) interactions in a single-molecule magnet. Nat.Commun. 2014, S, 5243. (d) Lin, P.-H.; Burchell, T.J.; Clérac, R.;Murugesu, M. Dinuclear Dysprosium(III) Single-Molecule Magnetswith a Large Anisotropic Barrier. Angew. Chem., Int. Ed. 2008, 47,8848-8851. (e) Chen, Q; Li, J.; Meng, Y.-S.; Sun, H.-L.; Zhang, Y.-Q; Sun, J.-L.; Gao, S. Tuning Slow Magnetic Relaxation in a Two-Dimensional Dysprosium Layer Compound through Guest Molecules.Inorg. Chem. 2016, SS, 7980-7987. (22) (a) Bi, Y.; Guo, Y.-N.; Zhao, L.; Guo, Y.; Lin, S.-Y.; Jiang, S.-D.;Tang, J.-K.; Wang, B.-W.; Gao, S. Capping Ligand Perturbed SlowMagnetic Relaxation in Dysprosium Single-Ion Magnets. Chem.-Eur.J. 2011, 17, 12476-12481. (b)Wu,J; Jung, J.; Zhang, P.; Zhang, H.;Tang, J; Le Guennic, B. Cis-trans isomerism modulates the magneticrelaxation of dysprosium single-molecule magnets. Chem. Sci. 2016, 7,3632-3639. (c) Tuna, F.; Smith, C. A.; Bodensteiner, M.; Ungur, L.;Chibotaru, L. F.; McInnes, E. J.L.; Winpenny, R. E. P.; Collison, D.;Layfield, R. A. A High Anisotropy Barrier in a Sulfur-BridgedOrganodysprosium Single-Molecule Magnet. Angew. Chem., Int. Ed.2012,51,6976-6980. (23) Lucaccini, E.; Briganti, M.; Perfetti, M.; Vendier, L.; Costes, J.-P.; Totti, F.; Sessoli, R.; Sorace, L. Relaxation Dynamics and MagneticAnisotropy in a Low-Symmetry Dy"Complex. Chem.-Eur. J. 2016,22,5552-5562. (24) Chen, Q; Meng, Y.-S.; Zhang, Y.-Q.; Jiang, S.-D.; Sun, H.-L.;Gao, S. A 1D dysprosium chain with slow magnetic relaxationconstructed from a pyridine-N-oxide ligand. Chem. Commun. 2014, SO,10434-10437. ( (25)( a ) Jiang, S.-D.; Wang, B.-W.; Su, G.; Wang, Z.-M.; Gao, S. A Mononuclear D y sprosium Co m plex Feat u ring Single-Molecule-Mag-net Behavior. Angew. Chem., Int. Ed. 2010, 49, 7448-7451. ( b) Feng,X.; Mathoniere, C.; Jeon, I .-R.; Rouzieres, M.; Ozarowski, A.; Aubrey, M. L.; Gonzalez, M. I.; Cl e rac, R.; Long, J. R. T ri s tability in a Lig h t-Actuated Single-Molecule M agnet. J . Am. C hem. S o c. 2 0 13, 13S, 1 5880-15884.(c) Liu,T.; Z heng, H.; Kang , S.; Shio t a, Y.; H ay a mi, S.; Mito, M .; S ato, O.; Y oshizawa, K . ; Kanegawa, S. ; Duan, C. A light- induced s pin c rossover actuated s ingle-chain magnet. N at. C ommun. 2013,4,2826-2833.(d) Selvanathan, P.; Hu a ng, G.; Guizouarn, T.; R oisnel, T .; Fernandez-Garcia, G.; Totti, F .; Le Guennic, B . ; C a lvez,G.; B ernot, K.; Norel, L.; Rigaut, S. Highly Axial Magnetic Anisotropyin a N,0s Dysprosium(III) Coordination Environment Generated bya Merocyanine L igand. Chem.-E u r. J. 2016, 2 2,15222-15226. ) (26) Xiang, Y.; Tong, A.; Jin, P.;Ju, Y. New Fluorescent RhodamineHydrazone Chemosensor for Cu(II) with High Selectivity andSensitivity. Org. Lett. 2006, 8, 2863-2866. (27) Karlstrom, G.; Lindh, R.; Malmqvist, P.-A.; Roos, B. O.; Ryde,U.; Veryazov, V.; Widmark, P.-O.; Cossi, M.; Schimmelpfennig, B.;Neogrady, P.; Seijo, L. MOLCAS: a program package for computa-tional chemistry. Comput. Mater. Sci. 2003, 28,222-239. CS Publications @ American Chemical SocietyDOl: acs.inorgchem.bnorg. Chem. norg. Chem. Three new dysprosium(III) complexes [Dy 2 (HL 1 -o) 2 (L 1 )-(NO 3 ) 3 ][Dy(NO 3 ) 5 ]·1.5ACE·0.5Et 2 O (1), [Dy(L 1 ) 3 ]·2.5MeOH·MeCN(2), and [Dy(L 2 ) 3 ]·MeOH·MeCN (3) (HL 1 = rhodamine Bsalicylaldehyde hydrazine, HL 2 = rhodamine B 3-methylsalicylaldehydehydrazine) were synthesized and characterized. Purple complex 1contains two ring-open ligands HL 1 -o and shows fluorescence of therhodamine amide moiety, whereas yellow complexes 2 and 3 arecomprised of ring-close ligands (L 1/2 ) − and display fluorescence of thesalicylaldehyde Schiff base part. For 2 and 3, Dy(III) ions are ninecoordinated by the six oxygen and three nitrogen atoms of three chelate(L 1/2 ) − ligands, but the arrangements of the three ligands are differentowing to the methyl substituent on HL 2 . There are three shortpredominant Dy−O phenoxy bonds in 2 and 3. The largest O phenoxy −Dy−O phenoxy angle is 148.64(17)° for 2 and 89.63(13)° for 3. Magnetic studiesreveal that complex 2 is a field-induced single-molecule magnet (U eff = 104.2 K under a dc magnetic field of 2000 Oe), and 3exhibits only a magnetic relaxation behavior owing to the quantum tunneling of magnetization (QTM). Furthermore, ab initio
确定






还剩7页未读,是否继续阅读?
上海棱光技术有限公司为您提供《Rhodamine Salicylaldehyde Hydrazone Dy(III) Complexes: Fluorescence and Magnetism》,该方案主要用于畜禽肉及副产品中Fluorescence and Magnetism检测,参考标准--,《Rhodamine Salicylaldehyde Hydrazone Dy(III) Complexes: Fluorescence and Magnetism》用到的仪器有棱光技术F98荧光分光光度计(2017)
推荐专场
相关方案
更多