凭借激光粒度仪A22大的测量范围、高分辨率以及稳定的测量结果,对氧化铝粉研磨后(研磨4小时和12小时)的粒径分布进行测量,用于后续合成复合材料提供评估。
方案详情

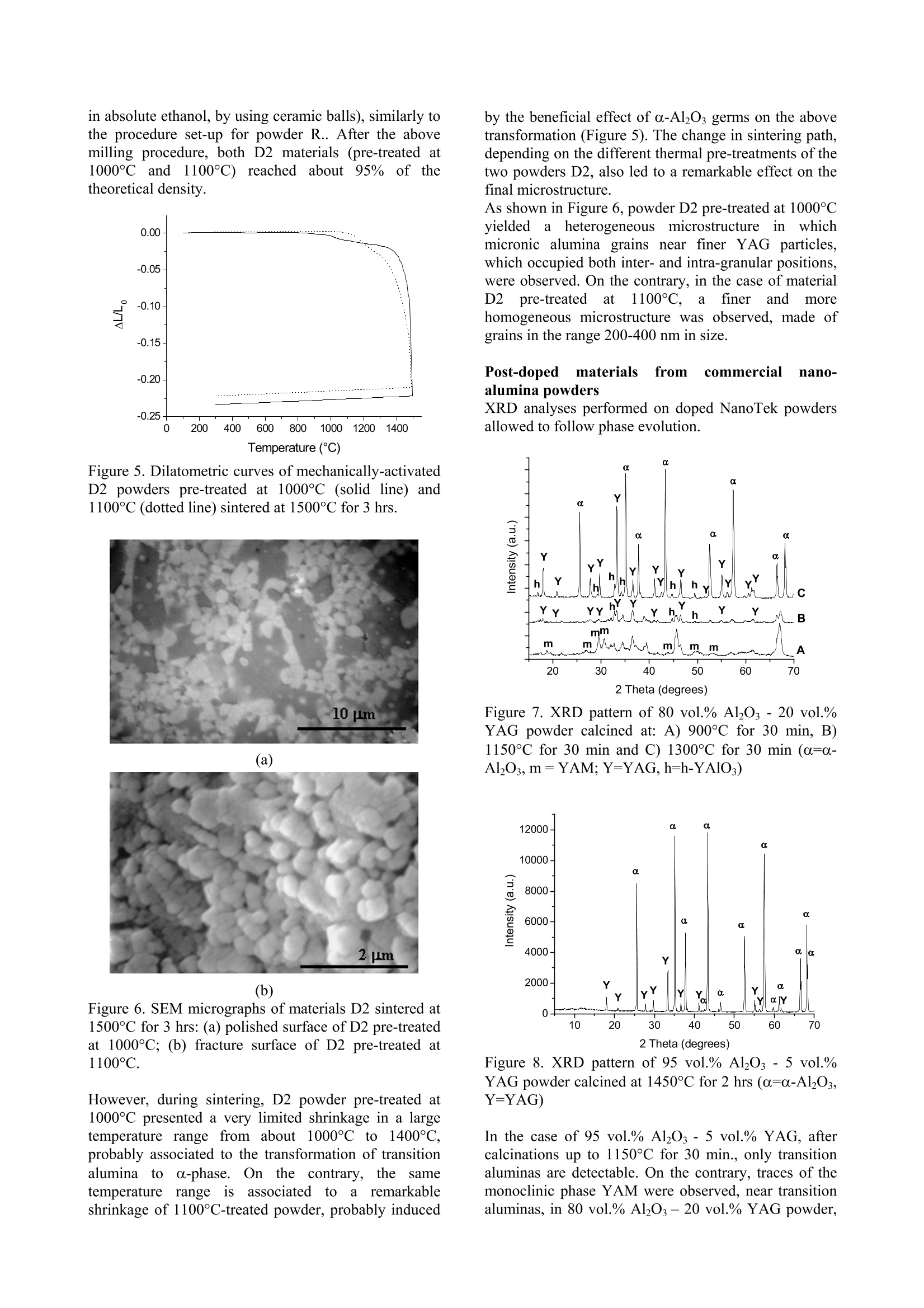
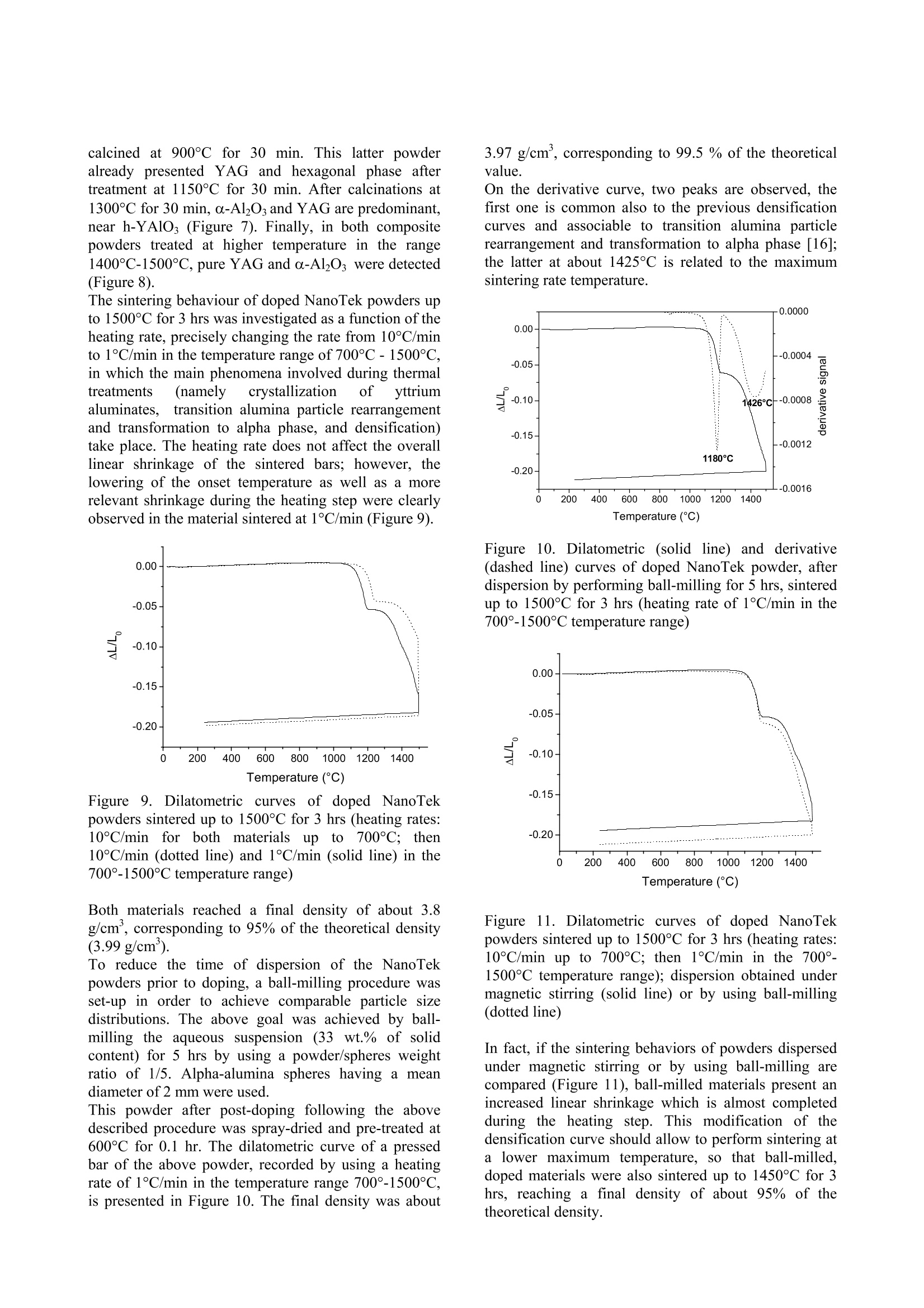
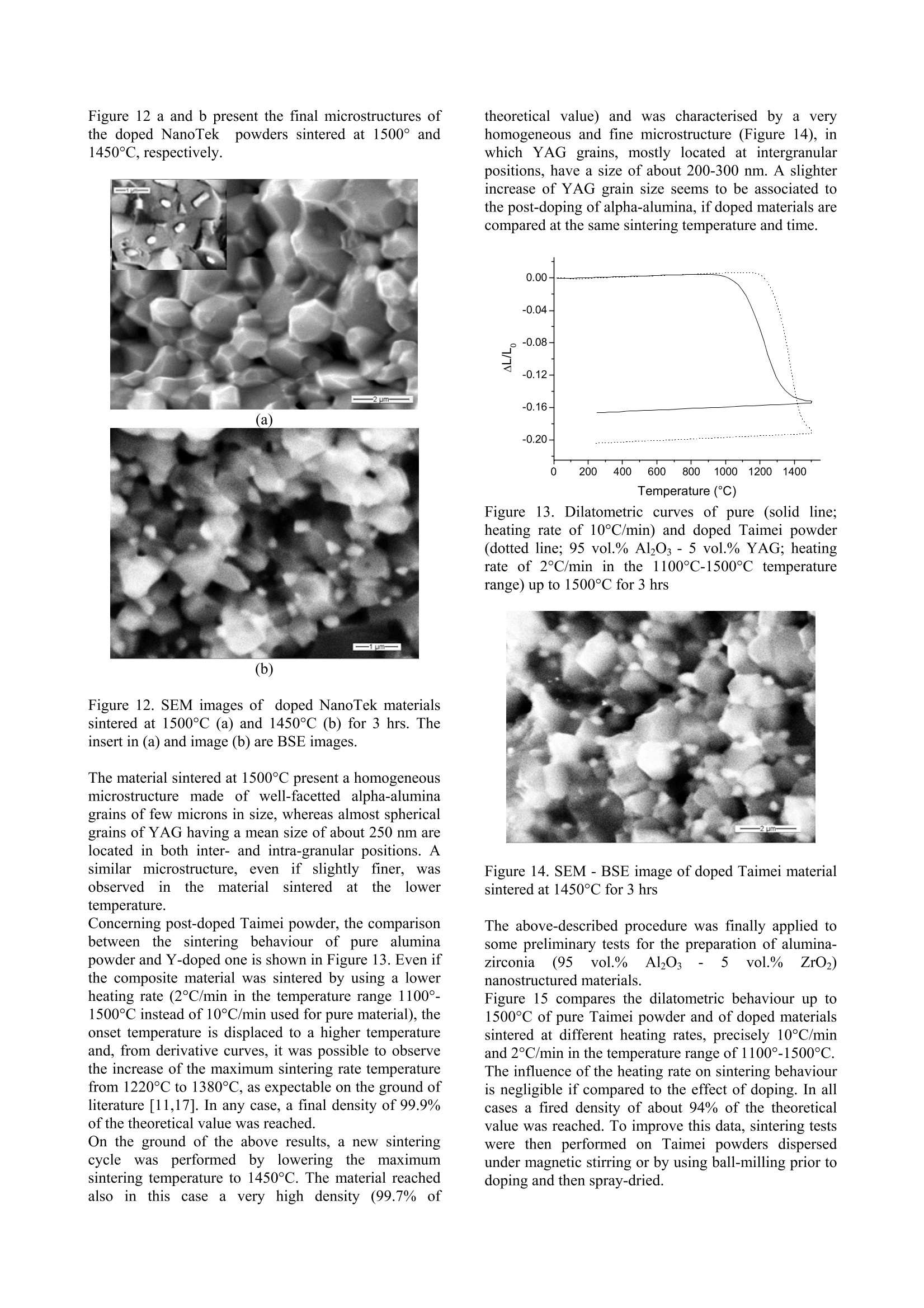
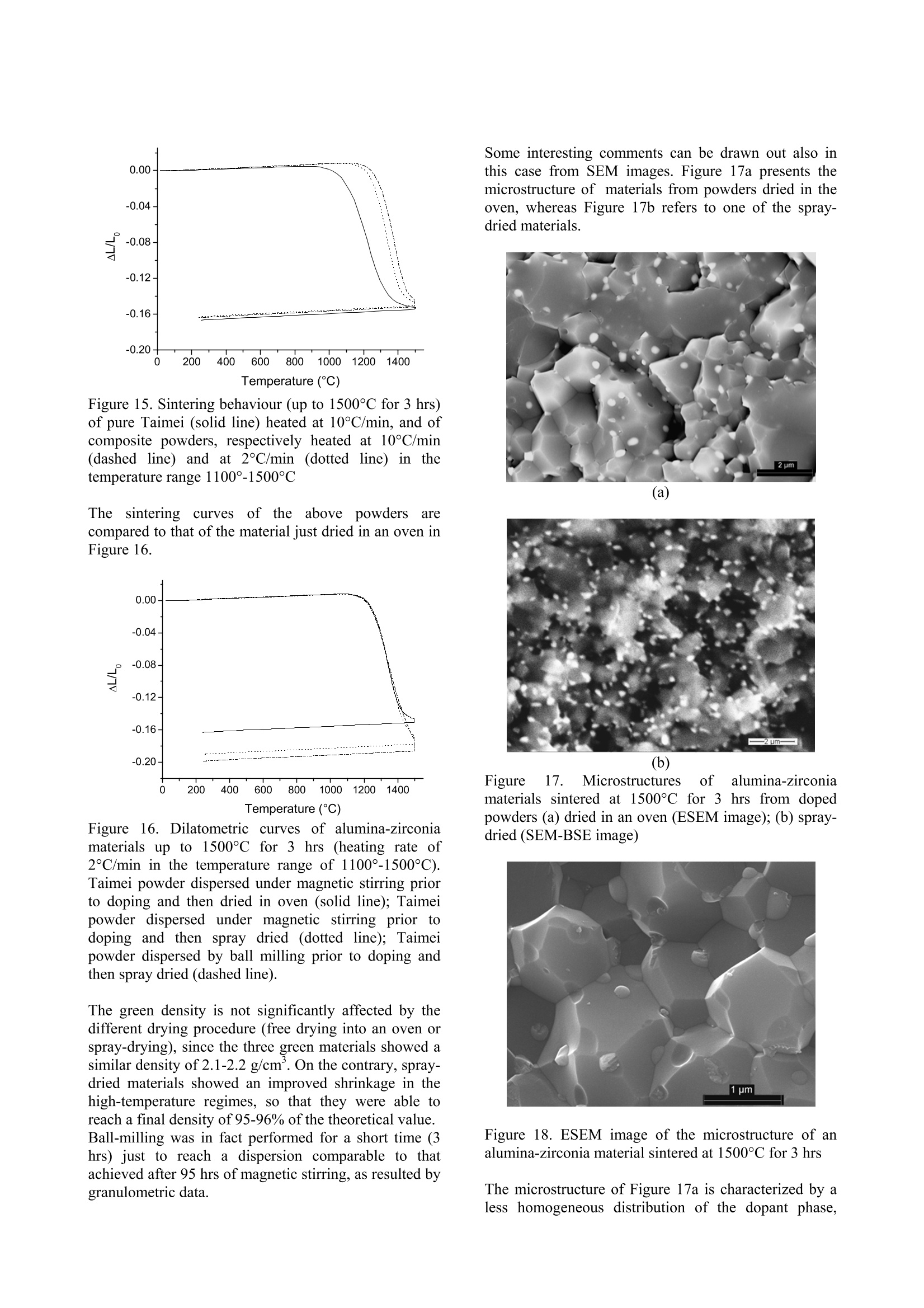
ResearchGateAvailable from: Laura MontanaroRetrieved on: 06 September 2015 See discussions, stats, and author profiles for this publication at:http://www.researchgate.net/publication/265751905 A Comparison among Different ProcessingRoutes towards Ceramic NanocompositesDevelopment ARTICLE CITATION DOWNLOADS VIEWS 39 21 A Comparison among Different Processing Routes towards CeramicNanocomposites Development Laura Montanaro*, Paola Palmero*,Gilbert Fantozzi**,Jerome Chevalier** (*) Dept. SMIC-Politecnico of Torino, INSTM-R.U. PoliTO-LINCE Lab., Corso Ducadegli Abruzzi, 24-10129 Torino, Italy (**) MATEIS, Bat. Blaise Pascal, 7 Av. Jean Capelle, INSA de Lyon, 69621 Villeurbanne,France In this paper, two different processing routes to preparealumina-based nanocomposites Were compared.Sintered bodies were prepared by : densification co-precipitatedalumina-YAGcomposite powdersafter suitablelecombination ofthermal pre-treatment and milling; -uusing alumina powders (derived from a laboratorysynthesis or from an industrial scale production) post-doped with yttrium or zirconium inorganic salt solutionsto develop alumina-YAG and alumina-zirconiananocomposites. In all cases natural sintering was performed to developdense bodies. Keywords: alumina-YAG nanocomposites, alumina-zirconia nanocomposites, wet-chemical synthesis,natural sintering, thermo-mechanical treatments. Introduction Ceramic nanocomposites can be elaborated by usingvarious processing routes starting from nanopowdermixing [1-3] or by nanocomposite powders obtained bycoprecipitation of complex compositions [4-8] as wellas by post-doping the matrix powder with a chemicalprecursor of the reinforcement phase [9-11]. Recently,for instance, the post-doping of an alpha-aluminapowder by using a zirconium alkoxide has beensuccessfully exploited to prepare an alumina-zirconiamicro-nanocomposite material [9]. This paper deals with the comparison among variousprocessing routes to develop nanostructured ceramiccomposites. 50 vol.% Al2O3-Y3Al5O12 (YAG) powders were firstsynthesized by following two different wet-chemicalroutes. A reverse-strike co-precipitation was used in onecase, since it is able to assure a highly-homogeneous,fine-grained product [12,13]. For a sake of comparison,the second composite powder was prepared startingfrom a pure alumina powder precipitated by using areverse-strikeprocedureeandthendopedwith astoichiometric amount ofyttriumnitrate. Thesynthesizedpowders were thenpprocessedbyperforming several treatments and sometimes couplingthem to an extensive mechanical activation by millingprior to dry pressing and natural sintering, in order toinvestigate the best thermal/mechanical pre-treatment toachieve dense and very fine composite materials. Finally the latter procedure was also applied to theproduction of both alumina-YAG and alumina-zirconia(95 vol% alumina-5vol% 2"d phase) nanocomposites,by post-doping commercial transition and/or alpha-alumina nanopowders by using metal chloride or nitratesolutions. Experimental Reverse-strike co-precipitated powders An alumina-YAG composite powder was prepared via areverse-strike co-precipitation starting from an aqueoussolution of AlCl3.6H2O and YCl3.6H2O. More