方案详情
文
Cyclodextrin-enhanced room-temperature phosphorescence (CD-RTP) shows promise as a sensitive analytical technique. Sample preparation is simple, data-acquisition and processing are easily automated with a Fluorolog® spectrometer, and the result is an intense, well-structured RTP signal. CD-RTP offers the advantages of increased selectivity based on molecular geometry, and partial immunity to quenching by dissolved O2 molecules.
方案详情

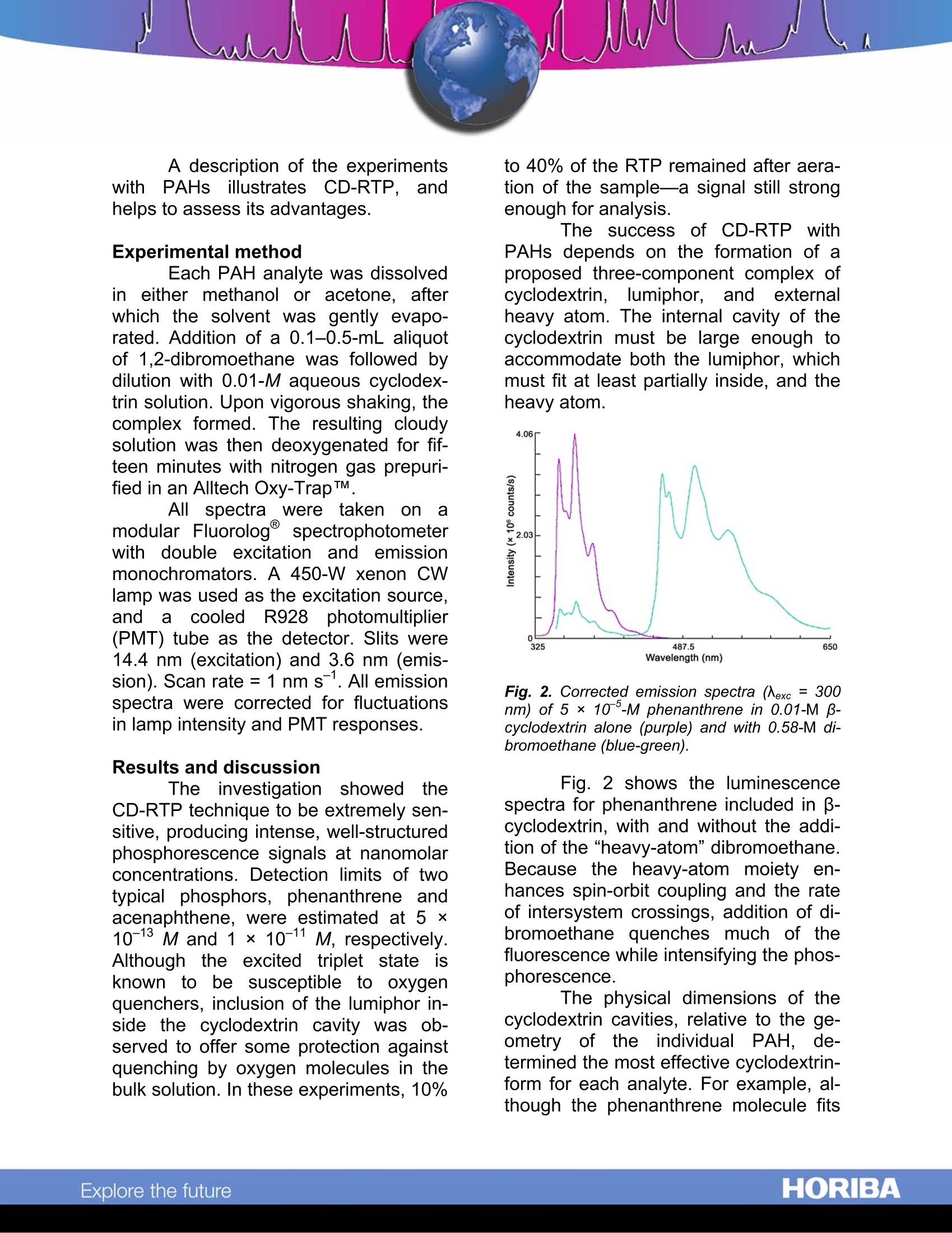
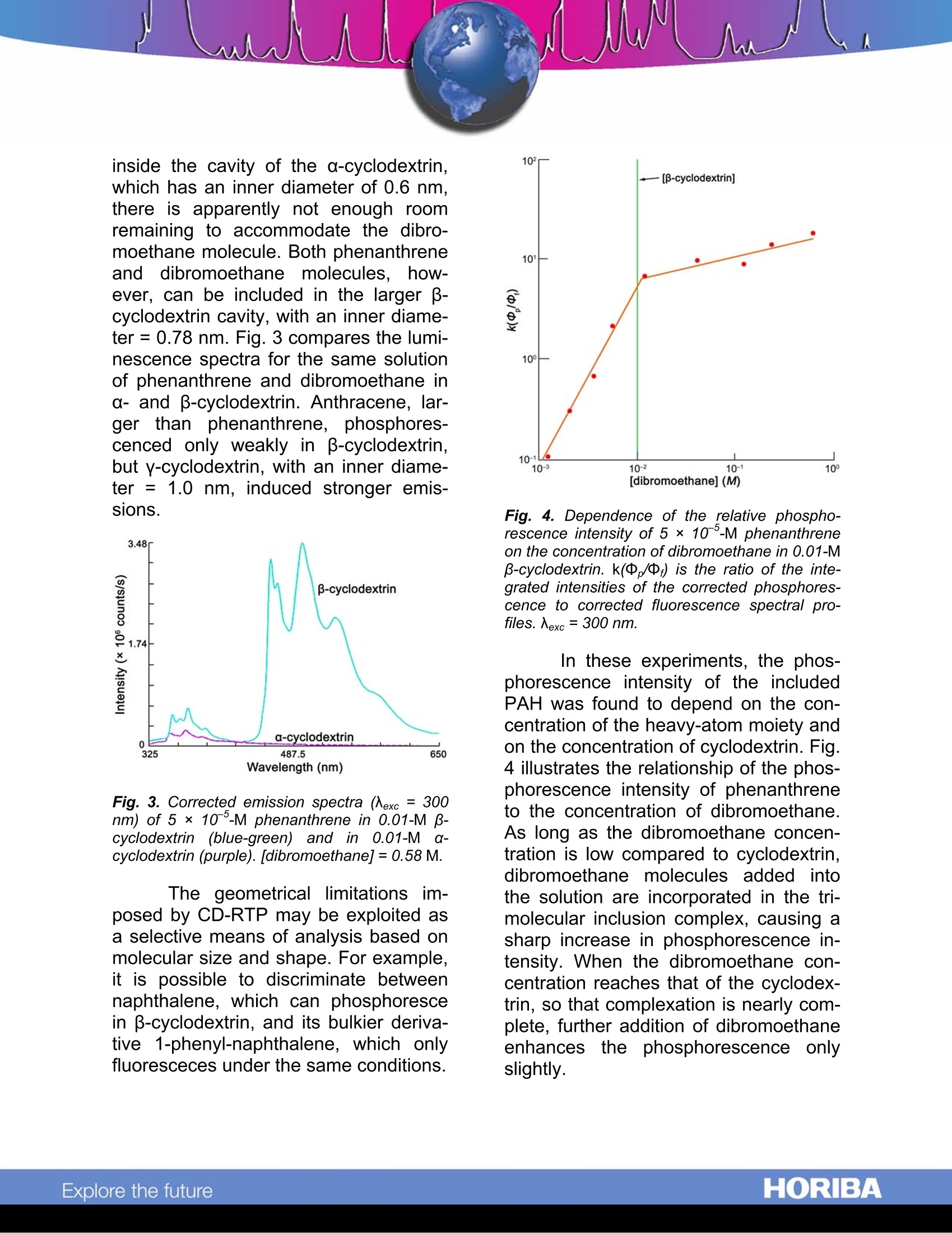
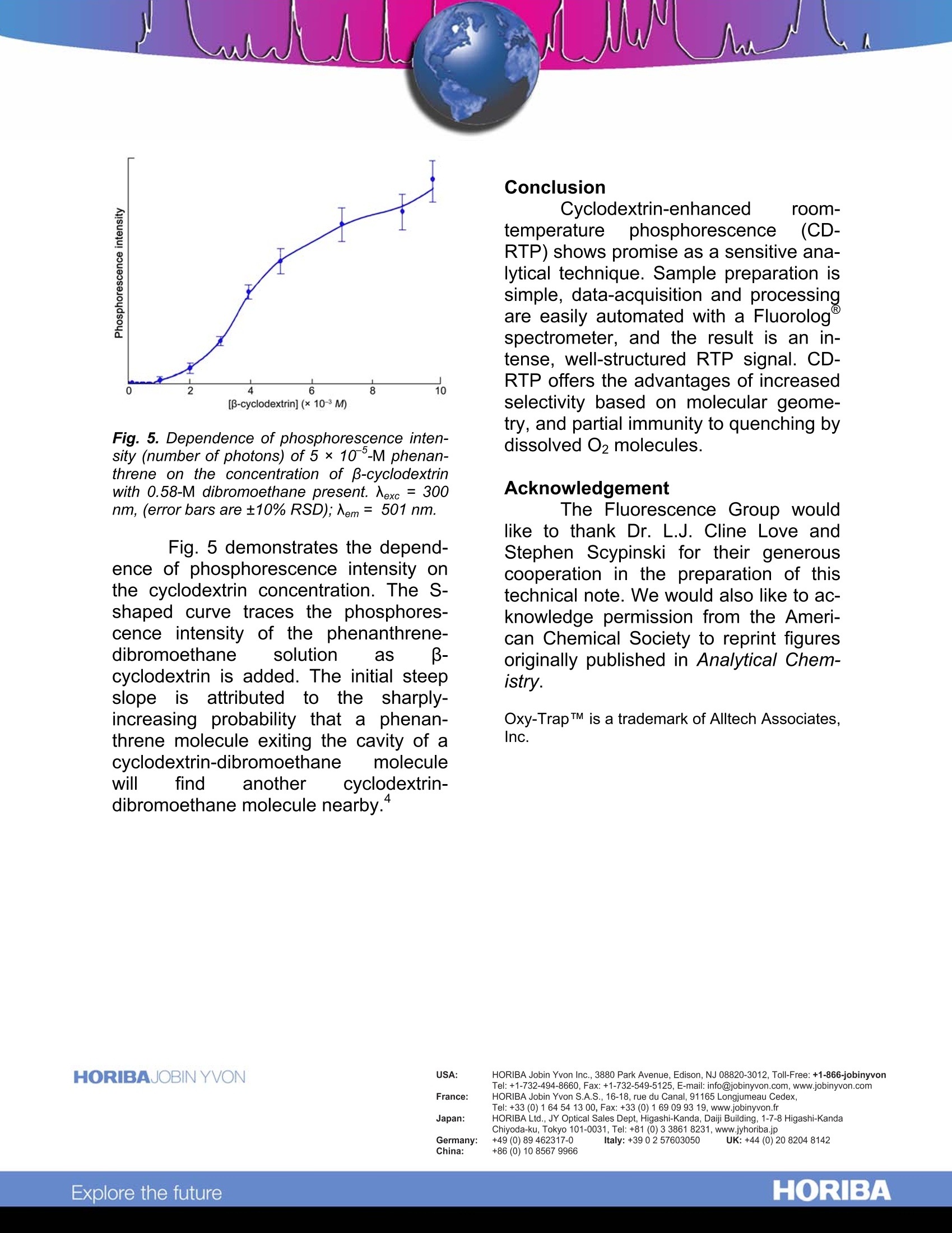
Cyclodextrin-Enhanced Room-TemperaturePhosphorescence Abstract Cyclodextrin-enhanced room-emperature phosphorescence (CD-RTP) is an analytical technique that of-fers well-resolved spectra and subpico-gram detection limits. When polynucleararomatic hydrocarbons (PAHs) are in-cluded in cyclodextrin molecules in thepresence of a heavy-atom moiety suchas1,2-dibromoethane, they exhibit in-tense,well-defined phosphorescenceemissions, often with distinct vibrationalstructure. Moreover., the cyclodextrinmoiety inhibits quenching of PAH phos-phorescence by O2 present in the bulksolution. Introduction Cyclodextrins (CDs) are made upof glucose monomers coupled to form arigid, conical structure with an interiorhydrophobic cavity. The most commoncyclodextrins, a, B, and y, are com-posed, respectively, of six, seven, andeight monomers. Initially, each cyclo-dextrin contains one or more watermolecules, produced during the mono-mer coupling. Because these includedwater molecules are easily displaced byhydrophobic species that fit into thecyclodextrin interior, cyclodextrins havea unique ability to form stable inclusioncomplexes with a variety of molecules. This inclusion capability has ledto an important use of cyclodextrins inluminescence spectroscopy. Cyclodex-trins have been found to enhance fluo-rescent and phosphorescent emissionsfrom molecules present in the CD cav- ity.1,2,3,4,5’Of particular interestit is thephenomenon