
液体颗粒制剂作为一种提高水溶性差药物溶出率的技术已被引入。本研究旨在将碳酸氢钠(NaHCO3)加入到液体颗粒制剂中,探讨其对药物释放速度的潜在影响。成功制备了NaHCO3含量为5%、12%、22%、32%、42% w/w的萘普生液体颗粒,考察了其理化性质,并与物理混合颗粒配方进行了比较。
方案详情

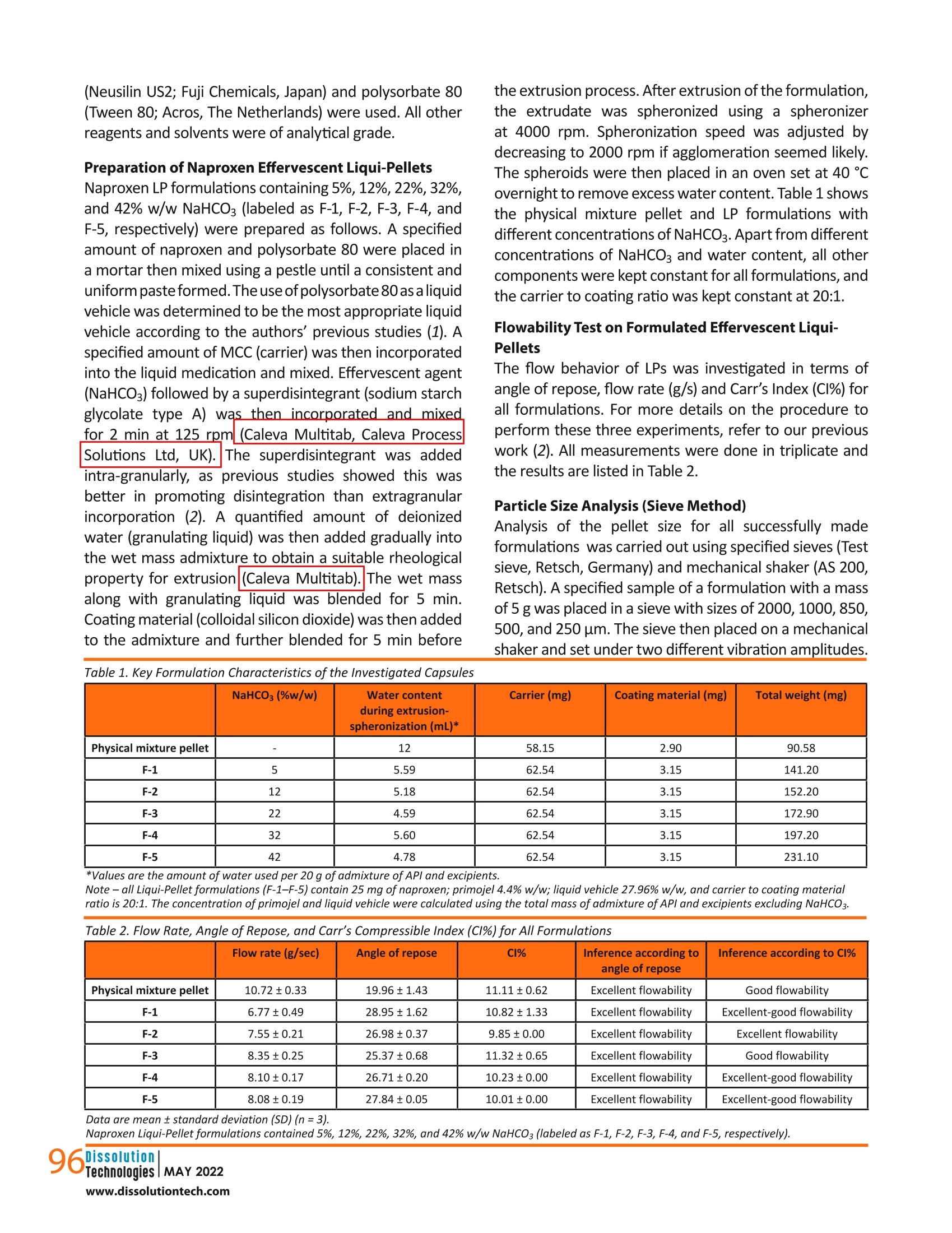
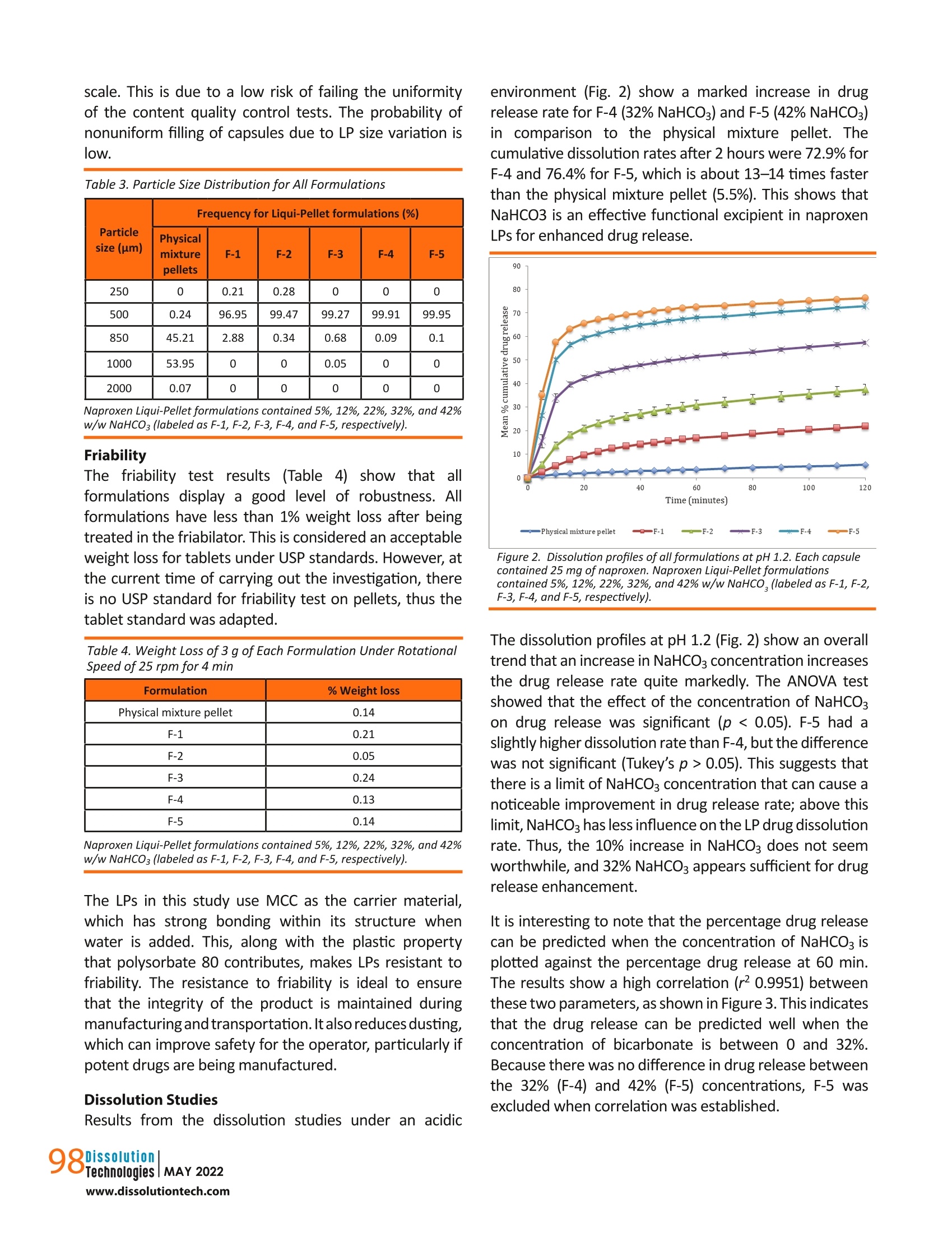
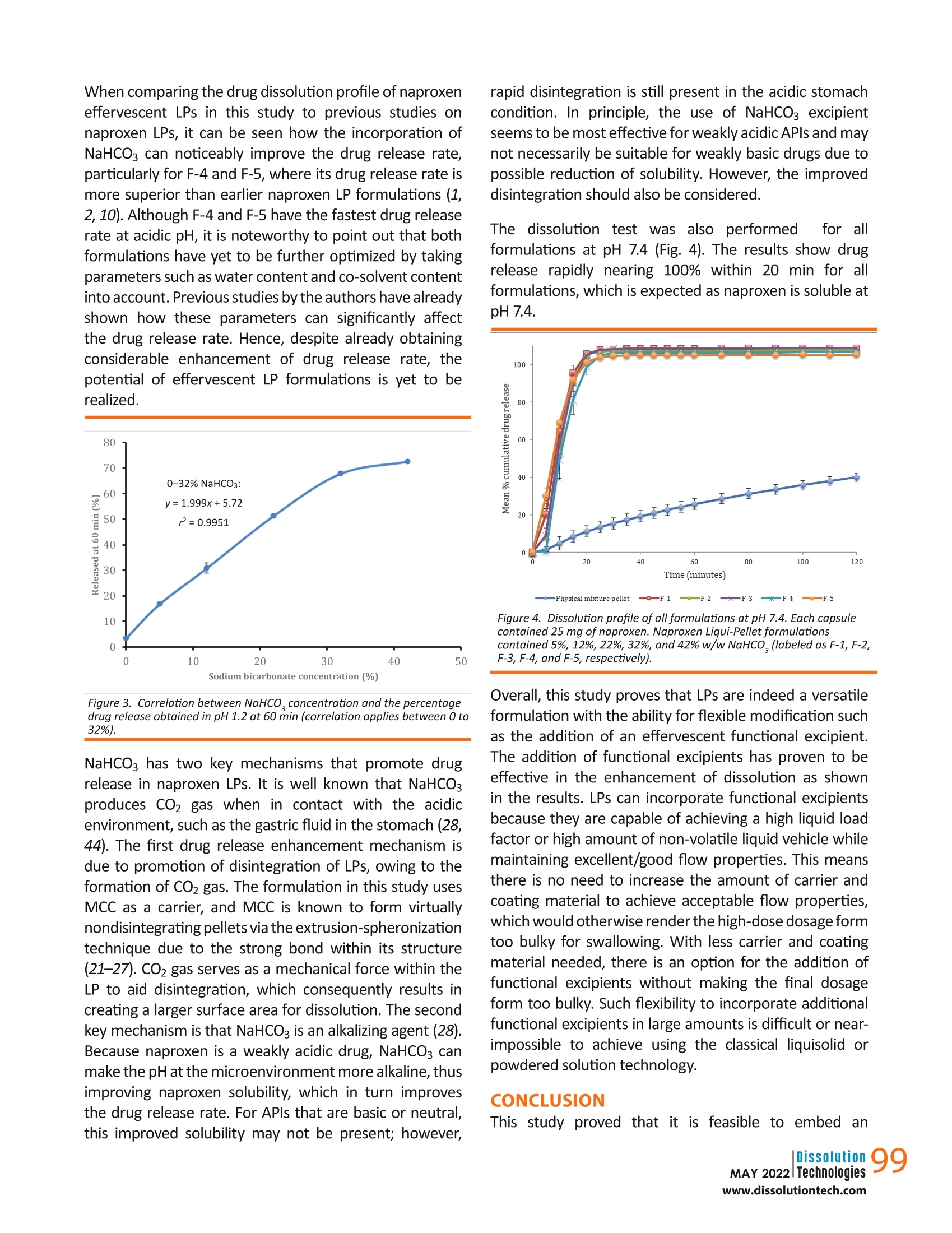
摘要: 液体颗粒制剂作为一种提高水溶性差药物溶出率的技术已被引入。本研究旨在将碳酸氢钠(NaHCO3)加入到液体颗粒制剂中,探讨其对药物释放速度的潜在影响。成功制备了NaHCO3含量为5%、12%、22%、32%、42% w/w的萘普生液体颗粒,考察了其理化性质,并与物理混合颗粒配方进行了比较。由于重量和尺寸的限制,特别是对于高剂量药物,在传统的液体固体制剂中,不可能或几乎不可能加入如此大量的功能性赋形剂。结果表明,NaHCO3是提高药物溶出率的有效功能赋形剂。NaHCO3浓度为42% w/w的液体颗粒剂在ph值为1.2的条件下,2 h释药率为76.4%,比物理混合颗粒剂快14倍(2 h释药率为5.5%),总体上NaHCO3浓度越高,释药速度越快;然而,这种效果是有限的。在一定限度以上,NaHCO3对溶解速率的影响减小。流动性试验表明,各配方均具有良好至优异的流动性。总的来说,这项研究证明了液体颗粒在配方设计方面的灵活性,这反过来又进一步支持了液体颗粒作为下一代口服剂型的潜力。 关键词:液球、液质体系、液固、泡腾剂、溶出促进剂 结论本研究证明,在LP配方中嵌入泡腾剂,既能获得优异或良好的流动性能,又能保持其稳定性是可行的. 总的剂量形式小而轻,足以吞咽。在配方中加入42%的NaHCO3,使每个含有LPs的胶囊单元的总重量仅为231 mg。如果采用液体固体技术,这是非常困难或几乎不可能实现的,特别是在高剂量药物中,由于需要增加载体和涂层材料,高液体负载系数将导致配方沉重而笨重,更不用说添加功能性赋形剂了。 溶出度试验结果显示,与NaHCO3的药物释放率有相当大的增加,其中这种配方在2小时后比物理混合颗粒快14倍。随着NaHCO3浓度的增加,药物释放速率也随之增加;然而,这是有限度的.在NaHCO3对药物释放速率的影响减小之前,可以加入多少NaHCO3。因此,有必要了解这个限度,以平衡胶囊的重量和药物释放性能,使其成为理想的剂型。实验结果表明,在较窄的粒径分布条件下,泡腾LPs具有较好的鲁棒性和优良的流动性能。总的来说, 结果支持在商业方向上采用固体基质剂型的液体原料药的概念的潜力。未来的研究将包括调查泡腾LPs在大剂量制剂中的可行性。 dx.doi.org/10.14227/DT290222P94 Ma t thew Lam* and A l i N okhodch i Pharmaceutics Research Laboratory, School of Life Sciences, University of Sussex, Brighton, UK. e -mail: m.lam@sussex.ac.uk ABST R ACT Liqui-Pellet formulations have been introduced as a technology to improve the dissolution rate of poorly water-soluble drugs. This study aimed to incorporate sodium bicarbonate (NaHCO3) into the Liqui-Pellet formulation to explore the potential impact on the drug release rate. Naproxen Liqui-Pellet formulations containing 5%, 12%, 22%, 32%, and 42%w/w of NaHCOs were successfully produced and their physicochemical properties were investigated and compared with a physical mixture pellet formulation. Incorporation of such a large amount of functional excipient is impossible or near impossible in the classical liquisolid formulation due to weight and size limitations, particularly for high-dose drugs. The results showed that NaHCOs is an effective functional excipient to enhance the drug dissolution rate. The Liqui-Pellet formulation with 42% w/w NaHCO3 released 76.4% of drug after 2 hours at pH 1.2, which was 14 times faster than the physical mixture pellet (5.5% drug release in 2 h). In general, a faster dissolution rate was observed with increasing NaHCO3 concentration; however, there was a limit to this effect. Above a certain limit, the influence of NaHCO3 on the dissolution rate lessened . The flowability test showed that all formulations have good to excellent flowability. Overall,this study demonstrates the flexibility of Liqui-Pellets in terms of formulation design, which in turn further supports the potential of Liqui-Pellets as the next generation oral dosage form. KEYWORDS: Liqui-Pellet, Liqui-Mass system, liquisolid, effervescent agent, dissolution enhancement IN TRO D UC T ION iqui-Pellets (LPs)are a recently developed oral dosage form that uses a wet mass system called the Liqui-Mass system. The primary purpose of this technology is to improve drug efficacy and reduce the risk of side effects, with manufacturing cost and feasibility in mind. The Liqui-Mass system brings versatility to formulation design and is essential to overcome the technological issues of the classical liquisolid technology (1-3). A diagram of the Liqui-Mass system and processes are shown in Figure 1. In brief, the Liqui-Mass system can produce LPs, liqui-tablets, free granules, and moldable wet mass dosage forms. It should