In analytical HPLC good resolution is required over the entire gradient; in preparative HPLC it is only important to achieve good resolution around the target compound, which should be collected in highest purity. With a small set of optimized gradient methods a much better resolution can be achieved than with a single generic method. The generation of a small method set based on retention time windows in the pre-preparative analytical run is described in this Application Note. Using Agilent standards with known elution properties makes this an easy four-step process, which can be accomplished in less than one hour. An application example demonstrating how the resolution of the target compound can be improved is also shown.
方案详情

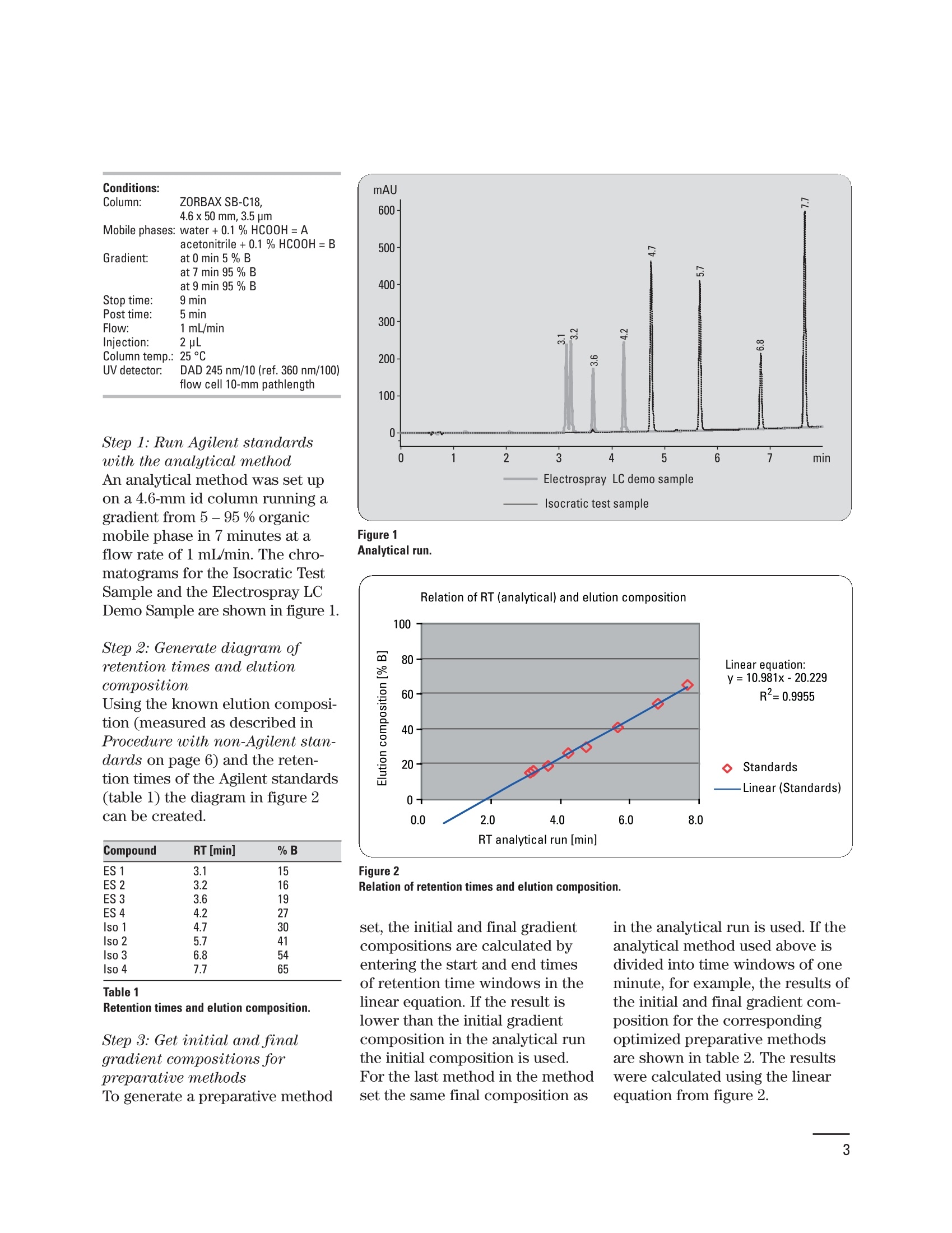
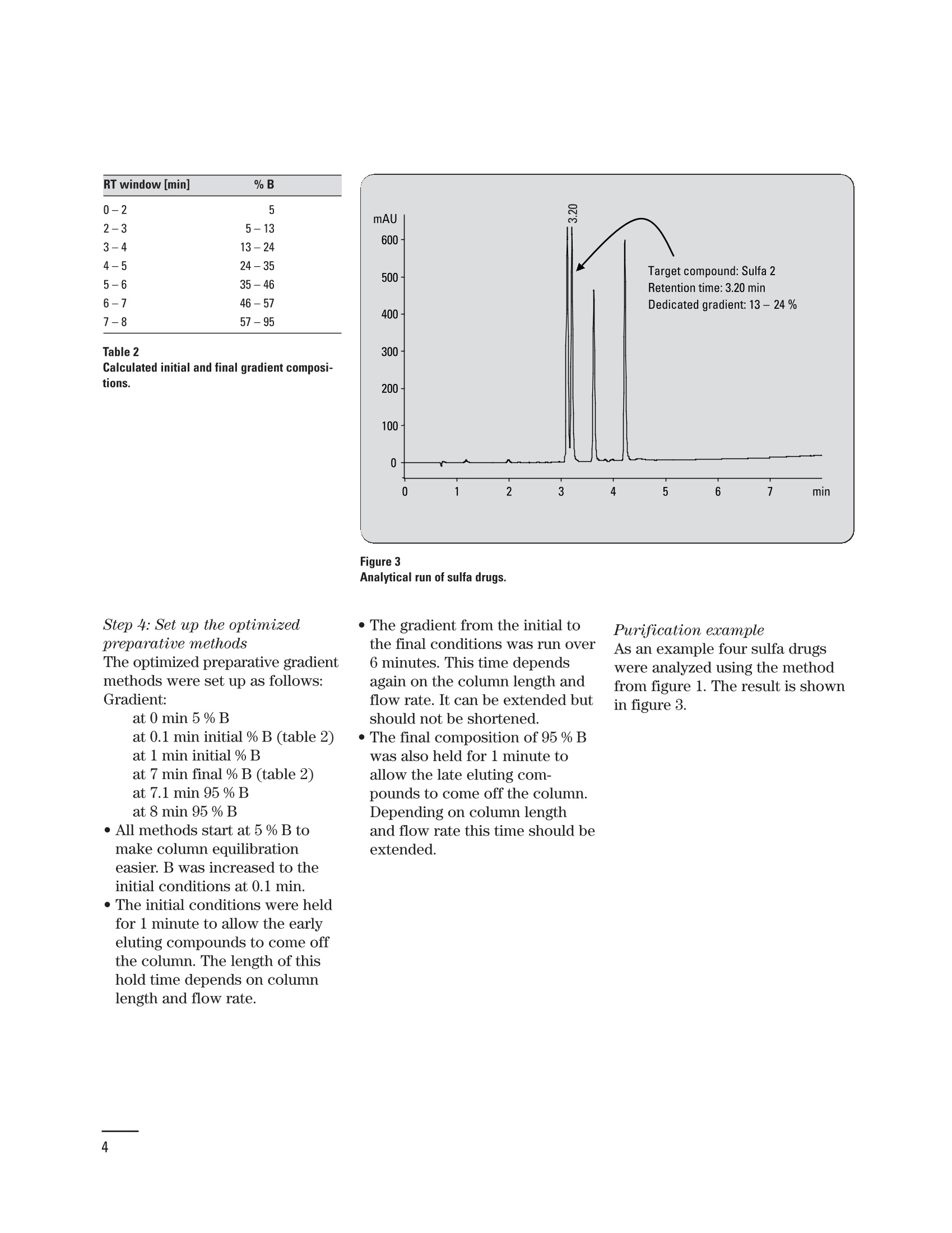
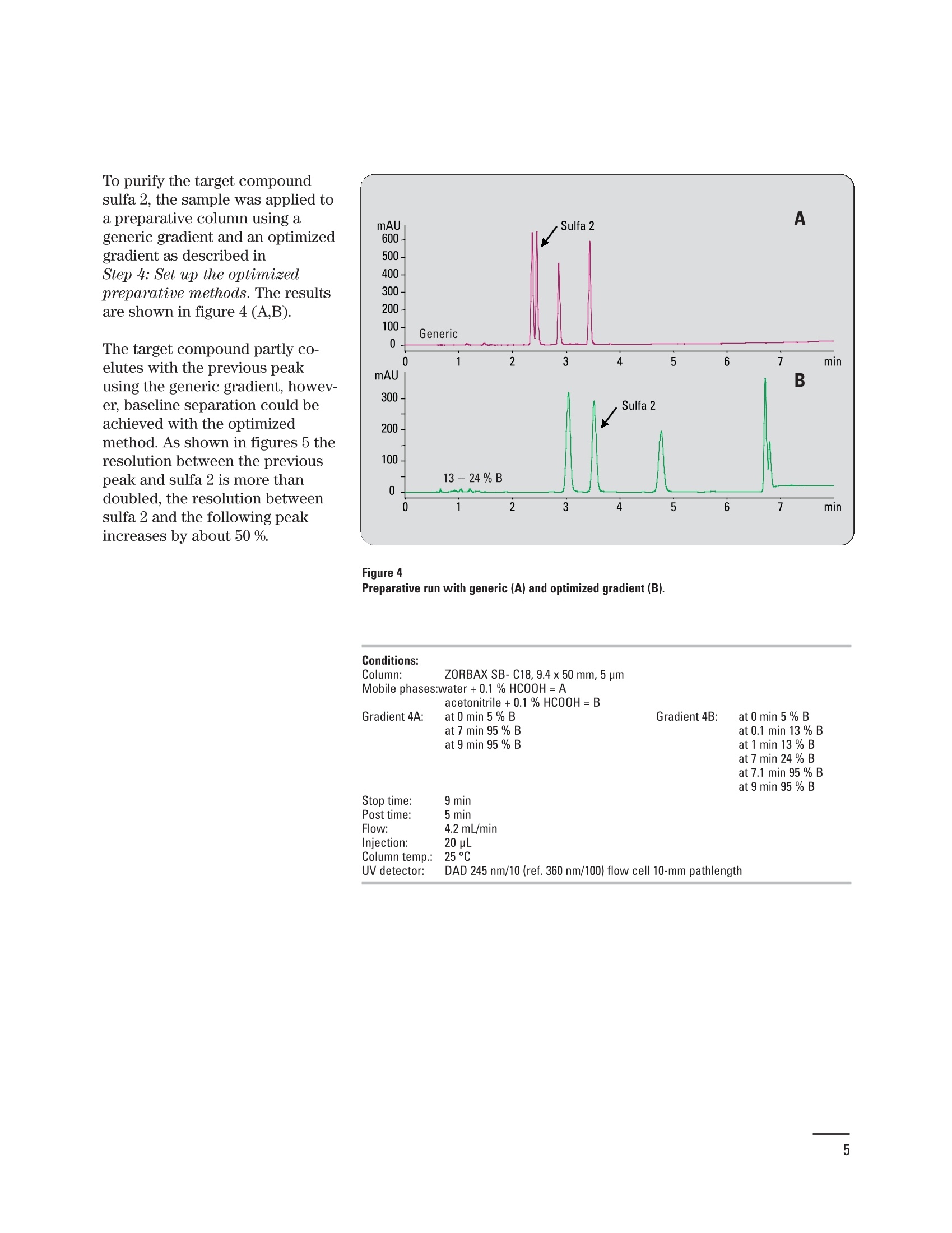
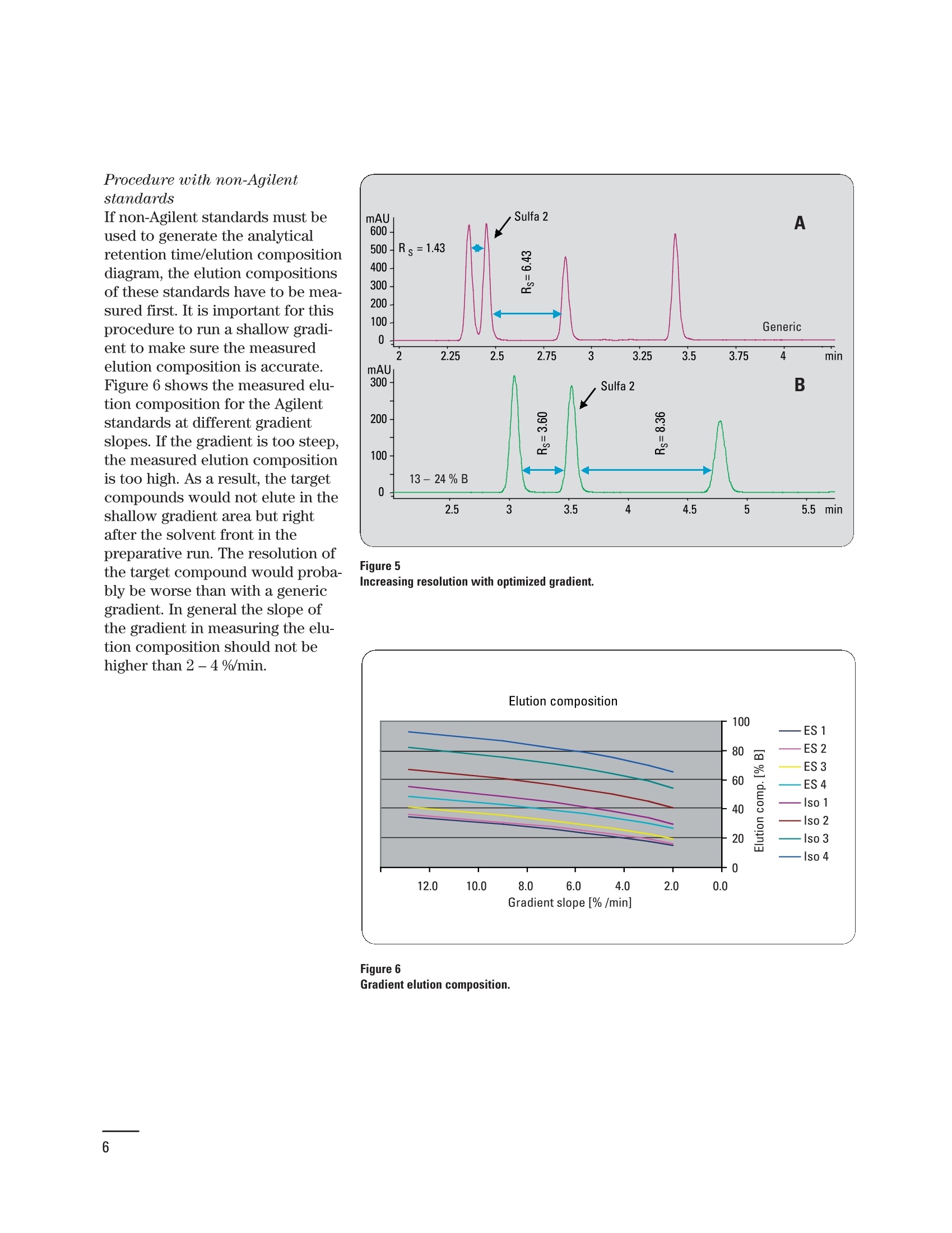
Creating an optimized preparative method setbased on a pre-preparative analytical run Application Note Udo Huber Abstract In analytical HPLC good resolution is required over the entire gradient;in preparative HPLC it is only important to achieve good resolutionaround the target compound, which should be collected in highestpurity. With a small set of optimized gradient methods a much betterresolution can be achieved than with a single generic method. The gen-eration of a small method set based on retention time windows in thepre-preparative analytical run is described in this Application Note.Using Agilent standards with known elution properties makes this aneasy four-step process, which can be accomplished in less than onehour. An application example demonstrating how the resolution of thetarget compound can be improved is also shown. Agilent Technologies Baseline separation of peaks isrequired for accurate quantifica-tion of compounds in analyticalHPLC. Therefore, a resolution ofabout 1.5 is needed