The Agilent 1290 Infinity II Preparative Open-Bed Fraction Collector was developed and optimized to enable efficient fraction collection with extended capacity and flexibility while providing the highest recovery and purity of the collected fractions. This Technical Overview demonstrates the performance of the 1290 Infinity II Preparative Open-Bed Fraction Collector when peak-based fraction collection is used. Using only the ultraviolet (UV) detection signal or a combination of UV and mass-selective detection (MSD) signals as a fraction trigger, purity and recovery of collected fractions are determined. Furthermore, the peak-based, collecting time slices fraction mode is discussed from a quantitative point of view.
方案详情

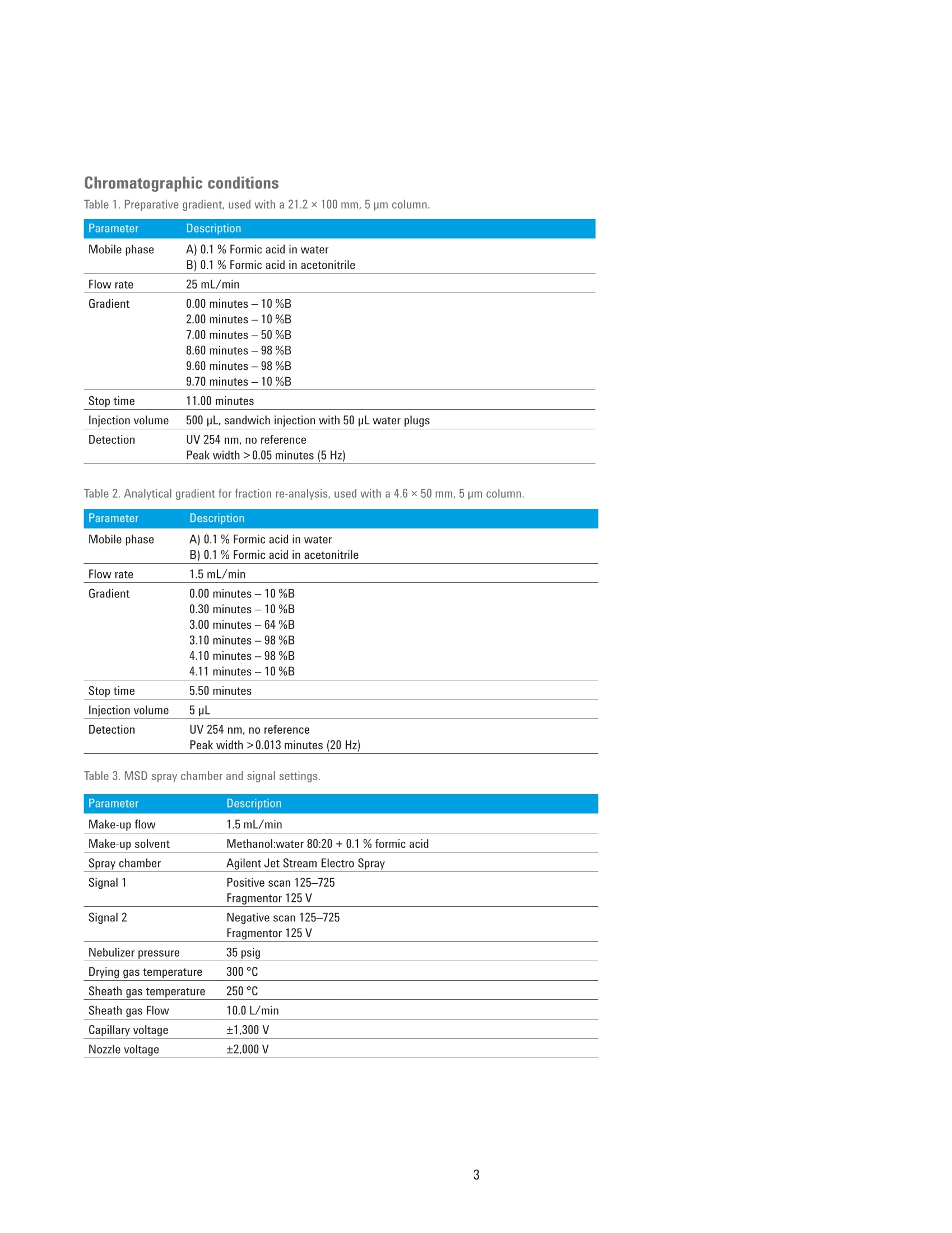
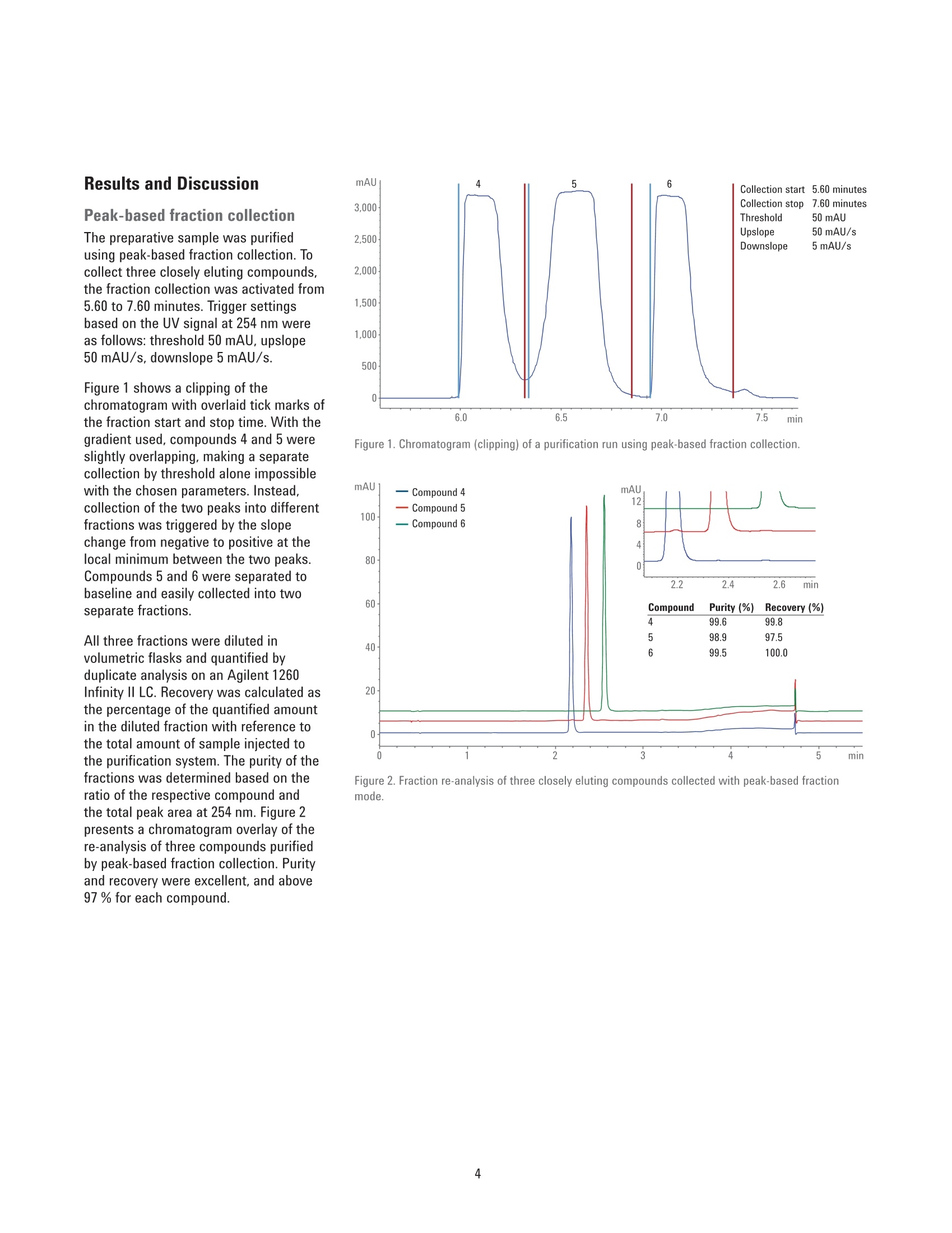
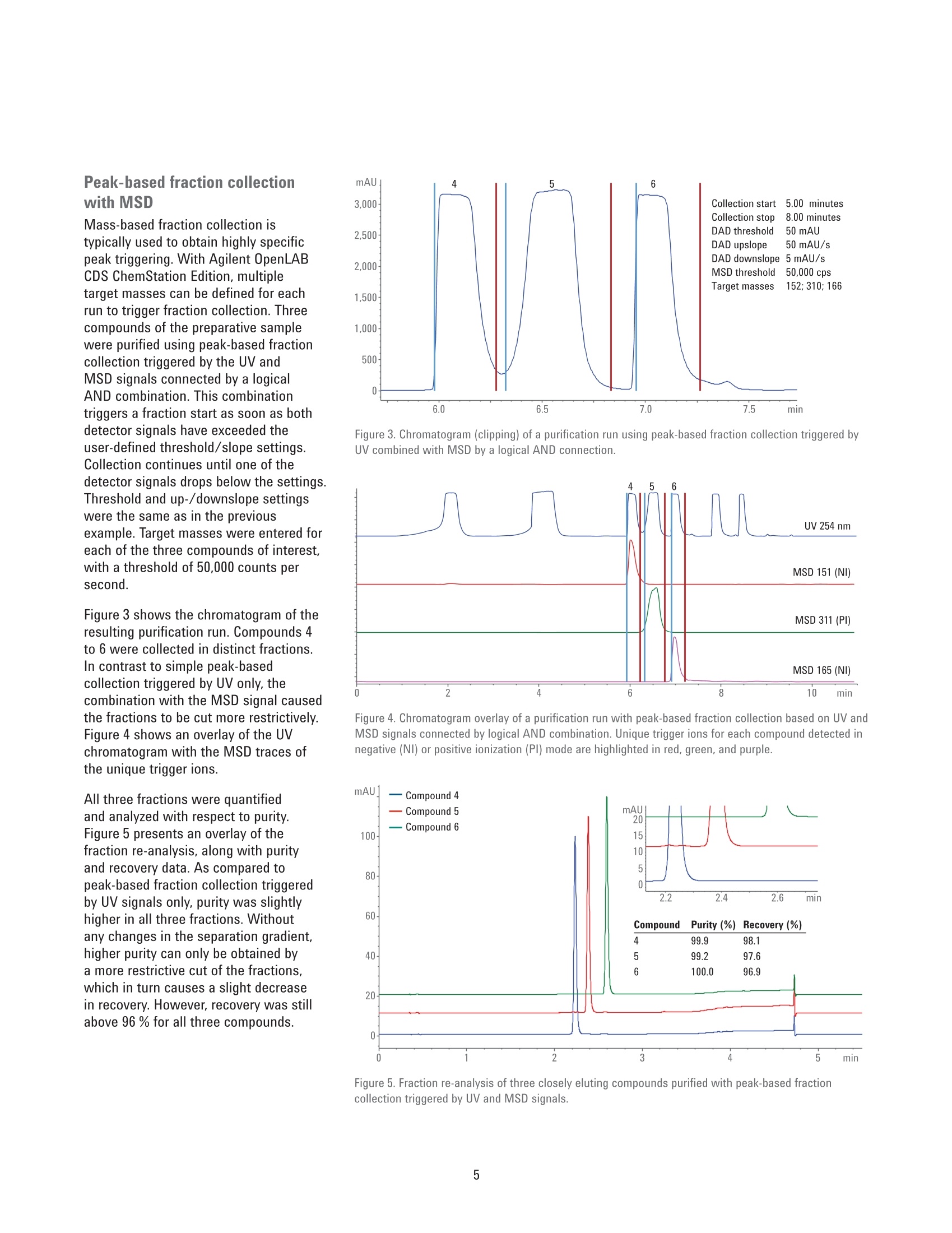
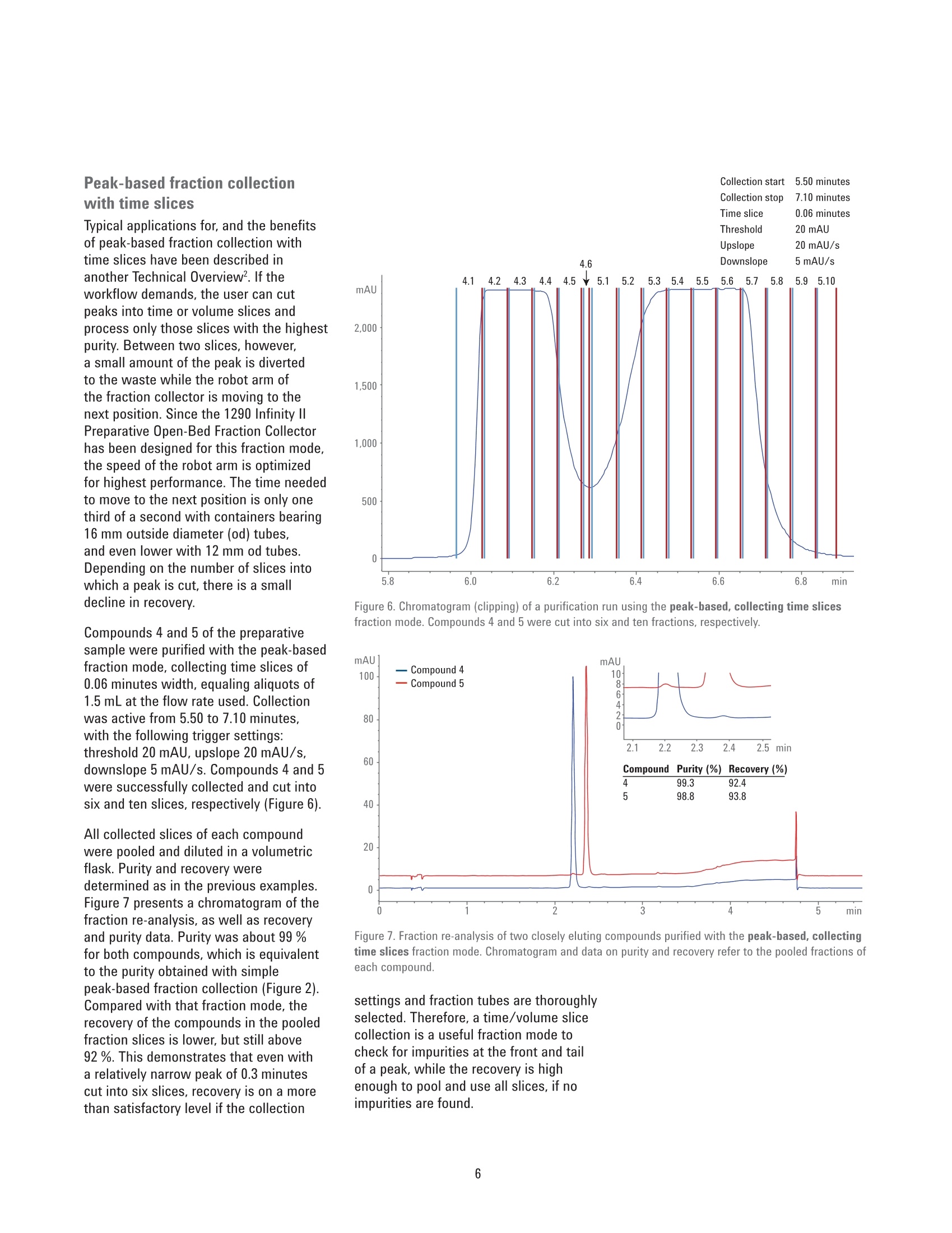
Performance Characteristics of theAgilent 1290 Infinity Il PreparativeOpen-Bed Fraction Collector Purity and Recovery of Purified Compounds Technical Overview Author Abstract Florian RieckAgilent Technologies, Inc. Waldbronn, Germany The Agilent 1290 Infinity II Preparative Open-Bed Fraction Collector was developedand optimized to enable efficient fraction collection with extended capacity andflexibility while providing the highest recovery and purity of the collected fractions.This Technical Overview demonstrates the performance of the 1290 Infinity lIPreparative Open-Bed Fraction Collector when peak-based fraction collection isused. Using only the ultraviolet (UV) detection signal or a combination of UV andmass-selective detection (MSD) signals as a fraction trigger, purity and recoveryof collected fractions are determined. Furthermore, the peak-based, collectingtime slices fraction mode is discussed from a quantitative point of view. Agilent Technologies Introduction Fraction collection in preparative-scalehigh-performance liquid chromatography(HPLC) can be challenging. Along with asuitable solvent gradient that separatesthe compounds of interest from unwantedimpurities, the delay between thedetector and the fraction collector playsa crucial role in the optimization process.Pure fractions with high recovery canonly be collected with accurate settingof the time needed for a substanceto migrate from the detector cell tothe fraction nozzle. The Agilent 1290Infinity II Preparative Open-Bed FractionCollector has been optimized in thisrespect to deliver