of CD-RTP, which sug-gests a highly selective analytical tech-nique based on the molecular geometryofof the lumiphor. Chromophores such1as PAHsexhibit virtually no phosphorescence inconventional fluid media. Scypinski andCline Love report, however, that intenseroom-temperature phosphorescenceoccurs when these carcinogens are pre-sent, with an external heavy atom, in thecavity of cyclodextrin. Fig. 1 depicts theinclusion of a phenanthrene moleculewithin the cavity ofa B-cyclodextrin.Scypinski and Cline Love also have ob-served CD-RTP in nitrogen heterocyclesand bridged biphenyls.’ Fig. 1. Axial and equatorial inclusions of phe-nanthrene within B-cyclodextrin. ( . 1 Hoshino, M.;I m amura, M.; Ikehara, K.;Hama, Y. J.Phys. Chem. 1981, 85,1820. ) ( Turro, N.J.; Bolt, J.D.; Duroda,Y.;Tabushi,I. Photo-chem. Photobiol. 1982,35, 69. ) °Turro, N.J.; Cox., G.S.; Li, X. Photochem. Photobiol.41983,37,149. " Scypinski, S.; Cline Love, L.J. Anal. Chem. 1984,56, 322. ° Scypinski, S.; Cline Love, L.J. Anal. Chem. 1984,56,331. A description of the experimentswit1h PAHs; illustrates; CD-RTP.,andhelps to assess its advantages. Experimental method Each PAH analyte was dissolvedin either methanol or acetone, afterwhich the solvent was gently evapo-rated. Addition of a 0.1-0.5-mL aliquotof 1,2-dibromoethane was followed bydilution with 0.01-M aqueous cyclodex-trin solution. Upon vigorous shaking,thecomplex formed. The resulting cloudysolution was then deoxygenated for fif-teen minutes with nitrogen gas prepuri-fied in an Alltech Oxy-TrapTM All. spectra were taken onamodular Fluorolog@ spectrophotometerwith double excitation and emissionmonochromators. A 450-W xenon CWlamp was used as the excitation source,anda cooledR928photomultiplier(PMT) tube as the detector. Slits were14.4 nm (excitation) and 3.6 nm (emis-sion). Scan rate =1 nm s. All emissionspectra were corrected for fluctuationsin lamp intensity and PMT responses. Results and discussion The investigation :showed theCD-RTP