
方案详情
文
A sweetness sensor with lipid/polymer membranes has been developed for evaluatingthe sweetness of sugars and sugar alcohols. Among the constituents of lipid/polymermembranes, gallic acid has been used as the main substance involved in sucrose responsein our group. In this study, as a step toward understanding the response mechanism ofthe sweetness sensor, functional groups of gallic acid, namely, carboxyl and hydroxylgroups, were focused on. Theresultsdemonstrated that the carboxyl group is essentialfor the sweetness sensor, whereas the hydroxyl group is not always necessary for thesucrose response. It was also revealed that the phosphate group may be a substitutefor the carboxyl group. Then, for one of the sensors with the highest response to a 300mM sucrose solution, named the sweetness sensor GL1, the basic characteristics suchas selectivity and correlation with sweetness were investigated. The behavior of GL1sensor outputs was relatively similar to the sweetness perception in humans.
方案详情

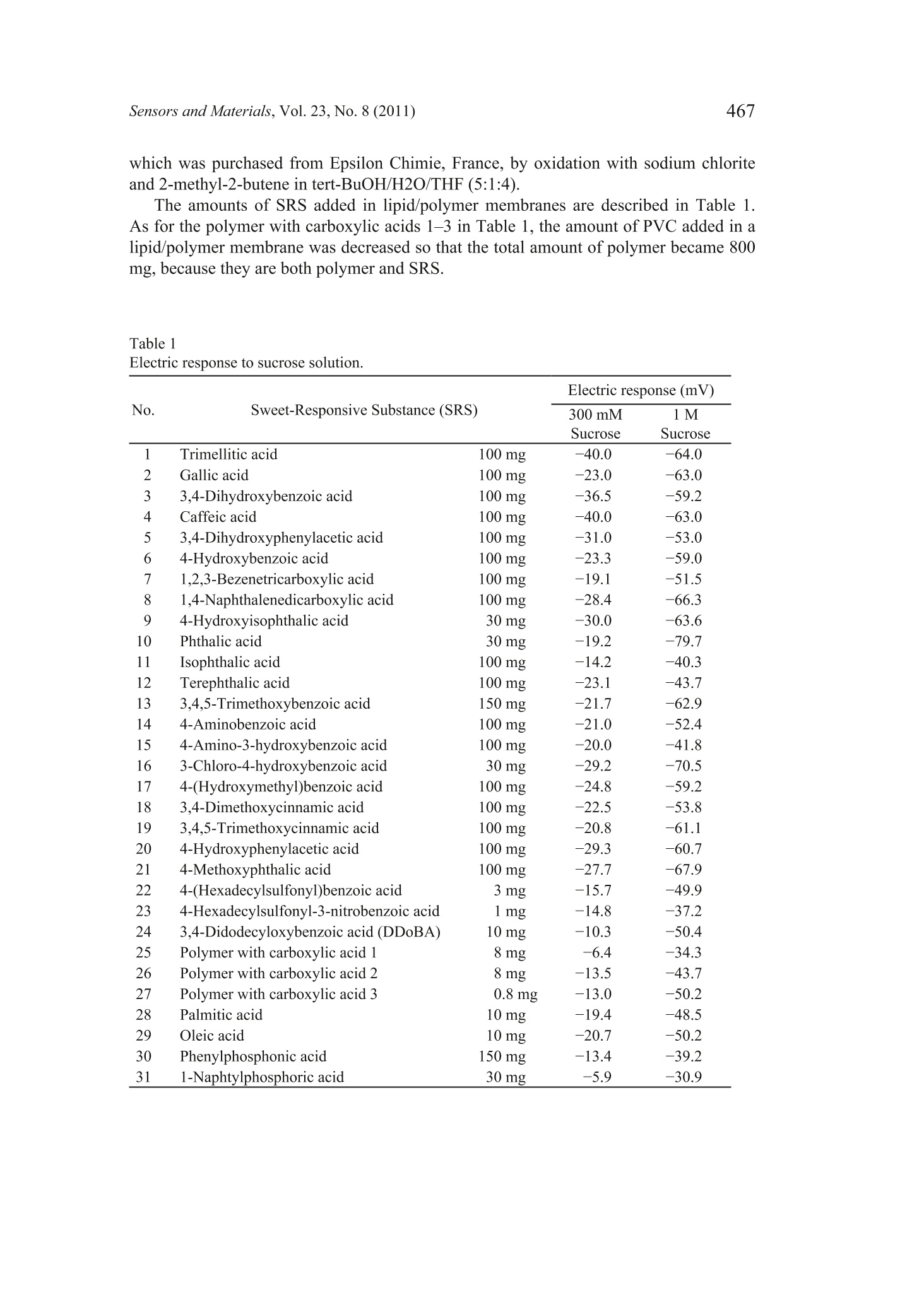
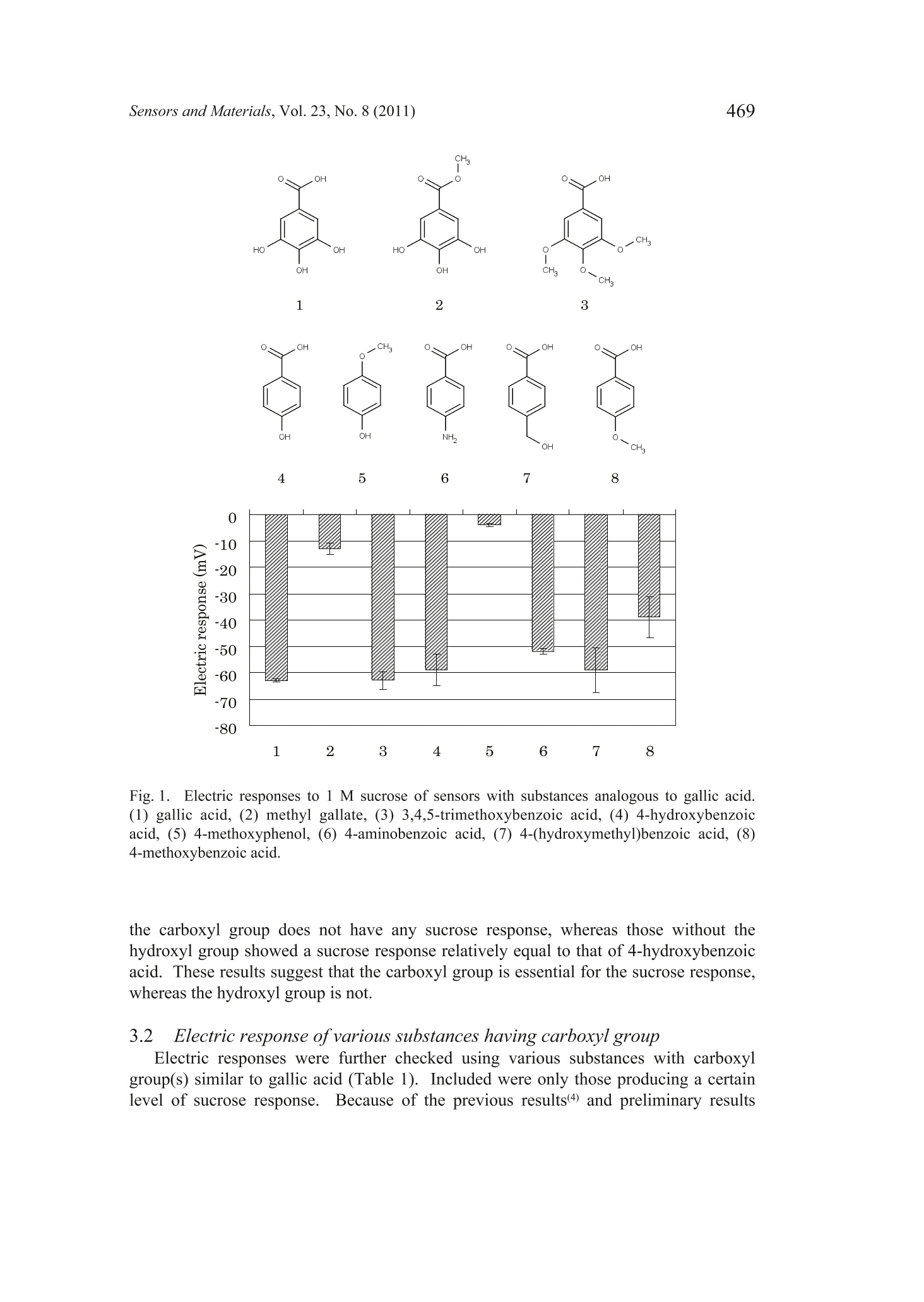
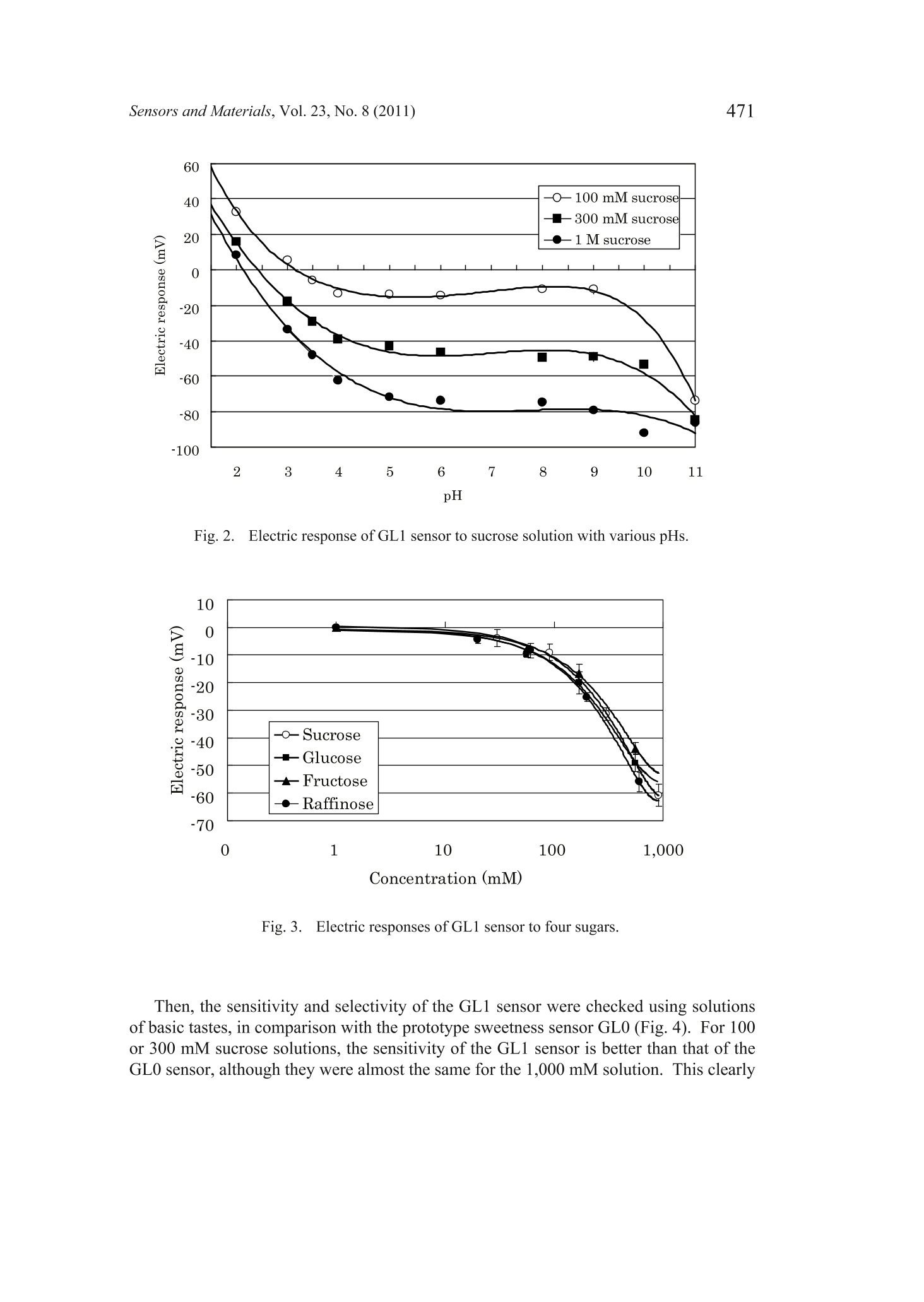
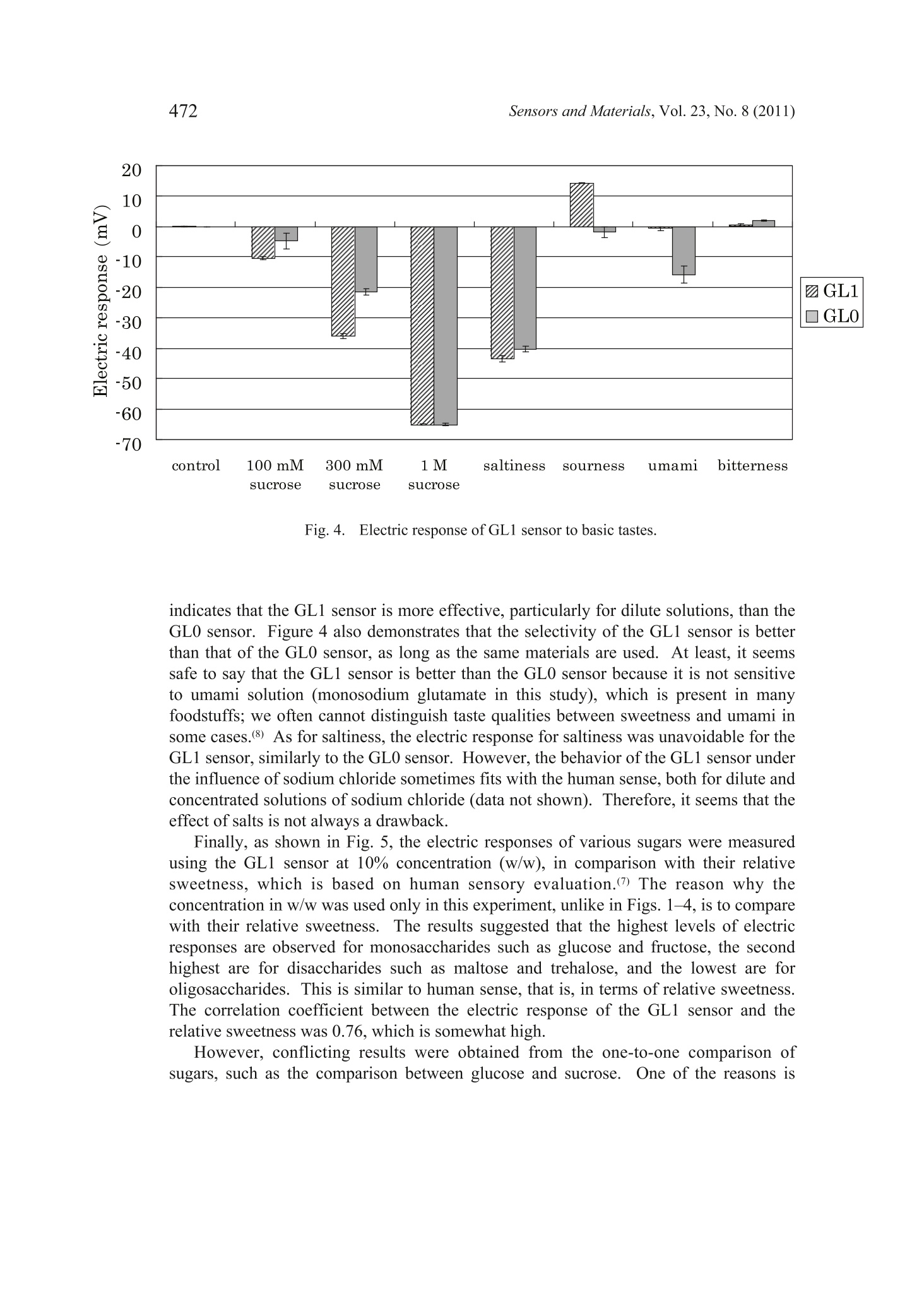
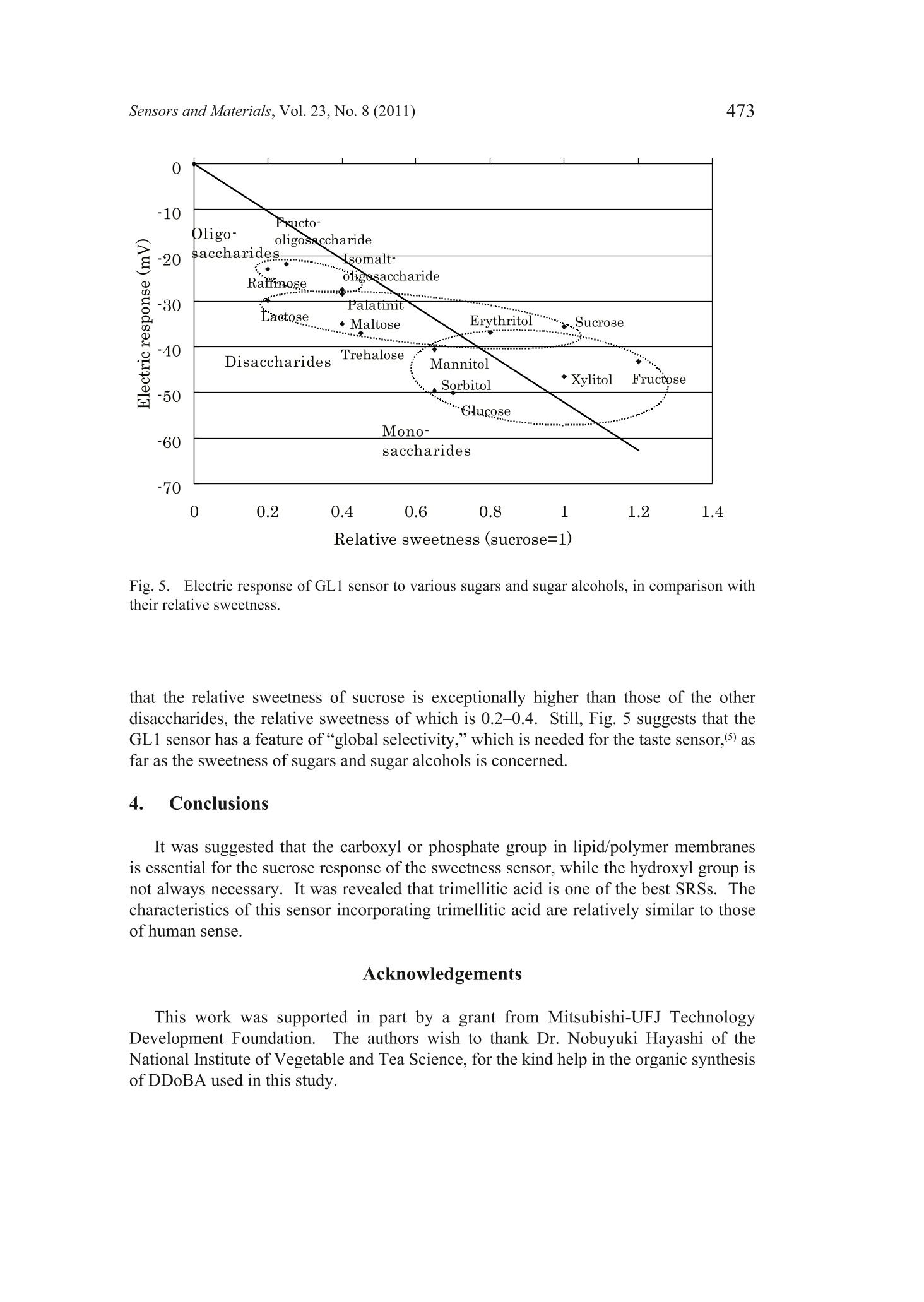
Sensors and Materials, Vol. 23,No. 8 (2011) 465-474MYU TokyoS & M 0859 466Sensors and Materials, Vol.23, No.8 (2011) 465 Sweetness Sensor with Lipid/Polymer Membranes:Sweet-Responsive Substances Kentaro Toyota, Hong Cui, Kentaro Abe, Masaaki Habara,Kiyoshi Tokol and Hidekazu Ikezaki Intelligent Sensor Technology, Inc., 5-1-1 Onna, Atsugi-shi, Kanagawa 243-0032, JapanGraduate School of Information Science and Electrical Engineering, Kyushu University,744Motooka, Nishi-ku, Fukuoka 819-0385,Japan (Received June 6, 2011; accepted August 29,2011) Keywords: sweetness sensor, lipid/polymer membrane, carboxyl group, hydroxyl group A sweetness sensor with lipid/polymer membranes has been developed for evaluatingthe sweetness of sugars and sugar alcohols..Among the constituents of lipid/polymermembranes, gallic acid has been used as the main substance involved in sucrose responsein our group. In this study, as a step toward understanding the response mechanism ofthe sweetness sensor, functional groups of gallic acid, namely, carboxyl and hydroxylgroups, were focused on. The results demonstrated that the carboxyl group is essentialfor the sweetness sensor, whereas the hydroxyl group is not