efficient and flexiblefraction collection with high purity andrecovery. A built-in delay sensor allowsprecise calibration of the delay betweeneach detector and the fraction valve. Thecalibration procedure does not requireany hardware modifications, and needsto be done only once on UV-basedsystems; when the flow rate changes, thedelay time is recalculated automatically,and adjusted accordingly. On systemsincluding an MSD, the delay time is notonly dependent on the flow rate, but alsoon the split ratio of the main flow and themake-up flow. Exchangeable tubing kits are availablewith two different inside diameters to fita wide range of flow rates. They can becut in length to minimize peak dispersionbetween the detector and fractioncollector. Fraction tubes, available infour different diameters (12, 16,25,and 30 mm) and two lengths (100 and150 mm), offer the right choice for anyflow rate and fraction size. Carefullychoosing fraction tubes is recommendedto ensure short pathways of the fractionnozzle between two fractions, or toprovide enough space for large fractionswithout the need to split them intomultiple tubes. Eight peak- and time-based modes,including two recovery modes, areavailable for fraction collection triggering.The different fraction modes, along withtheir typical applications,are describedin detail in another Technical Overview.This document focuses on the recoveryand purity of fractions that were collectedin peak-based mode. Fraction collectionis triggered by the UV signal only, or bya logical AND combination of UV andMSD signals. The collected fractions arere-analyzed and quantified. Experimental Instrumentation The Agilent 1260 Infinity Preparative-scalePurification System consisted of thefollowing modules: Agilent 1260 Infinity PreparativePump Cluster (G1361A andG1391A) Agilent 1260 Infinity QuaternaryPump (G1311B) for MSD makeupflow Agilent 1260 Infinity Dual-LoopAutosampler (G2258A) equippedwith 50 pL (lower) and 5 mL(upper) injection loops Agilent 1260 Infinity Diode ArrayDetector (G1315C) equipped witha 3 mm preparative flow cell(Option #22) Agilent 1290 Infinity II PreparativeOpen-Bed Fraction Collector(G7159B) equipped with fractioncontainers for 16 × 150 mm(G9312-60129) and 12×150 mm(G9321-60045) tubes Agilent Active Flow Splitter(G1968F) connected through anAgilent 1200 Infinity UniversalInterface Box II (G1390B) Agilent 6150 Single QuadrupoleLC/MS with Agilent Jet Streamtechnology (G6150B) For fraction re-analysis