文章中采用了Gamary电化学工作站,GAMRY Reference 600+软件功能强大,操作简便。硬件设计独特,性能稳定。GAMRY Reference 600+电化学综合测试仪可以满足电池、材料表征、生物传感器、电化学机理、点分析化学、腐蚀与防护、痕量物质检测、电化学合成等多种电化学研究领域。
方案详情

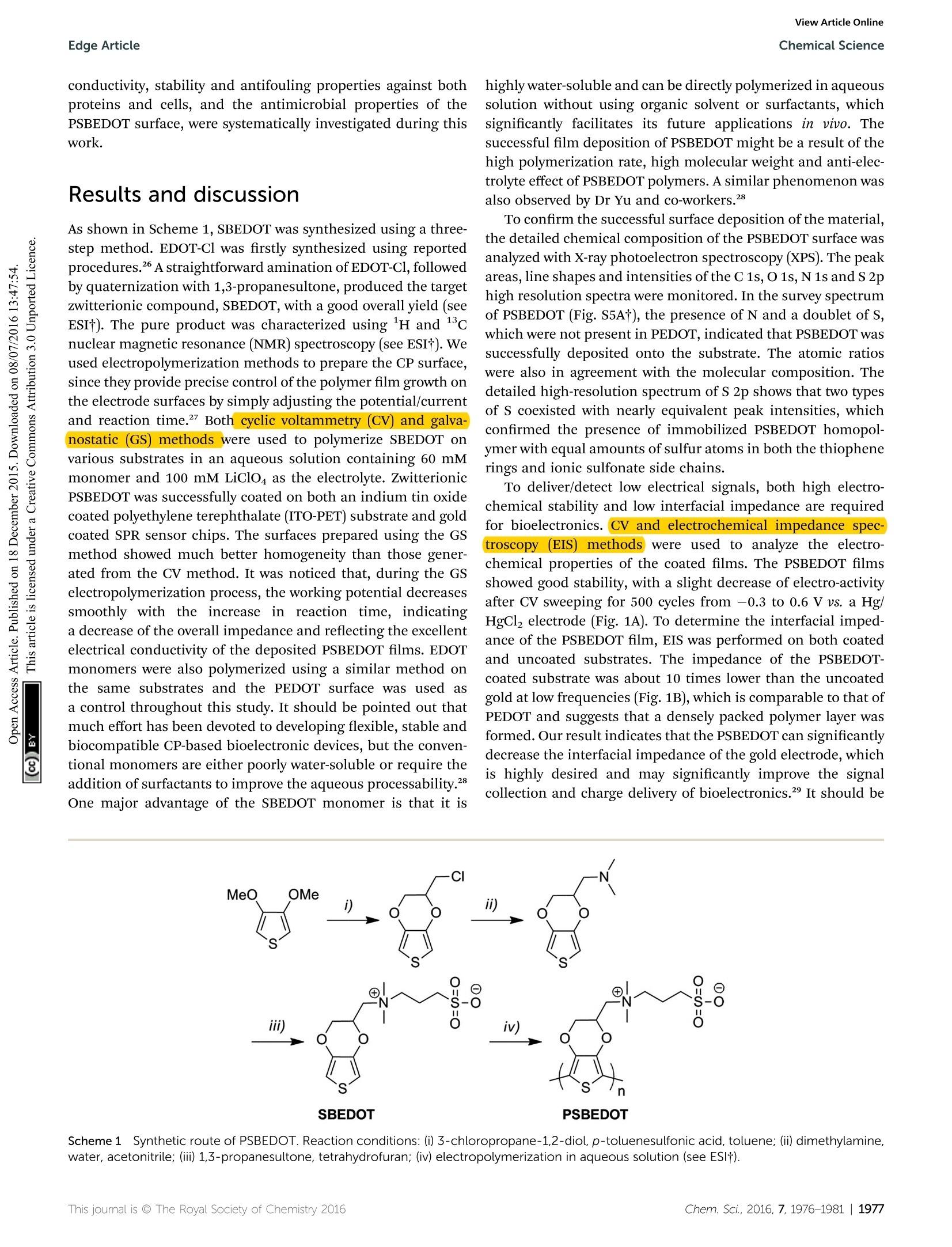
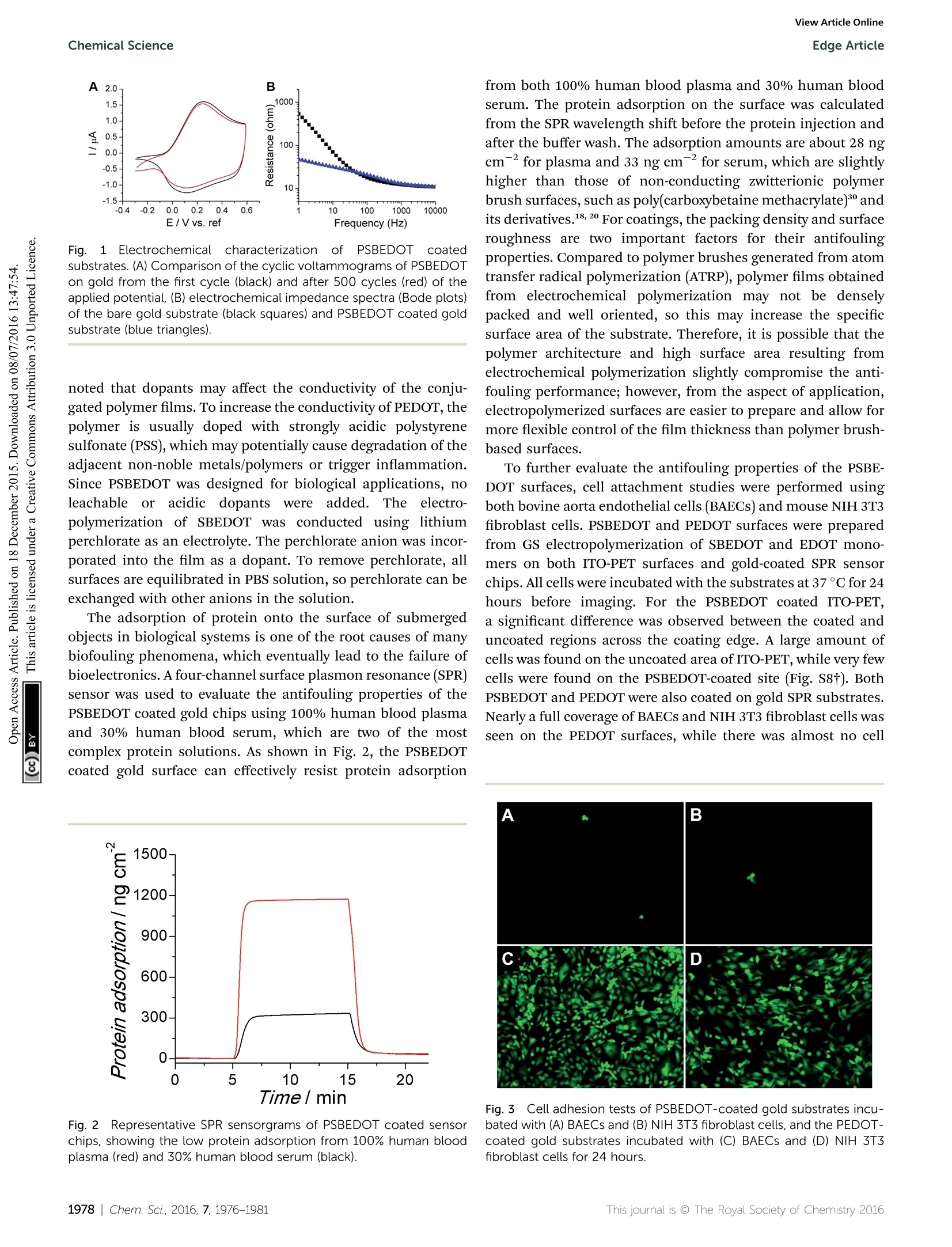
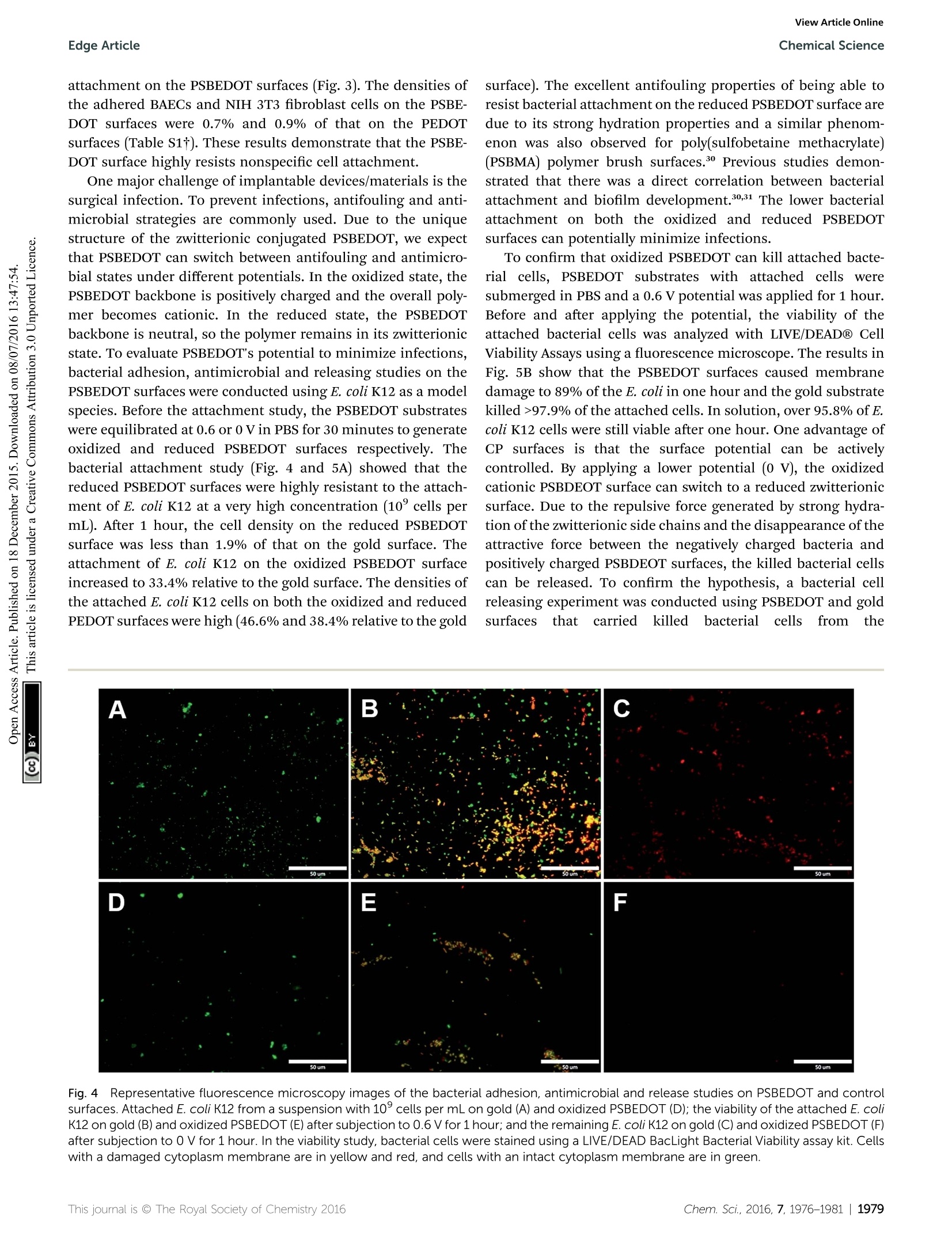
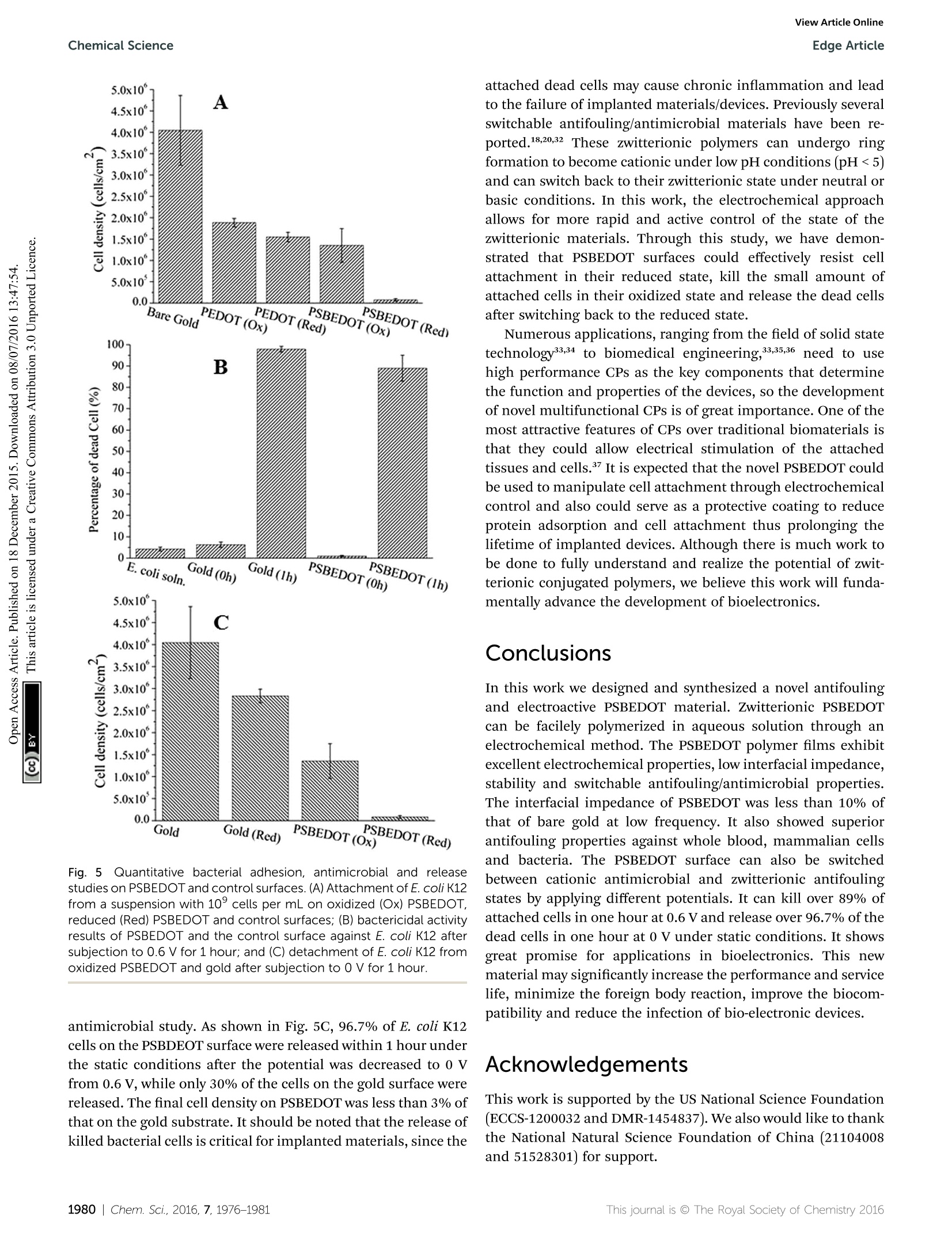
ChemicalScienceView Article OnlineView Journal|View Issue View Article OnlineEdge ArticleChemical Science CrossMark97.9% of the attached cells. In solution, over 95.8% ofE.coli K12 cells were still viable after one hour. One advantage ofCP surfaces is that the surface potential can be activelycontrolled. By applying a lower potential (0 V), the oxidizedcationic PSBDEOT surface can switch to a reduced zwitterionicsurface. Due to the repulsive force generated by strong hydra-tion of the zwitterionic side chains and the disappearance of theattractive force between the negatively charged bacteria andpositively charged PSBDEOT surfaces, the killed bacterial cellscan be released. To confirm the hypothesis, a bacterial cellreleasing experiment was conducted using PSBEDOT and goldsurfacestthatccarried killed bacterialcells fromnthe Fig. 4 Representative fluorescence microscopy images of the bacterial adhesion, antimicrobial and release studies on PSBEDOT and controlsurfaces. Attached E. coli K12 from a suspension with 10°cells per mL on gold (A) and oxidized PSBEDOT (D); the viability of the attached E. coliK12 on gold (B) and oxidized PSBEDOT (E) after subjection to 0.6 V for 1 