detailsabout this synthesis were already described elsewhere[4]. This powder, labelled R, was fully characterized bythermal analysis (DSC-TG,Netzsch STA 409) and X-ray diffraction (XRD, Philips PW 1710) performed aftercalcination at various temperatures and times, to followthe phase evolution as a function of the pre-treatmenttemperature. After treatment at 900°C for 30 minutes, abar uniaxially pressed at 300 MPa was fully densifiedby natural sintering at 1600°℃ for 3hrs, yielding ahighly-homogeneoussnmicrostructure, madee offa-alumina and YAG grains of about 1 um in size [4]. Tolower the sintering temperature and reduce the finalgrain size, a suitable thermal pre-treatment was coupledoannextensive milling, by followingapatentedprocedure [141. Post-doped materials from alumina powdersynthesised at a laboratory scale A pure alumina powder was synthesized by using theabove reverse-strike procedure, that is adding 2 Maqueous solution of AlCl3.6H2O to an ammonia solutionkept at a constant pH of 9 (±0.2) at 25°C. This powder,labelled D.. wasfully characterized as previouslydescribed for powder R. The dried precipitate was thenplanetary milled in absolute ethanol with agate balls andvessels for 4 (powder D1) and 12 (powder D2) hours.The mean particle size distribution was evaluated bylaser granulometry (Fritzsch analysette 22 compact),after ultrasonic dispersion in absolute ethanol for 10minutes: both powders showed almost super-imposableparticlesize distributions; and agglomerate sizescorresponding to 10, 50 and 90% of the cumulativedistribution of about 0.8, 4 and 11 um, respectively.Alumina-YAG (50 vol.%) composite powders werethen prepared by adding a stoichiometric amount ofyttrium nitrate (Y(NO3)3.6H2O) in alcoholic solution to the un-calcined powders D1 and D2 as well as topowder D1 treated at different temperatures on theground of XRD data and related phase evolution; also inthis case, a pre-germination of a-Al2Os by controlledthermal pre-treatments as well as the synergic effectinduced by coupling thermal treatments to a mechanicalactivation were investigated in view of achieving veryfine, dense sintered bodies. Post-doped materialIsS from commercial nano-alumina powders Two commercial alumina powders were used to set-upthis procedure. NanoTek, supplied by NanophaseTechnologies, USA, is a nano-crystalline transitionalumina prepared by Physical Vapor Synthesis (PVS). Itis characterized by an average crystallite size of about47 nm and it crystallizes into the alpha-phase at about1300°C (Figure 1). Figure 1.TG (dotted line) and DSC (solid line) curvesof the as-received NanoTek"powder The second powder, TM-DAR TAIMICRON, suppliedby Taimei Chemicals Co., Japan, is made of pure alphaalumina having a mean particle size of 350 nm. Post-doping was performed in order to develop 95vol.% alumina - 5 vol.% YAG composites, by partialreaction of the yttrium oxide precursor and the aluminapowder. NanoTek" powder was firstly dispersed in puredistilledwater (solid content of 33 wt.%) andmaintained under magnetic stirring for 120 hours. Anaqueous solution of YCl3.6H2O was also prepared andadded to the120 hrs-dispersedsuspension. Theobtained doped suspensioni was maintained undermagnetic stirring for 2 hrs before drying. Powdersuspensions diluted down to 4 wt.