an Agilent 1260Infinity II Binary LC was used in aconfiguration as follows: Agilent 1260 Infinity Il Binary Pump(G7112B) Agilent 1260 Infinity Il Vialsampler(G7129A) Agilent 1260 Infinity II Diode ArrayDetector (G7115A) equipped with a10 mm standard cell (G1315-60022) Columns Preparative columnAgilent ZORBAX SB-C18 PrepHT,21.2×100 mm, 5 pm (p/n 880966-122.01) Analytical columnAgilent ZORBAX SB-C184.6 ×50 mm, 5 pm (p/n 846975-902) Software The analytical system was controlled bythe Agilent OpenLAB CDS ChemStationEdition for LC and LC/MS Systems,version C.01.07 SR1 [110]. The preparativesystem was controlled by the samesoftware, version C.01.07 SR2 [257]. Solvents and samples LC grade acetonitrile was purchasedfrom Merck, Darmstadt, Germany. Freshultrapure water was obtained from aMilli-Q Integral system equipped witha 0.22 pm membrane point-of-usecartridge (Millipak). Formic acid, dimethylsulfoxide, and the eight components ofthe preparative sample were of analyticalgrade or higher, and purchased fromSigma-Aldrich,Taufkirchen, Germany. A sample was prepared froma mixture of acetaminophen,sulfamerazine sodium salt,caffeine,methylparaben, sulfadimethoxine,ethylparaben, propylparaben, andbenzyl-4-hydroxybenzoate, dissolved indimethyl sulfoxide at a final concentrationof 10 mg/mL. Chromatographic conditions Table 1. Preparative gradient, used with a 21.2×100 mm, 5 pm column. Parameter Description Mobile phase A) 0.1 % Formic acid in water B) 0.1 % Formic acid in acetonitrile Flow rate 25 mL/min Gradient 0.00 minutes -10 %B 2.00 minutes-10 %B 7.00 minutes-50 %B 8.60 minutes -98 %B 9.60 minutes-98 %B 9.70 minutes-10%B Stop time 11.00 minutes Injection volume 500 pL, sandwich injection with 50 pL water plugs Detection UV 254 nm, no reference Peak width >0.05 minutes (5 Hz) Table 2. Analytical gradient for fraction re-analysis, used with a 4.6×50 mm, 5 um column. Mobile phase A) 0.1 % Formic acid in water B) 0.1 % Formic acid in acetonitrile Flow rate 1.5 mL/min Gradient 0.00 minutes-10 %B 0.30 minutes -10 %B 3.00 minutes -64%B 3.10 minutes-98 %B 4.10 minutes -98 %B 4.11 minutes-10%B Stop time 5.50 minutes Injection volume 5pL Table 3. MSD spray chamber and signal settings. Parameter Description Make-up flow 1.5 mL/min Make-up solvent Methanol:water 80:20+0.1%formic acid Spray chamber Agilent Jet Stream Electro Spray Signal 1 Positive scan 125-725 Fragmentor 125 V Signal 2 Negative scan 125-725 Fragmentor 125 V Nebulizer pressure 35 psig