方案详情
文
Purifying a large number of compounds by liquid chromatography usually requires screening experiments and manual interaction during upscaling to preparative conditions. The Agilent Automated Purification software facilitates the workflow of analytical scouting to preparative purification by smart algorithms and calculated scale-up of separation gradients, all integrated in an intuitive interface. This Technical Overview demonstrates the purification of a compound library using the Agilent 1260 Infinity Automated LC/MSD Purification System controlled by the Automated Purification software.
方案详情

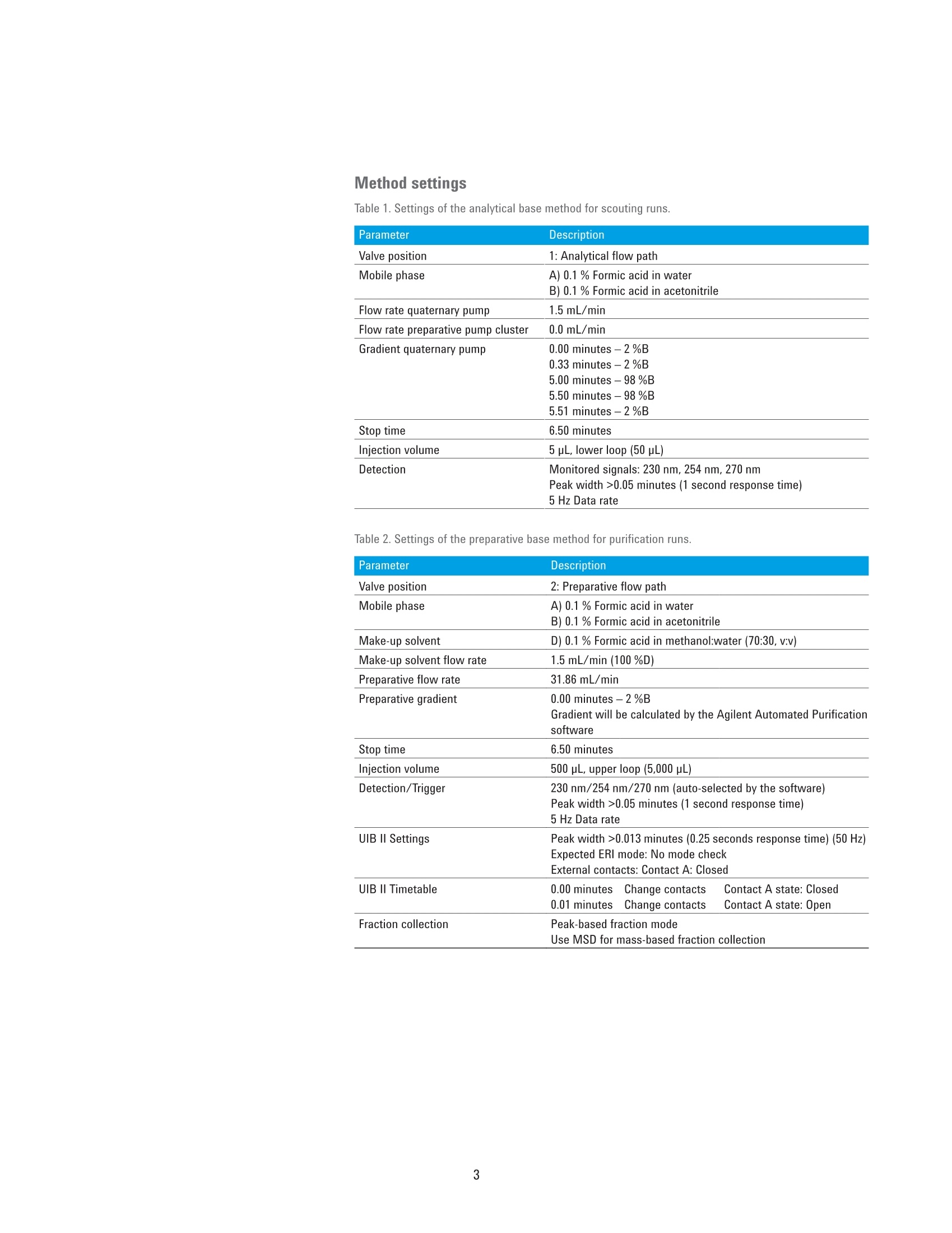
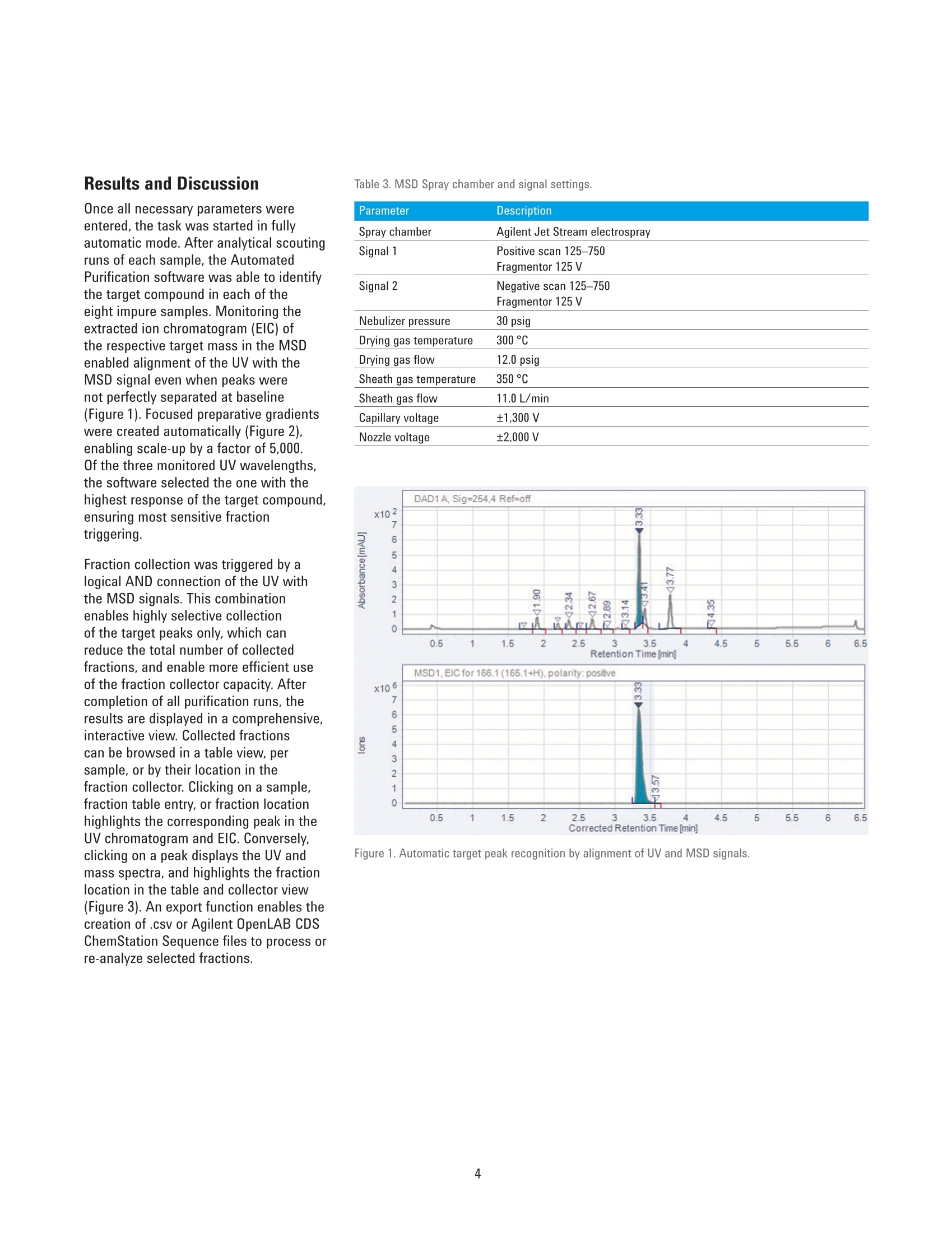
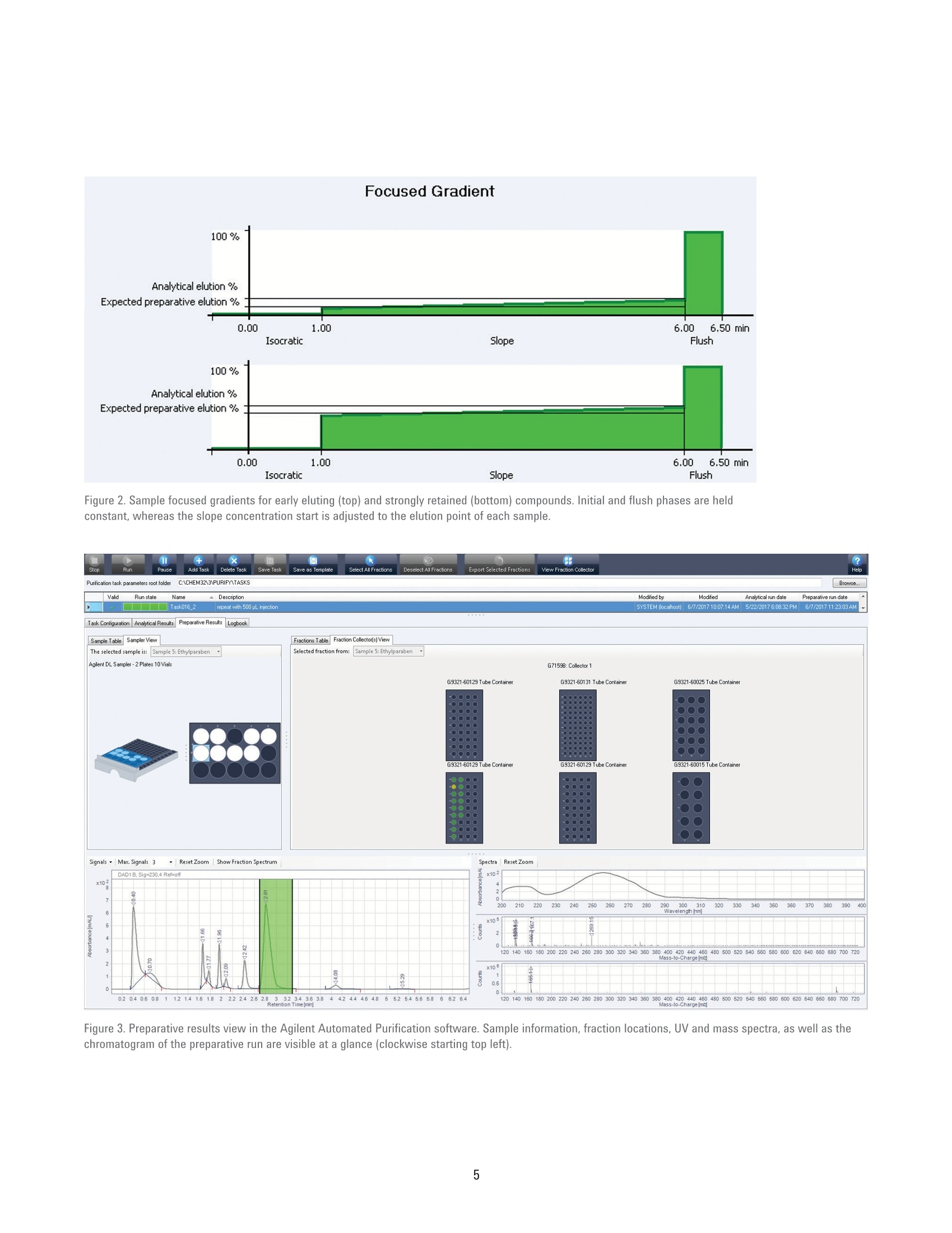
Automated Purification of CompoundLibraries Using the Agilent 1260Infinity Automated LC/MSDPurification System Technical Overview Author Abstract Florian RieckAgilent Technologies, Inc. Waldbronn, Germany Purifying a large number of compounds by liquid chromatography usually requiresscreening experiments and manual interaction during upscaling to preparativeconditions. The Agilent Automated Purification software facilitates the workflowof analytical scouting to preparative purification by smart algorithms andcalculated scale-up of separation gradients, all integrated in an intuitive interface.This Technical Overview demonstrates the purification of a compound libraryusing the Agilent 1260 Infinity Automated LC/MSD Purification System controlledby the Automated Purification software. Agilent Technologies Introduction Preparative liquid chromatography (LC)is a technique that is frequently appliedin pharmaceutical chemistry or earlystages of drug discovery. Dependingon the compound of interest, optimumchromatographic conditions usuallyneed to be determined by an analyticalscouting run before large amounts ofsample are purified. This process canbe time-consuming, especially withthe high number of samples typical inpharmaceutical chemistry. The Agilent Automated Purificationsoftware facilitates this scale-up process,and supports analysts in speeding uptheir workflows, providing true automaticscale-up from analytical to preparativegradients. An intuitive interface guidesthe user along the complete workflowincluding analytical scouting, scale-up,focused gradient generation, fractioncollection and review, and export ofresults. Smart algorithms check thevalidity of user inputs on-the-fly, andprevent the user from proceeding ifconflicting parameters are entered. Twodifferent views offer either the completesettings for method developers or arestricted set of parameters for operatorsor walk-up users (Easy Prep mode). Onceall details of the task are entered, theworkflow can be started either in fullyautomatic mode or with the