be noted that this technology is still at an early stage and its potential has yet to be fully realized. One of the key purposes of LPs i s to tackle drugs with low bioavailability through improving drug dissolution rates with consideration to commercial manufacturing (1, 2). Poor bioavailability due to poor dissolution is a major challenge in the pharmaceutical industry, with approximately 60% of drugs in the market considered poorly soluble in gastro-intestinal f luids, and around 40% of drugs in the development pipeline are poorly water-soluble according to the biopharmaceutical classification system (BCS) (4, 5). It has also been reported that up to 90% of drugs in the development pipeline could be poorly water-soluble (6). In this study, naproxen was selected as the model drug due to its poor watersolubility, particularly in acidic conditions. It is reported that naproxen solubility at 35 ℃ i n a dissolution medium with pH of 1.2 is 1.16×10-4 mol/L or 27 mg/L. However, in a dissolution medium with pH of 7.4, naproxen solubility is 1.455×10-2 mol/Lor approximately 3347 mg/L, which is considered extremely soluble (7). Figure 1. Diagram summarizing the novel Liqui-Mass system used to make Liqui-Pellets. In general, LP technology (also known as Liqui-Mass technology) stems from combining the concept similar to a powdered solution or liquisolid technology with pelletization technology (1, 2). Despite both sharing similarities, there is a crucial difference that distinguishes them. LP uses the more versatile Liqui-Mass system whereas liquisolid pellet uses a liquisolid system(1,2,8). To understand what is considered a liquisolid formulation, it is i mperative to know what exactly is the l iquisolid system.Spireas, who is the i nventor of liquisolid technology, along with the scientific community's extensive publications on liquisolid technology since 1998, define the liquisolid system as dry-looking and free-flowing powder containing liquid medication that should be compressible by adding carrier and coating materials (9). It is worth pointing out that the liquisolid system i s limited to free-flowing powder admixture, whereas the Liqui -Mass system is usually a wet mass or paste admixture, even before a granulating l iquid i s added, which will eventually evaporate. In LPs,the admixture becomes free-flowing once i t undergoes a pelletization process. This fundamental difference gives greater potential and versatility to LP compared to the l iquisolid formulation, as more liquid vehicle and additional excipients can be incorporated into the formulation while s t ill achieving commercially acceptable flowability and dosage form size (1, 2, 10). In addition, LPs are not restricted by the equation developed by Spireas for liquisolid formulation (9). The equation dictates the amount of carrier and coating material that are required depend on the amount of liquid medication to ensure acceptable flow property; however, this limits the classical liquisolid technology from producing high dose dosage forms with acceptable size for commercial use. LPs have demonstrated excellent flow property while having as high as 38% of total pellet mass made up of l iquid vehicle (2). Previous studies on LPs have proven it to be robust, capable of a having a smooth surface (i.e.,potential for application of coating technology), and having narrow particle size distribution. Furthermore, the method of manufacturing is simple, economical, capable of green technology, capable of versatile modification (i.e., apply coating technology, incorporation of functional excipients, and inherent versatility of being a multi-unit