Drying gas temperature 300°C Sheath gas temperature 250 °C Sheath gas Flow 10.0 L/min Capillary voltage ±1,300 V Nozzle voltage ±2,000V Results and Discussion Peak-based fraction collectionThe preparative sample was purifiedusing peak-based fraction collection. Tocollect three closely eluting compounds,the fraction collection was activated from5.60 to 7.60 minutes. Trigger settingsbased on the UV signal at 254 nm wereas follows: threshold 50 mAU, upslope50 mAU/s, downslope 5 mAU/s. Figure 1 shows a clipping of thechromatogram with overlaid tick marks ofthe fraction start and stop time. With thegradient used, compounds 4 and 5 wereslightly overlapping, making a separatecollection by threshold alone impossiblewith the chosen parameters. Instead,collection of the two peaks into differentfractions was triggered by the slopechange from negative to positive at thelocal minimum between the two peaks.Compounds 5 and 6 were separated tobaseline and easily collected into twoseparate fractions. All three fractions were diluted involumetric flasks and quantified byduplicate analysis on an Agilent 1260Infinity II LC. Recovery was calculated asthe percentage of the quantified amountin the diluted fraction with reference tothe total amount of sample injected tothe purification system. The purity ofthefractions was determined based on theratio of the respective compound andthe total peak area at 254 nm. Figure 2presents a chromatogram overlay of there-analysis of three compounds purifiedby peak-based fraction collection. Purityand recovery were excellent, and above97 % for each compound. 50 mAU 50 mAU/s 5 mAU/s Figure 1. Chromatogram (clipping) of a purification run using peak-based fraction collection. Figure 2. Fraction re-analysis of three closely eluting compounds collected with peak-based fractionmode. Peak-based fraction collectionwith MSD Mass-based fraction collection istypically used to obtain highly specificpeak triggering. With Agilent OpenLABCDS ChemStation Edition, multipletarget masses can be defined for eachrun to trigger fraction collection. Threecompounds of the preparative samplewere purified using peak-based fractioncollection triggered by the UV andMSD signals connected by a logicalAND combination. This combinationtriggers a fraction start as soon as bothdetector signals have exceeded theuser-defined threshold/slope settings.Collection continues until one of thedetector signals drops below the settings.Threshold and up-/downslope