if the peakareas are not too dissimilar. Agood resolution is only requiredfor the target compound in prepar-ative HPLC, and that compoundis required with high purity. Theseparation of the remainder ofcompounds in the chromatogramis not of interest. Generic gradi-ents of 5 -95 % organic mobilephase are frequently used forpurification runs, which do notalways lead to sufficient resolu-tion for the target compound. Toincrease the resolution an opti-mized method with a shallowergradient around the elution com-position of the target compoundcould be used. Since the prepara-tive method cannot be optimizedfor each compound this isachieved in practice by using asmall set of methods. The prepara-tive method is selected accordingto a retention time window, inwhich the compound elutes in thepre-preparative analytical run.The process of generating an opti-mized preparative method set forretention time windows in the pre-preparative analytical run isdescribed in an article by Blom etal."In this Application Note theprocess described in the article issimplified by using Agilent stan-dards but it can also be performedwith any other standard com- Experimental Equipment The experiments were done on anAgilent 1200 Series purificationsystem containing the followingmodules: Agilent 1200 Series quaternarypump with degasser ●Agilent 1200 Series well-plateautosampler ●· Agilent 1200 Series thermostat-ed column compartment · Agilent 1200 Series diode arraydetector (flow cell: 10-mm pathll6ength) · Agilent 1200 Series fraction col-lector AS The system was controlled usingthe Agilent ChemStation (rev. B.02.01). To produce a method set forretention time windows, the reten-tion times of several standardcompounds were measured usingan analytical method. A diagramwas generated showing the rela-tion of retention time and true elu-tion composition for these stan-dards. The elution composition ofany compound can be extractedor