
方案详情
文
从1970到1980,美国对塑料的需求从200亿磅增加到了500亿磅。塑料的成功主要是由于其广泛的应用,由于其广泛的性能,塑料可以替代金属、玻璃、陶瓷、纸张、天然纤维、包装材料、消费产品、管道、家具,等等……
塑料最重要的性能在于其重量轻,耐破损,能巩固功能部件,想对便宜,易于自动化生产,生产成本较低……
方案详情

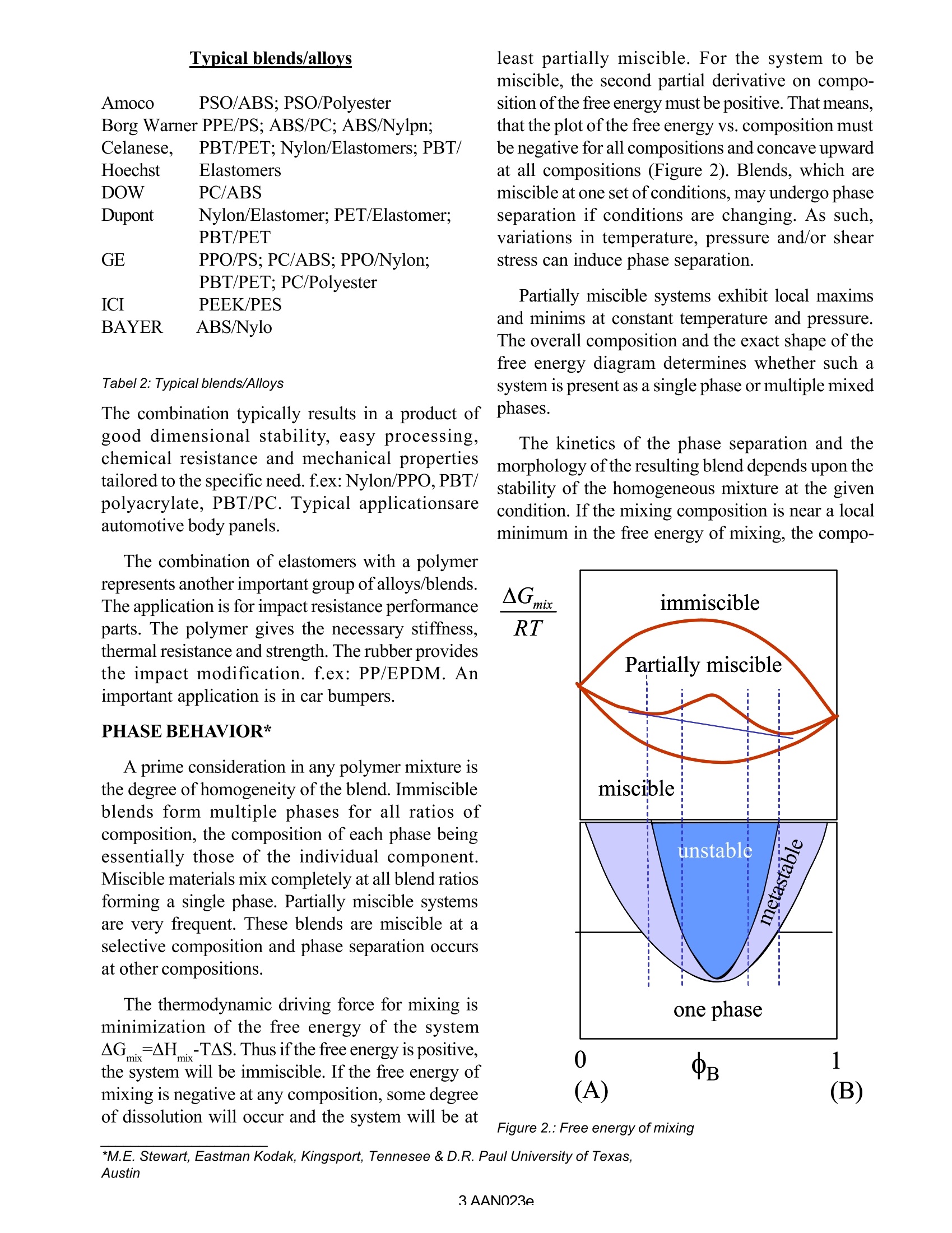
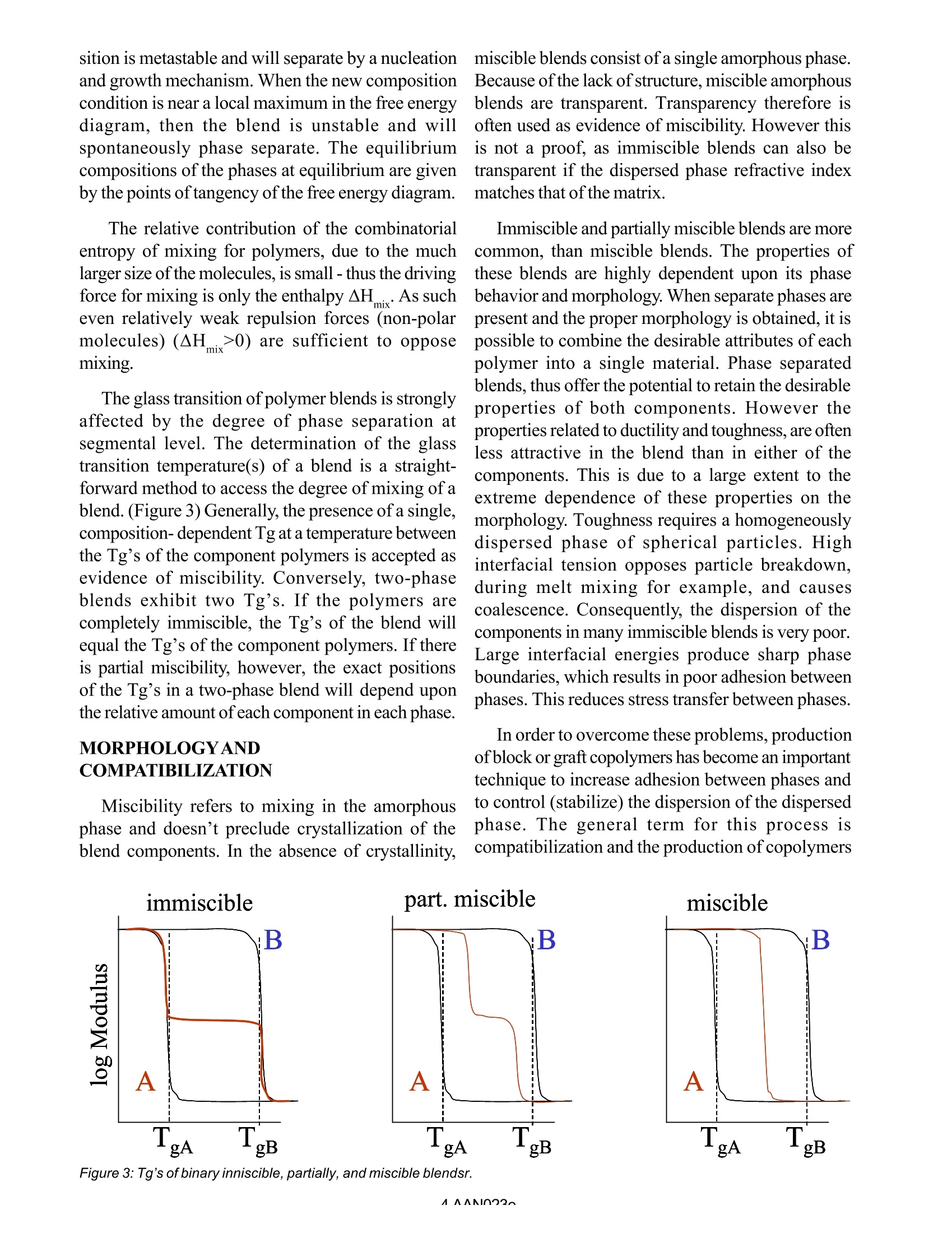
AAN023 Introduction to Polymer Blends and Alloys* A. Franck, TA Instruments Germany Keywords: blends, alloys, compatibilization, glass transition, mixing enthalpy, synergy, miscibility SCOPE From 1970 to 1980, the demand for plastics inthe United States increased from 20 to 50 billionpounds. The success of plastics is due to the broadspectrum of applications, attributable to a wide rangeof properties which made plastic replace metals,glass, ceramics, paper, natural fibers, packaging,consumer products,piping, furniture, etc... The important properties of plastics are lightweight, breakage resistWCIIance, ability to consolidatefunctional parts, relatively inexpensive, easyautomated production, lower costs of manu/facturing,.. With the accomplishment of substituting allpossible traditional materials, slower economic andpopulation growth in the developed regions of theword and polymer down grading (due to environ/mental protection, reinstitution of older values)becoming a significant trend, the polymer growthrates slowed down. Intercompetition amongpolymers in addition has increased and thedevelopment risk of new polymers often outweighsthe potential commercial reward. These factors favored the relative inexpensiveroute of combining two or more commercialavailable polymers through alloying or blending inorder to create product differentiation for newapplications. The blending technology turned out to be anexcellent tool for tailoring a polymer compound forspecific applications requirements, often at muchlower production costs then the current available 0B 0 1 Figure 1: Concentration diagramm of blend & alloys. material. Alloys and blends developments are marketdriven and demand an on going dialogue betweensupplier and customer. The continuous modificationand adjustment of blends and alloys for newapplications requires an extensive understanding ofthe relation of material structure, processing and end-use performance. Material testing instrumentsincluding Rheometers, dynamic mechanical ana/lyzers, etc...are a fundamental need o insure thisdevelopment ALLOYS AND BLEND TECHNOLOGY In order to mix two components and obtain aproduct, which doesn’t undergo complete phaseseparation during processing or enduse, a minimumthermodynamic compatibility is necessary. Beyondthis level of compatibility, greater attractive forces ( *Reference: A dvances in Polymer blends a n d Alloys Technology Volume 1; Ch a pter 1 by S.Y. Kienzle ) between the constituents serve to enhance theproperties of the final product. In general for two component mixture, anyproperty can be described