settingswere the same as in the previousexample. Target masses were entered foreach of the three compounds of interest,with a threshold of 50,000 counts persecond. Figure 3 shows the chromatogram of theresulting purification run. Compounds 4to 6 were collected in distinct fractions.In contrast to simple peak-basedcollection triggered by UV only, thecombination with the MSD signal causedthe fractions to be cut more restrictively.Figure 4 shows an overlay of the UVchromatogram with the MSD traces ofthe unique trigger ions. All three fractions were quantifiedand analyzed with respect to purity.Figure 5 presents an overlay of thefraction re-analysis, along with purityand recovery data. As compared topeak-based fraction collection triggeredby UV signals only,purity was slightlyhigher in all three fractions. Withoutany changes in the separation gradient,higher purity can only be obtained bya more restrictive cut of the fractions,which in turn causes a slight decreasein recovery. However, recovery was stillabove 96 % for all three compounds. Figure 3. Chromatogram (clipping) of a purification run using peak-based fraction collection triggered byUV combined with MSD by a logical AND connection. Figure 4. Chromatogram overlay of a purification run with peak-based fraction collection based on UV andMSD signals connected by logical AND combination. Unique trigger ions for each compound detected innegative (NI) or positive ionization (PI) mode are highlighted in red, green, and purple. Figure 5. Fraction re-analysis of three closely eluting compounds purified with peak-based fractioncollection triggered by UV and MSD signals. Peak-based fraction collectionwith time slices Typical applications for, and the benefitsof peak-based fraction collection withtime slices have been described inanother Technical Overview2. If theworkflow demands, the user can cutpeaks into time or volume slices andprocess only those slices with the highestpurity. Between two slices, however,a small amount of the peak is divertedto the waste while the robot arm ofthe fraction collector is moving to thenext position. Since the 1290 Infinity IlPreparative Open-Bed Fraction Collectorhas been designed for this fraction mode,the speed of the robot arm is optimizedfor highest performance. The time neededto move to the next position is only onethird of a second with containers bearing16 mm outside diameter (od)tubes,and even lower with 12 mm od tubes.Depending on the number