calculated from this diagram byknowing its retention time in theanalytical run. The same approachcan be taken for the initial andfinal gradient composition forretention time windows. As theelution composition for eight Agi-lent standards (Isocratic Test Sam-ple 01080-68704, Electrospray LCDemo Sample 59987-20033) is pro-vided in this Application Note, thegeneration of a preparativemethod set involves of four easysteps: 1. Run the Isocratic Test Sampleand the Electrospray LC DemoSample with the analyticalmethod. 2. Generate a diagram with themeasured retention times andthe known gradient elutioncompositions. 3. Calculate or extract the initialand final gradient compositionfor the optimized preparativemethods from the graph. 4. Set up the dedicated gradientfor each preparative method. Conditions: Column: ZORBAX SB-C18, 4.6x 50mm, 3.5 um Mobile phases: water +0.1 % HCOOH=A acetonitrile+0.1% HCOOH=B Gradient: at 0 min 5 % B at 7 min 95% B at 9 min 95% B Stop time: 9 min Post time: 5 min Flow: 1 mL/min Injection: 2 pL Column temp.: 25℃ UV detector: DAD 245nm/10 (ref. 360 nm/100) flow cell 10-mm pathlength Step 1: Run Agilent standardswith the analytical methodAn analytical method was set upon a 4.6-mm id column running agradient from 5 - 95 % organicmobile phase in 7 minutes at aflow rate of 1 mL/min. The chro-matograms for the Isocratic TestSample and the Electrospray LCDemo Sample are shown in figure 1. Step 2: Generate diagram ofretention times and elutioncomposition Using the known elution composi-tion (measured as described inProcedure with non-Agilent stan-dards on page 6) and the reten-tion times of the Agilent standards(table 1) the diagram in figure 2can be created. Compound RT [min] % B ES 1 ES 2 ES3 30 ES 4 Iso 1 Iso 2 Iso 3 Iso 4 Table 1 Retention times and elution composition. Step 3: Get initial and finalgradient compositions forpreparative methodsTo generate a preparative