option tomanually change parameters and reviewanalytical results before proceeding withthe preparative runs. The workflow in the AutomatedPurification software consists of ananalytical scouting run, an (automaticor manual) evaluation step, and apreparative run with fraction collection.During the scouting run, diluted samplesare analyzed by a generic gradient on theanalytical flow path of the Agilent 1260Infinity Automated LC/MSD PurificationSystem. In the evaluation phase, thecompounds of interest are identifiedby target masses specified in theanalytical sequence, and aligned withthe signal of the UV detector. Thesedata can be imported from any analyticalAgilent LC/MSD System. Based on thematching signals, the virtual elutionpoint of each target compound is calculated. Software algorithms generatea focused gradient that is optimized forthe calculated elution point of the targetcompound. The purification is then run onthe preparative flow path of the system,applying the calculated focused gradientfor each sample, enabling optimizedseparation and collection of the targetcompound. This Technical Overview demonstratesthe analytical-to-preparative workflowwith the Automated Purificationsoftware using a sample library of eightcompounds. Impure samples wereseparated and purified by peak-basedfraction collection triggered by acombination of ultraviolet (UV) and massselective detector (MSD) signals. Fractionpurity and recovery of the injected sampleamounts were measured as performancemarkers. Experimental Instrumentation The Agilent 1260 Infinity AutomatedLC/MSD Purification System consisted ofthe following modules: Agilent 1260 Infinity PreparativePump cluster (G1361A+ G1391A) Agilent 1260 Infinity Dual-LoopAutosampler (G2258A) Agilent 1260 Infinity Diode ArrayDetector (G1315C) with 3 mmpreparative flow cell (Option #022) Agilent 1290 Infinity II PreparativeOpen-Bed Fraction Collector(G7159B) Active Splitter (G1968F) Agilent 1260 Infinity QuaternaryPump (G1311B) Agilent 1260 Infinity Valve Drive(G1170A) with preparative2-position/10-port valve head(G4730A) Universal Interface Box (UIB) Il(G1390B) Agilent 6150 Single QuadrupoleLC/MS (G6150B) Columns Analytical column Agilent Prep C18 Scalar,4.6×50 mm, 5 pm (p/n 446905-902) Preparative column Agilent Prep C18, 21.2×50 mm PrepHTcartridge, 5 pm (p/n 446905-102) withPrepHT end fittings (p/n 820400-901) Software All experiments were conducted usingthe Agilent Automated Purificationsoftware A.01.04 [038], an add-on tothe Agilent OpenLAB CDS ChemStationEdition for LC and LC/MS Systems,version C.01.07 SR2 [263], controlled byLC Drivers A.02.15 [026]. Solvents and samples All solvents used were LC grade. Freshultrapure water was obtained from aMilli-Q Integral system equipped with a0.22-pm membrane