dosage form), and the potential for easy upscale of production and excipients used are common, generally safe, and easily obtainable. Hence, i t LPs may be attractive for commercial drug development (1, 2,10). In terms of drug safety, the technology uses small uniform size pellets, which make drug dispersion and distribution in the gastrointestinal tract more predictable than larger standard monolithic dosage forms (11). The improved drug distribution can prevent side effects caused byhaving high drug concentration at the local site (12). Additionally,the small size LPs have less variation in gastric emptying,which minimizes inter- and intra-variability of plasma drug profiles, thus improving drug safety and pharmacokinetic predictability (13,14). So far, in studies by the authors, microcrystalline cellulose (MCC) i s the only carrier material used for making LPs.This is because extrusion-spheronization is the key choice of the method used in producing LPs. In the extrusion-spheronizationtechnique, MCC is the gold standard carrier because it can form a wet mass with unique properties,such as good rheological properties, plasticity, and cohesiveness (15, 16). The unique properties are due to MCC having high internal porosity and l arge surface area,which allows a large amount of water to be absorbed and retained (17-20). Such properties are required for the successful production of pellets. Also, the MCC-based pellet has good sphericity, smooth surface, high density,and low friability (15).It is also well known that the MCC-based pellet is not suited for fast release formulation due to its resistance to disintegration (21-27). LP carrier composition is not restricted to only MCC. In this study, sodium bicarbonate (NaHCO3) will be applied to naproxen LP formulations as a func t ional material to enhance the drug release rate. NaHCO3 is considered an effervescent agent when in contact with acid (28). Its function is to enhance LP dissolution through promoting disintegration when in contact with the acidic gastric fluid (28). The increase in drug dissolution via promoting disintegration and increasing the surface area for dissolution is explain ed using the Noyes-Whitney equation (29). According to the equation, enhanced disintegration increases the surface area for drug release,which is proportional to the dissolution rate (29). Thus,greater surface area for disintegration results in an increased drug release rate (30). In addition to enhancing the dissolution rate, NaHCO3 can reduce gastric irritation of weakly acidic drugs due to i ts alkali property and is generally regarded as a non-toxic and non-i rritant material (28). MATER I ALS A N D M ET HODS Materials Naproxen (TCS Co,Japan), MCC (Avicel PH-101; FMC Corp.,UK), colloidal silicon dioxide (Aerosil 300; Evonik Industries AG, Hanau, Germany), sodium starch glycolate type A (Primojel; DFE Pharma, Goch, Germany), NaHCO3 (Acros,NJ, USA), synthetic magnesium alumino-metasilicate (Neusilin US2; Fuji Chemicals, Japan) and polysorbate 80(Tween 80; Acros, The Netherlands) were used. All other reagents and solvents were of analytical grade. Preparation of Naproxen Effervescent Liqui-Pellets the extrusion process. After extrusion of the formulation,the extrudate was spheronized using a spheronizer at 4000 rpm. Spheronization speed was adjusted by decreasing to 2000 rpm if agglomeration seemed likely.The spheroids were then placed in an oven set at 40℃overnight to remove excess water content. Table 1 shows the physical mixture pellet and LP formulations with different concentrations of NaHCO3. Apart from different concentrations of NaHCO3 and water