method Figure 1 Analytical run. set, the initial and final gradientcompositions are calculated byentering the start and end timesof retention time windows in thelinear equation. If the result islower than the initial gradientcomposition in the analytical runthe initial composition is used.For the last method in the methodset the same final composition as in the analytical run is used. If theanalytical method used above isdivided into time windows of oneminute, for example, the results ofthe initial and final gradient com-position for the correspondingoptimized preparative methodsare shown in table 2. The resultswere calculated using the linearequation from figure 2. RT window [min] %B 0-2 2-3 5-13 3-4 13-24 4-5 24-35 5-6 35-46 6-7 46-57 7-8 57-95 Table 2Calculated initial and final gradient composi-tions. Figure 3 Step 4: Set up the optimizedpreparative methodsThe optimized preparative gradientmethods were set up as follows:Gradient: at 0 min 5 % Bat 0.1 min initial % B (table 2)at 1 min initial % Bat 7 min final % B (table 2)at 7.1 min 95 % Bat 8 min 95 % B · All methods start at 5 % B tomake column equilibrationeasier. B was increased to theinitial conditions at 0.1 min. · The initial conditions were heldfor 1 minute to allow the earlyeluting compounds to come offthe column. The length of thishold time depends on columnlength and flow rate. · The gradient from the initial tothe final conditions was run over6 minutes. This time dependsagain on the column length andflow rate. It can be extended butshould not be shortened. · The final composition of 95 % Bwas also held for 1 minute toallow the late eluting com-pounds to come off the column.Depending on column lengthand flow rate this time should beextended. Purification escampleAs an example four sulfa drugswere analyzed using the methodfrom figure 1. The result is shownin figure 3. To purify the target compoundsulfa 2, the sample was