of slices intowhich a peak is cut, there is a smalldecline in recovery. Compounds 4 and 5 of the preparativesample were purified with the peak-basedfraction mode, collecting time slices of0.06 minutes width, equaling aliquots of1.5 mL at the flow rate used. Collectionwas active from 5.50 to 7.10 minutes,with the following trigger settings:threshold 20 mAU, upslope 20 mAU/s,downslope 5 mAU/s. Compounds 4 and 5were successfully collected and cut intosix and ten slices, respectively (Figure 6). All collected slices of each compoundwere pooled and diluted in a volumetricflask. Purity and recovery weredetermined as in the previous examples.Figure 7 presents a chromatogram of thefraction re-analysis, as well as recoveryand purity data. Purity was about 99 %for both compounds, which is equivalentto the purity obtained with simplepeak-based fraction collection (Figure 2).Compared with that fraction mode, therecovery of the compounds in the pooledfraction slices is lower, but still above92%. This demonstrates that even witha relatively narrow peak of 0.3 minutescut into six slices, recovery is on a morethan satisfactory level if the collection Collection stop 7.10 minutes Figure 6. Chromatogram (clipping) of a purification run using the peak-based, collecting time slicesfraction mode. Compounds 4 and 5 were cut into six and ten fractions, respectively. Figure 7. Fraction re-analysis of two closely eluting compounds purified with the peak-based, collectingtime slices fraction mode. Chromatogram and data on purity and recovery refer to the pooled fractions ofeach compound. settings and fraction tubes are thoroughlyselected. Therefore, a time/volume slicecollection is a useful fraction mode tocheck for impurities at the front and tailof a peak, while the recovery is highenough to pool and use all slices, if noimpurities are found. Conclusion The Agilent 1290 Infinity Il PreparativeOpen-Bed Fraction Collector is a versatileinstrument with enhanced fractionbed capacity and flexibility for diversecollection scenarios. Flow path andmechanics have been optimized for thehighest purity and recovery of collectedfractions. This Technical Overviewdemonstrates that fractions with highpurity and recovery (above 98 % and 96 %,respectively) can easily be collected withthe peak-based fraction mode triggered byUV signal only or a combination of the UVand MSD signals. When the peak-based,collecting time slices fraction mode isused to cut peaks into six or more