content, all other components were kept constant for all formulations, and the carrier to coating ratio was kept constant at 20:1. Flowability Test on Formulated Effervescent Liqui-Pellets The flow behavior of LPs was investigated in terms of angle of repose, flow rate (g/s) and Carr's Index (CI%) for all formulations. For more details on the procedure to perform these three experiments, refer to our previous work (2). All measurements were done in triplicate and the results are listed in Table 2. Particle Size Analysis (Sieve Method) Analysis of the pellet size for all successfully made formulations was carried out using specified sieves (Test sieve, Retsch, Germany) and mechanical shaker (AS 200,Retsch). A specified sample of a formulation with a mass of 5 g was placed in a sieve with sizes of 2000, 1000,850,500, and 250 um. The sieve then placed on a mechanical shaker and set under two different vibration amplitudes. NaHCO3 (%w/w) Water contentduring extrusion- spheronization (mL)* Carrier (mg) Coating material (mg) Total weight (mg) Physical mixture pellet 12 58.15 2.90 90.58 F-1 5 5.59 62.54 3.15 141.20 F-2 12 5.18 62.54 3.15 152.20 F-3 22 4.59 62.54 3.15 172.90 F-4 32 5.60 62.54 3.15 197.20 F-5 42 4.78 62.54 3.15 231.10 *Values are the amount of water used per 20 g of admixture of API and excipients. Note - all Liqui-Pellet formulations (F-1-F-5) contain 25 mg ofnaproxen; primojel 4.4% w/w; l iquid vehicle 27.96% w/w, and carrier to coating material ratio i s 20:1. The concentration of primojel and l iquid vehicle were calculated using the total mass of admixture of API and excipients excluding NaHCO3. Table 2. Flow Rate, Angle of Repose, and Carr's Compressible Index (C1%) for Al l Formulations Flow rate (g/sec) Angle of repose CI% Inference according toangle of repose Inference according to CI% Physical mixture pellet 10.72±0.33 19.96±1.43 11.11±0.62 Excellent flowability Good flowability F-1 6.77±0.49 28.95±1.62 10.82±1.33 Excellent flowability Excellent-good flowability F-2 7.55±0.21 26.98±0.37 9.85±0.00 Excellent flowability Excellent flowability F-3 8.35±0.25 25.37±0.68 11.32±0.65 Excellent flowability Good flowability F-4 8.10±0.17 26.71±0.20 10.23±0.00 Excellent flowability Excellent-good flowability F-5 8.08±0.19 27.84±0.05 10.01±0.00 Excellent flowability Excellent-good flowability Data are mean ± standard deviation (SD) (n=3). Naproxen Liqui -Pellet formulations contained 5%, 12%, 22%, 32%, and 42% w/w NaHCO3 (labeled as F-1, F-2, F-3, F-4, and F-5, respectively). 96 The first amplitude was set at 60 for only 1 min to quickly separate the particles. Such high amplitude generates high force that could damage the pellets if exposed for a prolonged length of time, so the process proceeded with a lower amplitude of 40 for a further 9 min. The collected fractions were weighed, and the size distribution was recorded for each formulation. Friability Test on Formulated Effervescent Liqui-Pellets To investigate the robustness of the manufactured formulations, a friability test was carried out. Briefly,weighing a few grams of specified pellets (around 3 g) was placed in a friabilator (D-63150, Erweka, Germany) along with the equivalent mass of glass beads. The friabilator container was sealed to stop pellets from leaving the drum and was set to rotate for 100 times (4 min set at 25rpm). The weight of the sample before (initial weight) and after (final weight) the test was used determined and the percentage weight loss due to friability testing. Dissolution Test Dissolution profiles were obtained from all feasible formulations. The in vitro drug dissolution test was carried out with a