hour; and the remaining E. coli K12 on gold (C) and oxidized PSBEDOT (F)after subjection to 0 V for 1 hour. In the viability study, bacterial cells were stained using a LIVE/DEAD BacLight Bacterial Viability assay kit. Cellswith a damaged cytoplasm membrane are in yellow and red, and cells with an intact cytoplasm membrane are in green. Fig. 5 Quantitative bacterial adhesion, antimicrobial and releasestudies on PSBEDOT and control surfaces. (A) Attachment of E. coli K12from a suspension with 10° cells per mL on oxidized (Ox) PSBEDOT,reduced (Red) PSBEDOT and controlsurfaces; (B) bactericidal activityresults of PSBEDOT and the control surface against E. coli K12 aftersubjection to 0.6 V for 1 hour; and (C) detachment of E. coli K12 fromoxidized PSBEDOT and gold after subjection to 0 V for 1 hour. antimicrobial study. As shown in Fig. 5C, 96.7% of E. coli K12cells on the PSBDEOT surface were released within 1 hour underthe static conditions after the potential was decreased to 0 Vfrom 0.6 V, while only 30% of the cells on the gold surface werereleased. The final cell density on PSBEDOT was less than 3% ofthat on the gold substrate. It should be noted that the release ofkilled bacterial cells is critical for implanted materials, since the attached dead cells may cause chronic inflammation and leadto the failure of implanted materials/devices. Previously severalswitchable antifouling/antimicrobial materials have been re-ported.18,20,32 These zwitterionic polymers can undergo ringformation to become