% were then spray-dried (Buchi Mini Spray Dryer B-290) in order to limitsegregation of the doping agent during drying. Thedried doped powders were submitted to various thermalpre-treatments to induce the chloride decomposition aswell as the solid state reaction to yield the final phases,pure YAG and a-Al2O3. X-Ray diffraction analyses were performed on thecalcined powderss to follow tthe phase evolution.However, the low volume percentage of the YAG phase(5 vol.%) in the composite powders does not allow toclearly detect the phase evolution by XRD, in particular for the low-temperature treated powders. For thisreason, a Y-doped NanoTek material, having a finalcomposition corresponding to 80 vol.% Al2O3 - 20vol.%6YAG was aalso preparedwithi the aim offollowing its thermal evolution till to the final, stablephases. Also Taimei alumina powder was post-doped followingthe above procedure. Both doped powders were thenpre-treated at 600°C for 0.1 hr prior to uniaxial pressingat 400 MPa and natural sintering. Finally, the same procedure was5extended to theproduction of an alumina-zirconia nanocomposite, i.e.,of a composition not involving a solid-state reactionduring its thermal treatment. Taimei alumina powder was again firstly dispersed indistilled water and post-doped by using ZrCl4 aqueoussolution. The doped dispersion was then dried (in anoven overnight or by spray-drying) and pre-treated at500℃ without any soaking time. Results and Discussions Reverse-strike co-precipitated powders Powder R, after calcination at 900°℃ for 30 minutes,was planetary milled for 8 hrs with agate vessels andballs in absolute ethanol and then ball-milled for 24 hrswith ceramic balls. Milling procedure led to a significant lowering of themaximum densification temperature (from 1600℃ toabout 1420℃) as well as of the temperatures associatedto h-YA10s (from 1060C to about 980℃), YAG (from1180°℃ to about 960℃) and a-Al2O3 (from 1250℃ atabout 1140℃) crystallization. More details on thisbehaviour were already published [15]. The lowering of the above characteristic temperatureswas not imputable to pollution due to extensive milling,as it was demonstrated by a statistic observation of alarge number of grain boundaries of sintered materialsperformed by High-Resolution Transmission ElectronMicroscope (HR-TEM, Jeol 2010FEG, Figure 2). Figure 2. HR-TEM micrograph of a grain boundarybetween a-alumina and YAG grain (scale: 1 cm=6 nm) An almost fully dense material (density of about 95% ofthe theoretical value) was produced by sintering themilled powder R at 1420°℃ for 2 hrs. The sintered composite presented an homogeneous microstructuremade of a-Al2O and YAG grains having a mean size ofabout 250 nm (Figure 3). Figure 3. TEM micrograph of material R, pre-treated at900℃ for 30 minutes and mechanically activated, afternatural sintering at 1420°C for 2hrs Post-doped materials from alumina powdersynthesised at a laboratory scale Powder D11wasfifirstly calcinedd atthree differenttemperatures andtimes, in order to obtain onlytransition alumina (8-AlzO, by calcination at 500°℃ for azero soaking time; powder D1-500),, an incipientcrystallization of a-Al2Os (by calcination at 900°℃ for30 minutes, powder D1-900) and a well crystallized α-Al203 (powders were rapidly plunged into a verticalfurnace kept at 1280°℃ for 5 minutes, powder D1-1280), as stated by XRD. Alumina-YAG composite powders were then prepared by adding a solution of a stoichiometric amount ofyttrium nitraattee ttoo the un-calcined and differentlycalcined powders D1. These doped powders were thentreated in the temperature range