
方案详情
文
该方法描述了通过水平衡法,使用同位素质谱测定葡萄酒中的水的O18/O16的方法,来鉴别是否葡萄酒中有掺水。
方案详情

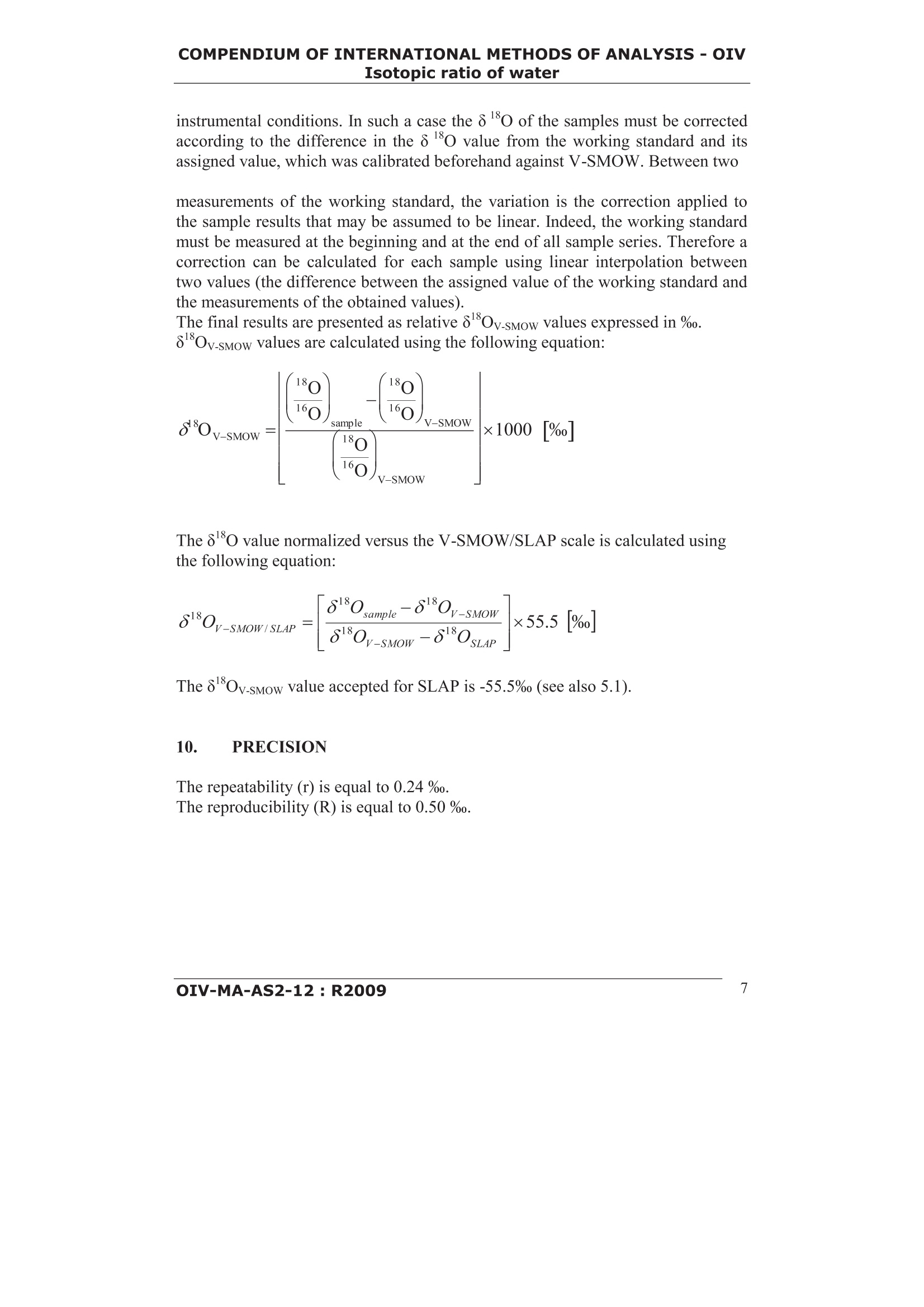
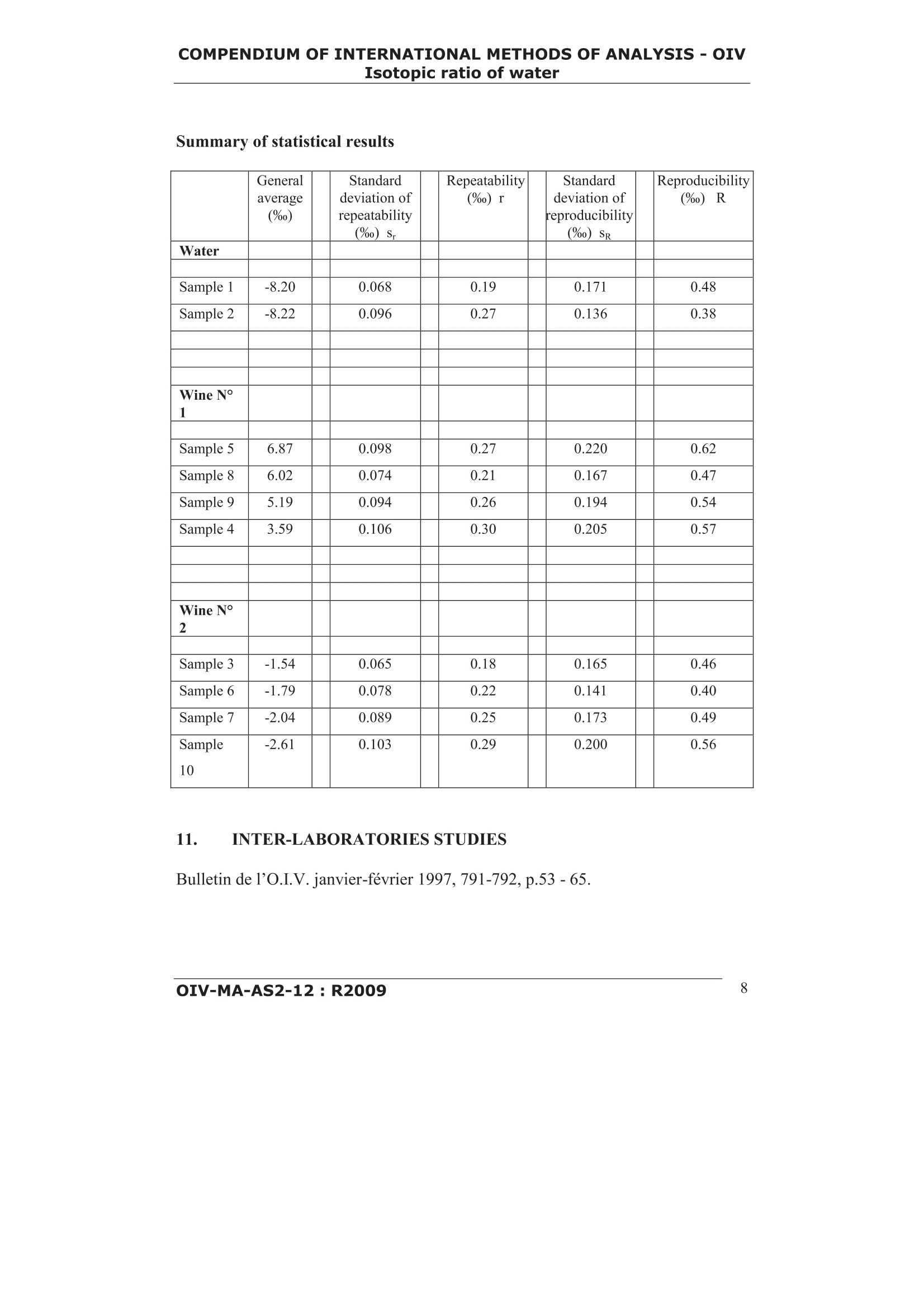
COMPENDIUM OFINTERNATIONAL METHODS OF ANALYSIS-OIVIsotopic ratio of water Method OIV-MA-AS2-12 Type II method 18Method for 0/o isotope ratio determinationof water in wines and must (Resolution OIV-Oeno 353/2009) 1. SCOPE The method describes the determination of the18o/160isotope ratio of water fromwine and must after equilibration with CO2, using the isotope ratio massspectrometry (IRMS). 2. REFERENCE STANDARDS ISO 5725:1994: Accuracy (trueness and precision) of measurement methodsand results: Basic method for the determination ofrepeatability and reproducibility of a standard measurementmethod.V-SMOW: Vienna-Standard Mean Ocean Water(0/0=Rv-sMow=0.0020052) GISP Greenland Ice Sheet Precipitation SLAP Standard Light Antarctic Precipitation 3. DEFINITIONS 18o/6o Isotope ratio of oxygen 18 to oxygen 16 for a given sample8*Ov-sMow Relative scale for the expression of the isotope ratio of oxygen 18to oxygen 16 for a given sample.8Ov-sMow is calculated using thefollowing equation: OIV-MA-AS2-12:R2009 COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIVIsotopic ratio of water using the V-SMOW as standard and as reference point for therelative 8 scale. BCR Community Bureau of Reference IAEA International Atomic Energy Agency (Vienna, Austria) IRMM Institute for Reference Materials and Measurements IRMS Isotope Ratio Mass Spectrometry m/z mass to charge ratio NIST National Institute of Standards & Technology RM Reference Material 4. PRINCIPLE The technique described thereafter is based on the isotopic equilibration of waterin samples of wine or must with a CO2 standard gas according to the following1isotopic exchange reaction: After equilibration the carbon dioxide in the gaseous phase is used for analysis bymeans of Isotopic Ratio Mass Spectrometry (IRMS) where the 18o/0 isotopicratio is determined on the CO2 resulting from the equilibration. 5. REAGENTS AND MATERIALS The materials and consumables depend on the method used (see chapter 6). Thesystems generally used are based on the equilibration of water in wine or mustwith CO2. The following reference materials, working standards and consumables can beused: 5.1 Reference materials Name issued by 80 versus V-SMOW V-SMOW,RM 8535 IAEA/NIST 0 %0 BCR-659 IRMM -7.18%o GISP.RM8536 IAEA/NIST -24.78%0 SLAP.RM 8537 IAEA/NIST -55.5%o COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIVIsotopic ratio of water 5.2 Working Standards 5.2.1 Carbon dioxide as a secondary reference gas for measurement (CAS 00124-38-9). 5.2.2 Carbon dioxide used for equilibration (depending on the instrument this gascould be the same as 5.2.1 or in the case of continuous flow systems cylinderscontaining gas mixture helium-carbon dioxide can also be used) 5.2.3 Working Standards with calibrated 8Ov-sMow values traceable tointernational reference materials. 