Abstract
An organic–inorganic poly(3,4-ethylenedioxy-thiophene) (PEDOT)/RuO2·xH2O nanocomposite (approxi-mately 1 wt.% RuO2) has been successfully prepared for the first time under microwave irradiation within 5 min with power 900 W via in situ chemical polymerization. The morphology and structure of the resultant material is characterized by transmission electron microscope and Fourier transform infrared. Moreover, the electrochemical properties of the synthesized nanocomposite can be controlled by adjusting the annealing temperature, which is definitely illustrated by cyclic voltammetry, galvanostatic charge–discharge, and electrochemical impedance spectra. Electrochemical data have shown that the PEDOT/RuO2·xH2O nanocomposite annealed at 150 °C possesses the most favorable charge/discharge ability with a specific capacitance of 153.3 F g?1at a current density of 150 mA g?1 and the high efficient utilization of PEDOT at various current densities. Furthermore, such composite has a less capacitance degradation of 23.8% after 1,000 continuous cycles. The improved electrochemical performance are mainly attributed to the large electroactive surface of nanocomposite and the existence of amorphous RuO2·xH2O particles as well as a synergistic effect of the polymer PEDOT and annealed RuO2·xH2O. Thus, the PEDOT/RuO2·xH2O nanocomposite annealed at 150 °C can act as a promising electroactive material for supercapacitor application
方案详情

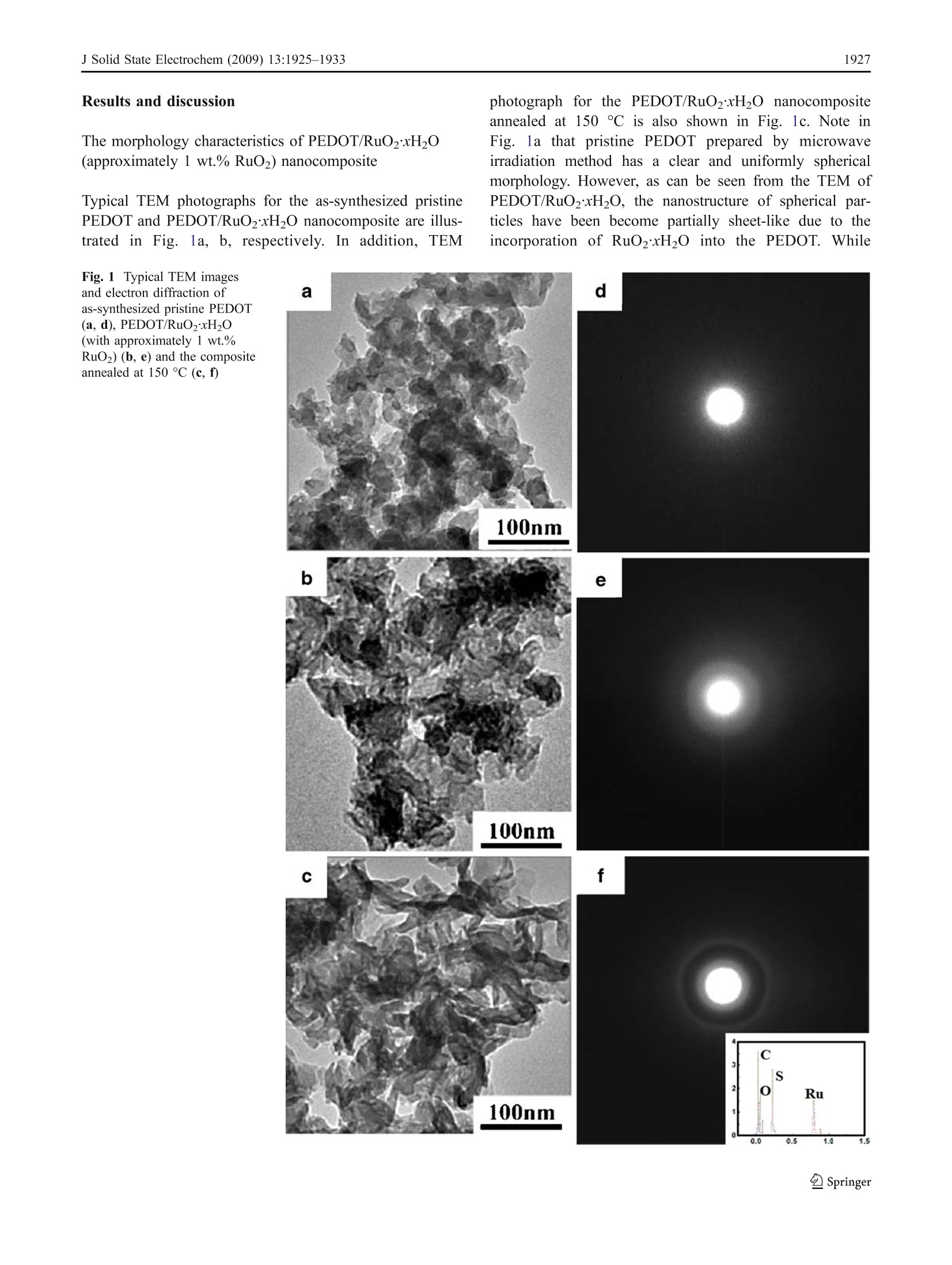
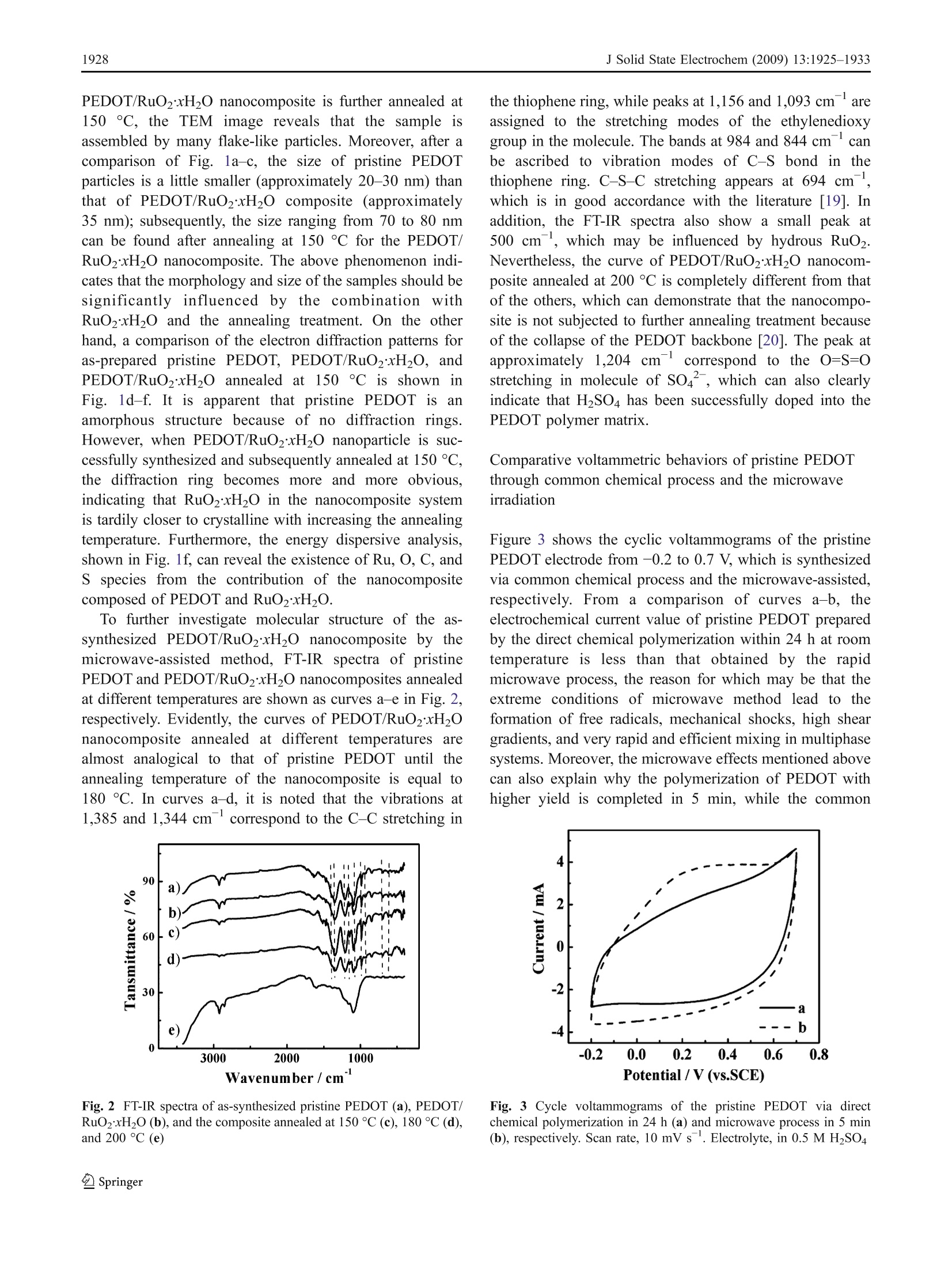
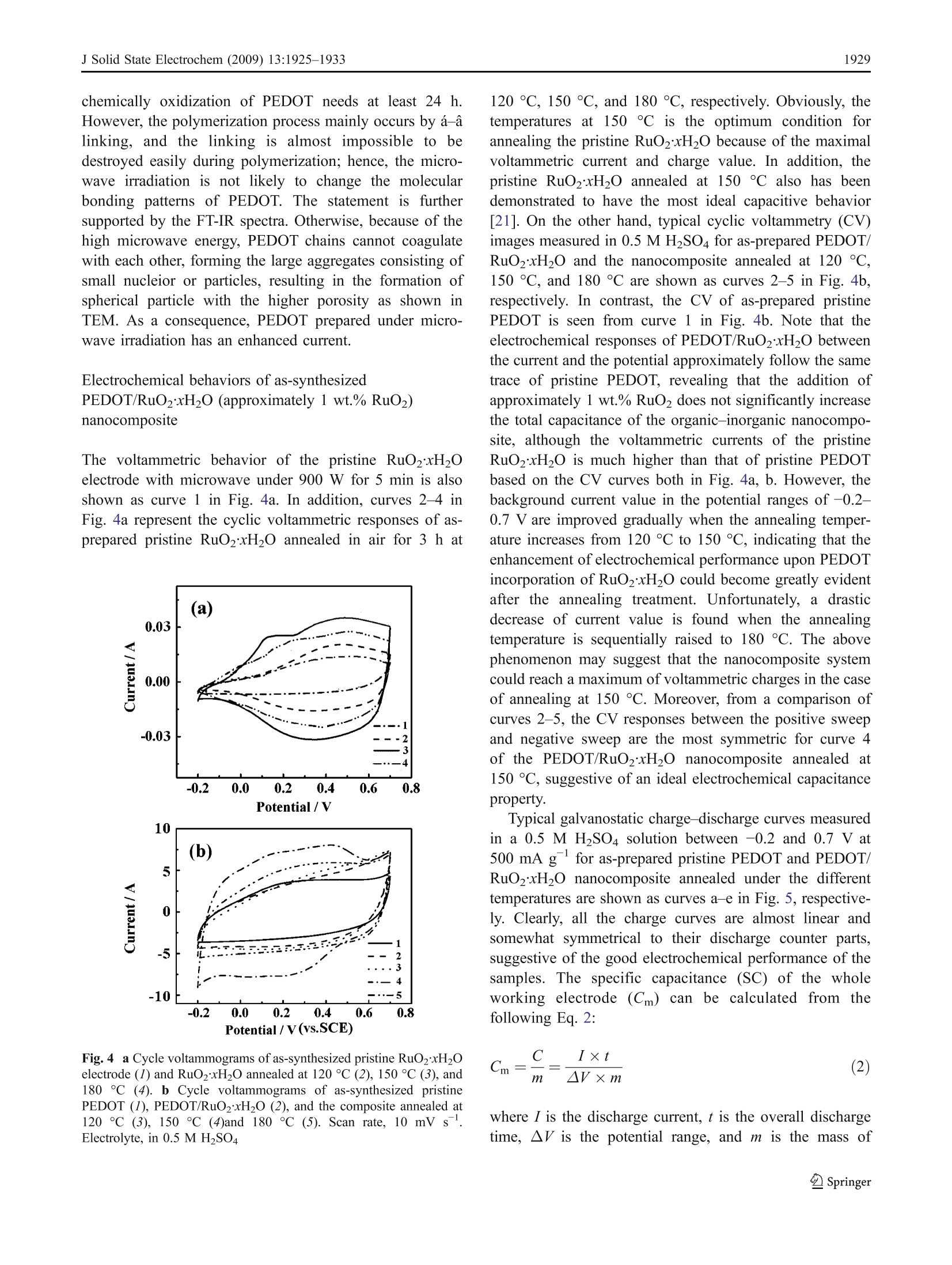
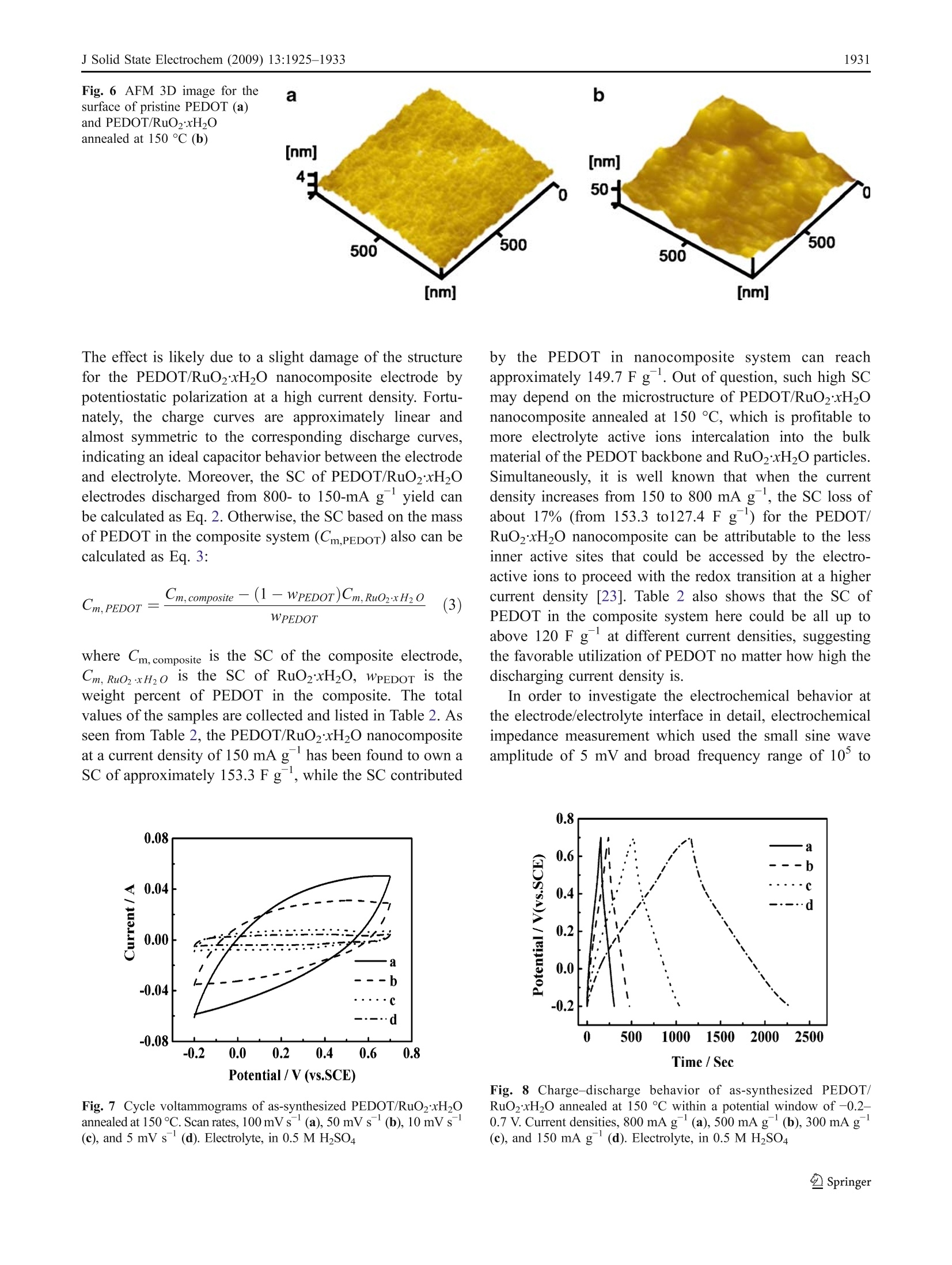
J Solid State Electrochem (2009) 13:1925-1933DOI 10.1007/s10008-008-0777-yORIGINAL PAPER J Solid State Electrochem (2009) 13:1925-19331926 Microwave-assisted synthesis of organic-inorganicpoly(3,4-ethylenedioxythiophene)/RuO2xH2Onanocomposite for supercapacitor Li Chen·Changzhou Yuan·Bo Gao· Shengyao Chen·Xiaogang Zhang Received: 16 August 2008 /Revised: 14 December 2008 /Accepted: 17 December 2008 /Published online: 14 January 2009C Springer-Verlag 2009 Abstract An organic-inorganic poly(3,4-ethylenedioxy-thiophene) (PEDOT)/RuO2xH2O nanocomposite (approxi-mately 1 wt.% RuO2) has been successfully prepared forthe first time under microwave irradiation within 5 minwith power 900 W via in situ chemical polymerization. Themorphology and structure of the resultant material ischaracterized by transmission electron microscope andFourier transform infrared. Moreover, the electrochemicalproperties of the synthesized nanocomposite caln lbecontrolled by adjusting the annealing temperature, whichis definitely illustrated by cyclic voltammetry, galvanostaticcharge-discharge, and electrochemical impedance spectra.Electrochemical data have shown that the PEDOT/RuO2xH2O nanocomposite annealed at 150 C possessesthe most favorable charge/discharge ability with a specificcapacitance of153.3 Fgat a current density of 150 mAgand the high efficient utilization of PEDOT at variouscurrent densities. Furthermore, such composite has a lesscapacitance degradation of 23.8% after 1,000 continuouscycles. The improved electrochemical performance aremainly attributed to the large electroactive surface of nano-composite and the existence of amorphous RuO2xH2Oparticles as well as a synergistic effect of the polymer PEDOTand annealed RuO2xH2O. Thus, the PEDOT/RuO2xH2Onanocomposite annealed at 150 ℃ can act as a promisingelectroactive material for supercapacitor application. Keywords Poly(3-4-ethylenedioxythiophene)· RuO2xH2O·Microwave-assisted·Nanocomposite ( L. Chen· C. Yuan· B. Gao· S. Chen·X. Zhang ( 区 )College o f Material Science & Engineering, N anjing University of Aeronautics and Astronautics, N anjing 210016, P e ople’s R e public of China e-mail : azhangxg @ 163.com ) Introduction Recently, there has been a great deal of interest in thesynthesis of nanocomposite by using some simple routesfor several applications, such as 1light emitting diodes,chemical sensors, supercapacitors, fuel cells, and so on[1-5]. In particular, the composite combined conjugatedpolymers with metal oxides at the “nanoscale”level ownsmuch enhanced charge-storage ability for the applicationof electrochemical capacitors due to