USP apparatus 2 (paddle) (708-DS Dissolution Apparatus and Cary 60 UV-Vis, Agilent Technologies, USA)using identical parameters as in previous studies on LPs with naproxen as a drug model (1, 2). Each hard gelatin capsule was filled with the specified pellet equivalent to 25 mg of naproxen. The filled capsules (n=3) underwent a dissolution test. The chosen dissolution media were HCI buffer solution at pH 1.2 and phosphate buffer solution at pH 7.4, which simulates gastrointestinal fluid without the enzymes. The volume of dissolution medium was 900 mL, maintained at 37.0±0.5℃ and 50 rpm. At set time intervals, the dissolution medium was automatically pumped into the ultraviolet spectrophotometer, and the absorbance of the solution was determined at 271nm. Yuksel et al used one-way analysis of var i ance (ANOVA)-based dependent and independent models to investigate which method is more reliable and discriminative for comparison of dissolution profiles (31). They showed that ANOVA-based tests and model-dependent techniques are more discriminative than independent models,such as similarity and difference factor analysis, despite independent models being recommended by the US FDA and implemented in various guidance documents published by the FDA (32-35). Therefore, the ANOVA test was used in the current study. RESULTS AN D DISCUSSION Preparation of Naproxen Effervescent Liqui -Pellets All formulations were successfully made into pellet form. Formulations F-4 (32% NaHCO3) and F-5 (42%NaHCO3)required a relatively higher amount of deionized water (i.e., granulating liquid) than the other formulations. This is because these two formulations have the two highest amounts of NaHCOg content, consequently a larger amount of total powder admixture; thus, more deionized water is required to obtain a reasonable rheological property of wet mass for successful extrusion. The plastic property is essential to allow for shaping and retaining the desired shape of extrudate. This ideal rheological property is primarily due to moisture i n the powder admixture, which has been subjected to much research (36-41). The fact that LPs can contain 42% of NaHCOs in the dosage form total weight is interesting. Such a large amount of functional excipients while maintaining good dosage form size and weight for swallowing would be difficult or i mpossible with classical liquisolid technology. This i s because theliquisolid formulation requires a large amount of carr i er and coating material when the liquid load factor (Lf) (i .e., the weight ratio of the liquid medication and the carrier material used in the formulation) is high as 1.Yet, LP formulation F-5 contains 42% of NaHCO3 and the dosage weight is only 231 mg, which shows the promising commercial potential of LP. Flowability The flow data reported in Table 2 indicate that all formulations have excellent, excellent-good, or good flow properties. Similar results were also observed in previous flowability studies on LPs (1, 2, 10), verifying that flowability is not a major issue for LPs as it is for liquisolid formulation. Hence, Spirea's mathematical equation,w hich determines the amount of APl and excipients to be used in maintaining a reasonable flow property, does not apply to LPs (9, 38). Without such a restriction, the formulation design with LPs can be more flexible. Particle Size Distribution Al l LP formulations had a narrow particle size distribution,which falls under 500 um (Table 3). This suggests that increasing the NaHCO3 content in the formulation does not appear to have an effect on i ts size. LP size is inherently small, allowing fast gastric emptying similar