5.3 Consumables Helium for analysis (CAS 07440-59-7) 6. APPARATUS 6.1 Isotope ratio mass spectrometry (IRMS) The Isotope ratio mass spectrometer (IRMS) enables the determination of therelative contents of ’c ofCOz gas naturally occurring with an internal accuracy of:0.05%. Internalaccuracy here iisdefinedas the differencebetween2measurements of the same sample of CO,. The mass spectrometer used for the determination of the isotopic composition ofCO2 gas is generally equipped with a triple collector to simultaneously measure thefollowing ion currents: m/z=44(2c010) m/z=45(c00 andco0) m/z=46(2c6010,12c0o and3co0) By measuring the corresponding intensities, the18o/0 isotopic ratio isdetermined from the ratio of intensities of m/z= 46 and m/z = 44 after correctionsfor isobaric species (co''0and 1c00) whose contributions can becalculated from the actual intensity observed for m/z= 45 and the usual isotopicabundances for 13 and ’o in Nature. The isotope ratio mass spectrometry must either be equipped with: -a double introduction system (dual inlet system) to alternately measure theunknown sample and a reference standard. -or a continuous flow system that transfers quantitatively the CO2 from the samplevials after equilibration but also the CO2 standard gas into the mass spectrometer. COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIVIsotopic ratio of water 6.2 Equipment and Materials All equipments and materials used must meet stated requirements of the usedmethod / apparatus (as specified by the manufacturer). However, all equipmentsand materials can be replaced by items with similar performance. 6.2.1 Vials with septa appropriate for the used system 6.2.2 Volumetric pipettes with appropriate tips 6.2.3 Temperature controlled system to carry out the equilibration at constanttemperature, typically within ±1 ℃ 6.2.4 Vacuum pump (if needed for the used system) 6.2.5 Autosampler (if needed for the used system) 6.2.6 Syringes for sampling (if needed for the used system) 6.2.7 GC Column to separate CO2 from other elementary gases (if needed for theused system) 6.2.8 Water removal device (e.g. cryo-trap, selective permeable membranes) 7. SAMPLING Wine and must samples as well as reference materials are used for analysis withoutany pre-treatment. In the case of the possible fermentation of the sample, benzoicacid (or another anti-fermentation product) should be added or filtered with a witha 0,22 um pore diameter filter. Preferably, the reference materials used for calibration and drift-correction shouldbe placed at the beginning and at the end of the sequence and inserted after everyten samples. 