the synergistic effects[6, 7]. Commonly, among all kinds of conjugated polymer,poly(3,4-ethylenedioxythiophene) (PEDOT), a derivative ofpolythiophene, has been paid more attention [8]. Owing tothe moderate band gap, unique structural properties, andreaction mechanism, PEDOT has several advantages, suchas high transparency in visible regime, excellent environ-mental stability, low redox potential, good thermal stability,and high conductivity in doped state either n-type or p-type[9, 10]. In addition, the combination of PEDOT and metaloxides also has been demonstrated to offer the improvedelectrochemical performance. For example, the intercalatedPEDOT/MoO3 composite has been successfully preparedwith the enhanced electrochemical properties for super-capacitor [11]. Moreover, Liu and Lee [12] have reportedthat the PEDOT/MnO2 composite with the 1D nano-structure can exhibit extreme excellent capacitive andmechanical properties for energy storage applications. On the other hand, in order to broaden the scope of thisorganic-inorganic hybrid material based on the polymerPEDOTand enhance their electrochemical redox behaviors,many families of noble transition metal oxides can beconsidered. Particularly, hydrous ruthenium oxide(RuO2xH2O) in either crystalline or amorphous hydrousform has been already recognized as the most promisingelectrode material for electrochemical capacitor [13, 14] based on the fact that RuO2xH2O with the porous structuremay show much better reversible faradic capacitiveperformance in acidic electrolyte according to the followingnon-stoichiometric reaction [15]. Consequently, they can offer a remarkable specificcapacitance value ranging from 720 to 760 F g[16]. Todate, the combination PEDOT with RuO2xH2O can enablethe distinct elevation of electrochemical properties com-pared with the common polymer electrode itself. Hong etal. [17] have demonstrated that the PEDOT/RuO2 compos-ite electrode has an enhanced capacitance of 420 F gbydepositing RuO2 on the conducting polymer PEDOT.Huang et al. [18] have reported that PEDOT-PSS/RuO2film can show the enhancement of optical and electrochem-ical properties because PEDOT-PSS can act as a three-dimensional, random, and electronically conducting templatewhich allows the formation of relatively uniform RuO2xH20particles. Unfortunately, the main obstacle for applicationsat present is that the cost of noble metal is high, thepreparation of the organic-inorganic material often is lowyield, and the procedure is time-consuming, involvingseveral steps, some additive and higher energy. Hence, in this study, the PEDOT/RuO2xH20 nano-composite (the mere approximately 1 wt.% RuO2) can belargely synthesized by in situ chemical polymerization withthe simple microwave-assisted within 5 min under power900 W. To the best of our knowledge, the fast-growing fieldof synthesizedPEDOT-based nanocomposite withRuO2xH2O to enhance the electrochemical performance ofmaterials for supercapacitor using microwave-assisted poly-merization has not been explored yet. In addition, theinfluence of annealing temperature upon the electrochemicalperformance of the resultant PEDOT/RuO2xH20 nano-composite is also investigated here, and the electrochemcialresults have illuminated that the PEDOT/RuO2xH20 nano-composite (the mere presence of approximately 1 wt.%RuO)annealed at 150 ℃ can display the favorable electrochemicalproperties due to the large electroactive surface, the existenceof RuO2xH2O particles, and a synergistic effect for thenanocomposite. Consequently, the as-synthesized PEDOT/RuO2xH20 nanocomposite can be considered as a promis-ing candidate material for supercapacitor application. Experimental Synthesis of PEDOT/RuO2xH2O (approximately1 wt.