to liquid, which would expose the weakly acidic naproxen to the more alkaline f luid in the small intestine quicker (12).Note that weakly acidic drugs tend to be more soluble in alkaline conditions; hence, the dissolution rate can be enhanced (43). The narrow particle size distribution of LP formulations is ideal for manufacturing, particularly on an industrial scale. This is due to a low risk of failing the uniformity of the content quality control tests. The probability of nonuniform filling of capsules due to LP size variation is IOW. Table 3. Particle Size Distribution for All Formulations Particle size (um) Frequency for Liqui-Pellet formulations (%) Physicalmixturepellets F-1 F-2 F-3 F-4 F-5 250 0 0.21 0.28 0 0 0 500 0.24 96.95 99.47 99.27 99.91 99.95 850 45.21 2.88 0.34 0.68 0.09 0.1 1000 53.95 0 0 0.05 0 0 2000 0.07 0 0 0 0 0 Naproxen Liqui -Pellet formulations contained 5%, 12%, 22%, 32%, and 42%w/w NaHCO3 (labeled as F-1, F-2, F-3, F-4, and F-5, respectively). Friability The friability test results (Table 4):show that all formulations display a good level of robustness. All formulations have less than 1% weight loss after being treated in the friabilator. This i s considered an acceptable weight loss for tablets under USP standards. However, at the current time of carrying out the i nvestigation, there is no USP standard for fr i ability test on pellets, thus the tablet standard was adapted. Table 4. Weight Loss of 3 g of Each Formulation Under Rotational Speed of 25 rpm for 4 min Table 4. Weight Loss of 3 g of Each Formulation Under Rotational Speed of 25 rpm for 4 min Formulation % Weight loss Physical mixture pellet 0.14 F-1 0.21 F-2 0.05 F-3 0.24 F-4 0.13 F-5 0.14 Naproxen Liqui -Pellet formulations contained 5%, 12%, 22%, 32%, and 42%w/w NaHCO3 (labeled as F-1, F-2, F-3, F-4, and F-5, respectively). The LPs in this study use MCC as the carrier material,which has strong bonding within its s tructure when water is added. This, along with the plastic property that polysorbate 80 contributes, makes LPs resistant to friability. The resistance to friability is ideal to ensure that the integrity of the product i s maintained during manufacturing andtransportation. It also reduces dusting,which can improve safety for the operator, particularly if potent drugs are being manufactured. Dissolution Studies Results from the dissolution studies under an acidic environment (Fig. 2) show a marked increase in drug release rate for F-4 (32%NaHCO3) and F-5 (42%NaHCO3)in comparison to the physical mixture pellet. The cumulative dissolution rates after 2 hours were 72.9%for F-4 and 76.4% for F-5, which is about 13-14 times faster than the physical mixture pellet (5.5%). This shows that NaHCO3 i s an effective functional excipient in naproxen LPs for enhanced drug release. Figure 2. Dissolution profiles of all formulations at pH 1.2. Each capsule contained 25 mg of naproxen. Naproxen Liqui-Pellet formulations contained 5%, 12%, 22%, 32%, and 42% w/w NaHCO,(labeled as F-1, F-2,F-3, F-4, and F-5,respectively). The dissolution profiles at pH 1.2 (Fig.2) show an overall trend that an increase in NaHCO3 concentration increases the drug release rate quite markedly. The ANOVA test showed that the effect of the concentration of NaHCO3on drug release was significant (p<0.05). F-5 had a slightly higher dissolution rate than F-4, but the difference was not significant (Tukey's p > 0.05). This suggests that there i s a limi t of NaHCO3 concentration that can cause a noticeable i mprovement i n drug release rate; above this limit, NaHCO3 has less influence on the LP drug dissolution rate. Thus, the 10% increase