point-of-use cartridge(Millipak).Acetaminophen, acetanilide,benzocaine, caffeine, ethylparaben,propylparaben, salicylic acid,sulfamerazine sodium salt, and dimethylsulfoxide (DMSO) were purchased fromSigma-Aldrich, Taufkirchen, Germany.Samples were prepared in DMSO at aconcentration of 10 mg/mL of the targetcompound and seven impurities atapproximately 1 mg/mL each. Analyticalscouting samples were diluted 50x inDMSO. The analytical-to-preparative purificationworkflow in the Automated Purificationsoftware is called a task. Each task canhold a single sample or a sequence ofmultiple samples, and needs input ofbasic parameters such as sample nameand location, target formula or mass,injection volume, and fraction startlocation. A generic analytical gradient isused for all scouting runs within a task;preparative gradients are calculatedspecifically for each sample after targetidentification. Method settings Table 1. Settings of the analytical base method for scouting runs. Parameter Description Valve position 1: Analytical flow path Mobile phase A) 0.1 % Formic acid in water B) 0.1 % Formic acid in acetonitrile Flow rate quaternary pump 1.5 mL/min Flow rate preparative pump cluster 0.0 mL/min Gradient quaternary pump 0.00 minutes-2 %B 0.33 minutes-2%B 5.00 minutes -98 %B 5.50 minutes -98 %B 5.51 minutes -2 %B Stop time 6.50 minutes Injection volume 5 pL, lower loop (50 pL) Detection Monitored signals: 230 nm, 254 nm, 270 nm Peak width >0.05 minutes (1 second response time) 5 Hz Data rate Table 2. Settings of the preparative base method for purification runs. Parameter Description Valve position 2: Preparative flow path Mobile phase A) 0.1 % Formic acid in water B) 0.1 % Formic acid in acetonitrile Make-up solvent D) 0.1% Formic acid in methanol:water (70:30,v:v) Make-up solvent flow rate 1.5 mL/min (100%D) Preparative flow rate 31.86 mL/min Preparative gradient 0.00 minutes-2%B Gradient will be calculated by the Agilent Automated Purification software Stop time 6.50 minutes Injection volume 500 pL, upper loop (5,000 pL) Detection/Trigger 230 nm/254 nm/270 nm (auto-selected by the software) Peak width >0.05 minutes (1 second response time) 5 Hz Data rate UIB II Settings Peak width >0.013 minutes (0.25 seconds response time) (50 Hz) Expected ERl mode: No mode check External contacts: Contact A: Closed UIB II Timetable 0.00 minutes Change contacts Contact A state: Closed 0.01 minutes Change contacts Contact A state: Open Fraction collection Peak-based fraction mode Use MSD for mass-based fraction collection Use MSD for mass-based fraction collection Results and Discussion Once all necessary parameters wereentered, the task was started in fullyautomatic mode. After analytical scoutingruns of each sample, the AutomatedPurification software