% RuO2) 3,4-Ethylenedioxythiophene (EDOT, 99%) and RuClsxH2Osolution were both obtained from Aldrich. Ammonium persulfate (APS), sulfuric acid (H2SO4), and ethanol wereanalytical grade and purchased from Shanghai ChemicalReagent. Aqueous solution was prepared with doublydistilled water. The procedure employed for preparing the PEDOT/RuO2xH20 nanocomposite was as follows: in a typicalsynthesis, 0.75 mL EDOT was dispersed in H2SO4 solution(30 mL, 0.2 M), followed by slowly adding 1 mLRuClsxH2O solution (0.01 g/mL). Then, APS was mixedwith a magnetic stirring and homogenization was continuedfor 2 h in order to disperse effectively at room temperature.Subsequently, the above mixture was moved into the XH-100A microwave instrument (Haoxiang, Beijing China)which was equipped with a reflux condenser and conductedfor a fixed time (5 min) with adequate power (900 W). Theresult composite was washed several times with distilledwater and alcohol by a centrifugal filtration method anddried at 60 ℃ overnight under vacuum. Finally, the product(the mere presence of approximately 1 wt.% RuO2) wasannealed in air for 3 h at the different temperatures rangingfrom 100 ℃ to 200 ℃. For comparison, the pristinePEDOT and pristine RuO2xH2O were prepared by thesame procedure as described above except that the precursorsolution was only monomer EDOT and RuClsxH2Osolution, respectively. Morphologycharacterization The morphologies and electron diffraction patterns of thesamples were examined by transmission electron micro-scope (TEM, FEI TECNAI-20). Fourier transform infrared(FT-IR) spectra were recorded with a model 360 NicoletAVATAR spectrophotometer. Atomic force microscopy(AFM, SPA 3300HV, Japan) was used to obtain andreconstruct a 3D image of the surface of the samples. Electrochemical measurements The working electrode, containing 5 mg as-preparedactive material, 0.6 mg acetylene black, and 0.3 mgpolytetrafluoroethylene was mixed well for 15 min.Then, the solvent (alcohol) was dropped into the abovemixture and ground to form the coating slurry which wassmeared onto the pretreated graphite substrates (area,1 cm²). Before the electrochemical test, the preparedelectrode was dried in air at 50 °C overnight. Electro-chemical characterization was carried out in a convention-al three-electrode cell with 0.5 M H2SO4 as the electrolyte.The platinum foil and saturated calomel electrode wereused as the counter and reference electrode, respectively.All electrochemical measurements were performed byCHI660 electrochemical analyzer system (Chenhua, ShanghaiChina). Results and discussion The morphology characteristics of PEDOT/RuO2xH2O(approximately 1 wt.% RuO2) nanocomposite Typical TEM photographs for the as-synthesized pristinePEDOT and PEDOT/RuO2xH2O nanocomposite are illus-trated in Fig.1a, b, respectively. In addition, TEM photograph for the PEDOT/RuO2xH20 nanocompositeannealed at 150 C is also shown in Fig. 1c. Note inFig. 1a that pristine PEDOT prepared by microwaveirradiation method has a clear and uniformly sphericalmorphology. However, as can be seen from the TEM ofPEDOT/Ru02xH2O, the nanostructure of spherical par-ticles have been become partially sheet-like due to theincorporation of RuO2xH2O into the PEDOT. While Fig. 1 Typical TEM imagesand electron diffraction ofas-synthesized pristine PEDOT(a, d), PEDOT/RuO2xH20(with approximately 1 wt.%RuO2) (b, e) and the compositeannealed at 150 °C (c,f) PEDOT/RuO2xH20 nanocomposite is further annealed at150 C, the TEM image reveals that the sample isassembled by many flake-like particles. Moreover, after acomparison of Fig. la-c, the size of pristine PEDOTparticles is a little smaller (approximately 20-30 nm) thanthat of PEDOT/RuO2xH2O composite (approximately35 nm); subsequently, the size ranging from 70 to 80 nmcan be found after annealing at 150 ℃ for the PEDOT/RuO2xH2O nanocomposite. The above phenomenon indi-cates that the morphology and size of the samples should besignificantly influenced by tthe combination withRuO2xH20 and the annealing treatment. On the otherhand, a comparison of the electron diffraction patterns foras-prepared pristine PEDOT, PEDOT/RuO2xH2O, andPEDOT/RuO2xH2O annealed at150 °C is shown inFig.. 