applied toa preparative column using ageneric gradient and an optimizedgradient as described inStep 4: Set up the optimizedpreparative methods. The resultsare shown in figure 4 (A,B). The target compound partly co-elutes with the previous peakusing the generic gradient, howev-er, baseline separation could beachieved with the optimizedmethod. As shown in figures 5 theresolution between the previouspeak and sulfa 2 is more thandoubled. the resolution betweensulfa 2 and the following peakincreases by about 50 %. Figure 4Preparative run with generic (A) and optimized gradient (B). Conditions: Column: ZORBAX SB-C18, 9.4x50 mm, 5 um Mobile phases:water +0.1% HCOOH=A Gradient 4A: acetonitrile+0.1% HCOOH= B at 0 min 5 % B Gradient 4B: at 0 min 5 % B at 7 min 95% B at 0.1 min 13 % B at 9 min 95 % B at 1 min 13%B at 7 min 24% B at 7.1 min 95 % B at 9 min 95% B Stop time: 9 min Post time: 5 min Flow: 4.2 mL/min Injection: 20 pL Column temp.: : 225°C UV detector: DAD 245 nm/10 (ref. 360 nm/100) flow cell 10-mm pathlength Procedure with non-Agilent standardsIf non-Agilent standards must beused to generate the analyticalretention time/elution compositiondiagram, the elution compositionsof these standards have to be mea-sured first. It is important for thisprocedure to run a shallow gradi-ent to make sure the measuredelution composition is accurate.Figure 6 shows the measured elu-tion composition for the Agilentstandards at different gradientslopes. If the gradient is too steep,the measured elution compositionis too high. As a result, the targetcompounds would not elute in theshallow gradient area but rightafter the solvent front in thepreparative run. The resolution ofthe target compound would proba-bly be worse than with a genericgradient. In general the slope ofthe gradient in measuring the elu-tion composition should not behigher than 2-4 %/min. Figure 5Increasing resolution with optimized gradient. Figure 