slices,the purity of the pooled slices is on thesame level as in peak-based collection.Sample loss caused by movement ofthe fraction valve between the slices isonly marginal, yielding recoveries of thepooled fractions above 92 %. References 1. Agilent 1290 Infinity ll PreparativeOpen-Bed Fraction Collector-Collect Each and Every Drop,Agilent Technologies Brochure,publication number 5991-7225EN,2016. 2. RRieck, F. Time-, Peak-,andMass-Based Fraction Collectionwith the 1290 Infinity II PreparativeOpen-Bed Fraction Collector.Agilent Technologies TechnicalOverview, publication number5991-7654EN, 2016. www.agilent.com/chem This information is subject to change without notice. C Agilent Technologies, Inc.,2016Published in the USA, December1, 2016 5991-7655EN Agilent Technologies Abstract The Agilent 1290 Infinity II Preparative Open-Bed Fraction Collector was developed and optimized to enable efficient fraction collection with extended capacity and flexibility while providing the highest recovery and purity of the collected fractions. This Technical Overview demonstrates the performance of the 1290 Infinity II Preparative Open-Bed Fraction Collector when peak-based fraction collection is used. Using only the ultraviolet (UV) detection signal or a combination of UV and mass-selective detection (MSD) signals as a fraction trigger, purity and recovery of collected fractions are determined. Furthermore, the peak-based, collecting time slices fraction mode is discussed from a quantitative point of view.Introduction Fraction collection in preparative-scale high-performance liquid chromatography (HPLC) can be challenging. Along with a suitable solvent gradient that separates the compounds of interest from unwanted impurities, the delay between the detector and the fraction collector plays a crucial role in the optimization process. Pure fractions with high recovery can only be collected with accurate setting of the time needed for a substance to migrate from the detector cell to the fraction nozzle. The Agilent 1290 Infinity II Preparative Open-Bed Fraction Collector1 has been optimized in this respect to deliver efficient and flexible fraction collection with high purity and recovery. A built-in delay sensor allows precise calibration of the delay between each detector and the fraction valve. The calibration procedure does not require any hardware modifications, and needs to be done only once on UV-based systems; when the flow rate changes, the