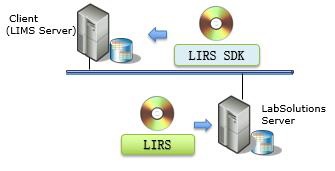
方案详情
文
Synthesizing novel compounds or isolating natural products can be a laborious and time-consuming process. After analyzing the precious sample on an analytical UHPLC system, the crucial step is to transfer the method to a preparative system with a minimal risk of losing valuable work or collecting impure compounds.
This Technical Overview describes a practical way to optimize the scale-up process for reversed-phase chromatography from an analytical UHPLC system to preparative LC systems using a focused gradient to increase the sample load on the preparative column to achieve optimum purity.
方案详情

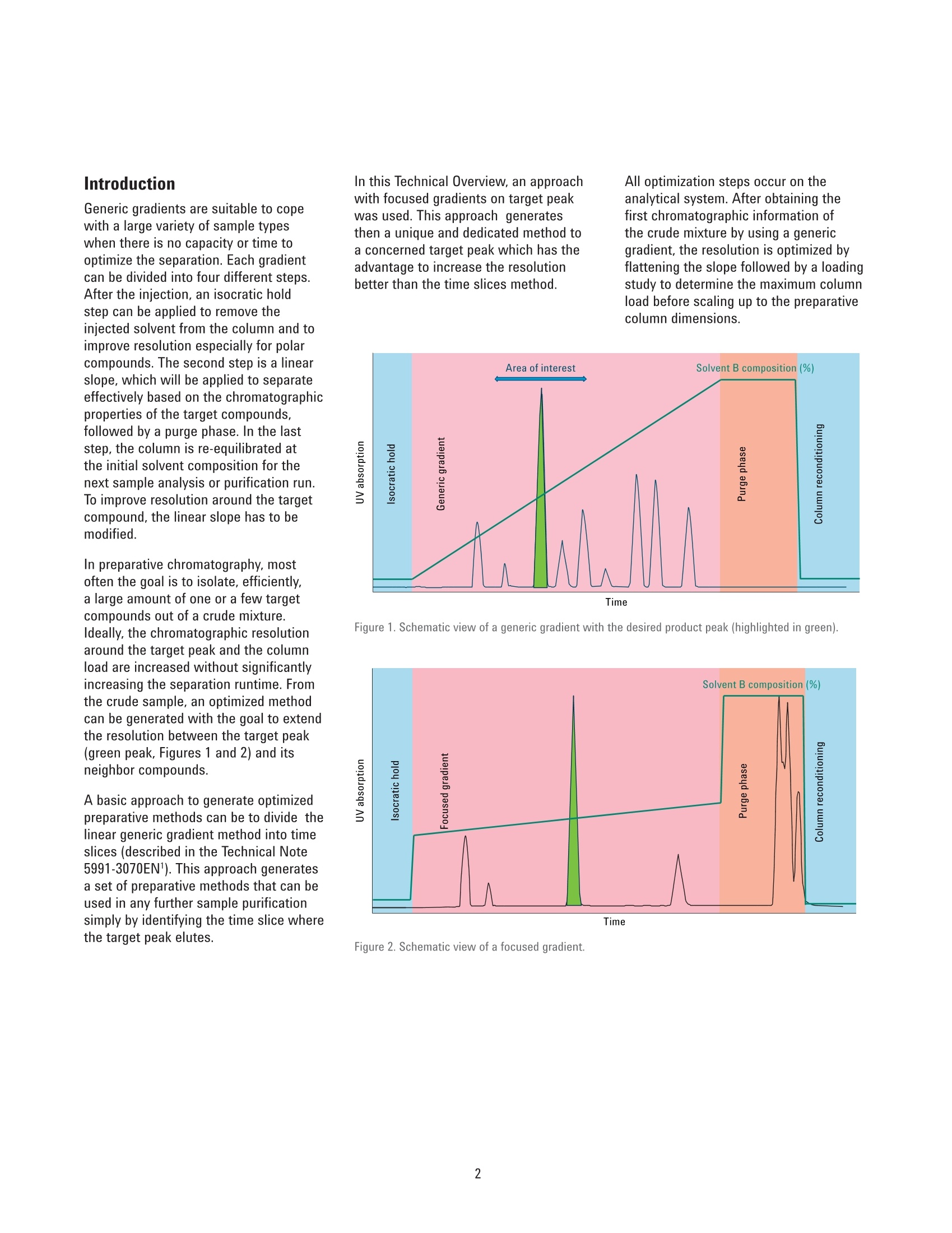
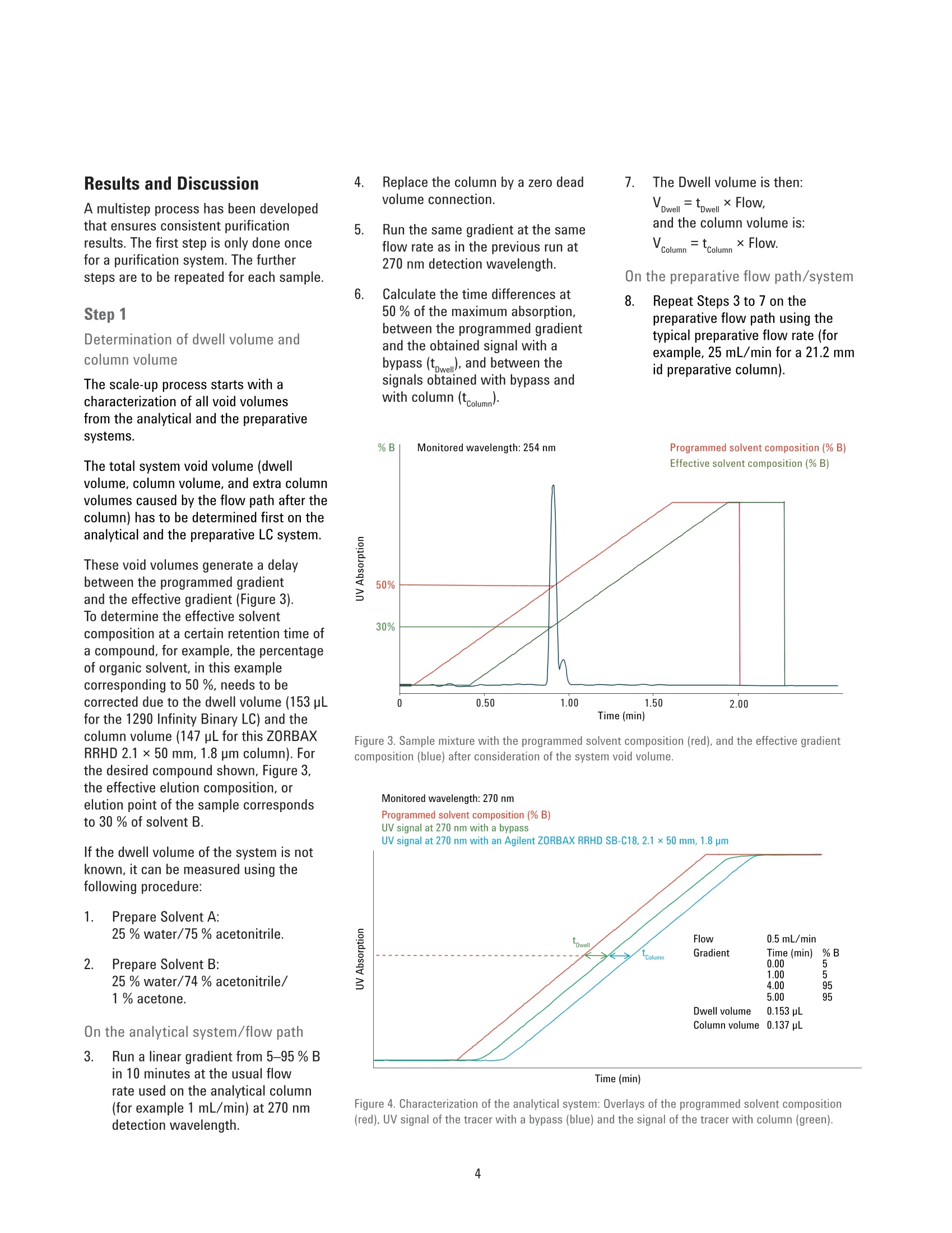
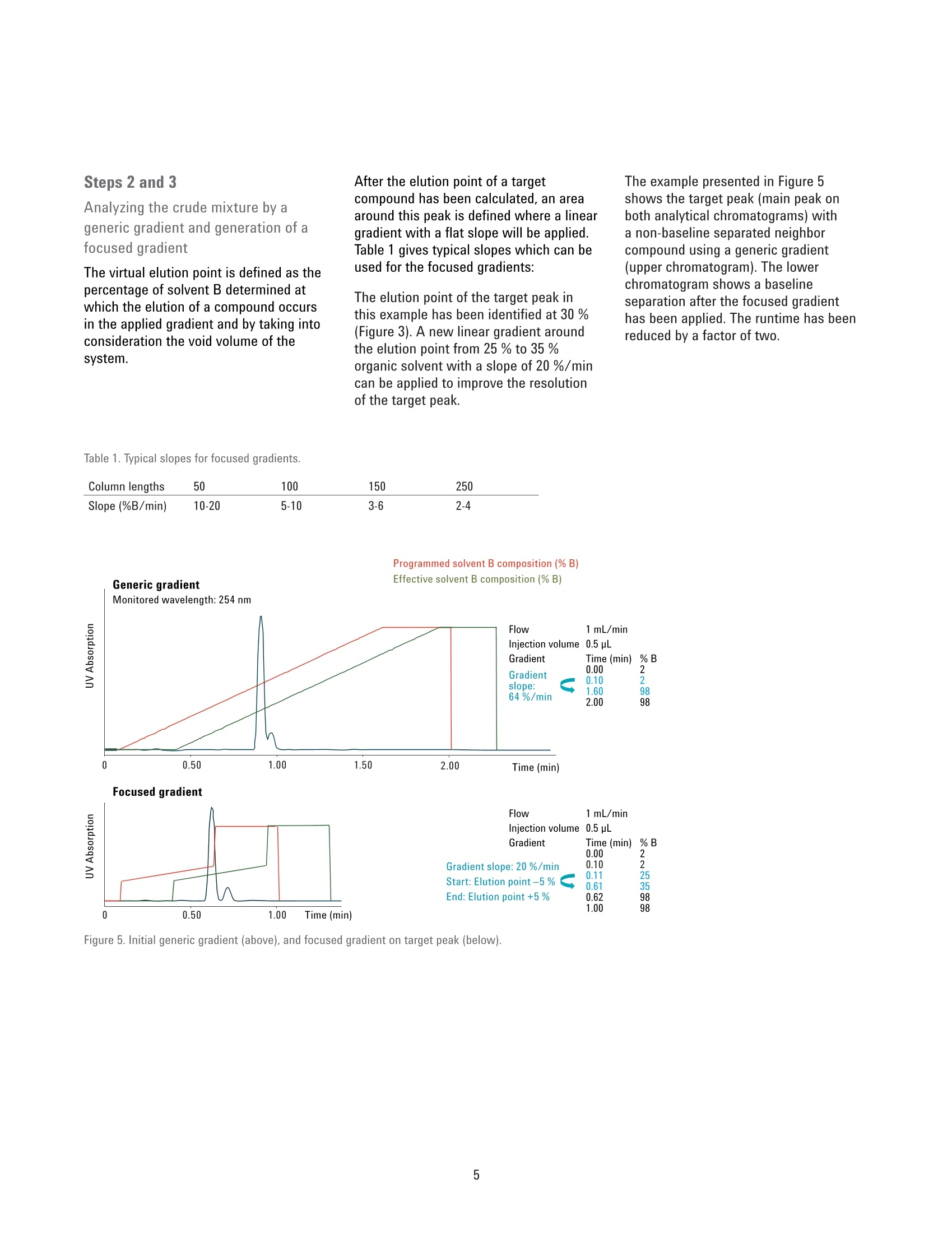
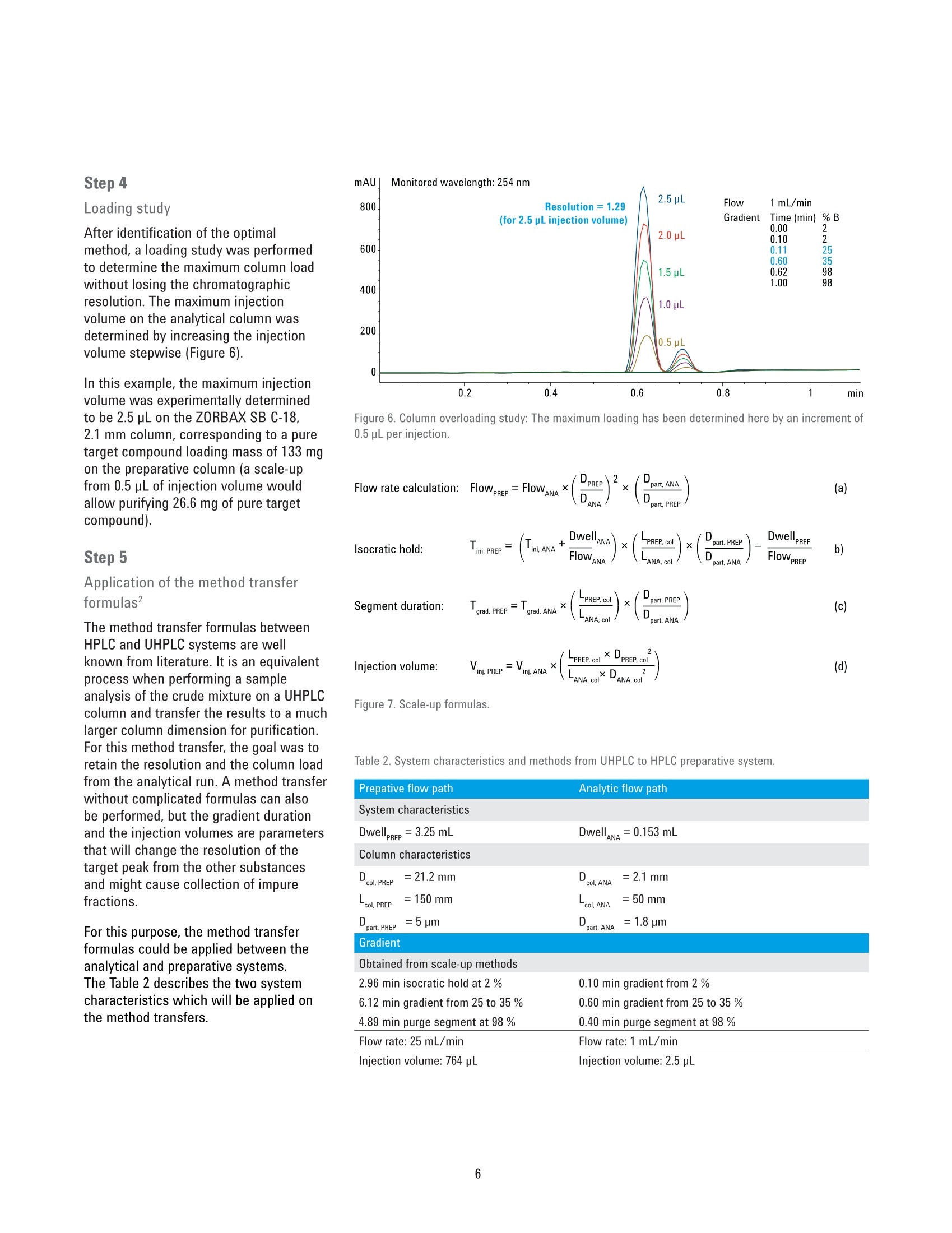
Analytical to Preparative HPLCMethod Transfer An easy way to scale up from UHPLC to preparativeHPLC using focused gradients Technical Overview Author Abstract Pierre Penduff Agilent Technologies, Inc. Waldbronn, Germany Synthesizing novel compounds or isolating natural products can be a laboriousand time-consuming process. After analyzing the precious sample on an analyticalUHPLC system, the crucial step is to transfer the method to a preparative systemwith a minimal risk of losing valuable work or collecting impure compounds.This Technical Overview describes a practical way to optimize the scale-upprocess for reversed-phase chromatography from an analytical UHPLC system topreparative LC systems using a focused gradient to increase the sample load onthe preparative column to achieve optimum purity. Agilent Technologies Introduction Generic gradients are suitable to copewith a large variety of sample typeswhen there is no capacity or time tooptimize the separation. Each gradientcan be divided into four different steps.After the injection, an isocratic holdstep can be applied to remove theinjected solvent from the column and toimprove resolution especially for polarcompounds. The second step is a linearslope, which will be applied to separateeffectively based on the chromatographicproperties of the target compounds,followed by a purge phase. In the laststep, the column is re-equilibrated atthe initial solvent composition for thenext sample analysis or purification run.To improve resolution around the targetcompound, the linear slope has to