6 Gradient elution composition. Conclusion References In this Application Note the gener-ation of a small set of optimizedpreparative methods based onretention time windows in thepre-preparative run was explained.By using the Isocratic Test Sampleand the Electrospray LC Demo 2.Sample the complete process wasdone in four easy steps taking lessthan one hour to accomplish. Theimproved resolution of or near thetarget compound using optimizedgradients was presented in anapplication example. 1.“Principles in preparative HPLC”,Agilent Technologies primer,publication number 5989-0652EN, 2004. ( Karl F. Blom, Brian Glass, Richard Sparks, and Andrew P. Combs, J.Comb. C hem., 6,874-883, 2004. ) Udo Huber is Senior ApplicationChemist at Agilent Technologies,Waldbronn, Germany. www.agilent.com/chem/1200 Copyright C 2010 Agilent TechnologiesAll Rights Reserved. Reproduction, adaptationor translation without prior written permissionis prohibited except as allowed under thecopyright laws. Published June 15, 2010Publication Number 5989-4844EN Agilent Technologies Abstract In analytical HPLC good resolution is required over the entire gradient; in preparative HPLC it is only important to achieve good resolution around the target compound, which should be collected in highest purity. With a small set of optimized gradient methods a much better resolution can be achieved than with a single generic method. The generation of a small method set based on retention time windows in the pre-preparative analytical run is described in this Application Note. Using Agilent standards with known elution properties makes this an easy four-step process, which can be accomplished in less than one hour. An application example demonstrating how the resolution of the target compound can be improved is also shown.Introduction Baseline separation of peaks is required for accurate quantification of compounds in analytical