delay time is recalculated automatically, and adjusted accordingly. On systems including an MSD, the delay time is not only dependent on the flow rate, but also on the split ratio of the main flow and the make-up flow. Exchangeable tubing kits are available with two different inside diameters to fit a wide range of flow rates. They can be cut in length to minimize peak dispersion between the detector and fraction collector. Fraction tubes, available in four different diameters (12, 16, 25, and 30 mm) and two lengths (100 and 150 mm), offer the right choice for any flow rate and fraction size. Carefully choosing fraction tubes is recommended to ensure short pathways of the fraction nozzle between two fractions, or to provide enough space for large fractions without the need to split them into multiple tubes.Eight peak- and time-based modes, including two recovery modes, are available for fraction collection triggering. The different fraction modes, along with their typical applications, are described in detail in another Technical Overview2 . This document focuses on the recovery and purity of fractions that were collected in peak-based mode. Fraction collection is triggered by the UV signal only, or by a logical AND combination of UV and MSD signals. The collected fractions are re-analyzed and quantified.Conclusion The Agilent 1290 Infinity II Preparative Open-Bed Fraction Collector is a versatile instrument with enhanced fraction bed capacity and flexibility for diverse collection scenarios. Flow path and mechanics have been optimized for the highest purity and recovery of collected fractions. This Technical Overview demonstrates that fractions with high purity and recovery (above 98 % and 96 %, respectively) can easily be collected with the peak-based fraction mode triggered by UV signal only or a combination of the UV and MSD signals. When the peak-based, collecting time slices fraction mode is used to cut peaks into six or more slices, the purity of the pooled slices is on the same level as in peak-based collection. Sample loss caused by movement of the fraction valve between the slices is only marginal, yielding recoveries of the pooled fractions above 92 %.
确定




还剩6页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《混合标样中乙酰氨基酚,磺胺甲嘧啶钠盐等检测方案(制备液相色谱)》,该方案主要用于其他中其他检测,参考标准--,《混合标样中乙酰氨基酚,磺胺甲嘧啶钠盐等检测方案(制备液相色谱)》用到的仪器有Agilent 1290 Infinity II 制备型液相色谱、Agilent 1290 Infinity II Multisampler
推荐专场
相关方案
更多
该厂商其他方案
更多

















