
方案详情
文
Interactions between water and component materials take place in all steps of dosage from manufacture. These interactions can affect the mechanical, physical and chemical properties and consequently also the behaviour of the material of interest.
方案详情

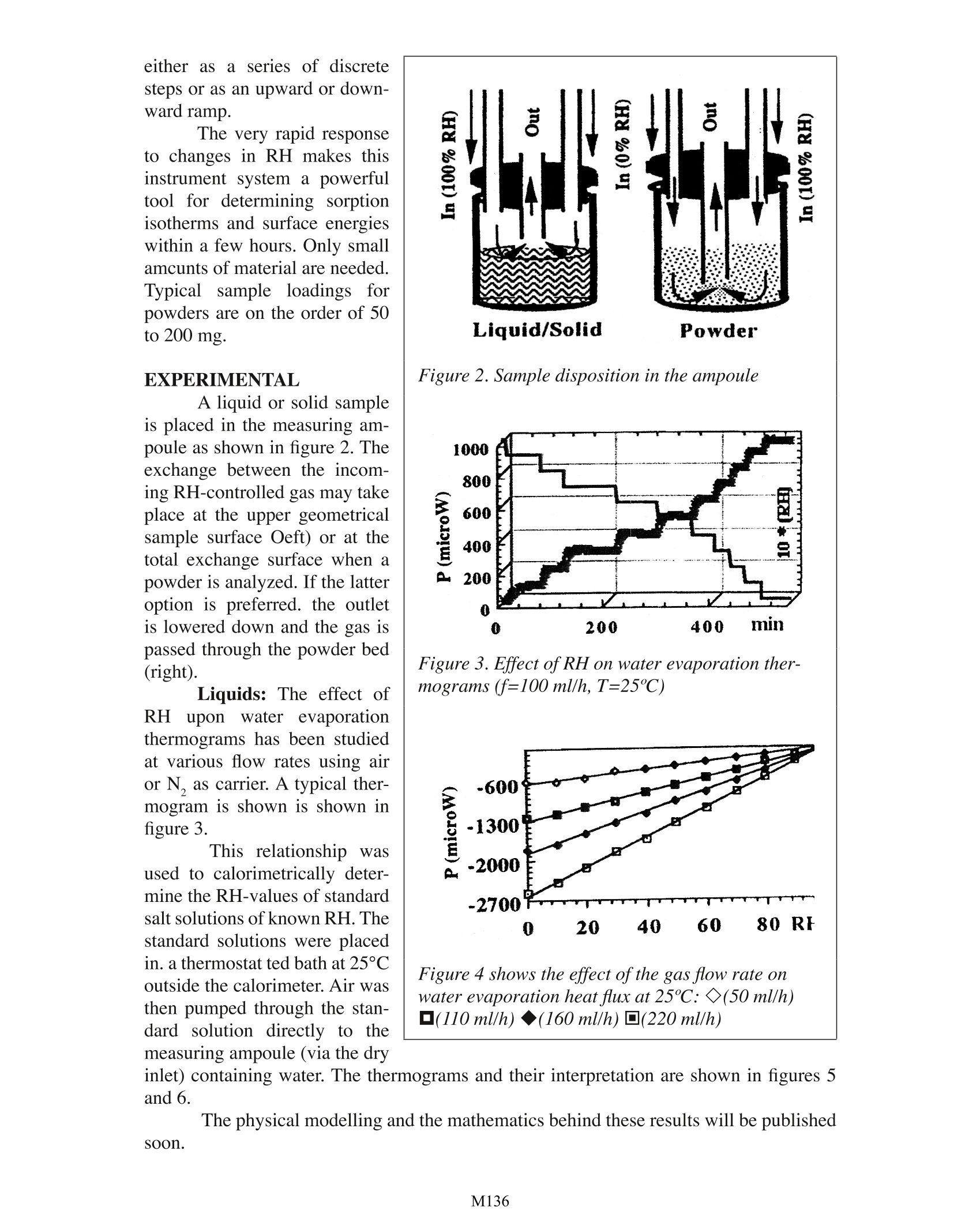
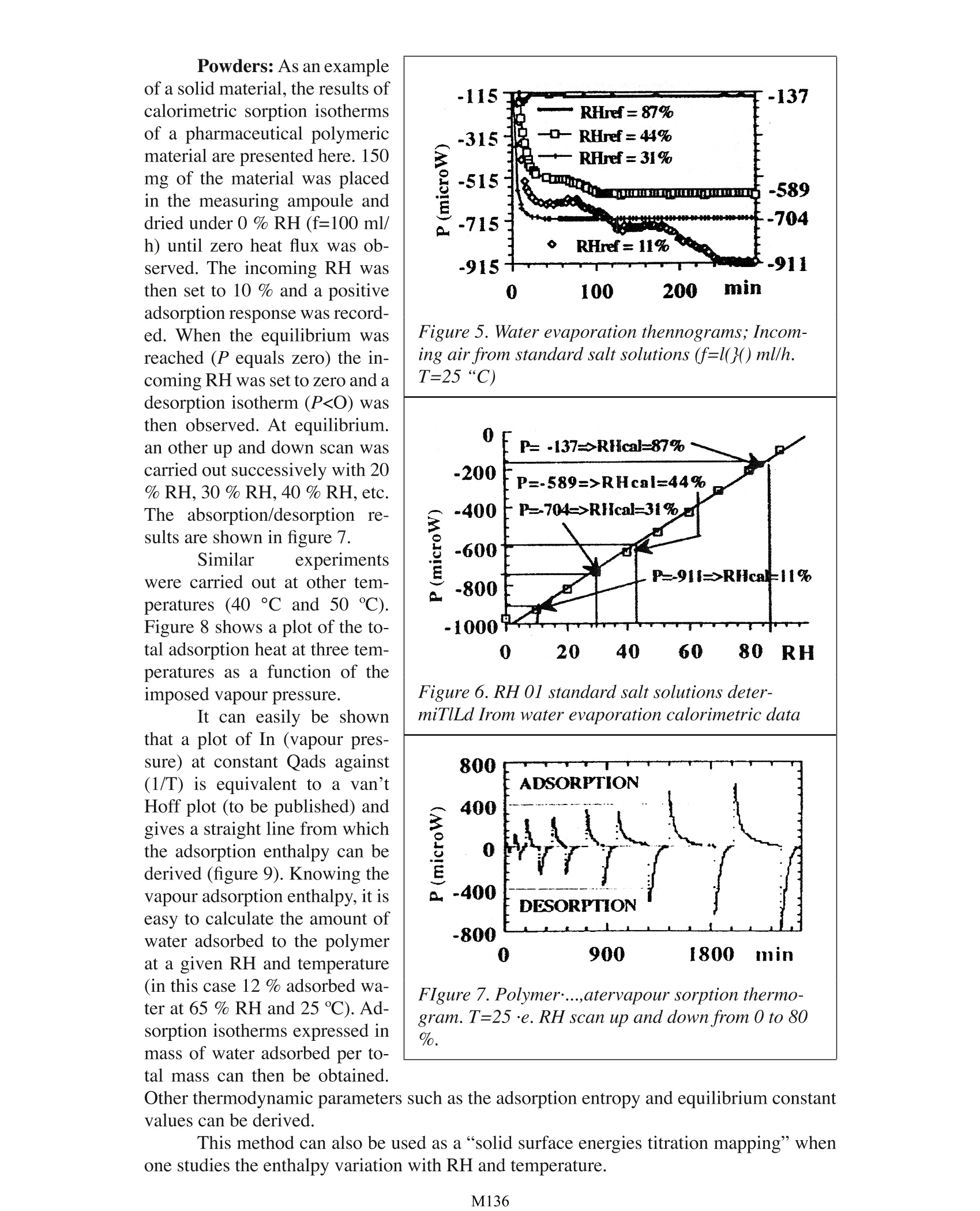
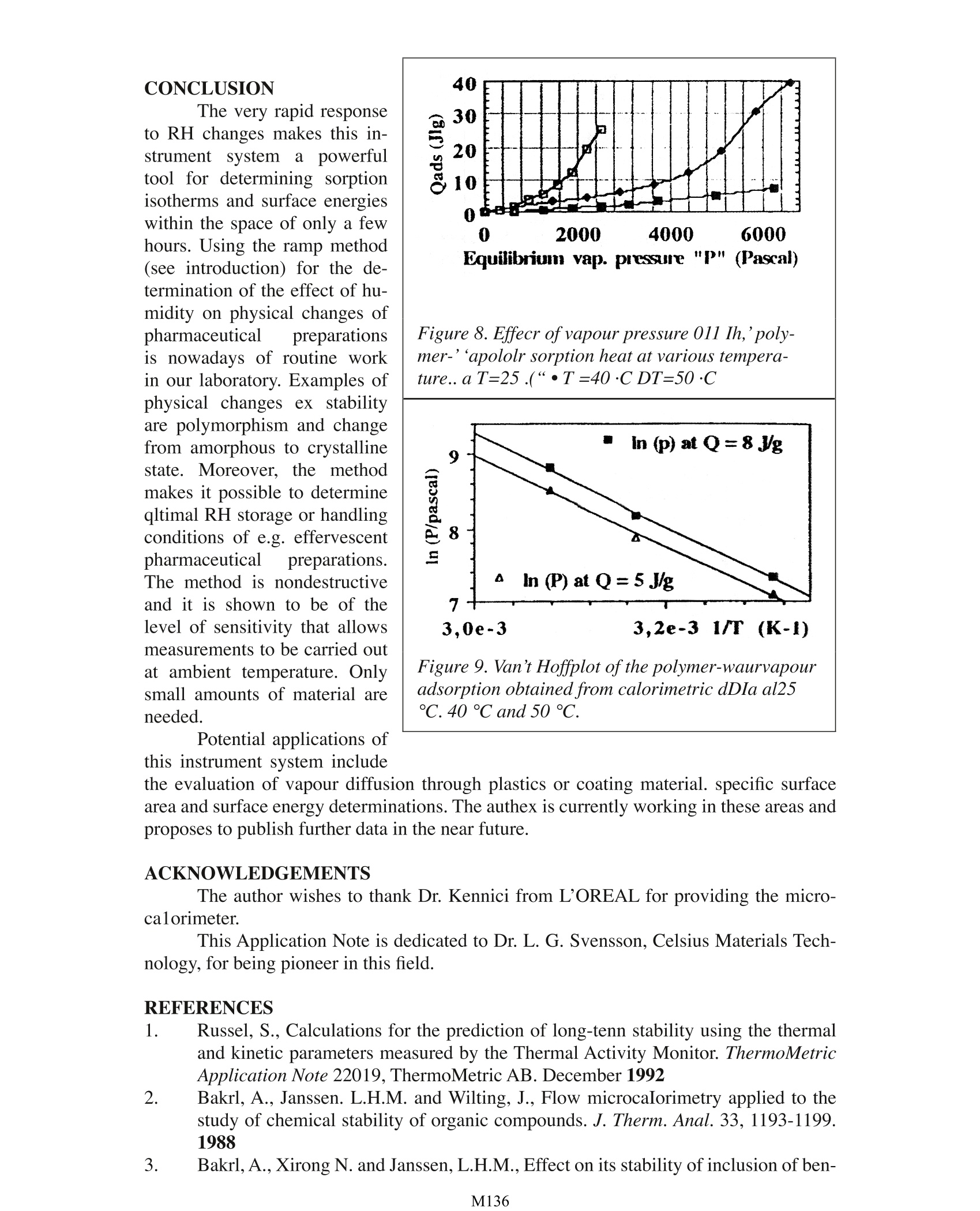
Design, Testing and Pharmaceutical Applications of a Gas PressureController Device for Solid-Gas Microcalorimetric Titration A. Bakri University Joseph Fourier Faculty of Pharmacy PharmaceuticalEngineering Avenue de Verdun 38240 Meylan, France INTRODUCTION Interactions between water and component materials take place in all steps of dos-age from manufacture. These interactions can affect the mechanical, physical and chemicalproperties and consequently also the behaviour of the material of interest. The use of the Thermal Activity Monitor (TAM) is already well established in thefield of compatibility and stability testing of pharmaceutical preparations and other chemi-cals in general. The sample is loaded into a sealed ampoule which is placed in the measur-ing position of the TAM. The recorded heat flux (P) can be related to the reaction rate andthe reaction enthalpy (refs. 1-4). However, such a closed system does not allow any controlor change of experimental factors such as oxygen pressure or water vapour pressure in theampoule during an ongoing experiment. In order to overcome this limitation, the Gas Pres-sure Controller device has been specifically developed to control, in particular the relativehumidity (RH) within the sample ampoule (fig.1). During a calorimetric experiment. drygas (1) is delivered by a flow rate controller (2) to a switching valve (3). In one position,a, the