always necessary for thesucrose response..It was also revealed that the phosphate group may be a substitutefor the carboxyl group. Then, for one of the sensors with the highest response to a 300mM sucrose solution, named the sweetness sensor GL1, the basic characteristics suchas selectivity and correlation with sweetness were investigated. The behavior of GL1sensor outputs was relatively similar to the sweetness perception in humans. 1. Introduction It has been difficult to develop sweetness sensors with a lipid/polymer membranebecause sweet-tasting substances like sucrose do not have an electric charge. To date,by changing the compositions of sensors with a lipid/polymer membrane, the selectivityand sensitivity to a sucrose solution have been gradually improved by our group.(1-3) Oneof the recent results was that the contact of sucrose to the surface of a lipid/polymermembrane depends on the chemical structure of the surface-modifying phenoliccompound like gallic acid.(4) For all the data obtained in these studies, the surface-modified lipid/polymer membranes were used. ( *Corresponding author: e-mail: Toyota.Kentaro@insent.co.jp ) Gallic acid, which is an important constituent of the sweetness sensor, has twofunctional groups, i.e., carboxyl and hydroxyl groups. In this paper, from the viewpointthat functional groups play important role(s) in the interaction between the sweetnesssensor and sugar samples, substances that are similar to gallic acid and have variousfunctional group(s) were checked for their sucrose response. The substances capable ofproducing a sucrose response in the sweetness sensor were tentatively named SRS, for“sweet-responsive substance."72 It is important to determine the necessary and sufficient conditions for the sweetnesssensor, which may provide hints for understanding the response mechanism. This maybe a basis for creating sensors that can detect neutral substances other than sugars. 2. Materials and Methods 2.1Sensor with lipid/polymer membrane Sensors with lipid/polymer membranes were made using tetradodecylammoniumbromide (TDAB), 1.5 mL of di-n-octylphenylphosphonate (DOPP), and 800 mg ofpolyvinyl chloride (PVC) as lipid, plasticizer, and polymer, respectively, as describedpreviously.