by the following equation: P. is the property value, c. the concentration and Ithe interaction coefficient. For I>0, the resultantpolymer has better properties then the arithmeticaverage of the property. For I-0, the properties are additive For I=, the resultant polymer has properties,worse than the arithmetic average ofthe components. Alloys are synergistic polymer combinations(I>0), if the components are thermodynamicallycompatible, or strong intermolecular forces make themixture behave as a single phase material with onesingle transition (TG). f.ex: PS modified PEO(Noryl) or PS modified PPE (Prevex). For theseblends, PS reduces the melt viscosity sufficiently toallow easy processing and largely retainingstrength(modulus), impact resistance, hydrolyticstability of the PEO or PPE. O Blends show less intense thermodynamiccompatibility and therefore exhibit discrete polymerphases with multiple glass transitions. In general,the properties of blends reflect the weightedarithmetic average of the properties of the consti/tuents (I=0). f.ex: PC/ABS blends. Specific gravity,coefficient of thermal expansion, flexural modulus,etc... are arithmetic averages. Crystallinepolymers Amorphous polymers properties ·chemical resistance ·dimensional stability and · sharp melting point warpage resistance polymers ·low viscosity melt clarity good flexural and heat good impact strength distorsion properties with ·No property changes at high temperatures reinforcements ·melting range PP ABS Nylon 1PC PBT poly sulfone poly acrylate Tabel 1: Crystalline and amorphous properties and polymers There are different ways to mix polymers, meltblending, solution blending, latex or dispersionblending. The melt blending using an extruder is pre-dominately used method to prepare blends.Compatibilizing agents may be incorporated in thesame time in order to enhance the synergism(compounds with functional groups that mate withthe dissimilar polymers) to prevent phase separationand to enhance the physical and mechanicalproperties; for example: block co-polymers. SELECTINGPOLYMER COMBINATIONS Polymer components, which make the blend, aregenerally selected to complement each other in oneor more of the following properties,costs,processability, mechanical properties, warpage,chemical resistance, thermal performance Blends are typically viewed as cost savings: -the more expensive polymer is combined with theless expensive product to provide adequateperformance at a significant reduced price (ABS/PC, ABS/Polysulfone, PES/PEEK). In addition, thedevelopment of a synergistic property profile (I>0)is the goal. Very often crystalline and amorphous polymersare blended to achieve a specific property range.Crystalline polymers have excellent chemicalresistance, good mechanical properties (especiallywith reinforcements), low viscosity whereasamorphous