cationic under low pH conditions (pH<5)and can switch back to their zwitterionic state under neutral orbasic conditions. In this work, the electrochemical approachallows for more rapid and active control of the state of thezwitterionic materials. Through this study, we have demon-strated that PSBEDOT surfaces could effectively resist cellattachment in their reduced state, kill the small amount ofattached cells in their oxidized state and release the dead cellsafter switching back to the reduced state. Numerous applications, ranging from the field of solid statetechnology33,34 to biomedical engineering,33,35,36 need to usehigh performance CPs as the key components that determinethe function and properties of the devices, so the developmentof novel multifunctional CPs is of great importance. One of themost attractive features of CPs over traditional biomaterials isthat they could allow electrical stimulation of the attachedtissues and cells. It is expected that the novel PSBEDOT couldbe used to manipulate cell attachment through electrochemicalcontrol and also could serve as a protective coating to reduceprotein adsorption and cell attachment thus prolonging thelifetime of implanted devices. Although there is much work tobe done to fully understand and realize the potential of zwit-terionic conjugated polymers, we believe this work will funda-mentally advance the development of bioelectronics. Conclusions In this work we designed and synthesized a novel antifoulingand electroactive PSBEDOT material. Zwitterionic PSBEDOTcan be facilely polymerized in aqueous solution through anelectrochemical method. The PSBEDOT polymer films exhibitexcellent electrochemical properties, low interfacial impedance,stability and switchable antifouling/antimicrobial properties.The interfacial impedance of PSBEDOT was less than 10% ofthat of bare gold at low frequency. It also showed superiorantifouling properties against whole blood, mammalian cellsand bacteria. The PSBEDOT surface can also be switchedbetween cationic antimicrobial and zwitterionic antifoulingstates by applying different potentials. It can kill over 89% ofattached cells in one hour at 0.6 Vand release over 96.7% of thedead cells in one hour at 0 V under static conditions. It showsgreat promise for applications in bioelectronics. This newmaterial may significantly increase the performance and servicelife, minimize the foreign body reaction, improve the biocom-patibility and reduce the infection of bio-electronic devices. Acknowledgements This work is supported by the US National Science Foundation(ECCS-1200032 and DMR-1454837). We also would like to thankthe National Natural Science Foundation of China (21104008and 51528301) for support. Notes and references ( 1 G. Lanzani, N ature Mater., 2014, 1 3,775-776. ) ( 2 P. J. Molino and G. G. Wallace, APL Mater.,2015,3,014913. ) ( 3 R. A. Green, S. Baek, L. A. Poole-Warren and P . J . Martens,Sci. Technol. Adv. Mater., 2010,11, 014107. ) ( 4 R. A. Green, N. H. Lovell, G. G. Wallace and