(1,3)Basically, the amount of TDAB added in lipid/polymer membranes was1 mg, by which the maximum electric response was obtained. However, some lipid/polymer membranes showed a maximum electric response with 0.5 to 2 mg of TDAB;0.5 mg of TDAB was added for lipid/polymer membranes with 4-hexadecylsulfonyl-3-nitrobenzoic acid, 1.2 mg of TDAB for those with 3,4-didodecyloxybenzoic acid (DDoBA),and 1.25 mg of TDAB for those with polymer with carboxylic acid 1. Moreover, 1.5mg of TDAB was added for those with 4-(hexadecylsulfonyl)be、aci13nzoic acid, polymerwith carboxylic acid 3, and phenylphosphonic acid, and 2 mg of TDAB was addedfor those with gallic acid, 4-methoxyphenol, 4-methoxybenzoic acid, phthalic acid,4-methoxyphthalic acid, and 1-naphtylphosphoric acid. 2.2 Sweet-responsive substances (SRSs) For sweet-responsive substances (SRSs), trimellitic acid, gallic acid, 3,4-dihydroxybenzoicacid, caffeic acid, 1,2,3-benzenetricarboxylic acid,4-hydroxyisophthalic acid, phthalicacid, isophthalic acid, terephthalic acid, 3,4,5-trimethoxybenzoic acid, 4-methoxyphthalicacid, oleic acid, phenylphosphonic acid, and 1-naphtylphosphoric acid were purchasedfrom Tokyo Chemical Industry Co., Ltd., Japan. Methyl gallate, 4-methoxyphenol,4-methoxybenzoic acid, 3,4-dihydroxyphenylacetic acid, 4-hydroxybenzoic acid, 1,4-naphthalenedicarboxylic acid, 4-aminobenzoic acid, 4-amino-3-hydroxybenzoic acid,3-chloro-4-hydroxybenzoic acid, 4-(hydroxymethyl) benzoic acid, 3,4-dimethoxycinnamicacid, 3,4,5-trimethoxycinnamic acid, 4-hydroxyphenylacetic acid, 4-(hexadecylsulfonyl)benzoic acid, 4-hexadecylsulfonyl-3-nitrobenzoic acid, polymer with carboxylic acid1 (poly(methyl methacrylate-co-methacrylic acid)), polymer with carboxylic acid2 (poly(ethylene-co-acrylic acid)), and polymer with carboxylic acid 3 (poly[2,6-bis(hydroxymethyl)-4-methylphenol-co-4-hydroxybenzoic acid]) were purchasedfrom Sigma-Aldrich, USA.Palmitic acid was purchased from Wako Pure ChemicalIndustries, Ltd., Japan. DDoBA was synthesized from 3',4'-(didodecyloxy)benzaldehyde, which was purchased from Epsilon Chimie, France, by oxidation with sodium chloriteand 2-methyl-2-butene in tert-BuOH/H2O/THF (5:1:4). The amounts of SRS added in lipid/polymer membranes are described in Table 1.As for the polymer with carboxylic acids 1-3 in Table 1, the amount of PVC added in alipid/polymer membrane was decreased so that the total amount of polymer became 800mg, because they are both polymer and SRS. Table 1Electric response to sucrose solution. 