bemodified. In preparative chromatography, mostoften the goal is to isolate, efficiently,a large amount of one or a few targetcompounds out of a crude mixture.Ideally, the chromatographic resolutionaround the target peak and the columnload are increased without significantlyincreasing the separation runtime. Fromthe crude sample, an optimized methodcan be generated with the goal to extendthe resolution between the target peak(green peak, Figures 1 and 2) and itsneighbor compounds. A basic approach to generate optimizedpreparative methods can be to divide thelinear generic gradient method into timeslices (described in the Technical Note5991-3070EN1). This approach generatesa set of preparative methods that can beused in any further sample purificationsimply by identifying the time slice wherethe target peak elutes. In this Technical Overview, an approachwith focused gradients on target peakwas used. This approach generatesthen a unique and dedicated method toa concerned target peak which has theadvantage to increase the resolutionbetter than the time slices method. All optimization steps occur on theanalytical system. After obtaining thefirst chromatographic information ofthe crude mixture by using a genericgradient, the resolution is optimized byflattening the slope followed by a loadingstudy to determine the maximum columnload before scaling up to the preparativecolumn dimensions. Figure 1. Schematic view of a generic gradient with the desired product peak (highlighted in green). Time Experimental Instrumentation Analytical System Agilent 1290 Infinity Binary LC: Agilent 1290 Infinity Binary Pump(G4220A) Agilent 1290 Infinity Autosamplerwith Thermostat (G4226A,G1330B) Agilent 1290 Infinity ThermostattedColumn Compartment (G1316B) Agilent 1290 Infinity Diode-ArrayDetector, including a Max-Lightstandard cartridge cell with 10-mmpath length, V(o) 1 pL (G4212A) Preparative System Agilent 1260 Infinity Preparative-scalePreparative System: Agilent 1260 Infinity PreparativeBinary Pumps (G1361A, G1391A) Agilent 1260 Infinity PreparativeAutosampler (G2260A) Agilent 1260 Infinity MultipleWavelength Detector (G1365D)equipped with a Quartz flow cell0.06-mm path length for MWD(G1365D#026) Column organizer module (G1383A) Agilent 1260 Infinity FractionCollector PS (G1364B) ( Columns ) Agilent ZORBAX RRHD SB-C18,2.1×50 mm, 1.8 pm (857700-902) Agilent ZORBAX