L. A. Poole-Warren, Biomaterials, 2008,29,3393-3399. ) ( 5 X. Y. Cui and D . C. Martin, Sens. Actuators, A, 2003, 103,384- 394. ) ( .6 H. Shirakawa,E. J.Louis, A. G. Macdiarmid, C. K. Chiang and A. J. Heeger, J . Chem. Soc., Che m . Commun., 1977,578-580, DOI: 10.1039/C39770000578. ) ( 7 W. S. Huang, B . D . H umphrey and A. G. Ma c diarmid, J.Chem. S oc., Faraday Trans. 1,1986,82 , 2385-2400. ) ( 8 A. F . D iaz, J. I . Castillo, J. A. Logan a n d W. Y. L ee, J. Electroanal. Chem., 1981, 129,115-132. ) ( 9 R.J. Waltman,J. Bargon and A. F . D iaz,J. Phys. Ch e m., 1983, 87,1459-1463. ) ( 10 M. Gerritsen, A. . Kros, V . \ S p rakel, J. A. Lutterman. R. J . M. Nolte and J. A. Jansen, Biomaterials,2000, 21,71-78. ) ( 11 B . D . R atner, Biomaterials science: an introduction to materials in medicine, Elsevier Academic P ress, Amsterdam, Boston, 2nd edn, 2004. ) ( 12 K . P. R. Nilsson and O. Inganas, Nat. M a ter., 2003, 2, 419. ) ( 13 L. M. Lira and S . I . C. de Torresi, Electrochem. Commun., 2005, 7 ,717-723. ) ( 14 S. Brahim, D. N a rinesingh an d A. Gui s eppi-Elie, Biosens. Bioelectron., 2002, 17,53-59. ) ( 15 M. C. Cushing and K. S. Anseth, Science, 2007,316,1133- 1134. ) ( 16 N . M ano,J. E. Yoo, J. Tarver, Y . L. Loo a n d A. Heller, J. Am.Chem. Soc., 2007, 129, 7006-7007. ) ( 17 L. J. Pan, G. H. Yu, D. Y. Zhai, H. R. Lee, W. T. Zhao, N. Liu,H. L . Wang, B . C. K . Tee, Y. Shi, Y. C ui and Z. N . B a o, Proc. Natl. Acad. Sci. U. S. A., 2012,109, 9 287-9292. ) ( 18 B . Cao,L. L. Li, Q. Tang