valve delivers dry gas directly to the sample ampoule. In the other position. b, thegas is passed through two humidifier reservoirs (4) in thermal contact with the calorimetric bath (5), and the gas it is thensaturated with water (100 %RH) before reaching the sample(6). Both dry (0 % RH) and wet(100%RH) gas pass separatelythrough heat exchangers (7) forthermal equilibration. The in-coming RH to the sample canbe controlled from the valveand varied from 0 to 100 % bymixing various ratios of dry andwet gas. The device operates witha precision better than ±0.1%RH within the range of 0% and100% RH throughout the oper-ating temperature of the TAM.It is possible to program the RHthrough the Digitam software Figure 1 Schematic view oithe Gas Pressure Con-troller units fitting in TAM. either asa series of discretesteps or as an upward or down-ward ramp. The very rapid responseto changes in RH makes thisinstrument system a powerfultool for determining sorptionisotherms and surface energieswithin a few hours. Only smallamcunts of material are needed.Typical sample loadings forpowders are on the order of 50to 200 mg. EXPERIMENTAL A liquid or solid sampleis placed in the measuring am-poule as shown in figure 2. Theexchange between the incom-ing RH-controlled gas may takeplace at the upper geometricalsample surface Oeft) or at thetotal exchange surface when apowder is analyzed. If the latteroption is preferred. the outletis lowered down and the gas ispassed through the powder bed(right). Liquids:: The effect ofRH uponwater evaporationthermograms has been studiedat various flow rates using airor N, as carrier. A typical ther-mogram is shown is shown infigure 3. This relationship wasused to calorimetrically deter-mine the RH-values of standardsalt solutions of known