SB-C18, Prep HTCartridge 21.2×150 mm,5 pm(870150-902) with end fittings(820400-901) Software Agilent OpenLAB CDS ChemStationEdition for LC & LC/MS Systems, Rev.C.01.05 [36] Solvents and SamplesSolvent A Water +0.1 % formic acid Solvent B Acetonitrile +0.1 % formic acid Purification mixture for preparative runsO-acetyl salicylic acid (0.07 g/mL)Salicylic acid (0.175 g/mL) in DMSO:acetonitrile 3:1 Purification mixture for analytical runs(dilution by 50 in water:acetonitrile 1:2)O-acetyl salicylic acid (1.4 pg/pL),Salicylic acid (3.5pg/pL) All solvents used were LC grade. Freshultrapure water was obtained from aMilli-Q Integral system equipped with a0.22-pm membrane point-of-use cartridge(Millipak) Results and Discussion A multistep process has been developedthat ensures consistent purificationresults. The first step is only done oncefor a purification system. The furthersteps are to be repeated for each sample. Step 1 Determination of dwell volume andcolumn volume The scale-up process starts with acharacterization of all void volumesfrom the analytical and the preparativesystems. The total system void volume (dwellvolume, column volume,and extra columnvolumes caused by the flow path after thecolumn) has to be determined first on theanalytical and the preparative LC system. These void volumes generate a delaybetween the programmed gradient1tand the effective gradient (Figure 3).To determine the effective solventcomposition at a certain retention time ofa compound, for example, the percentageof organic solvent, in this examplecorresponding to 50 %, needs to becorrected due to the dwell volume (153 pLfor the 1290 Infinity Binary LC) and thecolumn volume (147 pL for this ZORBAXRRHD 2.1×50 mm, 1.8 pm column). Forthe desired compound shown, Figure 3,the effective elution composition, orelution point of the sample correspondsto 30 % of solvent B. If the dwell volume of the system is notknown, it can be measured using thefollowing procedure: 1. Prepare Solvent A:25% water/75% acetonitrile. 2. Prepare Solvent B:25 % water/74% acetonitrile/1% acetone. On the analytical system/flow path 3. Run a linear gradient from 5-95 % Bin 10 minutes at the usual flowrate used on the analytical column(for example 1 mL/min) at 270 nmdetection wavelength. 4. Replace the column by a zero deadvolume connection. 7. 