and G. Cheng, Biomaterials,2013,34, 7592-7600. ) ( 19 B . Cao,L. L. Li, H. Y. Wu, T. Qiong, B. B. Sun, H. Dong,J. Zhe and G. Cheng, Chem. Commun., 2014, 50,3234-3237. ) ( 20 B. Cao, Q. Tang, L . L. Li, J . Humble, H. Wu, L . L iu a nd G. Cheng, Adv. H ealthcare Mater., 2013, 2, 1096-1102. ) 21 B. Cao, Q. Tang, L. L. Li, C. J. Lee, H. Wang, Y. Q. Zhang,H. Castaneda and G. Cheng, Chem. Sci., 2015,6,782-788. ( 22 B . Y. Ouyang, C. W. Chi, F. C. Chen,Q. F. Xi and Y. Yang,AdvFunct. Mater.,2005,15,203-208. ) ( 23 G . A. Sotzing, J. R. Reynolds and P. J. Steel, Adv. Mater.,1997, 9, 795-798. ) ( 24 C. L. Gaupp, K. W. Z ong, P . S chottland, B. C. Thompson,C. A. Thomas and J . R . R eynolds, M acromolecules, 2000, 3 3, 1132-1133. ) ( 25 T. Erb, U. Zhokhavets, G. Gobsch, S . R aleva, B. S tuhn, P. Schilinsky, C. Waldauf a n d C. J. Br a bec, Adv. Fun c t. Mater.,2005, 1 5,1193-1196. ) ( 26 J . L. Segura,R. Gomez, R. Blanco, E. Reinold and P. Bau e rle,Chem. Mater., 2006, 18,2834-2847. ) ( 27 X . Y . C ui and D . C . M a rtin, Sens. Actuators, B, 2 0 03, 89, 92- 102. ) ( 28 B . Z hu, S.-C. L uo, H. Zhao, H . -A. L i n, J. Sekine, A. Nakao, C. Chen, Y. Yamashita and H.-h. Yu, Nat. Commun., 2014, 5,4523. ) ( 29 M. R. Abidian andD. C. Martin, Biomaterials,2008,29,1273- 1283. ) ( 30 G . C heng,Z. Zh a ng, S. F. Chen, J. D . Bryers and S. Y. Jiang, Biomaterials, 2007, 28, 4192-4199. ) ( 31 G. C heng, G. Z . Li, H. Xue, S . F. Chen, J. D . Bryers and S. Y. Jiang, Biomaterials, 2009, 30, 5234-5240. ) ( 32 Z. Q. Cao, L . Mi, J . Mendiola, J . R. Ella-Menye, L. Zhang,H. X ue a n d S. Y. Jiang, Angew. Ch e m., Int. Ed., 20 1 2, 51, 2602-2605. ) ( 33 A. Facchetti, Chem. Mater., 2011,23,733-758. ) ( 34 Y .