between 1000°C and1300°℃ (no soaking time at the maximum temperature)and XRD was performed to follow phase evolution. Aclear influence of the thermal pre-treatment of powderD1 on the crystallization pathwaiss stated.YAGcrystallization temperature increases with increasing thepre-treatment temperature of powder D1. In addition,when powder D1 is calcined at the higher temperatures(i.e..D,1-900aanddD1-1280),thee appearance Oofsecondary, stable as well as metastable phases (likeY4Al2O9, YAM; orthorhombic YAlO3, YAP; Y203) wasobserved. On the contrary, calcination of powders D1and D1-500 led firstly to the crystallization of thehexagonal phase, h-YAlO3, that transforms into YAGphase at relatively low temperature, and then to pure a-alumina and YAG composite powders, similarly topowder R. The pure, final phases were yielded between1100°℃ and 1200°C in doped-D1 powder and between1200°℃ and 1300°℃ in doped D1-500 powder. Analumina-YAG composite powder was also obtained bydoping powder D2 following the above procedure. Purea-alumina-YAG composite were prepared by calciningdoped-D2 powders at a lower temperature than thatrequired for powders D1, as shown in Figure 4, in which XRD patterns of the doped powders treated at1000°C and 1100°℃ are compared. In fact, calcinationof doped-D2 powder at 1000℃ led to a relevantcrystallization of h-YAlO, and YAG, near a-aluminatraces, while h-phase and YAG were just detected intraces in powder D1 treated at the same temperature. At1100°C, well-crystallized YAG and a-Al2O3 weredetected in doped-D2 powder, whereas the same phaseswere less crystallized and h-YAlOs amount was stillobserved in doped-D1 powder. 2 Theta (degrees) (a) (b) Figure 4. XRD patterns of doped D1 (a) and D2 (b)powders calcined at 1000°℃ and 1100℃ (a=a-Al2O3,8=8-A1203,Y=YAG,h=h-YAlO3) Powder D2 calcined at 1100℃ presented the samephase composition than powder R heat-treated at asignificantly higher temperature (1215℃), so to inducea relevant growth of YAG grains and reduced powdersinterability. The best performing doped powders, thatis powders D2 pre-treated at 1000℃ and1100℃, weremilled prior to sinter at 1500℃ for 3 hrs. The bestresults were achieved by combining a preliminaryplanetary milling (for 4 hrs in absolute ethanol, by usingagate vessels and balls) to a ball milling step (for 24 hrs, in absolute ethanol,by using ceramic balls), similarly tothe procedure set-up for powder R.. After the abovemilling procedure, both D2 materials (pre-treated at1000℃ and 1100°C) reached1about95% of thetheoretical density. Figure 5. Dilatometric curves of mechanically-activatedD2 powders pre-treated at 1000℃ (solid line) and1100C (dotted line) sintered at 1500°℃ for 3 hrs. (a) (b) Figure 6. SEM micrographs of materials D2 sintered at1500°C for 3 hrs: (a) polished surface of D2 pre-treatedat 1000°℃; (b) fracture surface of D2 pre-treated at1100°C. However, during sintering, D2 powder pre-treated at1000℃ presented a very limited shrinkage in a largetemperature range from about 1000°℃ to 1400℃,probably associated to the transformation of transitionalumina toa-phase. COn the contrary, thesametemperature,rangeis associatedtctoaremarkableshrinkage of 1100°C-treated powder,probably induced by the beneficial effect of o-Al2O3 germs on the abovetransformation (Figure 5). The change in sintering path,depending on the different thermal pre-treatments of thetwo powders D2, also led to a remarkable effect on thefinal