方案详情
文
聚乳酸微球粒径为纳米级,需要分辨率高的激光粒度仪对微球进行粒径分布分析,A22 NanoTec测量范围宽(0.01-2100μm),分辨率高,灵敏度高,可以满足更高水平的测试需求。
方案详情

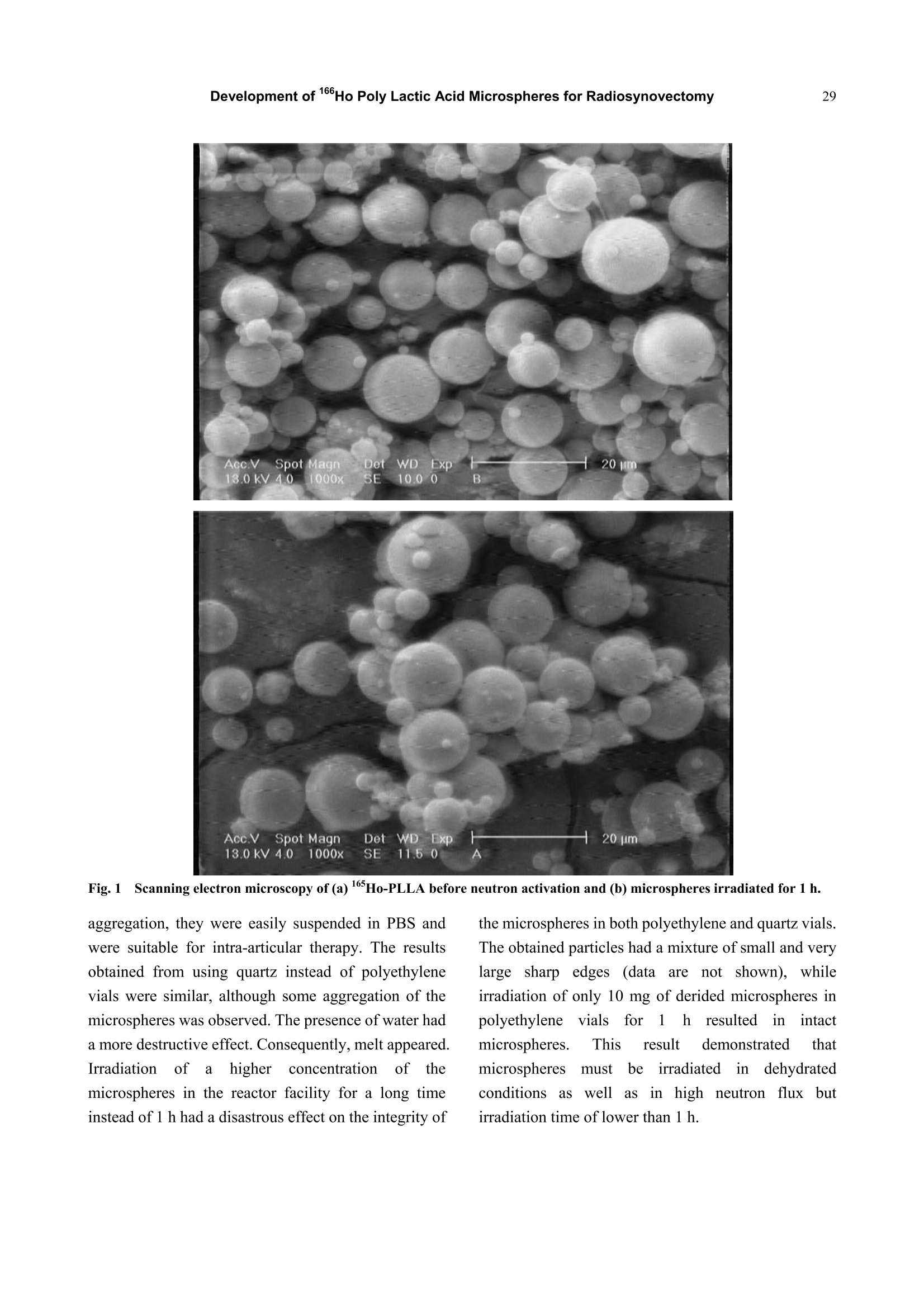
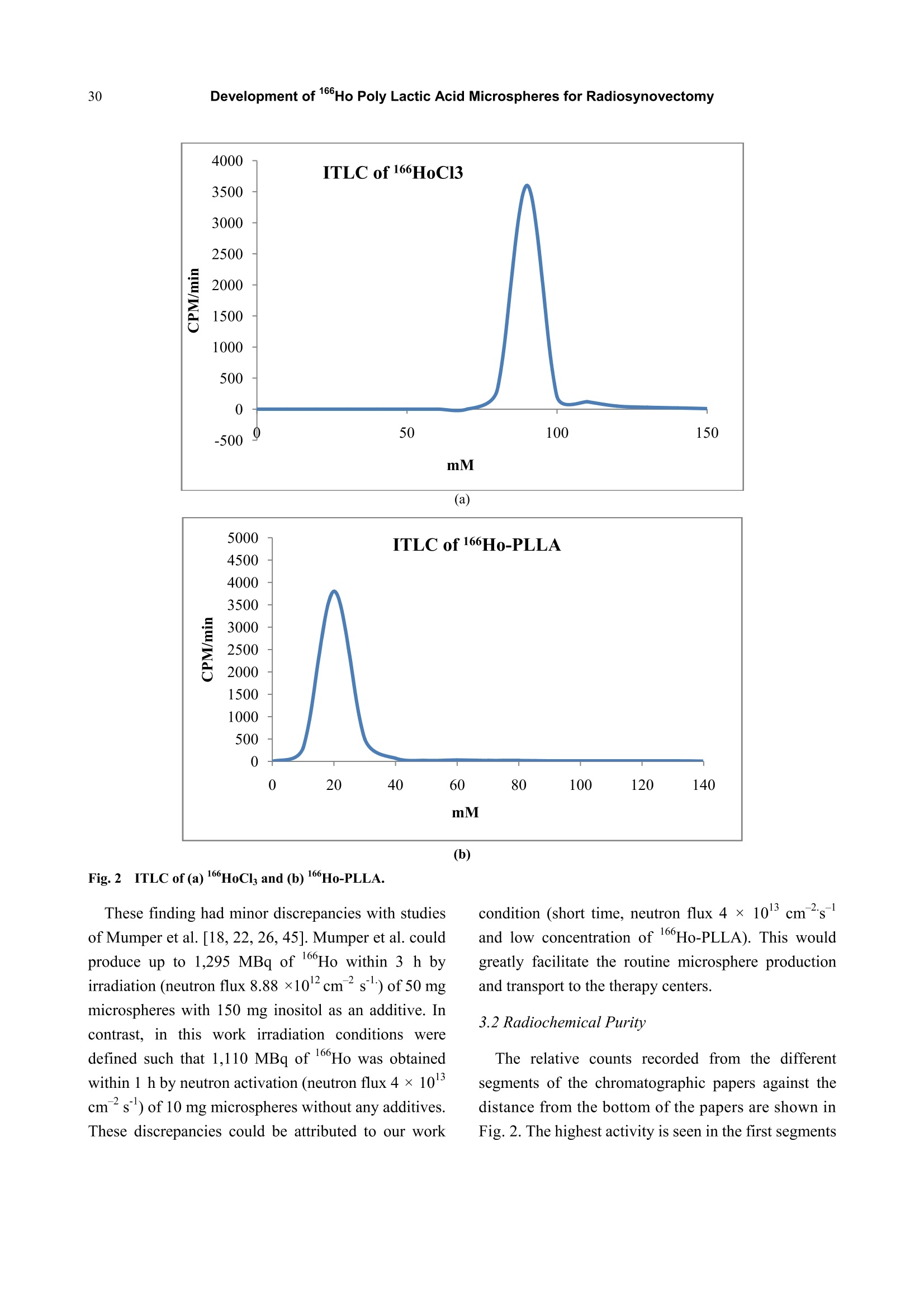
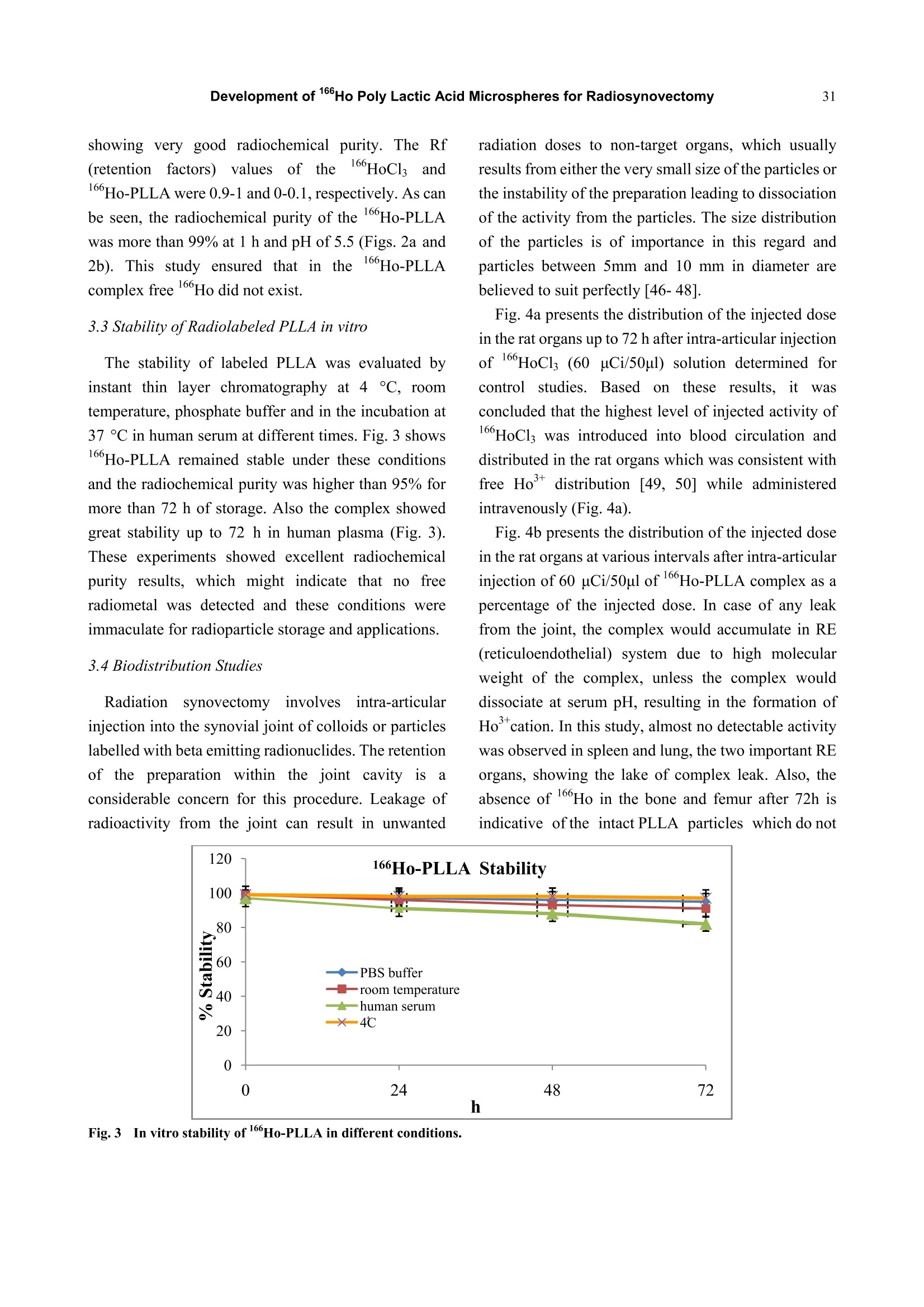
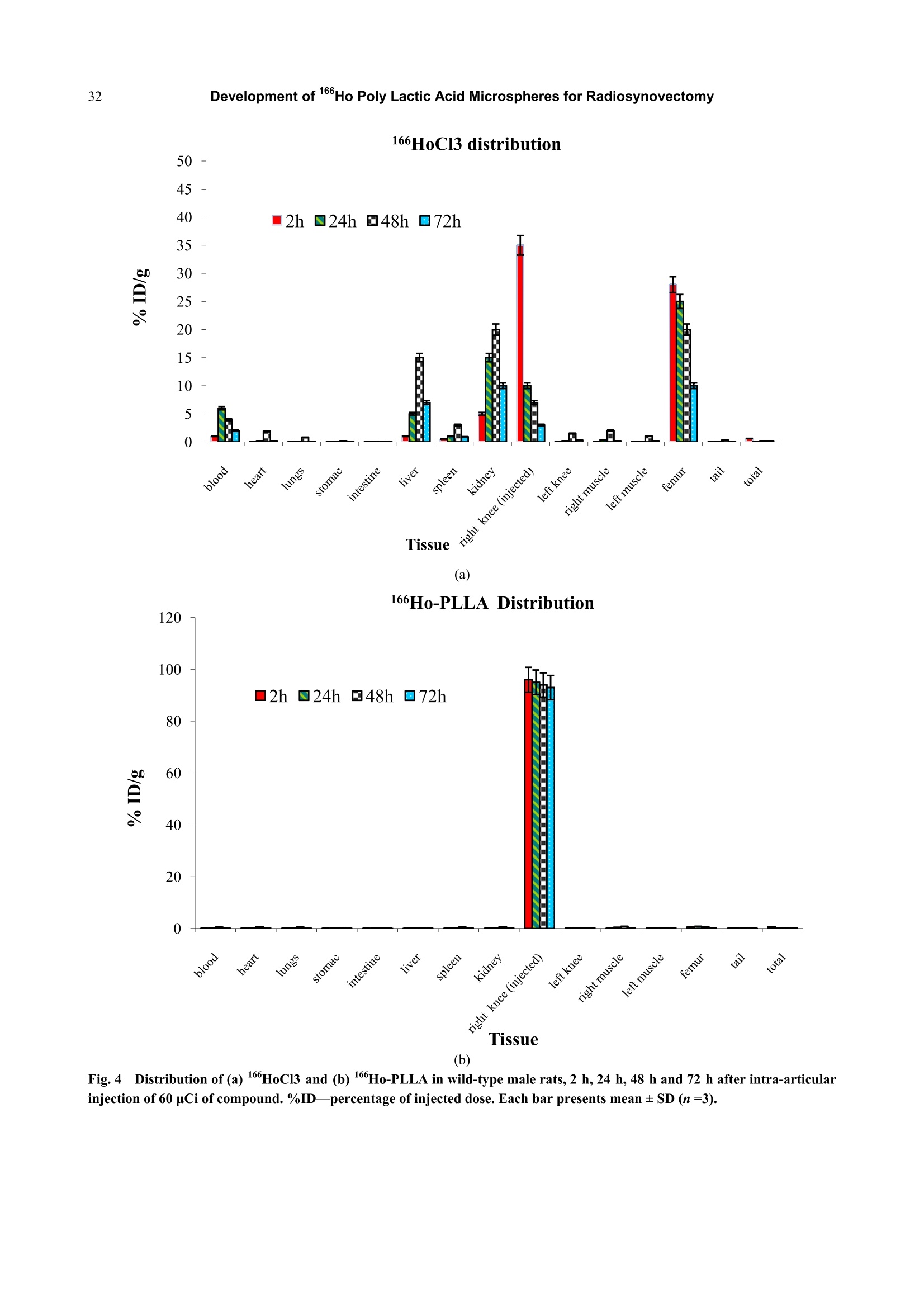
Journal of Pharmacy and Pharmacology 1 (2013) 25-35 26Development of 166Ho Poly Lactic Acid Microspheres for Radiosynovectomy Development of 1Ho Poly Lactic Acid Microspheres forRadiosynovectomy Kamal Yavari , Khodabandehloo Hadi , Taghikhani Mohamad , Majid Mazidi , Kalantari Bagher, Reza Solhifam Asl and Mohammad Ghannadi Maragheh 1. Department of Radiochemistry, Nuclear Sciences and Technology Research Institute, Tehran, PO Box 113658686, Iran 2. Department of Clinical Biochemistry, Faculty of Medicine, University of Tarbiat Modares, Tehran, Iran Received: June 24, 2013/ Accepted: August 22, 2013/Published: December 31, 2013. Abstract: Microsphere and particle technology with selective transport of radiation represents a new generation of therapeutics inradiosynovectomy.166Ho-PLLA-MS (Poly L-lactic acid microspheres loaded with holmium-166 acetylacetonate) are novelmicrodevices for intra-cavital radiosynovectomy in this work. 16Ho-PLLA agent was developed and quality controls of the compoundwere described.16HoAcAc-PLLA microparticles were prepared by dissolving holmium acetylacetonate and incorporating into PLLAspheres by the solvent evaporation technique. Microspheres were irradiated at TRR (tehran research reactor). The diameter and surfacemorphology were characterized by particle sizer and SEM (scanning electron microscopy) before and after irradiation. The complexstability, radiochemical purity and in vivo biodistribiotion were checked in the final solution up to 3 days. In this study,6Ho-PLLAspherical particles with a smooth surface and diameter of 5-10 um were obtained, which were stable in vitro and in vivo studies.Neutron irradiation did not damage the particles, and heightened activity stimulated radiosynovectomy.No significant leakage of dosefrom injection site and its distribution in organs was observed up to 3 days for 160Ho-PLLA. The ease with which the PLLA spherescould be made in the optimal size range for later irradiation and their ability to retain the 166Ho made them attractive agents forradionuclide synovectomy. Key words: Holmium-166, poly L-lactic acid, radiosynovectomy, artrit romatoid, drug delivery. 1.Introduction Developing new radiosynovectomy agents is ofgreatimportancedduee tothee aging ofhumanpopulations throughout the world and increasingoutbreak of inflammatory diseases [1, 2]. In the treatment of rheumatoid arthritis, a surgical,chemical or radiation synovectomy may be done.Surgical synovectomy suffers from the risks of surgeryand anesthesia, the need for hospitalization and aprolonged period of rehabilitation, albeit to someextent [2, 3]. In chemical synovectomy highly toxicagents such as osmic acid, alkylating substances suchas nitrogen mustards, methotrexate and cobra venom ( Corresponding author: Ka m al Yav a ri, Ph.D., research field: clinical biochemistry and pharmacology. E-mail:kyavari@aeoi.org.ir. ) were used initially but were then abandoned because ofpossible joint tissue damage [4, 5]. A well designed controlled drug delivery system canbe regarded as a solution to conventional therapyproblems and enhance the therapeutic efficacy ofactive pharmaceutical compounds. There are variousapproaches in delivering a therapeutic substance to thetarget site in a sustained controlled release fashion; andsuchlike approach is utilizing microspheres as carriersfor drugs [6,7]. A promising local radionuclide therapy employsradioactive microspheres for nonoperable group ofpatients suffering from rheumatoid arthritis. Two 90Ymicrospheres products are currently commerciallyavailable in clinical use: glass-based TheraSpheres andresin-based