8. PROCEDURE The descriptions that follow refer to procedures generally used fordetermination of the 18o/60 isotopic ratios by means of equilibration of waterwith a CO2 working standard and the subsequent measurement by IRMS. Theseprocedures can be altered according to changes of equipment and instrumentationprovided by the manufacturers as various kind of equilibration devices areavailable, implying g vavrarious conditions of operation. Two main technicalprocedures can be used for introduction of CO into the IRMS either through adual inlet system or using a continuous flow system. The description of all thesetechnical systems and of the corresponding conditions of operation is not possible. COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIVIsotopic ratio of water Note: all values given for volumes, temperatures, pressures and time periods areonly indicative. Appropriate values must be obtained from specifications providedby the manufacturer and/or determined experimentally. 8.1 Manual equilibration A defined volume of the sample/standard is transferred into a flask using a pipette.The flask is then attached tightly to the manifold. Each manifold is cooled down to below - 80℃ to deep-freeze the samples(manifold equipped with capillary opening tubes do not require this freezing step).Subsequently, the whole system is evacuated. After reaching a stable vacuum thegaseous CO2 working standard is allowed to expand into the various flasks. For theequilibration process each manifold is placed in a temperature controlled water-bath typically at 25℃ (±1℃) for 12 hours (overnight). It is crucial that thetemperature of the water-bath is kept constant and homogeneous. After the equilibration process is completed, the resulting CO2 is transferred fromthe flasks to the sample side bellow of the dual inlet system. The measurements areperformed by comparing several times the ratios of the CO2 contained in thesample side and the standard side (CO2 reference standard gas) of the dual inlet.This approach is repeated till the last sample of the sequence has been measured. 8.2 Use of an automatic equilibration apparatus A defined volume of the sample/standard is transferred into a vial using a pipette.The sample vials are attached to the equilibration system and cooled down tobelow - 80℃ to deep-freeze the samples (systems equipped with capillaryopening tubes do not require this freezing step). Subsequently, the whole system isevacuated. After reaching a stable vacuum the gaseous CO2 working standard is expanded intothe vials. Equilibrium is reached at a temperature of typically 22±1 °℃ after aminimum period of 5 hours and with moderate agitation (if available). Since theequilibration duration depends on various parameters (e.g. the vial geometry,temperature, applied agitation ...), the minimum equilibrium time should bedetermined experimentally. After the equilibration process is completed, the