microstructure. As shown in Figure 6, powder D2 pre-treated at 1000℃yielded1 a heterogeneous microstructure inwhichmicronic alumina grains near finer YAG particles,which occupied both inter- and intra-granular positions,were observed. On the contrary, in the case of materialD2ppre-treatedat 1100°C.afiner andd morehomogeneous microstructure was observed, made ofgrains in the range 200-400 nm in size. Post-doped materials from commercial nano-alumina powders XRD analyses performed on doped NanoTek powdersallowed to follow phase evolution. Figure 7.XRD pattern of 80 vol.% Al2O3 - 20 vol.%YAG powder calcined at: A) 900℃ for 30 min, B)1150°C for 30 min and C) 1300°C for 30 min (α=0-Al203, m=YAM; Y=YAG,h=h-YAlO3) Figure 8. XRD pattern of 95 vol.% Al203-5 vol.%YAG powder calcined at 1450°C for 2 hrs (a=a-AlO3,Y=YAG) In the case of 95 vol.% Al203-5vol.% YAG, aftercalcinations up to 1150°℃ for 30 min., only transitionaluminas are detectable. On the contrary, traces of themonoclinic phase YAM were observed, near transitionaluminas, in 80 vol.% Al203-20 vol.% YAG powder, calcined at 900C for 30 min. This latter powderalready presented YAG and hexagonal phase aftertreatment at 1150°C for 30 min. After calcinations at1300°℃ for 30 min, a-Al2Os and YAG are predominant,near h-YAlO3 (Figure 7). Finally, in both compositepowders treated at higher temperature in the range1400℃-1500℃, pure YAG and a-Al203 were detected(Figure 8). The sintering behaviour of doped NanoTek powders upto 1500℃ for 3 hrs was investigated as a function oftheheating rate, precisely changing the rate from 10°C/minto 1°C/min in the temperature range of 700℃-1500C,in which the main phenomena involved during thermaltreatments (namely crystallization of yttriumaluminates, transition alumina particle rearrangementand transformation to alpha phase, and densification)take place. The heating rate does not affect the overalllinear shrinkage of the sintered bars; however, thelowering of the onset temperature as well as a morerelevant shrinkage during the heating step were clearlyobserved in the material sintered at 1°C/min (Figure 9). Figure 9. Dilatometriccurves of doped NanoTekpowders sintered up to 1500°℃ for 3 hrs (heating rates:10°C/minfor both materialsup to 700℃; then10°C/min (dotted line) and 1°C/min (solid line) in the700°-1500°C temperature range Both materials reached a final density of about 3.8g/cm’, corresponding to 95% of the theoretical density(3.99 g/cm). To reduce the time of dispersion of the NanoTekpowders prior to doping, a ball-milling procedure wasset-up in order to achieve comparable particle sizedistributions. The above goal was achieved by ball-milling the aqueous suspension (33 wt.% of solidcontent) for 5 hrs by using a powder/spheres weightratio of 1/5. Alpha-alumina spheres having a meandiameter of 2 mm were used. This powder after post-doping following the abovedescribed procedure was spray-dried and pre-treated at600°C for 0.1 hr. The dilatometric curve of a pressedbar of the above powder, recorded by using a heatingrate of 1°C/min in the temperature range 700°-1500°C,is presented in Figure 10. The final density was about 3.97 g/cm’, corresponding to 99.5 % of the theoreticalvalue. On the derivative curve, two peaks are observed, thefirst one is common also to the previous densificationcurves and associable to transition alumina particlerearrangement and transformation to alpha phase[16];the latter at about 1425°C is related to