microspheres [5-8]. Radionuclide synovectomy consists oftltheintra-articular administration of a therapeuticradionuclide such asstbeta emitter radionuclide incolloidal or particulate form to a diseased joint toreduce the inflamed synovium [8, 9]. This procedurewas first reported by Fellinger et al. [10] in 1952 andhas been used extensively in Europe [11-14]. The selection criteria for radionuclide synovectomyhave toinclude thephysicalaandd chemicalcharacteristics of radionuclide and microspheres. Theideal properties for radio labeled micro-spheres orparticlessfor intra-cavitaltherapyareas: highmechanicalstabilityto resistst bbreakdown, highchemical stability to resist elution of radioactive label,macrophage removal or radiolysis, uniform size, unitdensity to prevent settling or streaming, relative ease oflabelingwith high-energy betaparticle, lowphotofraction and intermediate (days) half-life [15]. Theparticulate material usedaslsvehicle forradionuclides may be based on synthetic or naturalbiodegradable polymers, glass, resins, plastics, etc.[16]. The first plastic microspheres were labeled withY and showed an unpredictable and catastrophicleaching of yttrium, which brought them into question[17]. This problem was solved later by the use of glassand resin-based microspheres. Although the glassspheres have several advantages, their high density [18]and their non biodegradability are major drawbacks[18-22]. Different resins such as Bio-Rex 70, Cellex-P,Chelex 100, Sephadex SP and AG 50W-X8 have beeninvestigatedrevealingencouraginggrresults[231.However, the obvious disadvantages of these systemsare theirr nonbiodegradability, llimited surfaceadsorption and low labeling capacity [17, 23-25]. Polymer-based microspheres have many advantagesover other materials, in particular their near-plasmadensity, biodegradability and biocompatibility [26, 27].Polylactic acid which can be irradiated is regarded as avector. Polylactic acid, a synthetic polymer, issabiodegradableand biocompatiblee ppolymerrwithinteresting semi-crystalline properties [28]. Poly lactic acid hasbeen recently chosennas amatrixx ofmicroparticles,s,lincorporating eitherr(before theirpreparation) the neutron-activatable16sholmium-complexes or metallic 185-187 rhenium orbeing llabeled (after theirpreparation) byy ttheradioactive 90 yttrium or aa188 rhenium salt forintra-cavital therapy [29-37]. In addition tomicrospheres, thetype of radionuclideeplaysasignificant role on the success of the therapeuticstrategy.Yttrium90, rhenium-188 and holmium-166radioisotopes have characteristics, which make thempotentially suitable for the treatment of inflamedarthritis [38-41]. However, Yttrium-90 has a majord dvantage as a radioisotope for therapy..Thebiodistribution of microspheres loaded with 90Y cannotbe directly determined in clinical trials, since Y is apure B-emitter and does not produce imageable y-rays[16-18,29,30]. Natural rhenium is composed of two isotopes(185Re and 187Re) that form -emitting 186Re and188Ree rradioisotopesrespectively,uponn rneutronactivation. The nuclear and dosimetric properties of the18Re and 188Re radioisotopes are comparable to thoseof Y, but they have imageable y-photons [38-41].Holmium-166 (160Ho) is a very attractive candidate,since this radionuclide emits gamma rays in addition tohigh-energy beta particles, allowing both nuclearimaging and radioablation, respectively. Moreover,holmium can be visualized by CT and MRI due to itshigh attenuation icoefficient and paramagneticproperties [37, 41-44]. Also, favorable its half-life of26.9 hr is long enough to eliminate logistic problemsencountered with the short-lived radionuclides issufficient to provide a high radiation dose rate. Its crosssection is comparable with rhenium, but 1Ho has anatural abundance of 100%) andd thus only oneradioisotope, 166Ho. is formed by neutronbombardment. Taking theseecharacteristics intoconsideration. 