2.3 Measurement conditions Sensors with lipid/polymer membranes were preconditioned with the reference solution(30 mM KCl and 0.3 mM tartaric acid, pH 3.5) for 48 h before use. Measurements wereperformed using the Taste Sensing System SA402B (Intelligent Sensor Technology,Inc., Japan), as described previously.(5) For cleaning the sensors, a positively chargedmembrane-washing solution (10 mM NaOH, 100 mM NaCl, 30% EtOH, pH 12.5) wasused. This solution is important not only for washing but also for optimizing the sensor.Because the surfaces of sensors always have a positive electric charge, data are obtainedas negative values; the higher the concentration of the sucrose solution, the morenegative the sensor output value. Data are shown as average values from the last threeout of five cycles of measurement, except for DDoBA (the last three out of ten cycles ofmeasurement),because sensor outputs from the early stage of measurement tend to beunstable. 2.4 Measurement samples Sample solutions of sugars were prepared by adding them to the reference solution(30 mM KCl and 0.3 mM tartaric acid, pH 3.5). Sucrose, glucose, fructose, xylitol,erythritol, sorbitol, mannitol, maltose, lactose, trehalose, palatinit, raffinose, isomalt-oligosaccharide, and fructo-oligosaccharide were purchased from Wako Pure ChemicalIndustries, Ltd., Japan. For sample solutions of basic tastes, potassium chloride (300mM), tartaric acid (3 mM), monosodium glutamate (10 mM), and quinine hydrochloride (0.1mM) were used as salty, sour, savoriness (umami), and bitter samples, respectively. 3. Results and Discussion 3.1 Electric response of sensors with gallic acid and its derivatives In contrast to the previous studies,(1-4) a new method was used in this paper,in whichno surface modification of the sensor is performed before measurement. This is becauseof the preliminary results, suggesting that surface-modified lipid/polymer membranesand the membranes proposed here often have different sucrose responses. Since somephenolic compounds showed a sucrose response only when they were contained withinthe lipid/polymer membranes, one of the reasons for the difference was considered to bethe difference in the efficiency of surface modification among the phenolic compounds. As a functional group, the carboxyl and hydroxyl groups from the chemical structureof gallic acid were focused on. The results are summarized in Fig. 1. Firstly, to evaluatethe importance of the carboxyl group, methyl gallate