1d-f. It is apparent that pristine PEDOT issanamorphous structure because of no diffraction rings.However, when PEDOT/RuO2xH2O nanoparticle is suc-cessfully synthesized and subsequently annealed at 150℃,the diffraction ring becomes more and more obvious,indicating that RuO2xH2O in the nanocomposite systemis tardily closer to crystalline with increasing the annealingtemperature. Furthermore, the energy dispersive analysis,shown in Fig. 1f, can reveal the existence of Ru, O, C, andS species from the contribution of the nanocompositecomposed of PEDOT and RuO2xH20. To further investigate molecular structure of the as-synthesized PEDOT/RuO2xH20 nanocomposite by themicrowave-assisted method, FT-IRspectra of pristinePEDOT and PEDOT/RuO2xH20 nanocomposites annealedat different temperatures are shown as curves a-e in Fig. 2,respectively. Evidently, the curves of PEDOT/RuO2xH2Onanocomposite annealed at different temperatures arealmost analogical to that of pristine PEDOT until theannealing temperature of the nanocomposite is equal to180 °C. In curves a-d, it is noted that the vibrations at1,385 and 1,344 cmcorrespond to the C-C stretching in Fig. 2 FT-IR spectra of as-synthesized pristine PEDOT (a), PEDOT/RuO2xH2O (b), and the composite annealed at 150℃(c), 180°C (d),and 200 C (e) the thiophene ring, while peaks at 1,156 and 1,093 cmareassigned to the stretching modes of the ethylenedioxygroup in the molecule. The bands at 984 and 844 cmcanbe ascribed to vibration modes of C-S bond in thethiophene ring. C-S-C stretching appears at 694 cm,which is in good accordance with the literature [19]. Inaddition, the FT-IR spectra also show a small peak at500 cm, which may be influenced by hydrous RuO.Nevertheless, the curve of PEDOT/RuO2xHO nanocom-posite annealed at 200°℃ is completely different from thatof the others, which can demonstrate that the nanocompo-site is not subjected to further annealing treatment becauseof the collapse of the PEDOT backbone [20]. The peak atapproximately 1,204 cm correspond to the O=S=0stretching in molecule of SO4, which can also clearlyindicate that HSO4 has been successfully doped into thePEDOT polymer matrix. Comparative voltammetric behaviors of pristine PEDOTthrough common chemical process and the microwaveirradiation Figure 3 shows the cyclic voltammograms of the pristinePEDOT electrode from -0.2 to 0.7V, which is synthesizedvia common chemical process and the microwave-assisted,respectively. From a comparison of curves a-b, theelectrochemical current value of pristine PEDOT preparedby the direct chemical polymerization within 24 h at roomtemperature isis less than that obtained by the rapidmicrowave process, the reason for which may be that theextreme conditions of microwave method lead to theformation of free radicals, mechanical shocks, high sheargradients, and very rapid and efficient mixing in multiphasesystems. Moreover, the microwave effects mentioned abovecan also explain why the polymerization of PEDOT withhigher yield is completed in 5 min, while the common Fig. 3 Cycle voltammograms of the pristine PEDOT via directchemical polymerization in 24 h (a) and microwave process in 5 min(b), respectively. Scan rate, 10 mV s. Electrolyte, in 0.5 M H2SO4 chemically oxidization of PEDOT needs at least 24 h.However, the polymerization process mainly occurs by á-alinking, and the linking is almost impossible to bedestroyed easily during polymerization; hence, the micro-wave irradiation is not likely to change the molecularbonding patterns of PEDOT. The statement isfurthersupported by the FT-IR spectra. Otherwise, because of thehigh microwave energy, PEDOT chains cannot coagulatewith each other, forming the large aggregates consisting ofsmall nucleior or particles, resulting in the formation ofspherical particle with the higher porosity as shown inTEM. As a consequence, PEDOT prepared under micro-wave irradiation has an enhanced current. Electrochemical behaviors of as-synthesizedPEDOT/RuO2xH2O (approximately 1 wt.% RuO2)nanocomposite The voltammetric behavior of the pristine RuO2xH2Oelectrode with microwave under 900 W for 5 min is alsoshown as curve 1 in Fig. 4a. In addition, curves 2-4 inFig. 