5. Run the same gradient at the sameflow rate as in the previous run at270 nm detection wavelength. The Dwell volume is then:Vowel =towellx Flow,and the column volume is;Vcolumn =columnx Flow. On the preparative flow path/system 6. Calculate the time differences at50 % of the maximum absorption,between the programmed gradientand the obtained signal with abypass (t w), and between thesignals obtained with bypass andwith column (tcolumn). 8. Repeat Steps 3 to 7 on thepreparative flow path using thetypical preparative flow rate (forexample, 25 mL/min for a 21.2 mmid preparative column). %B Monitored wavelength: 254 nm Programmed solvent composition (%B)Effective solvent composition (%B) coo Figure 3. Sample mixture with the programmed solvent composition (red), and the effective gradientcomposition (blue) after consideration of the system void volume. Monitored wavelength: 270 nm Time (min) Figure 4. Characterization of the analytical system: Overlays of the programmed solvent composition(red), UV signal of the tracer with a bypass (blue) and the signal of the tracer with column (green). Steps 2 and 3 Analyzing the crude mixture by ageneric gradient and generation of afocused gradient The virtual elution point is defined as thepercentage of solvent B determined atwhich the elution of a compound occursin the applied gradient and by taking intoconsideration the void volume of thesystem. After the elution point of a targetcompound has been calculated, an areaaround this peak is defined where a lineargradient with a flat slope will be applied.Table 1 gives typical slopes which can beused for the focused gradients: The elution point of the target peak inthis example has been identified at 30 %(Figure 3). A new linear gradient aroundthe elution point from 25 % to 35 %organic solvent with a slope of 20 %/mincan be applied to improve the resolutionof the target peak. The example presented in Figure 5shows the target peak (main peak onboth analytical chromatograms) witha non-baseline separated neighborcompound using a generic gradient(upper chromatogram). The lowerchromatogram shows a baselineseparation after the focused gradienthas been applied. The runtime has beenreduced by a factor of two. Figure 5. Initial generic gradient (above), and focused gradient on target peak (below). Step 4 Loading study After identification of the optimalmethod, a loading study was performedto determine the maximum column loadwithout