RH. Thestandard solutions were placedin. a thermostat ted bath at 25°Coutside the calorimeter. Air wasthen pumped through the stan-dard solution directly to themeasuring ampoule (via the dry Figure 2. Sample disposition in the ampoule Figure 3. Effect of RH on water evaporation ther-mograms (f=100 ml/h, T=25℃) Figure 4 shows the effect ofthe gas flow rate onwater evaporation heat flux at 25℃: ◇(50 ml/h)口(110 ml/h):)◆(160 ml/h) 口(220 ml/h) inlet) containing water. The thermograms and their interpretation are shown in figures 5and6.The physical modelling and the mathematics behind these results will be publishedsoon. Similar experimentswere carried out at other tem-peratures (40°C and 50 ℃).Figure 8 shows a plot of the to-tal adsorption heat at three tem-peratures as a function of theimposed vapour pressure. tal mass can then be obtained. Powders: As an exampleof a solid material, the results ofcalorimetric sorption isothermsof a pharmaceutical polymericmaterial are presented here. 150mg of the material was placedin the measuring ampoule anddried under 0 % RH (f=100 ml/h) until zero heat flux was ob-served. The incoming RH wasthen set to 10 % and a positiveadsorption response was record-ed. When the equilibrium wasFigure 5. Water evaporation thennograms; Incom-reached (P equals zero) the in-ing air from standard salt solutions (f=l(}()ml/h.coming RH was set to zero and aT=25“C)desorption isotherm (PRHcal=87%carried out successively with 20-200P=-589=>RHcal=44%% RH, 30 % RH, 40 %RH, etc.The absorption/desorption re-(-400P=-704->RHcal=31%Msults are shown in figure 7.0J-!600w)P=-911=>RHcat=11%-800.