HPLC. Therefore, a resolution of about 1.5 is needed if the peak areas are not too dissimilar. A good resolution is only required for the target compound in preparative HPLC1, and that compound is required with high purity. The separation of the remainder of compounds in the chromatogram is not of interest. Generic gradients of 5 – 95 % organic mobile phase are frequently used for purification runs, which do not always lead to sufficient resolution for the target compound. To increase the resolution an optimized method with a shallower gradient around the elution composition of the target compound could be used. Since the preparative method cannot be optimized for each compound this is achieved in practice by using a small set of methods. The preparative method is selected according to a retention time window, in which the compound elutes in the pre-preparative analytical run. The process of generating an optimized preparative method set for retention time windows in the prepreparative analytical run is described in an article by Blom et al. In this Application Note the process described in the article is simplified by using Agilent standards but it can also be performed with any other standard compounds.Conclusion In this Application Note the generation of a small set of optimized preparative methods based on retention time windows in the pre-preparative run was explained. By using the Isocratic Test Sample and the Electrospray LC Demo Sample the complete process was done in four easy steps taking less than one hour to accomplish. The improved resolution of or near the target compound using optimized gradients was presented in an application example.
确定



还剩6页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《混合标样中磺胺检测方案(制备液相色谱)》,该方案主要用于化药制剂中含量测定检测,参考标准--,《混合标样中磺胺检测方案(制备液相色谱)》用到的仪器有Agilent 1260 Infinity II 制备型液相色谱、Agilent 1290 Infinity II Multisampler、OpenLAB 软件
推荐专场
相关方案
更多
该厂商其他方案
更多