losing the chromatographicresolution. The maximum injectionvolume on the analytical column wasdetermined by increasing the injectionvolume stepwise (Figure 6). In this example, the maximum injectionvolume was experimentally determinedto be 2.5 uL on the ZORBAX SB C-18,2.1 mm column, corresponding to a puretarget compound loading mass of 133 mgon the preparative column (a scale-upfrom 0.5 uL of injection volume wouldallow purifying 26.6 mg of pure targetcompound). Step 5 Application of the method transferformulas? The method transfer formulas betweenHPLC and UHPLC systems are wellknown from literature. It is an equivalentprocess when performing a sampleanalysis of the crude mixture on a UHPLCcolumn and transfer the results to a muchlarger column dimension for purification.For this method transfer, the goal was toretain the resolution and the column loadfrom the analytical run. A method transferwithout complicated formulas can alsobe performed, but the gradient durationand the injection volumes are parametersthat will change the resolution of thetarget peak from the other substancesand might cause collection of impurefractions. For this purpose, the method transferformulas could be applied between theanalytical and preparative systems.The Table 2 describes the two systemcharacteristics which will be applied onthe method transfers. Figure 6. Column overloading study: The maximum loading has been determined here by an increment of0.5 pL per injection. Segment duration:. grvaad, PREP=Tgaad, AN(A*(wmn)(m) (c) Figure 7.Scale-up formulas. Table 2. System characteristics and methods from UHPLC to HPLC preparativesystem. Prepative flow path Analytic flow path System characteristics DwellPREP=3.25 mL DwellaNA=0.153 mL Column characteristics col,PREP=21.2 mm col,ANA =2.1 mm =col, PREP150 mm col.ANA =50 mm Dpart, PREP=5 pm part,ANA =1.8 pm Gradient Obtained from scale-up methods 2.96 min isocratic hold at 2% 0.10 min gradient from 2 % 6.12 min gradient from 25 to 35 % 0.60 min gradient from 25 to 35 % 4.89 min purge segment at 98 % 0.40 min purge segment at 98 % Flow rate: 25 mL/min Flow rate: 1 mL/min Injection volume: 764 pL Injection volume: 2.5 pL No. Step 1 Determination of dwell volume and column volume for the system 2 Sample analysis to determine the elution point of the target compound 3 Creation of a focused gradient to increase resolution for