was used as an alternative to gallicacid, which has a methylester moiety instead of a carboxyl group. The electric responseof the sensor with methyl gallate was considerably reduced compared with that withgallic acid (Fig.1). Secondly, to evaluate the importance of the hydroxyl group, 3,4,5-trimethoxybenzoicacid was used as an alternative to gallic acid, which has a methoxy group instead of ahydroxyl group. The electric response of the sensor with 3,4,5-trimethoxybenzoic acidwas almost the same as that with gallic acid (Fig.1). To evaluate again the importanceof the carboxyl and hydroxyl groups, 4-hydroxybenzoic acid (and its derivatives) wasalso used as an alternative to gallic acid. The results indicated that the derivative without -10o -20 -30 -40 -50 -60 -70 -80 Fig. 1. Electric responses to 1 M sucrose of sensors with substances analogous to gallic acid.(1) gallic acid, (2) methyl gallate, (3) 3,4,5-trimethoxybenzoic acid, (4) 4-hydroxybenzoicacid, (5) 4-methoxyphenol, (6) 4-aminobenzoic acid, (7) 4-(hydroxymethyl)benzoic acid, (8)4-methoxybenzoic acid. the carboxyl group does not have any sucrose response, whereas those without thehydroxyl group showed a sucrose response relatively equal to that of 4-hydroxybenzoicacid. These results suggest that the carboxyl group is essential for the sucrose response,whereas the hydroxyl group is not. 3.2 Electric response ofvarious substances having carboxyl group Electric responses were further checked using various substances with carboxylgroup(s) similar to gallic acid (Table 1). Included were only those producing a certainlevel of sucrose response. Because of the previous results(4 and preliminary results showing that almost all the substances with a carboxyl group neighboring hydroxylgroup(s) on the benzene ring, such as 2,6-dihydroxybenzoic acid, seldom give a sucroseresponse, they were not included as test substances. For each substance tested, several (ormore) sensors were made so that the maximum response was obtained; in some cases, a30 mg amount gave the highest response. The results clearly showed that the sucrose response is observed not only insubstances with both carboxyl group and hydroxyl group on the benzene ring but alsoin those with only the carboxyl group such as 1,4-naphthalenedicarboxylic acid andphthalic acid. It was also indicated that the phosphate group is also capable of producingthe sucrose response, as