was able to identifythe target compound in each of theeight impure samples. Monitoring theextracted ion chromatogram (EIC) ofthe respective target mass in the MSDenabled alignment of the UV with theMSD signal even when peaks werenot perfectly separated at baseline(Figure 1). Focused preparative gradientswere created automatically (Figure 2),enabling scale-up by a factor of 5,000.Of the three monitored UV wavelengths,the software selected the one with thehighest response of the target compound,ensuring most sensitive fractiontriggering. Fraction collection was triggered by alogical AND connection of the UV withthe MSD signals. This combinationenables highly selective collectionof the target peaks only, which canreduce the total number of collectedfractions, and enable more efficient useof the fraction collector capacity. Aftercompletion of all purification runs, theresults are displayed in a comprehensive,interactive view. Collected fractionscan be browsed in a table view, persample, or by their location in thefraction collector.Clicking on a sample,fraction table entry, or fraction locationhighlights the corresponding peak in theUV chromatogram and EIC. Conversely,clicking on a peak displays the UV andmass spectra, and highlights the fractionlocation in the table and collector view(Figure 3). An export function enables thecreation of .csv or Agilent OpenLAB CDSChemStation Sequence files to process orre-analyze selected fractions. Parameter Description Spray chamber Agilent Jet Stream electrospray Signal 1 Positive scan 125-750 Fragmentor 125 V Signal 2 Negative scan 125-750 Fragmentor 125V Nebulizer pressure 30 psig Drying gas temperature 300°C Drying gas flow 12.0 psig Sheath gas temperature 350°C Sheath gas flow 11.0 L/min Capillary voltage ±1,300 V Nozzle voltage ±2,000 V 品 区 人 SeA Figure 1. Automatic target peak recognition by alignment of UV and MSD signals. Figure 2. Sample focused gradients for early eluting (top) and strongly retained (bottom) compounds. Initial and flush phases are heldconstant, whereas the slope concentration start is adjusted to the elution point of each sample. Retention Time [min] Mass-to-Charge[m/a Figure 3. Preparative results view in the Agilent Automated Purification software. Sample information, fraction locations, UV and mass spectra, as well as thechromatogram of the preparative run are visible at a glance (clockwise starting top left). All collected fractions were re-analyzedwith respect to purity and recovery of theinjected compound. Purity was 94 % orhigher throughout all fractions. With theexception of one compound, all sampleshad a recovery of 91 % or higher (Table 4).This demonstrates that even in fullyautomated operation, the AutomatedPurification software produces pure andreliable