the target compound 4 Determine the maximum column loadability 5 Scaling up to a preparative column using the available formula 6 Preparative injection and fraction collection Figure 8. Preparative chromatogram. Conclusion A scale-up from a 2.1-mm id columnon a UHPLC system to a 1260 InfinityPreparative scale system equipped witha 21.2-mm id column was successfullydeveloped. For all scale-up situations, a correctmethod transfer was required to keepthe resolution constant. This ensuredmaximum purity and recovery from theprecious sample. The steps in Table 3 summarize theprocess. References 1. Peptides Purification on the Agilent218 Bio-inert Binary PurificationSystem, Agilent Technical Overview,5991-3070EN. 2. Guidelines for the use of UHPLCInstruments. Requirements for UHPLCinstruments, method developmentin UHPLC and method transfer fromregular HPLC to UHPLC, Dr. DavyGuillarme, Prof. Jean-Luc Veuthey,Freeware download: http://www.unige.ch/sciences/pharm/fanal/Icap/telechargement.htm 3.U. Huber and R.E. Majors,"Principlesin preparative HPLC", AgilentTechnologies Primer, PublicationNumber 5989-6639EN,2007. www.agilent.com/chem/purification This information is subject to change without notice. C Agilent Technologies, Inc., 2013 Published in the USA, September 1, 2013 5991-2013EN Agilent Technologies Abstract Synthesizing novel compounds or isolating natural products can be a laborious and time-consuming process. After analyzing the precious sample on an analytical UHPLC system, the crucial step is to transfer the method to a preparative system with a minimal risk of losing valuable work or collecting impure compounds. This Technical Overview describes a practical way to optimize the scale-up process for reversed-phase chromatography from an analytical UHPLC system to preparative LC systems using a focused gradient to increase the sample load on the preparative column to achieve optimum purity.Introduction Generic gradients are suitable to cope with a large variety of sample types when there is no capacity or time to optimize the separation. Each gradient can be divided into four different steps. After the injection, an isocratic hold step can be applied to remove the injected solvent from the column and to improve resolution especially for polar compounds. The second step is a linear slope, which will be applied to separate effectively based on