-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2 0 09,109, 5868-5923. ) ( 35 C . Zhu, L. Liu, Q. Yang, F. Lv and S. Wang, Chem. Rev.,2012, 112, 4687-4735. ) ( 36 D . C. M artin, MRS C o mmun., 2015, 1-2 3 , first view. ) ( 37 C . E. Schmidt, V. R. Shastri, J. P . Vacanti and R. Langer, Proc.Natl. Acad. Sci. U . S. A., 1997,94, 8948-8953. ) Chem. Sci., , his journal is O The Royal Society of Chemistry This journal is @ The Royal Society of Chemistry hem. Sci., GAMRY Reference 600+软件功能强大,操作简便。硬件设计独特,性能稳定。GAMRY Reference 600+电化学综合测试仪可以满足电池、材料表征、生物传感器、电化学机理、点分析化学、腐蚀与防护、痕量物质检测、电化学合成等多种电化学研究领域。对每种测试都提供了完美的解决方案。GAMRY Reference 600+技术优势:(1) 电流分辨率高,20 aA,有实验数据证实。低电流到pA。(2) 仪器的背景电流小,<2 μV rms。(3) 仪器的高阻抗测试/低阻抗测试性能好,100TOHM, 0.1 mOHM 等。(4) 软件友好,容易学习,和功能全;可以扩展任何需要的电化学测量。(5) 再加一台仪器,可以和SECM, IMPS/IMVS 等兼容。(6) 和RAMNAN, UV, TEM, EQCM, RDE 等联用。(7) 浮地设计最好,可以和高压釜合用。(8) 有温度测试模块。(9) 软件自动编程,进行电化学测量。(10) EIS阻抗频率范围:10μHz到5 MHzReference 600+是典型的科学研究级电化学分析仪器,它使用USB接口,可以直接与便携式计算机和台式计算机连接,支持功能强大的Gamry应用软件包,例如DC105™直流腐蚀软件包,PHE200™物理电化学软件包,EIS300™电化学阻抗谱软件包等,几乎涵盖所有电化学分析方法。Reference 600+电化学综合测试仪的输出电流范围从±600 mA至±60 pA,拥有11档电流范围和档位快速补偿能力。Reference 600+的输出电压为±22V,恒电位扫描电压为±11V。进行CV实验时,最快的扫描速率能达到1200V/s。这些特点保证了Reference 600+能够适应高腐蚀速率及具有良好导电能力溶液的电化学体系。Reference 600+的直接信号综合电路允许EIS频率扫描范围从10μHz到5 MHz,在1MHz情况下进行EIS测量时的误差小于2%,是真正意义上的高频电化学交流阻抗测试系统。