results. Conclusion The Agilent Automated Purificationsoftware is a versatile add-on to theAgilent OpenLAB CDS ChemStation thatfacilitates the workflows of purificationlaboratories. All crucial steps fromanalytical scouting to fraction collectionand data review are combined in anintuitive user interface. With trulyautomated scale-up,on-the-fly calculationof focused gradients, and import andexport functionalities, the AutomatedPurification software is a valuable tool tospeed up the process of LC purificationfor experienced and novice users. ThisTechnical Overview demonstrates thepurification of a compound library on anAgilent 1260 Infinity Automated LC/MSDPurification System controlled by theAutomated Purification software. Even infully automated operation, fraction purityand recovery are typically above 90 %. Compound Purity Recovery Acetaminophen >99 % 91% Acetanilide >99% 93% Benzocaine 99% 92% Caffeine 94% 96% Ethylparaben 97% 92% Propylparaben 99% 95% Salicylic acid 99% 95% Sulfamerazine 99% 81% AbstractPurifying a large number of compounds by liquid chromatography usually requires screening experiments and manual interaction during upscaling to preparative conditions. The Agilent Automated Purification software facilitates the workflow of analytical scouting to preparative purification by smart algorithms and calculated scale-up of separation gradients, all integrated in an intuitive interface. This Technical Overview demonstrates the purification of a compound library using the Agilent 1260 Infinity Automated LC/MSD Purification System controlled by the Automated Purification software.IntroductionPreparative liquid chromatography (LC) is a technique that is frequently applied in pharmaceutical chemistry or early stages of drug discovery. Depending on the compound of interest, optimum chromatographic conditions usually need to be determined by an analytical scouting run before large amounts of sample are purified. This process can be time-consuming, especially with the high number of samples typical in pharmaceutical chemistry.The Agilent Automated Purification software facilitates this scale-up process, and supports analysts in speeding up their workflows, providing true automatic scale-up from analytical to preparative gradients. An intuitive interface guides the user along the complete workflow including analytical scouting, scale-up, focused gradient generation, fraction collection and review, and export of results. Smart algorithms check the validity of user inputs on-the-fly, and prevent the user from proceeding if conflicting parameters are entered. Two different views offer either the complete settings for method developers or a restricted set of parameters for operators or walk-up users (Easy Prep mode). Once all details