in NaHCOs does not seem worthwhile, and 32% NaHCO3 appears sufficient for drug release enhancement . It is interesting to note that the percentage drug release can be predicted when the concentration of NaHCO3 i s plotted against the percentage drug release at 60 min.The results show a high correlation (r0.9951) between these two parameters, as shown in Figure 3. This indicates that the drug release can be predicted well when the concentration of bicarbonate is between 0 and 32%.Because there was no difference in drug release between the 32% (F-4) and 42% (F-5) concentrations, F-5 was excluded when correlation was established. When comparing the drug dissolution profile of naproxen effervescent LPs in this study to previous studies on naproxen LPs, it can be seen how the incorporation of NaHCO3 can noticeably i mprove the drug release rate,particularly for F-4 and F-5, where i ts drug release rate is more superior than earlier naproxen LP formulations (1,2,10). Although F-4 and F-5 have the fastest drug release rate at acidic pH, i t i s noteworthy to point out that both formulations have yet to be further optimized by taking parameters such as water content and co-solvent content into account. Previous studies by the authors have already shown how these parameters can significantly affect the drug release rate. Hence, despite already obtaining considerable enhancement of drug release rate, the potential of effervescent LP formulations is yet to be realized. 50 Figure 3. Correlation between NaHCO, concentration and the percentage drug release obtained in pH 1.2 at 60 min (correlation applies between 0 to 32%). NaHCO3 has two key mechanisms that promote drug release in naproxen LPs. It is well known that NaHCO3produces CO2 gas when in contact with the acidic environment, such as the gastric fluid i n the stomach (28,44). The first drug release enhancement mechanism is due to promotion of disintegration of LPs, owing to the formation of CO2 gas. The formulation in this study uses MCC as a carrier, and MCC is known to form virtually nondisintegrating pellets via the extrusion-spheronization technique due to the strong bond within i ts structure (21-27). CO2 gas serves as a mechanical force within the LP to aid dis i ntegration, which consequently results in creating a l arger surface area for dissolution. The second key mechanism is that NaHCOs is an alkalizing agent (28).Because naproxen is a weakly acidic drug, NaHCO3 can make the pH at the microenvironment more alkaline, thus improving naproxen solubility, which in turn i mproves the drug release rate. For APls that are basic or neutral,this improved solubility may not be present; however, rapid disintegration is still present in the acidic stomach condition. In principle, the use of NaHCo3 excipient seems to be most effective for weakly acidic APls and may not necessarily be suitable for weakly basic drugs due to possible reduction of solubility. However , the improved disintegration should also be considered. The dissolution test was also performed tor all formulations at pH 7.4 (Fig. 4). The results show drug release rapidly nearing 100% within 20 min for all formulations, which is expected as naproxen is soluble at pH 7.4. 