-10000 20 40 60 80RHIt can easily be shownthat a plot of In (vapour pres-sure) at constant Qads against(1/T) is equivalent to a van’tHoff plot (to be published) andgives a straight line from whichthe adsorption enthalpy can bederived (figure 9). Knowing thevapour adsorption enthalpy, it iseasy to calculate the amount ofwater adsorbed to the polymerat a given RH and temperature(in this case 12 % adsorbed wa-ter at 65 % RH and 25 ℃). Ad-sorption isotherms expressed inmass of water adsorbed per to- Figure 6. RH 01 standard salt solutions deter-miTlLd Irom water evaporation calorimetric data FIgure 7. Polymer...,atervapour sorption thermo-gram. T=25·e.RH scan up and down from 0 to 80 Other thermodynamic parameters such as the adsorption entropy and equilibrium constantvalues can be derived. This method can also be used as a “solid surface energies titration mapping”whenone studies the enthalpy variation with RH and temperature. CONCLUSION The very rapid responseto RH changes makes this in-strument system a powerfultool for determining sorptionisotherms and surface energieswithin the space of only a fewhours. Using the ramp method(see introduction) for the de-termination of the effect of hu-midity on physical changes ofpharmaceutical preparationsis nowadays of routine workin our laboratory. Examples ofphysical changes ex stabilityare polymorphism and changefrom amorphous to crystallinestate. Moreover, the methodmakes it possible to determineqltimal RH storage or handlingconditions of e.g. effervescentpharmaceutical preparations.The method is nondestructiveand it is shown to be of thelevel of sensitivity that allowsmeasurements to be carried outat ambient temperature. Onlysmall amounts of material areneeded. Potential applications ofthis instrument system include Figure 8. Effecr of vapour pressure 01 Ih,'poly-mer-'apololr sorption heat at various tempera-ture..aT=25.