well as the carboxyl group. Among the substances tested, on theother hand, those with the sulfo group on the benzene ring gave a low sucrose response (datanot shown), which may suggest that a weak acid is important for the sucrose response.Although lower than those of others, the sucrose response was also observed for palmiticacid and oleic acid, which suggests that the benzene ring is not always necessary.Interestingly, the results also demonstrated that even the sensors with carboxyl groups intheir polymer portion show the sucrose response, although the level is not high. All these results suggest that the carboxyl group or phosphate group is essentialfor the sucrose response and that whatever substance with such a functional groupmay function as SRS. Considering these results, to indicate substances producing thesucrose response such as gallic acid, the term “phenolic compound,”which has beenused before,(3,4) seems no longer applicable. Thus, the substances capable of producingthe sucrose response for the sweetness sensor were tentatively named SRS, for “sweet-responsive substance." Because it became clear that trimellitic acid is one of the best SRSs with regard toelectric response to a dilute solution of 300 mM sucrose, it was exclusively used for thefollowing experiments. Another reason is that the chemical structure of trimellitic acidis simple; it is composed of a benzene ring only with three carboxyl groups. To simplify,the sensor with trimellitic acid is hereafter named sweetness sensor GL1. 3.3 Basic characteristics ofGL1 sensor The sucrose response of the GL1 sensor was examined with regard to pH (Fig. 2).The results showed that the level ofelectric response was decreased at pH 4 or lower butincreased at pH 10 or higher. This behavior looks similar to the sweetness perception inhumans, as far as the pH of common foods, namely, around pH 2 to pH 7, is concerned.This is, in other words, concerned with the interaction between sweetness and sourness.In general, we are supposed to perceive sweet more weakly with the addition of sourness. Next, the concentration characteristics of the GL1 sensor were analyzed using foursugars (Fig.3). For all the sugar samples, electric responses start with approximately 10-30 mM and increase logarithmically with an increase in concentration to approximately100-300 mM. Then, they decrease at approximately 1,000 mM or higher. This behaviorof the GL1 sensor is quite similar to that of human perception; the human threshold forperceiving the sweet taste of a sucrose solution has been reported to be 10-30 mM,(6)and the concentration at which we can perceive most clearly is around 300 mM (ca. 10%w/w).