resulting CO2 is transferred fromthe vials to the sample side bellow of the dual inlet system. The measurements areperformed by comparing several times the ratios of the CO2 contained in thesample side and the standard side (CO2 reference standard gas) of the dual inlet.This approach is repeated till the last sample of the sequence has been measured. COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIVIsotopic ratio of water 8.3 Manual preparation manual and automatic equilibration and analysis with adual inlet IRMS A defined volume of sample / standard (eg. 200 pL) is introduced into a vialusing a pipette. The open vials are then placed in a closed chamber filled with theCO2 used for equilibration (5.2.2). After several purges to eliminate any trace ofair, the vials are closed and then placed on the thermostated plate of the samplechanger. The equilibrationii:s reached after at least 8 hours at 40 °C. Once theprocess of equilibration completed, the CO2 obtained is dried and then transferredinto the sample side of the dual inlet introduction system. The measurements areperformed by comparing several times the ratios of the CO2 contained in thesample side and the standard side (CO2 reference standard gas) of the dual inlet.This approach is repeated till the last sample of the sequence has been measured. 8.4 Use of an automatic equilibration apparatus coupled to a continuous flowsystem A defined volume of the sample/standard is transferred into a vial using a pipette.The sample vials are placed into a temperature controlled tray. Using a gas syringe the vials are flushed with mixture of He and CO. The COremains in the headspace of the vials for equilibration. Equilibrium is reached at a temperature typically of 30 ±1 ℃ after a minimumperiod of 18 hours.After the equilibration process is completed the resulting CO2 is transferred by means of the continuous flow system into the ion source of the mass spectrometer.CO2 reference gas is also introduced into the IRMS by means of the continuousflow system. The measurement is carried out according to a specific protocol foreach kind of equipment. 9. CALCULATION The intensities for m/z = 44, 45, 46 are recorded for each sample and referencematerials analysed in a batch of measurements. The 18o/60 isotope ratios are thencalculated by the computer and the software of the IRMS instrument according tothe principles explained in section 6.1. In practice the 18o/60 isotope ratios aremeasured against a working standard previously calibrated against the V-SMOW.Small variations may occur while measuring on line due to changes in the COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIVIsotopic ratio of water instrumental conditions. In such a case the 8180 of the samples must be correctedaccording to the difference in the 80 value from the working standard and