the chromatographic properties of the target compounds, followed by a purge phase. In the last step, the column is re-equilibrated at the initial solvent composition for the next sample analysis or purifi cation run. To improve resolution around the target compound, the linear slope has to be modifi ed. In preparative chromatography, most often the goal is to isolate, effi ciently, a large amount of one or a few target compounds out of a crude mixture. Ideally, the chromatographic resolution around the target peak and the column load are increased without signifi cantly increasing the separation runtime. From the crude sample, an optimized method can be generated with the goal to extend the resolution between the target peak (green peak, Figures 1 and 2) and its neighbor compounds. A basic approach to generate optimized preparative methods can be to divide the linear generic gradient method into time slices (described in the Technical Note 5991-3070EN1 ). This approach generates a set of preparative methods that can be used in any further sample purifi cation simply by identifying the time slice where the target peak elutes.In this Technical Overview, an approach with focused gradients on target peak was used. This approach generates then a unique and dedicated method to a concerned target peak which has the advantage to increase the resolution better than the time slices method. All optimization steps occur on the analytical system. After obtaining the fi rst chromatographic information of the crude mixture by using a generic gradient, the resolution is optimized by fl attening the slope followed by a loading study to determine the maximum column load before scaling up to the preparative column dimensions.Conclusion A scale-up from a 2.1-mm id column on a UHPLC system to a 1260 Infinity Preparative scale system equipped with a 21.2-mm id column was successfully developed. For all scale-up situations, a correct method transfer was required to keep the resolution constant. This ensured maximum purity and recovery from the precious sample. The steps in Table 3 summarize the process.
确定



还剩6页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《混合标样中O-乙酰水杨酸,水杨酸检测方案(制备液相色谱)》,该方案主要用于其他中O-乙酰水杨酸,水杨酸检测,参考标准--,《混合标样中O-乙酰水杨酸,水杨酸检测方案(制备液相色谱)》用到的仪器有Agilent 1260 Infinity II 制备型液相色谱、Agilent 1290 Infinity II Multisampler、OpenLAB 软件
推荐专场
相关方案
更多
该厂商其他方案
更多