4a represent the cyclic voltammetric responses of as-prepared pristine RuO2xH20 annealed in air for 3 h at Fig. 4 a Cycle voltammograms of as-synthesized pristine RuO2xH2Oelectrode (1) and RuO2xH20 annealed at 120 °℃ (2), 150°℃(3), and180℃ (4). b Cycle voltammograms of as-synthesized pristinePEDOT (I), PEDOT/RuO2xH2O(2), and the composite annealed at120 ℃ (3),150℃ (4)and 180 ℃ (5). Scan rate, 10 mV s .Electrolyte, in 0.5 M HzSO4 120℃, 150 C, and 180 °℃, respectively. Obviously, thetemperatures at 150 ℃ is the optimum condition forannealing the pristine Ru02xH2O because of the maximalvoltammetric current and charge value. In addition, thepristine RuO2xH20 annealed at 150 C also has beendemonstrated to have the most ideal capacitive behavior[21]. On the other hand, typical cyclic voltammetry (CV)images measured in 0.5 MH2SO4 for as-prepared PEDOT/RuO2xH2O and the nanocomposite annealed at 120 C,150 C, and 180 C are shown as curves 2-5 in Fig. 4b,respectively. In contrast, the CV of as-prepared pristinePEDOT is seen from curve 1 in Fig. 4b. Note that theelectrochemical responses of PEDOT/RuO2xH20 betweenthe current and the potential approximately follow the sametrace of pristine PEDOT, revealing that the addition ofapproximately 1 wt.% RuO2 does not significantly increasethe total capacitance of the organic-inorganic nanocompo-site, although the voltammetric currents of the pristineRuO2xH20 is much higher than that of pristine PEDOTbased on the CV curves both in Fig. 4a, b. However, thebackground current value in the potential ranges of-0.2-0.7 V are improved gradually when the annealing temper-ature increases from 120 ℃ to 150 °C, indicating that theenhancement of electrochemical performance upon PEDOTincorporation of RuO2xH2O could become greatly evidentafter the annealing treatment. Unfortunately, a drasticdecrease of current value is found when the annealingtemperature is sequentially raised to 180 °C. The abovephenomenon may suggest that the nanocomposite systemcould reach a maximum of voltammetric charges in the caseof annealing at 150 °C. Moreover, from a comparison ofcurves 2-5, the CV responses between the positive sweepand negative sweep are the most symmetric for curve 4of the PEDOT/RuO2xH20 nanocomposite annealed at150 C, suggestive of an ideal electrochemical capacitanceproperty. Typical galvanostatic charge-discharge curves measuredin a 0.5 M HSO4 solution between -0.2 and 0.7 V at500 mA gfor as-prepared pristine PEDOT and PEDOT/RuO2xH20 nanocomposite annealed under the differenttemperatures are shown as curves a-e in Fig. 5, respective-ly. Clearly, all the charge curves are almost linear andsomewhat symmetrical to their discharge counter parts,suggestive of the good electrochemical performance of thesamples. The specific capacitance (SC) of the wholeworking electrode (Cm)can1tbe calculated from thefollowing Eq. 2: where I is the discharge current,t is the overall dischargetime, AV is the potential range, and m is the mass of Fig. 5 Charge-discharge behavior of as-synthesized pristine PEDOT(a), PEDOT/RuO2xH2O (b), the composite annealed at 120 C (c),150℃ (e), and 180℃(d) at the current density of 500 mA g withina potential window of -0.2-0.7V. Electrolyte, in 0.5 M HSO4 electroactive material. Based on the curves a-e in Fig. 5,the SC of the electrode can be respectively calculatedaccording to Eq. 2 and listed in Table 1. As a whole, the SCof composite system is higher than that of PEDOT only(55.6F g) due to the pseudocapacitance of RuO2xH20nanoparticles. In addition, it is evident that the absolute SCvalues of the PEDOT/RuO2xH20 nanocomposite systemcan be enhanced with increasing the annealing temperatureand reach a maximum of approximately 132.4 F g in thecase of annealing at 150 C, which is in good agreementwith the above CV results. The above result can be favoredby three points. Firstly, PEDOT/RuO2xH20 nanocompo-site annealed at 150 °C with the flake-like structure is moreaccessible to the electrolyte, and more inner electroactivesites for the doping/dedoping process can occur in 3Dpolymer matrix. The more electroactive surface can be alsofavored by the AFM analysis in Fig. 6. The AFM analysesclearly indicate that the pristine PEDOT has a homoge-neous surface morphology with very low roughness(approximately 0.398 nm). Conversely, the surface mor-phology of PEDOT/RuO2xH2O nanocomposite significant- Table 1 The SC of PEDOT and PEDOT/RuO2xH2O composite (withapproximately 1 wt.