technique to be extremely sen-sitive, producing intense, well-structuredphosphorescence signals at nanomolarconcentrations. Detection limits of twotypical phosphors, phenanthrene andacenaphthene, were estimated at 5 x10-13 M and 1 ×10-11 M, respectively.Although the excited triplet stateisknown to be susceptible to oxygenquenchers, inclusion of the lumiphor in-side the cyclodextrin cavity was ob-served to offer some protection againstquenching by oxygen molecules in thebulk solution. In these experiments, 10% to 40% of the RTP remained after aera-tion of the sample-a signal still strongenough for analysis. The successofCD-RTP withPAHs depends on the formation of aproposed three-component complex ofcyclodextrin, lumiphor, and externalheavy atom. The internal cavity of thecyclodextrin must be large enough toaccommodate both the lumiphor, whichmust fit at least partially inside, and theheavy atom. Fig. 2. Corrected emission spectra (Nexc = 300nm) of 5 × 10 -M phenanthrene in 0.01-M p-cyclodextrin alone (purple) and with 0.58-M di-bromoethane (blue-green). Fig. 2 shows the luminescencespectra for phenanthrene included in -cyclodextrin, with and without the addi-tion of the"heavy-atom”dibromoethane.Because the heavy-atom moiety en-hances spin-orbit coupling and the rateof intersystem crossings, addition of di-bromoethane quenches much of thefluorescence while intensifying the phos-phorescence. The physical dimensions of thecyclodextrin cavities, relative to the ge-ometry of the iindividual PAH, de-termined the most effective cyclodextrin-form for each analyte.For example, al-though the phenanthrene molecule fits inside the cavity of the a-cyclodextrin,which has an inner diameter of 0.6 nm.there is apparently not enough roomremaining to accommodate the dibro-moethane molecule. Both phenanthreneand dibromoethane molecules, Ihow-ever, can be included in the larger -cyclodextrin cavity, with an inner diame-ter=0.78 nm. Fig.3 compares the lumi-nescence spectra for the same