166Ho is therefore an attractive candidatefor use in future treatments [38-44]. In this research, we have reported on the potential use of biodegradable neutron activated poly-L-lacticacid microspheres (5-10 um) containing 160Ho for theradionuclide synovectomy. In this novel application,PLLA microspheres containing sufficient amounts ofstable 16Ho, in the form of 16sHo-acetylacetona(Ho-AcAcc), prepared in the optimal size range andtheir physiochemical properties fully characterizedbefore and after becoming radioactive by neutronbombardment. 2. Material and Methods 2.1. Preparation of the Ho-acetylacetonate Complex The complex of holmium with acetylacetone(165HoAcAc)wasprepared aand characterized asdescribed by Nijsen et al. [44]. In short, acetylacetone(180 mL) was dissolved in 1,000 mL water. The pH ofthis solution was brought to 8 with an aqueous solutionof NH4 (28.4% v/v). Holmium chloride (10 g in 30 mLwater) was added to this solution, and HoAcAc crystalswere formed at room temperature in 24 h. The crystalswere collected by filtration, washed with water anddried off by nitrogen. 2.2 Preparation ofHoAcAc-loaded Microspheres 16sHoAcAc-loaded poly (L-lactic acid) microsphereswith different 16HoAcAc amounts were prepared bysolvent evaporation as described by Nijsen [44]. In thisstudy1HoAcAc (1 g) and PLLA (0.65 g) weredissolved in18.6 mL chloroform. The resultinghomogeneous solution was added to an aqueoussolution of PVA (100 mL water, 3% (w/w)). Themixture was stirred at 1200 rpm for 8 h, then theprecipitated spheres were centrifuged at 2500 rpm for10 min to remove the viscous continuous phase andlater on, they were filtered using a 3.0 um filter. Thefiltered spheres were resuspended in 800 mL 0.1N HClfor 2 min to remove the unincorporated 165Ho-AcAc,refiltered and washed with 100 mL of deionized H2O.Three subsequent filtrations using fresh 3.0 um filterpaper assured optimal particle size. The particles weredried off by nitrogen. 2.3 Size Distribution and Surface Characteristics The size distribution of the microspheres wasdetermined by a laser particle sizer from Fritsch GmbH(Analysette 22 Nanotec model). Surface morphologyof HoAcAc-loaded PLLA microspheres was evaluatedby scanning electron microscopy using a Philips XL30FEGSEM. Samples of PLLA microspheres weremounted on aluminium stubs and sputter-coated with aPt/Pd layer of about 10 nm. 2.4 Irradiation ofMicropariticles In order to stimulate heightened 16Ho activity, theprepared microsphere samples were irradiated inTehran Research Reactor installed in IAERI (IranAtomic Energy Research Institute). Samples consistingof 10 mg of microspheres in polyethylene tube(diameter range 5-10 microns) were irradiated in thereactor for 1h in a thermal neutron flux of 4 × 10'n/cm’s 2.5 Quality Control of the 166Ho-PLLA Microsphers Radionuclide Purity As for evaluating any radionuclide impurity of166Ho-PLLAproducts.160Ho-PPLA complexx wachecked by y and p spectrotometry by means ofHPGeand beta scintillation detection systems respectively. 2.6 Radiochemical Purity The labeling efficiency was evaluated by instantthin-layer chromatography (ITLC, Gelman ScienceInc.) utilizing whatman n°1 strips, eluted with 1 mMEDTA: (v/v) as solvent. 2.7 Stability of160Ho-PLLA Samples of 1 mL fresh human blood serum wereprepared. An aliquot of 25 uL of the 166Ho-PLLAcomplex (100 uCi; 100 ug microspheres) was added toeach sample, then the samples were incubated at 37°C.Later on, they were analyzed by ITLC after 0, 24 h, 48h and 72 h of incubation. The same procedure wasrepeated for incubating the complex at 4 ℃, room temperature and phosphate buffer conditions. 