% RuO2) annealed at different temperatures in0.5 M HSO4 electrolyte at the current density of 500 mA g Composites Cm (Fg PEDOT (without doped Ru) 55.6 PEDOT/RuO2xH2O 60 69.1 PEDOT/RuO2xH20 annealed at 120 83.5 different temperatures (C) 150 132.4 180 89.4 ly changes upon the heat treatment at 150 °C. At this stage,coalescence of the nuclei takes place, accompanied by theincrease of roughness of the film (approximately 11.4nm),resulting in the more electroactive surface. Secondly,according to Chen and Hu [22], the electronic conductivityof RuO2xH20 commonly may be poor because theamorphous nanostructure should make the electron pathwaydiscontinuous during the charge storage and deliveryprocesses. However, the discontinuity in the electronpathway probably may be improved by the annealingtreatment due to the sintering of amorphous RuO2xH2O;moreover, when amorphous RuO2xH20 particles areannealed at 150 °C, the samples may retain the most facilepathways for both electron and proton conduction and havethe best utilization of active species, resulting in theconsiderably high SC. As a consequence, the SC ofresultant PEDOT/RuO2xH2O nanocomposite is corre-spondingly promoted. Finally, there may be a synergisticeffect for the nanocomposite composed of annealedRuO2xH20 and PEDOT. In addition, as seen from Table 1, the SC value ofPEDOT/RuO2xH2O significantly degrades when theannealing temperature is at 180 C. The reason could besupported by the fact that the water content of RuO2 ismuch less and the crystallization of RuO2xH2O has beentransformed when further increasing the annealing temper-ature, which would inhibit the transportation of electrolyteions in the bulk. In summary, when the annealing temperature isat150 C, the capacitive behavior of as-prepared PEDOT/RuO2xH2O nanocomposite is better than that of compositesystem annealed at any other temperature. Therefore, itwould be investigated in detail in the next section. Curvesa-d in Fig. 7 show a comparison of the voltammetricbehavior for PEDOT/RuO2xH20 nanocomposite annealedat 150 °C measured at different scan rates. It is noticeablethat all the curves could exhibit the analogously rectangularshape. Meanwhile, the voltammeric charges integrated fromthe positive sweeps are very close to those integrated fromtheir corresponding negative sweeps, which can illuminatethat the electrode has an excellent reversibility. Further-more, although the scan rate is increasing discontinuously,no obvious distortions in CV curves is observed, indicativeof the high power property. All the above results demon-strate that the PEDOT/RuO2xH2O nanocomposite annealedat 150 °C can be considered as a potential candidate in theapplication of electrochemical supercapacitors. The galvanostatic charge-discharge of as-preparedPEDOT/RuO2xH20 nanocomposite annealed at 150 ℃measured in 0.5 M HSO4 between -0.2 and 0.7 V under150, 300, 500, and 800 mA gare shown as curves a-d inFig. 8, respectively. Note that the iR drop turns out to bemuch obvious with increasing the applied current density. The effect is likely due to a slight damage of the structurefor the PEDOT/RuO2xH20 nanocomposite electrode bypotentiostatic polarization at a high current density. Fortu-nately, the charge curves are approximately linear andalmost symmetric to the corresponding discharge curves,indicating an ideal capacitor behavior between the electrodeand electrolyte. Moreover, the SC of PEDOT/RuO2xH2Oelectrodes discharged from 800- to 150-mA gyield canbe calculated as Eq. 2. Otherwise, the SC based on the massof PEDOT in the composite system (Cm.PEDOT) also can becalculated as Eq. 3: where Cm,composite is the SC of the composite electrode,Cm, RuO2 xH,o is the SC of RuO2xH2O, WPEDoT is theweight percent of PEDOT in the composite. The totalvalues of the samples are collected and listed in Table 2. Asseen from Table 2, the PEDOT/RuO2xH2O nanocompositeat a current density of 150 mA ghas been found to own aSC of approximately 153.3 F g, while the SC contributed Fig. 7 Cycle voltammograms ofas-synthesized PEDOT/RuO2xH2Oannealed at 150℃. Scan rates, 100mVs(a), 50 mVs(b),10mVs(C), and 5 mVs (d). Electrolyte, in 0.5 M HSO4 by the PEDOT in nanocomposite system can reachapproximately 149.7Fg. Out of question, such high SCmay depend on the microstructure of PEDOT/RuO2xH2Onanocomposite annealed at 150 °C, which is profitable tomore electrolyte active ions intercalation into the bulkmaterial of the PEDOT backbone and RuO2xH20 particles.Simultaneously, it is well known that when the currentdensity increases from 150 to 