polymers provide good dimensionalstability (no warpage) and excellent impact strength. Tabel 2: Typical blends/Alloys The combination typically results in a product ofgood dimensional stability, easy processing,chemical resistance and mechanical propertiestailored to the specific need. f.ex:Nylon/PPO,PBT/polyacrylate, PBT/PC. Typical applicationsareautomotive body panels. The combination of elastomers with a polymerrepresents another important group of alloys/blends.The application is for impact resistance performanceparts. The polymer gives the necessary stiffness,thermal resistance and strength. The rubber providesthe impact modification. f.ex: PP/EPDM. Animportant application is in car bumpers. PHASE BEHAVIOR* A prime consideration in any polymer mixture isthe degree of homogeneity of the blend. Immiscibleblends form multiple phases for all ratios ofcomposition, the composition of each phase beingessentially those of the individual component.Miscible materials mix completely at all blend ratiosforming a single phase. Partially miscible systemsare very frequent. These blends are miscible at aselective composition and phase separation occursat other compositions. The thermodynamic driving force for mixing isminimization of the free energy of the systemAG=^H.-TAS. Thus if the free energy is positive,mixthe system will be immiscible. If the free energy ofmixing is negative at any composition, some degreeof dissolution will occur and the system will be at least partially miscible. For the system to bemiscible, the second partial derivative on compo-sition of the free energy must be positive. That means,that the plot of the free energy vs. composition mustbe negative for all compositions and concave upwardat all compositions (Figure 2). Blends, which aremiscible at one set ofconditions, may undergo phaseseparation if conditions are changing. As such,variations in temperature, pressure and/or shearstress can induce phase separation. Partially miscible systems exhibit local maximsand minims at constant temperature and pressure.The overall composition and the exact shape of thefree energy diagram determines whether such asystem is present as a single phase or multiple mixedphases. The kinetics of the phase separation and themorphology of the resulting blend depends upon thestability of the homogeneous mixture at the givencondition. If the mixing composition is near a localminimum in the free energy of mixing, the compo- Figure 2.: Free energy of mixing ( *M.E. Stewart, Eastman Kodak, Ki n gsport, Tennesee & D.R. Paul University of Texas, Austin ) sition is metastable and will separate by a nucleationand growth mechanism. When the new compositioncondition is near a local maximum in the free energydiagram, then the blend is unstable and willspontaneously phase separate. The equilibriumcompositions