the maximumsintering rate temperature. Figure 10. Dilatometric (solid line) and derivative(dashed line) curves of doped NanoTek powder, afterdispersion by performing ball-milling for 5 hrs, sinteredup to 1500℃ for 3 hrs (heating rate of 1°C/min in the700°-1500°C temperature range) Figure 11. Dilatometric curves of doped NanoTekpowders sintered up to 1500°℃ for 3 hrs (heating rates:10°C/min up to 700°C; then 1°C/min in the 700°-1500C temperature range); dispersion obtained undermagnetic stirring (solid line) or by using ball-milling(dotted line) In fact, if the sintering behaviors of powders dispersedunder magnetic stirring or by using ball-milling arecompared (Figure 11), ball-milled materials present anincreased linear shrinkage which is almost completedduring the heating step. This modification of thedensification curve should allow to perform sintering ata lower maximum temperature, so that ball-milled,doped materials were also sintered up to 1450℃ for 3hrs, reaching a final density of about 95% of thetheoretical density. Figure 12 a and b present the final microstructures ofthe doped NanoTek powders sintered at 1500° and1450°C, respectively. (a) (b) Figure 12. SEM images of doped NanoTek materialssintered at 1500°C (a) and 1450°C(b) for 3 hrs. Theinsert in (a) and image (b) are BSE images. The material sintered at 1500℃ present a homogeneousmicrostructure made of well-facetted alpha-aluminagrains of few microns in size, whereas almost sphericalgrains of YAG having a mean size of about 250 nm arelocated in both inter- and intra-granular positions. Asimilar microstructure, even if slightly finer, wasobservedin tthe materialhlsinteredat thelowertemperature. Concerning post-doped Taimei powder, the comparisonbetween the sintering behaviour of pure aluminapowder and Y-doped one is shown in Figure 13. Even ifthe composite material was sintered by using a lowerheating rate (2°C/min in the temperature range 1100°-1500℃ instead of 10°C/min used for pure material), theonset temperature is displaced to a higher temperatureand, from derivative curves, it was possible to observethe increase of the maximum sintering rate temperaturefrom 1220°℃ to 1380°C, as expectable on the ground ofliterature [11,17]. In any case, a final density of 99.9%of the theoretical value was reached. On the ground of the above results, a new sinteringcyclewasisperformedby loweringtthe: maximumsintering temperature to 1450°C. The material reachedalso in this case a very high density (99.7%of theoretical value) and was characterised by a veryhomogeneous and fine microstructure (Figure 14), inwhich YAG grains, mostly located at intergranularpositions, have a size of about 200-300 nm. A slighterincrease of YAG grain size seems to be associated tothe post-doping of alpha-alumina, if doped materials arecompared at the same sintering temperature and time. Figure 13. Dilatometric curves of pure (solid line;heating rate of 10°C/min) and doped Taimei powder(dotted line; 95 vol.% Al203-5 vol.% YAG; heatingrate of 2°C/min in the 1100C-1500°C temperaturerange) up to 1500℃ for 3 hrs Figure 14. SEM-BSE image of doped Taimei materialsintered at 1450°C for 3 hrs The above-described procedure was finally applied tosome preliminary tests for the preparation of alumina-zirconia (95 vol. Al203 5 vol.