800 mA g, the SC loss ofabout 17% (from 153.3 to127.4 F g ) for the PEDOT/RuO2xH20 nanocomposite can be attributable to the lessinner active sites that could be accessed by the electro-active ions to proceed with the redox transition at a highercurrent density [23]. Table 2 also shows that the SC ofPEDOT in the composite system here could be all up toabove 120 F g at different current densities, suggestingthe favorable utilization of PEDOT no matter how high thedischarging current density is. In order to investigate the electrochemical behavior atthe electrode/electrolyte interface in detail, electrochemicalimpedance measurement which used the small sine waveamplitude of 5 mV and broad frequency range of 10 to Fig. 8 Charge-discharge behavior of as-synthesized PEDOT/RuO2xH20 annealed at 150°C within a potential window of -0.2-0.7 V.Current densities,800 mA g (a), 500 mA g (b),300 mA g(c), and 150 mA g (d). Electrolyte, in 0.5 M HSO4 Table 2 The SC of PEDOT/RuO2xH2O (with approximately 1 wt.%RuO2) annealed at150°C and the SC based on the mass of PEDOT in0.5 M HSO4 electrolyte at different current densities Current densities Cm (Fg) Cm,PEDOT (F g ) (mAg) 800 127.4 124.4 500 132.4 129.4 300 142.5 139.1 150 153.3 149.7 10 Hz is carried out at open-circuit potentials in 0.5 MH2SO4 electrolyte. Complex plane plots of the impedanceof PEDOT/RuO2xH20 nanocomposite annealed at 150℃and pristine PEDOT electrodes via microwave process arepresented in Fig. 9, respectively. As shown in the inset, it isobvious that the nanocomposite has the smaller radius ofsemicircle in high-frequency region after the introduction ofRuO2xHO. Furthermore, the charge transfer resistance(Ret) related to the faradic process of electrodes can beestimated according to the radius of semicircle; hence, thenanocomposite electrode is demonstrated to have the lowerRet of0.5 dropping from 0.8 of pristine PEDOT, whichshould be ascribed to the more ion intercalation, emersion,and, subsequently, the increase of the effective active sitesfor faradic reactions. Asa consequence, the PEDOT/RuO2xH20 nanocomposite annealed at: 150°C has asignificant improvement on the conductivity in comparisonwith the pristine PEDOT. In the low-frequency region, theimaginary part of curve b is more vertical with the realpart than that of curve a, which can clearly present themore excellent ideal capacitive behavior of the annealednanocomposite. Fig. 9 Typical complex plane impedance plots for as-synthesizedpristine PEDOT (a), PEDOT/RuO2xH2O annealed at 150℃ (b)measured at open-circuit potential. Inset, data in the high-frequencyregion. Electrolyte, in 0.5 M HSO4 Fig. 10 Cycle life of as-synthesized PEDOT/RuO2xH20 annealed at150℃ (a), the pristine PEDOT gained from microwave-assisted (b).Electrolyte, in 0.5 M H2SO4 In order to test the cycle life of the nanocomposite, theSC against the CV cycle number in 0.5 M H2SO4 for thepure PEDOT and the PEDOT/RuO2xH20 nanocompositeannealed at 150℃ are shown in Fig.10. From curves a-b,the electrochemical stability of the nanocomposite electrodeis higher than that of the pristine PEDOT in the whole1,000 cycles. Importantly, a rapid loss in capacitance for thePEDOT/RuO2xH2O nanocomposite electrode is clearlyfound during the initial 400 cycles (from approximately160 to approximately 125 F g ), while the SC lossbecomes negligible when the cycle number of CV is above400. Therefore, though the recyclability of hydrous RuO2 ispoor in H2SO4 solution, the SC of nanocomposite system isonly decayed out approximately 23.8%, while the loss ofSC for the pristine PEDOT is up to approximately 33.3%during the whole consecutive cycle. Consequently, thecycle stability of the organic-inorganic hybrid PEDOT/RuO2xH20 nanocomposite annealed at 150 C is evenbetter than that of pristine PEDOT electrode. Conclusions In conclusion, PEDOT/RuO2xH20 nanocomposite (themere presence of approximately 1 wt.