2.8 Biodistribution of166Ho-PLLA in Wild Type Ratsafter Intra-articular Administration To determine the accumulation of 166Ho-PLLAmicrospheres in the intra-articular cavity, radiolabeledPLLA solution was carefully administered to wild-typerats. A volume (50 uL) of final radiolabeled PLLAsolution containing 60 ± 5 uCi radioactivities wasinjected intra-articularly into rats. The animals weresacrificed 2 h, 24 h, 48 h and 72h post injection. Thespecific activity of different organs was calculated andexpressed as %ID/g tissue. 3. Result and Discusion 3.1 Size Distribution and Surface Characteristics The size distribution and surface morphology of themicrospheres were characterized using a laser particlesizer and SEM. both before and after neutron activation.The particle size distribution was narrow, from 5 um to10 um, with an average microsphere size of 6.7 umwhen the microspheres were prepared at 1,200 rpm.The average particle size could be tailored by alteringthe stirring rate and %PVA during emulsifying. Thesize distribution Was narrow to control thebiodistribution of the microspheres and to reducepossible shunting of small microspheres to non-targetorgans after administration. A sieving step resulted inmore confined size distribution. More than 94% of themicrospheres had a size between 5-10 um after sieving.The particle size analyses of the166Ho-PLLA-MSshowed ttlhat the required size distributionwasguaranteed and small fragments due to radiationdamage were not seen. SEM analysis showed thatspherical particles with a smooth surface were formed(Figs. la and lb). Particle surfacedamage,agglomeration or fragments was not observed afterneutron activation of the microspheres for 1h. The sizedistribution and morphology findings indicated that themicrospheres were resistant to neutron irradiation andthere was no difference in suspending behavior of Ho-PLLA-MS before and after neutron irradiation.which indicated that the chemical composition of thesurface had not changed Formation of size and shape of microsphere dependson several parameters. In this study some of theseparametersincluding PVA .concentration, pH ofreaction.i, sStirring rate,,andstirringtime wereinvestigated. Increasing concentrations of PVA(from 1% to 3%) in continuous phase resulted in theformation of smaller spheres. Concentrations of 2%-3%PVA led to smooth microspheres. This could beattributed to higher viscosity in the continuous phase,leading to smaller droplet size in the dispersed phase.An increased stirring rate and time led to the smallerparticle formation. Stirring rates with 300 rpm, 600rpm, 900 rpm, 1,200 rpm and 1,600 rpm resulted inparticles 30-40 um, 20-30 um, 10-20 um, 5-10 um and0.5-4 um diameters respectively. Maximum extractionof16Ho-PLLA was observed with a pH of above 7.5.The parameters examined resulted inn astandardmethod under the following conditions: 3% PVA, 10mL 165HoAcAc in 180 mL chloroform, 25℃ and 1200rpm for 8 h, yielding 4-5g of microspheres with a 5-10um diameter. These results were comparable withthose of the studies of Mumper et al. [45]. In order to set up reactor conditions as comparable aspossible and then definenecessaryirradiationconditions toproduce1ttherapeuticddosages of16Ho-PLLA-MS while maintaining their integrity andsuitability for therapy, some irradiation parameterswere investigated including: (1) the facility; (2)irradiation time; (3) water content of the microspheres;(4) the amount of microspheres per vial and (5)material of the vial. Non-irradiated 165Ho-PLLA-MS showed a smoothand spherical appearance (Fig. 2a). After irradiation inthe reactor facility (neutron flux 4×10lcm.s) for lh in polyethylene vials, surface changes were not seenwith SEM (Fig. 2b). Overall structural integrity wasmaintained11in terms of form and size and themicrospheres did not showedtendencytowards Fig.1Scanning electron microscopy of (a) 165Ho-PLLA before neutron activation and (b) microspheres irradiated for 1 h. aggregation, they were easily suspended in PBS andwere suitable for intra-articular therapy. The resultsobtained from using quartz instead of polyethylenevials were