itsassigned value, which was calibrated beforehand against V-SMOW. Between two measurements of the working standard, the variation is the correction applied tothe sample results that may be assumed to be linear. Indeed, the working standardmust be measured at the beginning and at the end of all sample series. Therefore acorrection can be calculated for each sample using linear interpolation betweentwo values (the difference between the assigned value of the working standard andthe measurements of the obtained values). The final results are presented as relative 8Ov.sMow values expressed in %o.8 *Ov-sMow values are calculated using the following equation: The 80 value normalized versus the V-SMOW/SLAP scale is calculated usingthe following equation: The 8Ov.sMow value accepted for SLAP is -55.5% (see also 5.1). 10. PRECISION The repeatability (r) is equal to 0.24%o. The reproducibility (R) is equal to 0.50 %o. Summary of statistical results Generalaverage(%0) Standarddeviation ofrepeatability(%o) s. Repeatability(%o) r Standarddeviation ofeproducibility(%o) SR Reproducibility(%o) R Water Sample 1 -8.20 0.068 0.19 0.171 0.48 Sample 2 -8.22 0.096 0.27 0.136 0.38 Wine N°1 Sample 5 6.87 0.098 0.27 0.220 0.62 Sample 8 6.02 0.074 0.21 0.167 0.47 Sample 9 5.19 0.094 0.26 0.194 0.54 Sample 4 3.59 0.106 0.30 0.205 0.57 Wine N°2 Sample3 -1.54 0.065 0.18 0.165 0.46 Sample 6 -1.79 0.078 0.22 0.141 0.40 Sample 7 -2.04 0.089 0.25 0.173 0.49 Sample10 -2.61 0.103 0.29 0.200 0.56 11. INTER-LABORATORIES STUDIES Bulletin de P'O.I.V. janvier-fevrier 1997, 791-792,p.53-65. COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIVIsotopic ratio of water 12. BIBLIOGRAPHY [1] Allison, C.E., Francey, R.J. and Meijer., H.A., (1995) Recommendations forthe Reporting of Stable Isotopes Measurements of carbon and oxygen. Proceedingsof a consultants meeting held in Vienna, 1 -3. Dec.1993,IAEA-TECDOC-825,155-162,Vienna, Austria. [2] Baertschi, P., (1976) Absolute 180 Content of Standard Mean Ocean Water.Earth and Planetary Science Letters, 31, 341-344. [3] Breas, O,. Reniero, F. and Serrini, G., (1994) Isotope Ratio MassSpectrometry: Analysis of wines from different European Countries. Rap. Comm.Mass Spectrom., 8, 967-987. [4] Craig, H., (1957) Isotopic standards for carbon and oxygen and correctionfactors for mass spectrometric analysis of carbon dioxide. Geochim. Cosmochim.Acta, 12, 133-149. [5] Craig, H.,(1961) Isotopic Variations in Meteoric Waters. Science, 133, 1702-1703. [6] Craig, H., (1961) Standard for reporting concentrations of deuterium andoxygen-18 in natural waters. Science. 133. 1833-1834. [7] Coplen, T., (1988) Normalization of oxygen and hydrogen data. ChemicalGeology (Isotope Geoscience Section). 72. 