of the phases at equilibrium are givenby the points of tangency of the free energy diagram. The relative contribution of the combinatorialentropy of mixing for polymers, due to the muchlarger size of the molecules, is small- thus the drivingforce for mixing is only the enthalpyAH.. As sucheven relatively weak repulsion forces (non-polarmolecules) (^H m>ix0) are sufficient to opposemixing. The glass transition of polymer blends is stronglyaffected by the degree of phase separation atsegmental level. The determination of the glasstransition temperature(s) of a blend is a straight-forward method to access the degree of mixing of ablend. (Figure 3) Generally, the presence ofa single,composition-dependent Tg at a temperature betweenthe Tg’s of the component polymers is accepted asevidence of miscibility. Conversely, two-phaseblends exhibit two Tg's. If the polymers arecompletely immiscible, the Tg’s of the blend willequal the Tg’s of the component polymers. If thereis partial miscibility, however, the exact positionsof the Tg’s in a two-phase blend will depend uponthe relative amount of each component in each phase. MORPHOLOGYANDCOMPATIBILIZATION Miscibility refers to mixing in the amorphousphase and doesn’t preclude crystallization of theblend components. In the absence of crystallinity, miscible blends consist of a single amorphous phase.Because of the lack of structure, miscible amorphousblends are transparent. Transparency therefore isoften used as evidence of miscibility. However thisis not a proof, as immiscible blends can also betransparent if the dispersed phase refractive indexmatches that of the matrix. Immiscible and partially miscible blends are morecommon, than miscible blends. The properties ofthese blends are highly dependent upon its phasebehavior and morphology. When separate phases arepresent and the proper morphology is obtained, it ispossible to combine the desirable attributes of eachpolymer into a single material. Phase separatedblends, thus offer the potential to retain the desirableproperties of both components. However theproperties related to ductility and toughness, are oftenless attractive in the blend than in either of thecomponents. This is due to a large extent to theextreme dependence of these properties on themorphology. Toughness requires a homogeneouslydispersed phase of spherical particles. Highinterfacial tension opposes particle breakdown,during melt mixing for example, and causescoalescence. Consequently, the dispersion of thecomponents in many immiscible blends is very poor.Large interfacial energies produce sharp phaseboundaries, which results in poor adhesion betweenphases. This reduces stress transfer between phases. In order to overcome these problems, productionof block or graft copolymers has become an importanttechnique to increase adhesion between phases