similar, although some aggregation of themicrospheres was observed. The presence of water hada more destructive effect. Consequently, melt appeared.Irradiationn of alhigher concentrationi off ttlhemicrospheres in the reactor facility for a long timeinstead of 1 h had a disastrous effect on the integrity of the microspheres in both polyethylene and quartz vials.The obtained particles had a mixture of small and verylarge sharp edges (data are not shown), whileirradiation of only 10 mg of derided microspheres inpolyethylene vials for1 hresultediirn intactmicrospheres. This result demonstrated thatmicrospheresmust beirradiated in dehydratedconditions aswell as in high neutron flux butirradiation time of lower than 1 h. (a) (b) These finding had minor discrepancies with studiesof Mumper et al.[18, 22,26,45]. Mumper et al. couldproduce up to 1,295 MBq of 16Ho within 3 h byirradiation (neutron flux 8.88 ×10'cm’s) of 50 mgmicrospheres with 150 mg inositol as an additive. Incontrast, in this work irradiation conditions weredefined such that 1,110 MBg of 166Ho was obtainedwithin 1 h by neutron activation (neutron flux 4×10cm’s) of 10 mg microspheres without any additives.These discrepancies could be attributed to our work condition (short time, neutron flux 4 ×1013 cm’sand low concentration of 166Ho-PLLA). This wouldgreatly facilitate the routine microsphere productionand transport to the therapy centers. 3.2 Radiochemical Purity The relative counts recorded from the differentsegments of the chromatographic papers against thedistance from the bottom of the papers are shown inFig. 2. The highest activity is seen in the first segments showing very good radiochemical purity. The Rf(retentionfactors) values(of the166HoCl andHo-PLLA were 0.9-1 and 0-0.1, respectively. As canbe seen, the radiochemical purity of the 166Ho-PLLAwas more than 99% at 1 h and pH of 5.5 (Figs. 2a and2b). This study ensured that in thee 166Ho-PLLAcomplex free 166Ho did not exist 3.3 Stability ofRadiolabeled PLLA in vitro The stability of labeled PLLA was evaluated byinstant thin layer chromatography at 4 ℃, roomtemperature, phosphate buffer and in the incubation at37 ℃ in human serum at different times. Fig. 3 shows166Ho-PLLA remained stable under these conditionsand the radiochemical purity was higher than 95% formore than 72 h of storage. Also the complex showedgreat stability up to 72 h in human plasma (Fig. 3).These experiments showed excellent radiochemicalpurity results, which might indicate that no freeradiometal was detected and these conditions wereimmaculate for radioparticle storage and applications. 3.4 Biodistribution Studies Radiation ssynovectomy inivncvolves iintra-articularinjection into the synovial joint of colloids or particleslabelled with beta emitting radionuclides. The retentionof the preparation within the joint cavity isconsiderable concern for this procedure. Leakage ofradioactivity from the joint can result in unwanted radiation doses to non-target organs, which usuallyresults from either the very small size of the particles orthe instability of the preparation leading to dissociationof the activity from the particles. The size distributionof the particles is of importance in this regard andparticles between 5mm and 10 mm in diameter arebelieved to suit perfectly [46-48]. Fig. 4a presents the distribution of the injected dosein the rat organs up to 72 h after intra-articular injectionof 166HoCls (60 uCi/50ul) solution determined forcontrol studies.s.BBased ontthese results, itwaSconcluded that the highest level of injected activity of6HoCls was introduced into blood circulation anddistributed in the rat organs which was consistent withfree Hodistributionl[49, 50] while administeredintravenously (Fig. 4a). Fig. 4b presents the distribution of the injected dosein the rat organs at various intervals after intra-articularinjection of 60 uCi/50ul of 