% ZrO2)nanostructured materials. Figure 15 compares the dilatometric behaviour up to1500°℃ of pure Taimei powder and of doped materialssintered at different heating rates, precisely 10°C/minand 2°C/min in the temperature range of 1100°-1500°C.The influence of the heating rate on sintering behaviouris negligible if compared to the effect of doping. In allcases a fired density of about 94% of the theoreticalvalue was reached. To improve this data, sintering testswere then performed on Taimei powders dispersedunder magnetic stirring or by using ball-milling prior todoping and then spray-dried. Figure 15. Sintering behaviour (up to 1500℃ for 3 hrs)of pure Taimei (solid line) heated at 10°C/min, and ofcomposite powders, respectively heated at 10°C/min(dashed line) and at 2C/min (dotted line) in thetemperature range 1100°-1500°C Thesintering curves of the above powdersarecompared to that of the material just dried in an oven inFigure 16. Figure 16. Dilatometric curves of alumina-zirconiamaterials up to 1500℃ for 3 hrs (heating rate of2°C/min in the temperature range of 1100°-1500℃).Taimei powder dispersed under magnetic stirring priorto doping and then dried in oven (solid line); Taimeipowder dispersed under magnetic stirring prior todoping and then spray dried (dotted line); Taimeipowder dispersed by ball milling prior to doping andthen spray dried (dashed line). The green density is not significantly affected by thedifferent drying procedure (free drying into an oven orspray-drying), since the three green materials showed asimilar density of 2.1-2.2 g/cm’. On the contrary, spray-dried materials showed an improved shrinkage in thehigh-temperature regimes, so that they were able toreach a final density of 95-96% of the theoretical value.Ball-milling was in fact performed for a short time (3hrs) just to reach a dispersion comparable to thatachieved after 95 hrs of magnetic stirring, as resulted bygranulometric data. Some interesting comments can be drawn out also inthis case from SEM images. Figure 17a presents themicrostructure of materials from powders dried in theoven, whereas Figure 17b refers to one of the spray-dried materials. (a) (b) Figure 17. Microstructures ofalumina-zirconiamaterials sintered at 1500°C for 3 hrs from dopedpowders (a) dried in an oven (ESEM image); (b) spray-dried (SEM-BSE image) Figure 18. ESEM image of the microstructure of analumina-zirconia material sintered at 1500°C for 3 hrs The microstructure of Figure 17a is characterized by aless homogeneous distribution of the dopant phase, probably induced by the drying procedure (free dryingof the doped suspension into an oven) which shouldinduce a certain segregation. On the contrary, the fastdrying performed in the spray-drier on small suspensiondroplets is able to preserve the homogeneity of thecomposite dispersion ((Figure 17b).In the latter.particularly,itisppossibleto appreciateeaveryhomogeneous and fine distribution of zirconia grains inboth inter and intra-granular positions (Figure 18). Themean size of the zirconia grains is about 200 nm. Conclusions This research has demonstrated that post-doping ofcommercial, both transition and alpha, alumina powdersbyyusingyttriumor zirconium oxideeinorganicprecursors should be successfully used to produce veryfine and homogeneous YAG-alumina and ZrO2-aluminadense composites. Therefore, this route is an effectivealternative to the preparation of composite startingpowdersbyuusing, forinstance, reverse-strikecoprecipitation. By controlling some crucial processparameters, such as alumina powder dispersion route,post-doping procedure