andto control (stabilize) the dispersion of the dispersedphase. The general term for this process iscompatibilization and the production of copolymers B T gB Figure 3: Tg’s of binary inniscible, partially, and miscible blendsr. in situ during the melt mixing process is known asreactive compatibilization. The goal of mostcompatibilization schemes is to increase theproperties of the blends while retaining the two-phase nature and not to promote miscibility of theblend. MARKET FOR BLENDSANDALLOYS-LOOKOUT INTHE FUTURE Increased inter-polymer competition withresultant losses in market share and margins forcethe polymer suppliers to focus their attention onproviding polymer systems that offer uniquecompetitive advantage to their customers. With thedevelopment and commercialization of -8 years(compared to 15 to 20 years for new polymers) theblend market is very dynamic. Suppliers arecontinually developing new alloys and blend tosatisfy the changing applications and needs withbetter engineered products and lower cost products. Alloys and blends represent inexpensive routesto satisfy both end use material requirements andsuppliers desires for competitive product differen-tiation. Despite the recession in polymers, thecontinuous development effort of thesuppliers in agrowing market for blends creates a need foradvanced material testing instruments to characterizethe specific mechanical and other physical propertiesof blends in the product development environment. AAN AANI From 1970 to 1980, the demand for plastics in the United States increased from 20 to 50 billion pounds. The success of plastics is due to the broad spectrum of applications, attributable to a wide range of properties which made plastic replace metals, glass, ceramics, paper, natural fibers, packaging, consumer products, piping, furniture, etc...The important properties of plastics are light weight, breakage resistance, ability to consolidate functional parts, relatively inexpensive, easy automated production, lower costs of manufacturing,..With the accomplishment of substituting all possible traditional materials, slower economic and population growth in the developed regions of the word and polymer down grading (due to environmental protection, reinstitution of older values) becoming a significant trend, the polymer growth rates slowed down. Intercompetition among polymers in addition has increased and the development risk of new polymers often outweighs the potential commercial reward.These factors favored the relative inexpensive route of combining two or more commercial available polymers through alloying or blending in order to create product differentiation for new applications.
确定



还剩3页未读,是否继续阅读?
TA仪器为您提供《聚合物共混和合金中性能检测方案(流变仪)》,该方案主要用于其他中性能检测,参考标准--,《聚合物共混和合金中性能检测方案(流变仪)》用到的仪器有TA仪器Discovery流变仪
推荐专场
相关方案
更多
该厂商其他方案
更多