166Ho-PLLA complex as apercentage of the injected dose. In case of any leakfrom the joint, the complex would accumulate in RE(reticuloendothelial) system due to high molecularweight of the complex, unless the complex woulddissociate at serum pH, resulting in the formation ofHo cation. In this study, almost no detectable activitywas observed in spleen and lung, the two important REorgans, showing the lake of complex leak. Also, theabsence of 166Ho in the bone and femur after 72h isindicative ofthe intact PLLA particles which do not escape the joint space. Free166Ho, known as; abone-seeking element [49,50], could leave the jointspace and deposit in the skeleton system, however, thefemur and bone marrow uptake of l6Ho accounted foronly 0.03%-0.1%of the injected dose and only 2.7% ofthe total leached activity. 4. Conclusions In summary,166Ho-PLLA-MS containing sufficientmasses of neutron activatable Ho were prepared bymeans of the solvent evaporation technique with highradiochemical yield (>99 %) under non-hazardousconditions. Spherical particles with a smooth surfaceand diameter of 5-10 um were obtained and irradiatedlater to produce therapeutic amounts of 16Ho-PLLA.Neutron irradiation did not damage the particles, andadequate activity Was stimulated forr nuclearbiodistribution and radioablation of inflammationsthroughintral-cavital injections.The irradiatedparticles were stable in the final solution at roomtemperature, 37 ℃ and in the presence of humanserum, and could be used even 24 h after preparation.Also, the prepared complex showed a remarkableability to retain the encapsulated 16Ho that coulddeliver a high radiation dose to the diseased synovium,albeit low doses to other organs. Intra-articularinjection of 166Ho-PLLA complex to the wild malerats and investigation of leakage of activity in thebody showed that most of the injected dose hasremained in injection site 72 h after injection. Thehigh labeling yield (99%), radiochemical purity andexcellent in vitro and in vivo stability of the complex,lead to the conclusion that 166Ho-PLLA particleswould be useful as a therapeutic agent in the arthritistreatment. Also the potential clinical use of this agentis very promising since its preparation does notrequire the handling of heightened activity and thehalf-life of 166Ho removes the necessity of being closeto a reactor. Finally, a kit formulation was developedfor the in situ preparation of the radiopharmaceuticalin clinical centers. ( References ) ( [1] S.L D avid, W . F rederick, H.J.W. Tom, Rheumatoid arthritis, Lancet. 376 (2010)1094-110 8 . ) ( 21 I .R. Dell, Therapeutic strategies for rheumatoid arthritis,N. Engl. J. Med.350(2004)2591-602. ) ( [3] 3j C.B. Sledge, C o rrection of Arthritic Deformities in the Lower E x tremities a nd Sp ine, A rthritis and A lliedConditions, in: M cCarty (Ed.), 9th ed . , Lea and Feb i ger,Philadelphia, 1979. ) ( [4] W.U. Kampen, , W. B renner, N . C zech, E . Henze, Intraarticula r application o f f u nsealed b eta-emittingradionuclides i n t he t reatment course o f i n flammatory j oint diseases, Curr . Med. Chem. A nti-inflammatory & Anti-allergy Agents 1 ( 1) (2002) 77-87. ) ( [51 5] ( G. R. V o n, A . Sw e nsson, Int r a-articular i n jections ofosmic acid in painful joint a ffections, Acta. M ed. S c and. 259(1951)27-32. ) ( [6] M. Alagusundaram,C.C.S. Madhu,K. Umashankari, B . V. Attuluri , C . Lavanya, S. Ramkanth, Microspheres as anowel d rug delivery system-A rewiew, In t . J. C hem.Tech. R es. 1 (2009) 526-534. ) ( [7] J. Nidhi, G. Neha G, K. Divya, N. U pendra, Microspheres:Mucoadhesion based c o ntrolled drug delivery system.RGUHS.J. Pharm. Sci. 2 (2012) 28-40. ) ( [8] F. Melichar, M. Kropacek, J. Srank, Labelled compounds as radiopharmaceuticals for radiosynoviorthesis,J. R a diol.Nucl. Chem. 280 (2009) 353-358. ) ( 191 L. Miszczyk, G. W ozniak, B. Jochymek, Effectivenessevaluation of knee joint 90Y radiosynovectomy, Przegl.Lek. 64 (2007)450-453. ) ( [10] K . Fellinger, J Schmid, L ocal t h erapy of r h eumatic d iseases, Wien. Z. Inn. Med. 33(9)(1952) 3 51-363. ) ( [11] B.M. A nsdll, A. C rook, J .R. Mallard, E.G.L. Bywaten, Evaluation o f i n tra-articular c olloidal Au-198 in th e treatment of persistent knee effusions , Ann. Rheum. Dis. 22 (1963)435-439. ) ( [12] J J .M. Gumpel, T . C. B e er, J . C. Crawley, H .E . F arran,Yttrium-90 in p ersistent synovitis of the knee: A singlecenter comparison of four radiocolloids, Br. J. Radiol. 