and subsequent drying step,sintering cycle and particularly heating rate, ittiispossible to refine the obtained microstructure towardsthe preparation of micro-nanocomposite materials. Acknowledgments The Authors wish to thank the European Commissionandltthe ItalianilMinistry MIURRtcto)bhave partiallysupported this research in the framework of the IPproject NANOKER and of the project Legge 449/97“Materiali compositi per applicazioni strutturali dirilevante interesse industriale", respectively, and Prof.Claude Esnouf of INSA of Lyon to have performedTEM and HR-TEM observations. References A. Hirvonen, R. Nowak, Y. Yamamoto, T.Sekino, K. Niihara: Fabrication, Structure,Mechanical and Thermal Properties ofZirconia-based Ceramic Nanocomposites.J. Eur. Cer. Soc. 26(2006),1497-1505. 2. M.l.sSternitzke: Structural CeramicNanocomposites. J. Eur. Cer. Soc. 17(1997),1061-1082. 3. Y.K. Jeong, K. Niihara: Microstructureand Mechanical Properties of PressurelessSintered Al2O3/SiCNanocomposites.NanoStructured Materials 9 (1997),193-196. 4. P. Palmero, A. Simone, C. Esnouf, G.Fantozzi.l,L. Montanaro:ComparisonAmong Different Sintering Routes forPreparing Alumina-YAG Nanocomposites.J. Eur. Cer. Soc. 26 (2006), 941-947. 5. X.H. Jin, L. Gao: Microstructure andMechanical Properties of NdAlO3/Al2O3 Nanocomposits. Materials ScienceeandEngineering A 354 (2003),326-330. 6. Y.Q. Wu, Y.F. Zhang, S.W. Wang, J.K.Guo: In-situuSynthesisofiRoadlikeLaAl11O18inAl O3Powderirby aCoprecipitation Method.J. Eur. Cer.Soc.21 (2001),919-923. 7. W.Q. Li. L. Gao:Processing,Microstructure and Mechanical Propertiesof 25 vol.% YAG-Al2O: Nanocomposites.NanoStructured Materials 11 (1999),1073-1080. 8. H.Z. Wang, L. Gao, L.H. Gui, J.K. Guo:Preparation and Properties of IntergranularAl2O3-SiC Nanocomposites.NanoStructured Materials 10 (1998), 947-953. 9. M. Schehl, L.A. DIiaz, R. Torrecillas:Alumina Nanocomposites from Powder-Alkoxide Mixtures. Acta Materialia 50(2002),1125-1139. 10. S. Deville, J. Chevalier, G. Fantozzi, J.F.Bartolome, J. Requena, J.S. MOya, R.Torrecillas, L.A. Diaz: Low-TemperatureAgeing of Zirconia-Toughnened AluminaCeramicsS:and its Implications irBiomedical Implants. J. Eur. Cer. Soc. 23(2003),2975-2982. 11. R. Voytovych, I.Mac Laren, M.A. Gulgiin,Cannon, M. Ruhle: The Effect of Yttriumon Densification and Grain Growth in a-Alumina. Acta Materialia 50 (2002),3453-3463. 12. P. Apte, H. Burke, H. Pickup: Synthesis ofYttrium Aluminum Garnet by ReverseStrike Precipitation. Journal of MaterialsResearch 7 (1992),706-711. 13. H.Wang, L. Gao,K. Niihara: Synthesis ofNanoscaled Yttrium Aluminum GarnetPowder by the Co-Precipitation Method.Mater. Sci. Eng. A 288 (2000), 1-4. 14. L.Montanaro. P.Palmero. A.Simone.C.Stella: Europen Patent n°EP05105757.8(2005) 15. P. Palmero, L. Montanaro: Processing andSintering of Alumina-YAGNanocomposites. Advances in Science andTechnology 45 (2006), 1696-1703. 16. C. Legros, C. Carry, P. Bowen, H.Hoffmann: Sinteringg of a TransitionAlumina: Effect of Phase Transformation.Powder Characteristics and ThermalCycle. J. Europ.Ceram.Soc. 19 (1999),1967-1978 17. J. Fang, A.M. Thompson, M.P. Harmer,H.M. Chan:Effectt of Yttrium andLanthanum on the Final-Stage SinteringBehaviour of Ultrahigh-Purity Alumina. J.Am. Ceram. Soc. 80 (1997),2005-2012
确定


还剩7页未读,是否继续阅读?
北京飞驰科学仪器有限公司为您提供《不同纳米陶瓷复合材料中粒径分布检测方案(激光粒度仪)》,该方案主要用于其他中粒径分布检测,参考标准--,《不同纳米陶瓷复合材料中粒径分布检测方案(激光粒度仪)》用到的仪器有德国FRITSCH(飞驰)A22大量程纳米激光粒度仪
推荐专场
相关方案
更多
该厂商其他方案
更多