(“·T=40·C DT=50·C Figure 9. Van’t Hoffplot ofthe polymer-waurvapouradsorption obtained from calorimetric dDIa al25C. 40℃ and 50℃. the evaluation of vapour diffusion through plastics or coating material. specific surfacearea and surface energy determinations. The authex is currently working in these areas andproposes to publish further data in the near future. ACKNOWLEDGEMENTS The author wishes to thank Dr. Kennici from L'OREAL for providing the micro-calorimeter. This Application Note is dedicated to Dr.L. G. Svensson, Celsius Materials Tech-nology, for being pioneer in this field. REFERENCES 1 Russel, S., Calculations for the prediction of long-tenn stability using the thermaland kinetic parameters measured by the Thermal Activity Monitor. ThermoMetricApplication Note 22019, ThermoMetric AB. December 1992 2. Bakrl, A., Janssen. L.H.M. and Wilting, J., Flow microcaIorimetry applied to thestudy of chemical stability of organic compounds. J. Therm. Anal.33, 1193-1199.1988 3. Bakrl, A., Xirong N. and Janssen, L.H.M., Effect on its stability of inclusion of ben- zocaine in betacyc10dextrin as studied by conduction microcalorimetty. In: Minutesof the Fifth International Symposium on Cyclodextrins, Editions de Sante Publish-ers, Paris, pp.261-266,1990 4. Svensson, L.G., Microcalorimetric measurement of the rate of diffusion of waterthrough a plastic barrier: ThermoMetric Application Note 22001, ThermoMetricAB. December 1990 M Interactions between water and component materials take place in all steps of dosage from manufacture. These interactions can affect the mechanical, physical and chemical properties and consequently also the behaviour of the material of interest.
确定


还剩3页未读,是否继续阅读?
TA仪器为您提供《制药中控制检测方案(差示扫描量热)》,该方案主要用于原料药中化合物发现检测,参考标准--,《制药中控制检测方案(差示扫描量热)》用到的仪器有TA仪器+等温滴定微量热仪+ NANO ITC
推荐专场
相关方案
更多