48(1975)377-381. ) ( [13] M N . Oka, Radiation synovectomy of the rheumatoid kneewith yttrium-90, Ann. Clin. Res. 7 (1975)205-210. ) ( [14] C.M. Onetti, E . G utierrez, E. Hieba, C.R. A guirre, Synoviorthesis with Pcolloidal chromic p hosphate in rheumatoid arthritis, J . Rheumatol. 9 (1982) 229-238. ) ( [15] J J .C. Harbert, Nuclear Medicine: D iagnosis and Therapy,The M ed. Public. Inc., New York, 1996, pp. 1141-1155. ) ( [ 1 6] R. Sarlesh, A . P reeti, P. Ashish, A review on microspheres: Methods o f preparation and e v aluation, W . J. Pharm.Pharmcol. Sci. 1 (2012) 422-438. ) ( [17] G .J. Ehrhardt, D.E. D a y, Therapeutic use of 9 0y ) ( Microspheres, Nucl. Med. Biol. 1 4 (1987) 233-242. ) ( [18] R.J . Mumper, U.Y. R yo, M . J a y, Neutron activated holmium-166-Polyy (L-lactiicc acid) Microspheres : A potential agent for the internal radiation therapy of hepatic tumours, J. Nucl. Med. 32 (1991) 2139-2143. ) ( [19] J J . .H. . Turner, P.G. C laringbold, P.F.B. Klemp, P .J. Cameron, A.A. M a rtindale, R .J. J . ( Glancy, /, e1 al.. 166Ho-microsphere l iver r adiotherapy: A preclinical SPECT dosimetry study in t he pig, Nucl. Med. Comm. 1 5(1994) 5 45-553. ) ( [20] S.D. C onzone, U . O. Ha f eli, D.E. Da y , G.J. Eh r hardt,Preparation a nd properties of r a dioactive r h enium gl a ss microspheres i ntended for i n-vivo r adioembolization therapy,J. Biomed. Mat. R es. 42 (1998) 617-625. ) ( [21] H .N. Wagner, B.A. Rh o des, Y. Sasaki, J.P. Ryan, Studiesof the c irculation with r adioactive m icrospheres, I n vestRadiol. 4 (1969) 374-386. ) ( [22 ] M. 1 . Jay, S.S. K hare. R.S. ). M umper, U.Y. Ryo, Microencapsulation of activable radiotherapeutic agents, Biol. Synth. Mem. 292(1989) 293-300. ) ( [23 ] P P.A. Schubiger, H .F. Beer, L . Geiger, H . Rosler, A. Zimmermann,J. Tri l ler, et al.,90Y-resin particles-animal experiments on pigs w ith r egard t o t he i ntroduction ofsuperselective embolization t h erapy, Nucl. Med. Bio l . 18(1991) 305-311. ) ( [24] J J . . L . Kalliomaki, S. Jalava , M. Mottonen, On t hedevelopment t of a djuvant arthritis in the j oint intra-articularly irradiated by radioactive yttriumY resin,Scand. J. Rheumatol. 31(1974)325-331. ) ( [25] S S . Ho, W.Y. L au, T.W.T.Leung, M. C h an, Y . K. Ngar, P.J.Johnson, et al., Partition model for estimating radiationdoses f rom yttrium-90 mi c rospheres in t re a ting hepatic tumours, Eur. J . Nucl. Med. 23 ( 1996) 947-952. ) ( [26] R .J. Mumper, B. Mills, U .Y. Ryo, M. J a y, Polymeric microspheres f or r adionuclide synovectomy c o ntaining neutron-activated holmium-166, J. Nucl Med. 23 (1992) 398-402. ) ( [27] C . Vilos , L. A. Velasquez, Therapeutic strategies based onpolymeric microparticles, J. Biomed. Biotech. 1 0 ( 2012) 1-6. ) ( [28] S .H. Hyon, B i odegradable po l y (lactic acid) microspheresfor drug d elivery systems, Yonsei. M ed. J. 4 1 ( 2000) 720-734. ) ( [29] U. Hafeli, RRadioactive microspheres for ri m edical applications , Phys. Chem. B . Biotechnol. 7 ( 2002) 21 3 -248. ) ( [30]U. H afeli, R.W. Atcher, C . E. M o rris, B. Beresford, J. L .Humm, R . M. Ma c klis, Polymeric radiopharmaceuticaldelivery systems, Radioact. Radiochem. 