solutionof phenanthrene and dibromoethane ina- and B-cyclodextrin. Anthracene, lar-ger than phenanthrene, phosphores-cenced only weakly in B-cyclodextrin,but y-cyclodextrin, with an inner diame-ter = 1.0 nm, induced stronger emis-sions. Fig. 3. Corrected emission spectra (Aexc = 300nm) of 5× 10-M phenanthrene in 0.01-M B-cyclodextrin (blue-green) and in 0.01-M(0-cyclodextrin (purple). [dibromoethane]=0.58 M. The geometrical limitations im-posed by CD-RTP may be exploited asa selective means of analysis based onmolecular size and shape. For example,it is possible to discriminate betweennaphthalene, which can phosphorescein B-cyclodextrin, and its bulkier deriva-tive 1-phenyl-naphthalene, which onlyfluoresceces under the same conditions. Fig. 4. Dependence of the relative phospho-rescence intensity of 5×10 °-M phenanthreneon the concentration of dibromoethane in 0.01-M-cyclodextrin. k(中,/中;) is the ratio of the inte-grated intensities of the corrected phosphores-cence to corrected fluorescence spectral pro-files. Nexc=300 nm. In these experiments, the phos-phorescence intensity of the includedPAH was found to depend on the con-centration of the heavy-atom moiety andon the concentration of cyclodextrin. Fig.4 illustrates the relationship of the phos-phorescence intensity of phenanthreneto the concentration of dibromoethane.As long as the dibromoethane concen-tration is low compared to cyclodextrin,dibromoethane molecules added intothe solution are incorporated in the tri-molecular inclusion complex, causing asharp increase in phosphorescence in-tensity. When the dibromoethane con-centration reaches that of the cyclodex-trin, so that complexation is nearly com-plete, further addition of dibromoethaneenhancesthe phosphorescence onlyslightly. Fig. 5. Dependence of phosphorescence inten-sity (number of photons) of 5 ×10 -M phenan-threne on the concentration of p-cyclodextrinwith 0.58-M dibromoethane present. Nexc =300nm, (error bars are±10%RSD); Nem= 501 nm. Fig. 5 demonstrates