293-297 [8] Coplen, T. and Hopple, J., (1995) Audit of V-SMOW distributed by the USNational Institute of Standards and Technology. Proceedings of a consultantsmeeting held in Vienna, 1 - 3. Dec. 1993, IAEA-TECDOC-825,35-38 IAEA.Vienna, Austria. [9] Dunbar, J., (1982 Detection of added water and sugar in New Zealandcommercial wines.). Elsevier Scientific Publishing Corp. Edts. Amsterdam, 1495-501. [10] Epstein, S. and Mayeda, T.(1953) Variations of the 18o/60 ratio in naturalwaters. Geochim. Cosmochim. Acta,4,213. [11] Forstel, H. (1992) Projet de description d'une methode : variation naturelle durapport des isotopes0 et O dans l’eau comme methode danalyse physique duvin en vue du controle de lorigine et de laddition d'eau. OIV, FV n° 919,1955/220792. [12] Gonfiantini, R., (1978) Standards for stable isotope measurements in naturalcompounds. Nature, 271, 534-536. [13] Gonfiantini, R.,(1987) Report on an advisory group meeting on stable isotopereference samples for geochemical and hydrochemical investigations. IAEA,Vienna. Austria. [14] Gonfiantini, R., Stichler, W. and Rozanski, K., (1995) Standards andIntercomparisonnMaterialsdistributedby theeIIAEAfor StableIsotopesMeasurements. Proceedings of a consultants meeting held in Vienna, 1- 3. Dec.1993,IAEA-TECDOC-825, 13-29 Vienna,Austria. COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIVIsotopic ratio of water [15] Guidelines for Collaborative Study Procedures (1989) J. Assoc. Off. Anal.Chem., 72, 694-704. [16] Martin, G.J., Zhang, B.L., Day, M. and Lees, M.,(1993) Authentification desvins et des produits de la vigne par utilisation conjointe des analyses elementaire etisotopique. OIV, F.V., n°917, 1953/220792. ( [ 1 7] Martin, G .J., Forstel, H. and M oussa, I . (1995) La recherche du mouillage des vins par analyse isotopiqueH et 180. OIV, FVn° 1006, 2268/240595 ) ( [ 1 8] Martin, G .J. (1996) Recherche d u mouillage de s vins par la me s ure de l ateneur en 180 de l'eau des vi n s. OIV,FVn°1018, 2 325/300196. ) ( [ 1 9] Martin, G.J. and Lees, M., (1997) Detection de P e nrichissement des vins parconcentration des mouts au m oyen de l’analyse isotopique H et o d e l'eau desvins. OIV,FVn°1019,2326/300196. ) ( [20] Moussa,I., ( 1992) Recherche du mouillage dans les vi n s par spectrometrie demasse d es rapports isotopiques (SMRI). OIV , FVn°915,1937/130592. ) ( [21] Werner, R .A. and B r and, W., (2001) Re f erence Strategies and techniques instable isotope rati o analysis. Ra p . Comm. Mass Spectrom., 15, 501-519. ) ( [22] Zhang, B .L., F ourel, F., Naulet, N. and Martin, G.J., (1992) Influence del'experimentation e t d u t r aitement de Pe c hantillon sur la precision et la jus t esse des mesures des r apports isotopique s (D/H) e t (0/0). O I V, F.V. n ° 91 8 , 1954/220792. ) IV-MA-AS-R
确定







还剩8页未读,是否继续阅读?
艾力蒙塔贸易(上海)有限公司为您提供《葡萄酒中掺水检测方案 》,该方案主要用于葡萄酒及果酒中理化分析检测,参考标准--,《葡萄酒中掺水检测方案 》用到的仪器有elementar isoprime 100r稳定同位素质谱
推荐专场
相关方案
更多
该厂商其他方案
更多