(7) For a 1,000 mM sucrose solution, most people feel its sweetness too strongly todistinguish. Fig. 2. Electric response of GL1 sensor to sucrose solution with various pHs. Fig. 3. Electric responses of GL1 sensor to four sugars. Then, the sensitivity and selectivity of the GL1 sensor were checked using solutionsof basic tastes, in comparison with the prototype sweetness sensor GL0 (Fig. 4). For 100or 300 mM sucrose solutions, the sensitivity of the GL1 sensor is better than that of theGL0 sensor, although they were almost the same for the 1,000 mM solution. This clearly Z GLi GL0 Fig.4..Electric response of GL1 sensor to basic tastes. indicates that the GL1 sensor is more effective, particularly for dilute solutions, than theGL0 sensor. Figure 4 also demonstrates that the selectivity of the GL1 sensor is betterthan that of the GL0 sensor, as long as the same materials are used. At least, it seemssafe to say that the GL1 sensor is better than the GL0 sensor because it is not sensitiveto umami solution (monosodium glutamate in this study), which is present in manyfoodstuffs; we often cannot distinguish taste qualities between sweetness and umami insomecases.(8) As for saltiness, the electric response for saltiness was unavoidable for theGL1 sensor, similarly to the GL0 sensor. However, the behavior of the GL1 sensor underthe influence of sodium chloride sometimes fits with the human sense. both for dilute andconcentrated solutions of sodium chloride (data not shown). Therefore, it seems that theeffect of salts is not always a drawback. Finally, as shown in Fig. 5, the electric responses of various sugars were measuredusing the GL1 sensor at 10% concentration (w/w), in comparison with their relativesweetness, which is based on human sensory evaluation.(7) The reason why theconcentration in w/w was used only in this experiment, unlike in Figs. 1-4, is to comparewith their relative sweetness. The results suggested that the highest levels of electricresponses are observed for monosaccharides such as glucose and fructose, the secondhighest are for disaccharides such as maltose and trehalose, and the lowest are foroligosaccharides. This