of the task are entered, the workflow can be started either in fully automatic mode or with the option to manually change parameters and review analytical results before proceeding with the preparative runs.The workflow in the Automated Purification software consists of an analytical scouting run, an (automatic or manual) evaluation step, and a preparative run with fraction collection. During the scouting run, diluted samples are analyzed by a generic gradient on the analytical flow path of the Agilent 1260 Infinity Automated LC/MSD Purification System. In the evaluation phase, the compounds of interest are identified by target masses specified in the analytical sequence, and aligned with the signal of the UV detector. These data can be imported from any analytical Agilent LC/MSD System. Based on the matching signals, the virtual elution point of each target compound is calculated. Software algorithms generate a focused gradient that is optimized for the calculated elution point of the target compound. The purification is then run on the preparative flow path of the system, applying the calculated focused gradient for each sample, enabling optimized separation and collection of the target compound. This Technical Overview demonstrates the analytical-to-preparative workflow with the Automated Purification software using a sample library of eight compounds. Impure samples were separated and purified by peak-based fraction collection triggered by a combination of ultraviolet (UV) and mass selective detector (MSD) signals. Fraction purity and recovery of the injected sample amounts were measured as performance markers.Conclusion The Agilent Automated Purification software is a versatile add-on to the Agilent OpenLAB CDS ChemStation that facilitates the workflows of purification laboratories. All crucial steps from analytical scouting to fraction collection and data review are combined in an intuitive user interface. With truly automated scale-up, on-the-fly calculation of focused gradients, and import and export functionalities, the Automated Purification software is a valuable tool to speed up the process of LC purification for experienced and novice users. This Technical Overview demonstrates the purification of a compound library on an Agilent 1260 Infinity Automated LC/MSD Purification System controlled by the Automated Purification software. Even in fully automated operation, fraction purity and recovery are typically above 90 %.
确定


还剩4页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《混合标样中乙酰氨基酚、乙酰苯胺等检测方案(制备液相色谱)》,该方案主要用于其他中乙酰氨基酚、乙酰苯胺等检测,参考标准--,《混合标样中乙酰氨基酚、乙酰苯胺等检测方案(制备液相色谱)》用到的仪器有Agilent 1260 Infinity II 制备型液相色谱、Agilent 6100 系列单四极杆液质联用系统、Agilent 1290 Infinity II Multisampler
推荐专场
相关方案
更多
该厂商其他方案
更多
