% RuO2) have beensuccessfully prepared via the one-step microwave-assistedmethod, which is simple, easy, efficient, and environmen-tally friendly. The relationships between the annealingconditions and structural changes for the resultant materialshave been investigated based on the TEM and FT-IR data.In addition, the CV, galvanostatic charge-discharge, andelectrochemical impedance spectra have demonstrated thatthe organic-inorganic hybrid PEDOT/RuO2xH2O nano-composite annealed at 150 °C possess the best electro-chemical properties in three-electrode cell system, highlighting the SC of 153.3 F gat a current density of150 mAg, the high efficient utilization of PEDOT in thenanocomposite system at various current densities, and acapacitance degradation of 23.8% after 1,000 continuouscycles. Here, the improvement of electrochemical perfor-mance could be mainly attributed to the large electroactivesurface of nanocomposite for faradic reaction, the existenceof amorphous RuO2xH20 particles at150 C, and asynergistic effect of the polymer PEDOT and the annealedRuO2xH20. Therefore, all of above suggest that thePEDOT/RuO2xH20 nanocomposite annealed at 150 ℃ isa very promising supercapacitor electrode material. Fur-thermore, the microwave-assisted method described herecould be further developed to be versatile for the synthesisof other kinds of polymer-based materials. Acknowledgements TThis work was supported by National BasicResearch Program of China (973 Program no. 2007CB209703) andNational Natural Science Foundation of China (nos. 20633040,20873064),Graduate Innovation Plan of Jiangsu Province (CX07B-089Z). ( Reference ) ( 1 . Colvin VL, Schlamp M C, Alivisatos AP ( 1994) Nature 370:354. doi: 10.1038/37035 4 a0 ) ( 2. Arbizzani C , M astragostino M , Meneghello L, P araventi R (19 9 6) Adv Mater 8:331. doi: 10 . 1002/adma . 19960080409 ) ( 3. Baraton MI, Merhara L , W ang J, Gonsalves K E (1 9 9 8) Na n o-technology 9:356. d oi:1 0. 1 088/0957-4 4 84/9/4/0 1 0 ) ( 4. Rajesh B, Thambi KR, B onard JM, Xanthapolous N, Mathieu HJ,Viswanathan B ( 2 002) Electrochem Solid-State L e tt 5 : E71. doi: 10 . 1149 / 1 . 1518610 ) 5. Mattes BR, Knobbe ET, Fuqua PD, Nishid F, Chang EW, PierceBM, Dunn B, Karner RB (1991) Synth Met 43:3183. doi:10.1016/0379-6779(91)91262-9 ( 6. Rios EC, Rosario AV, Mello RM, Micaroni L (2007) J P o werSources 1 63:11 3 7. doi:1 0.1016/j .jp owsour.2006.09.056 ) ( 7 . Zhang L , Wang M (2003) J Phys Chem B 10 7 :6748. doi:10 .1021/jp034130g ) ( 8 . Zhang X, L ee J S, Lee GS, Cha D K, Moon JK, Yang DJ, ManoharSK (2006) Macromolecules 39:470. doi:1 0.1021/ m a051975c ) ( 9 . Gustafsson J C, L iedberg B, I n ganas O (1 9 94) So l id St a te Io n 69:145. doi: 10.1016/0167-2738(94)90403-0 ) ( 10. Chiu WW, S ejdic JT, Cooney RP, Bowmaker GA (2005) Synth M et 1 5 5:80. doi:1 0.1016/j .s ynthmet.2005.06.012 ) ( 1 1 . Muruganand AV, Viswanath A K (2 0 06) J A ppl Ph y s 10 0 :074319. doi: 10 .1 063 / 1.2356788 ) ( 12. Liu R, Lee SB ( 2008) J Am Chem So c 13 0 :2942. do i :10. 1021/ ja71 1 2382 ) 13. Wang YG, Zhang XG (2004) Electrochim Acta 49:1957.doi:10.1016/j.electacta.2003.12.023 ( 14. Panic V, V idakovic T, G ojkovic S , Dekanski A , Mi l onjic S , N ikolic B ( 2003) E lectrochim A cta 4 8:3805. d oi:10 .1 0 16/S0013 - 4686(03)00514-0 ) ( 15. Trasatti S, Kurzweil P ( 1994) Platin M et Rev 46:38 ) ( 16. Fang QL, Evans D A , Roberson SL , Zheng JP (2001) J Electro- chem Soc 148:A833. doi:1 0.1149/1. 1 379739 ) ( 1 7 . Hong JI, Yeo I H , P aika WK (2001) J E l ectrochem Soc 14 8 :A156. doi: 10. 1 149/ 1 .1342166 ) ( 18. Huang LM, W en T C , Gopalan A (2 0 06) Electrochim A c ta 51:3469.d o i:10 .1016/j.e l ect a cta.2005.09.049 ) ( 19. Han DX, Y ang GF, N i u L , I v aska A (2007) J E lectroanal Chem 602:24. d oi:1 0.1016/j.jelechem.2006.1 1 . 0 27 ) ( 2 0 . Wang JC, Yu XF, Li YX, Liu Q (2007) J Phy s Chem C111:18073.doi: 10.1021 / jp 0749468 ) ( 21. Hu CC, L iu M J, C hang KH (2007) J P o wer Sources 1 6 3:1126.doi: 10.1016/j.jpowsour.2006.09.060 ) ( 22. Chen W C, H u CC (2 0 04) J Power Sources 125:292. doi:10 .1016/ j.j powsour.2003.08.001 ) ( 23. Liang YY, Li HL, Zhang XG (2007) J P ower Sources 1 73:599doi: 10.1016/ j. jpowsour.2007. 0 8.01 0 ) Springer
确定




还剩7页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《Microwave-assisted synthesis nanocomposite for supercapacitor(微波辅助合成纳米材料)》,该方案主要用于纳米材料中--检测,参考标准--,《Microwave-assisted synthesis nanocomposite for supercapacitor(微波辅助合成纳米材料)》用到的仪器有祥鹄微波合成仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