is similar to human sense, that is, in terms of relative sweetness.The correlation coefficient between the electric response of the GL1 sensor and therelative sweetness was 0.76, which is somewhat high. However, conflicting results were obtained from the one-to-one comparison ofsugars, such as the comparison between glucose and sucrose.One of the reasons is Fig. 5. IElectric response of GL1 sensor to various sugars and sugar alcohols, in comparison withtheir relative sweetness. that the relative sweetness of sucrose is exceptionally higher than those of the otherdisaccharides, the relative sweetness of which is 0.2-0.4. Still, Fig. 5 suggests that theGL1 sensor has a feature of“global selectivity,”which is needed for the taste sensor,(5) asfar as the sweetness of sugars and sugar alcohols is concerned. 4. Conclusions It was suggested that the carboxyl or phosphate group in lipid/polymer membranesis essential for the sucrose response of the sweetness sensor, while the hydroxyl group isnot always necessary. It was revealed that trimellitic acid is one of the best SRSs. Thecharacteristics of this sensor incorporating trimellitic acid are relatively similar to thoseof human sense. Acknowledgements This work was supported in part by a grant from Mitsubishi-UFJ TechnologyDevelopment Foundation. The authors wish to thank Dr. Nobuyuki Hayashi of theNational Institute of Vegetable and Tea Science, for the kind help in the organic synthesisof DDoBA used in this study. References H. Cui, M. Habara, H. Ikezaki and K.Toko: Sens. Mater. 17 (2005)385. M. Habara, H. Cui, H. Ikezaki and K. Toko:6th East Asia Conference on Chemical Sensors2005 (EACCS, Guilin, China, 2005) 2S4-5, p. 166. 3 M. Habara, D. Beppu, H. Cui, H. Ikezaki and K. Toko: Sens. Mater. 19 (2007) 325. 4 H. Cui, M. Habara, H. Ikezaki and K. Toko: International Conference on Sensing Technology2008 (ICST, Tainan, Taiwan, 2008) p. 610. 5Y. Kobayashi, M. Habara, H. Ikezaki, R. Chen, Y. Naito and K.Toko: Sensors 10 (2010)3411. K. Paulus and A. M. Reisch: Chem. Senses 5 (1980) 11. R. S. Shallenberger: Taste Chemistry (Blackie Academic and Professional, London, 1993)Chap. 6. 8 J. Mojet, J. Heidema and E. C.-Hazelhof: Chem. Senses 29 (2004)671.
确定





还剩8页未读,是否继续阅读?
产品配置单
北京盈盛恒泰科技有限责任公司为您提供《电子鼻用于脂质高分子膜甜味传感器的响应物质》,该方案主要用于其他中--检测,参考标准--,《电子鼻用于脂质高分子膜甜味传感器的响应物质》用到的仪器有电子舌、日本INSENT味觉分析系统(电子舌)
推荐专场
感官智能分析系统(电子鼻/电子舌)
相关方案
更多