the depend-ence of phosphorescence intensity onthe cyclodextrin concentration. The S-shaped curve traces the phosphores-cence intensity of the phenanthrene-dibromoethane solution as -cyclodextrin is added. The initial steepslopeis attributedto the sharply-increasing probability that a phenan-threne molecule exiting the cavity of acyclodextrin-dibromoethane moleculewill find another cyclodextrin-hy 4dibromoethane molecule nearby." Conclusion Cyclodextrin-enhanced room-temperature phosphorescence (CD-RTP) shows promise as a sensitive ana-lytical technique. Sample preparation issimple, data-acquisition and processingare easily automated with a Fluorologspectrometer, and the result is an in-tense, well-structured RTP signal. CD-RTP offers the advantages of increasedselectivity based on molecular geome-try, and partial immunity to quenching bydissolved O2 molecules. Acknowledgement The Fluorescence Group wouldlike to thank Dr. L.J. Cline Love andStephen Scypinski for their generouscooperation in the preparation of thistechnical note. We would also like to ac-knowledge permission from the Ameri-can Chemical Society to reprint figuresoriginally published in Analytical Chem-istry. Oxy-TrapTM is a trademark of Alltech Associates,Inc. Copyright C HORIBA Jobin Yvon; version .HORIBAExplore the future HORIBAExplore the future Cyclodextrin-enhanced room-temperature phosphorescence (CD-RTP) shows promise as a sensitive analytical technique. Sample preparation is simple, data-acquisition and processing are easily automated with a Fluorolog® spectrometer, and the result is an intense, well-structured RTP signal. CD-RTP offers the advantages of increased selectivity based on molecular geometry, and partial immunity to quenching by dissolved O2 molecules.
确定

还剩2页未读,是否继续阅读?
HORIBA(中国)为您提供《多环芳香烃中环湖精(Cyclodextrin)增强的室温磷光技术检测方案(分子荧光光谱)》,该方案主要用于其他中环湖精(Cyclodextrin)增强的室温磷光技术检测,参考标准--,《多环芳香烃中环湖精(Cyclodextrin)增强的室温磷光技术检测方案(分子荧光光谱)》用到的仪器有HORIBA Fluorolog®-3科研级荧光光谱仪
推荐专场
相关方案
更多
该厂商其他方案
更多