3(1992) 1- 1 4. ) ( [31] C. N. s hanthi, G. Rakesh, M. K . Arun, T r aditional and emerging applications of microspheres: A Review, Int. J. Pharm. T ech. 2 ( 2010) 6 75-681. ) ( [32] K. Kothari, S. Suresh, H. S arma, V. Meera, M. Pillai, 1 88 Re-labeled hydroxyapatite p articles for r adiation synovectomy, A p pl R a diat I s ot. 58 (2003)463-468. ) ( [33] C.Y.Shin, M . Son, J.L. Ko, 18 8 rh e nium-tin colloid, as anew therapeutic a gent o f r h eumatoid a r thritis, Arch.Pharm Res. 26 (2003) 1 68-172. ) ( [34] F I . W ebb, J . L owe, R . B l uestone, Uptake o f colloidalradioactive yttrium by synovial membrane, A nn. Rheum. Dis. 28 (1969) 300-302. ) ( [35] S .J. Wang, W.Y. Lin, M.N. C hen, J.T. Chen, W.L. H o,B.T. Hsieh, et al., Histologic study o f effects of radiationsynovectomy with Rhenium-188 microsphere, Nucl. Med.Boil.28 (2001)727-732. ) ( [36] O. S chweeger, K . Weiss, H . Sinzinger, C . P i rich,Radiation synovectomy with 1 6 Ho-Ferric h y droxide: A f irst experience, J. Nucl Med. 4 3 ( 2002) 1 4 89-1494. ) ( [37] T1 .C. Karagiannis, Comparison of di f ferent classes o f radionuclides f or potential use in radioimmunotherapy, J.Nucl. Med.10 (2007) 82-88. ) ( [38] R .P. Spencer, Short-lived radionuclides i n therapy, Nucl.Med. Biol. 14 (1987) 537-538. ) ( [39] M. N eves, F. W aerenborgh, L . Patricio, Palladium-109 and holmium-166 potential radionuclides for synoviotherapy-radiation absorbeddose ca lculations Appl. Radiat.Isot. 3 8 (1987) 745-749. ) ( [40] I I . Vergote, R. H. L arsen, L.D. V os, J.M. N esland, O. Bruland, J . B jorgum, e t a l ., Therapeutic e f ficacy o f the alpha-emitte r 211At bound on microspheres compared with 9Y a n d 2P colloids in a murine intraperitoneal tumor model, Gynecol. Oncol. 47 (1992) 366-372. ) ( [41] S .J. Wang, W.Y. Li n , M.N. Ch e n, B.T. Hsieh, L.H. She n ,Z.T. T sai, e t al., Rhenium-188 m icrospheres: A n e wradiation synovectomy agent , Nucl. Med . Commun. 19 (1998)427-433. ) ( [42] J J .F.W. Nijsen, J .H. Seppenwoolde, T . H a venith, C. B o s,C.J. Bakker, A .D. v an h et S chip, Liver T umors: M R imaging o f r adioactive holmium m icrospheres-phantom and rabbit s tudy, Radiol. 231 ( 2004) 491-499. ) ( [43] F. Hosain, M. H addon, H . H o sain, J.K. Drost, R .P. Spencer, R adiopharmaceuticals for diagnosis and treatment of arthritis, Nucl. Med. Biol. 1 7 (1990) 1 5 1-155. ) ( [44] J .F. Nijsen, B.A. Zonnenberg, J.R. Woittiez, D.W. R ook,I.A. Swildens-van W oudenberg, P. P . Va n Rij k , et al. , Holmium-166 poly lactic acid m icrospheres applicable for intra-arterial radionuclide therapy of hepatic malignancies: Effects of preparation and n eutron activation techniques, E ur. J. Nucl. Med. 26 (1999) 699-704. ) ( [45] R .J. Mumper, M. J a y, Poly (L-lactic Acid) microspheres containing n eutron-activatable holmium-165: A s t udy o f the physical characterization of microspheres beffor andafter irradiations i n a nuclear reactor, Pharma. R es. 9 (1992) 1 49-154. ) ( [46] M. Davis, In: R adiopharmaceutical Science C ouncil Newsleuer, M ay 1989, p.6 ) ( [47] J .H. R atdilife, I.M. Hunneyball, A. S m ith, C.G. Vi l son,S.S. Davis, Preparation and evaluation of biodegradablepolymeric systems for intra-articalarde livery of drugs, JPharm. P harmacol. 3 6 (1984) 431-436. ) ( [48]J. NobleJ, A.G. J ones, M .A. Davies, C. B . Sledge, R. I .Kramer, E. Livni, L eakage of radioactive particle systemsfrom a synovial joint s tudied w ith a gamma camera: Its ) application to radiation synovectomy, I. Bone. Joint. Surg..65 (1983)381-389. ( [49] A . M oghadam, A. R. Jalilian, K . Yavari, Production a n d .quality control of [160Ho ] DOTA-bevacizumab for therapeutic applications, J. Radioanal. Nucl. C hem. 2 9 2(2012) 1 065-1073. ) ( [50] H H .B. B reitz, R.E . Wendt, M.S. Stabin, 166Ho-DOTMP radiation-absorbed d ose e s timation f o r skeletal t a rgeted radiotherapy, J. Nucl. Med.47 (2006) 534-542. )
确定







还剩9页未读,是否继续阅读?
北京飞驰科学仪器有限公司为您提供《聚乳酸微球中粒径分布检测方案(激光粒度仪)》,该方案主要用于其他中粒径分布检测,参考标准--,《聚乳酸微球中粒径分布检测方案(激光粒度仪)》用到的仪器有德国FRITSCH(飞驰)A22大量程纳米激光粒度仪
推荐专场
相关方案
更多