强大的电压和电流信号过滤系统能保证在嘈杂的环境中得到稳定安静的测试环境。优异的稳定性保证即使电脑运行其他程序时也可正常工作。Reference 600+提供了独一无二的结合能力,具有电流中断和阳极反馈IR补偿能力。 Reference 600+ 的电化学软件Gamry为Reference600设计了一套完备的电化学应用软件程序。电化学试验在Gamry FrameworkTM上进行,其数据在电化学分析软件Echem AnalysTM中进行处理。PWR800电化学能源软件包PWR800软件包为测试和表征能源存储和转化期间以及材料领域提供了标准测试技术。我们将大功率循环伏安,充电,放电,循环充放电,以及其他常用大电流测试组合起来。当然,您同样也可以采用Gamry序列测试将所需要的特殊测试按顺序组合起来。EIS电化学阻抗谱EIS对各种各样的应用来说是一个功能强大的工具,对PWR800软件极好的补充。Gamry开发的EIS软件—易于使用、程序简洁。它提供了许多不同EIS测试技术,包括恒电位、恒电流、混合控制模式。 可使用单一正弦波测量,也可利用多重正弦波进行快速EIS的测量。同样来自于Gamry’s扩展软件包PHE200 物理电化学—一般电化学研究软件包。PV220 脉冲伏安—标准和可定制的脉冲实验。DC105 直流腐蚀—腐蚀测试过程中遇到的素有标准技术。ESA410 电化学信号分析—电化学噪声信号的获取和分析。EN120 电化学噪声—简单的噪声软件。EFM140 EFM—模拟Tafel常数和腐蚀速率。CPT110 临界点蚀温度—ASTM标准化测试。 VFP600 可视前面板—基础电化学控制,成本低廉。REFERENCE 600+恒电位仪/恒电流仪/零电阻电流仪/浮地设计(与地绝缘) 重量3 公斤尺寸9 (W) x 19 (H) x 27 (D) 27 厘米 系统电极连接2,3, 4或者5电极最大电流±600 mA电流范围11 (60 pA - 600 mA)电流量程(包括内部增益)13最小电流分辨率20 aA最大施加电位±11 V上升时间<250 ns最小时间基数3.333 μs噪声和纹波(典型的)<2 μV rms 控制放大器槽压± 22 V最大电流> ± 600 mA速度设定5 EIS 测量频率范围10 μHz - 5 MHz施加电压振幅3 V max施加电流振幅600 mA max 电位计输入阻抗 > 1014 Ω || < 0.2 pF输入电流 < 10 pA带宽 > 15 MHz at -3 dB 电位施加精度±1mV±0.2% 设置范围施加分辨率200 μV, 50 μV, 12.5 μV/bit 测量精度±1mV±0.2% 读数范围测量分辨率1μV,10μV,100μV, 400 μV /bitt 电流施加/测量精度±10 pA±0.05% ;量程±0.2%值(600 mA-6nA)或者0.75%值(600 pA) 或者1.5%值(60 pA)施加/测量分辨率0.003%带宽> 10 MHz (600 mA – 600 μA),>0.15 MHz (6 μA)
确定


还剩4页未读,是否继续阅读?
北京亿诚恒达科技有限公司为您提供《磺基甜菜碱-3,4-亚乙基二氧噻吩中导电性,稳定性检测方案(电化学工作站)》,该方案主要用于原料药中理化性质检测,参考标准--,《磺基甜菜碱-3,4-亚乙基二氧噻吩中导电性,稳定性检测方案(电化学工作站)》用到的仪器有电化学工作站Reference 600+
推荐专场
相关方案
更多
