一 P hys ic a l m ixture pe ll e t —Q 一F -1 一一 F -2 —一 F -3 一米一 F -4 一 F-5 Figure 4. Dissolution profile of all formulations at pH 7.4. Each capsule contained 25 mg of naproxen. Naproxen Liqui-Pellet formulations contained 5%, 12%, 22%, 32%, and 42% w/w NaHCO, (labeled as F-1, F-2,F -3, F -4, and F-5,respectively). Overall, this study proves that LPs are indeed a versatile formulation with the ability for flexible modification such as the addition of an effervescent functional excipient.The addition of functional excipients has proven to be effective in the enhancement of dissolution as shown in the results. LPs can incorporate functional excipients because they are capable of achieving a high liquid load factor or high amount of non-volatile liquid vehicle while maintaining excellent/good flow properties. This means there is no need to increase the amount of carrier and coating material to achieve acceptable flow properties,which would otherwise render the high-dose dosage form too bulky for swallowing. With less carrier and coating material needed, there is an option for the addition of functional excipients without making the final dosage form too bulky. Such flexibility to incorporate additional functional excipients in large amounts is difficult or near-impossible to achieve using the classical liquisolid or powdered solution technology. CONCLUSION This study proved that it is feasible to embed an effervescent agent into LP formulation, whilst obtaining excellent or good flow property and yet keeping the overall dosage form small and l ight enough for swallowing.The i ncorporation of 42% NaHCOs in the formulation made the total weight of each capsule unit containing LPs to be only 231 mg. This would have been very difficult or near i mpossible to achieve with liquisolid technology,particularly in a high-dose drug, where a high liquid load f actor would result in a heavy and bulky formulation due to increase carrier and coating mater i al being required,let alone the addition of functional excipients. The dissolution test results show a considerable increase in the drug release rate with NaHCO3, where such formulations are around 14 times faster than a physical mixture pellet after 2 hours. As NaHCOs concentration increases, so does the drug release rate; however, there i s a limit to how much NaHCO3 can be added before its influence on the drug release rate lessens. Therefore, it i s i mperative to know this limit to balance the weight of capsule and drug release performance into an ideal dosage form. The data from this investigation verifies that the effervescent LP can achieve good robustness and excellent or good flow properties with narrow particle size distribution. Overall,the results support the potential for taking the concept of a liquid APl in a solid matrix dosage form in a commercial direction. Future study will include investigating the feasibility of effervescent LPs in high-dose formulation. F UNDING The authors disclosed no financial support related to this article. CON F L I CTS OF IN T EREST This technology is protected by International patent WO2020/021254 A1 (f iled July 24,2019, published January 30,2020). RE F ERENCES 1. Lam, M.; Ghafourian, T.; Nokhodchi, A. Liqui-Pellet: the emerging next-generation oral dosage form which stems from liquisolid concept in combination with pelletization technology.AAPS PharmSciTech. 2019, 20 (6), 231. DOI: 10.1208/s12249-019-1441-9. 2. Lam, M.; Ghafourian, T .; Nokhodchi , A. Optimis i ng the release rate of naproxen liqui-pellet: a new technology for emerging novel oral dosage form. Drug Deliv. Transl. Res. 2020, 10 (1),43-58. DOI: 10.1007/s13346-019-00659-6. 3. Lam, M.; Nokhodchi, A.Pharmaceutcal methodIsS and compositions International patent WO/2020/021254, January 30, 2020. https://patentscope.wipo.int/search/en/detail.jsf?docld=WO2020021254 (accessed April 13, 2022). 4. Lipinski , C. A.; Lombardo, F.; Dominy, B. W.; Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3-26. DOI: 10.1016/S0169-409X(00)00129-0. 5. Merisko -Liversidge, E. Nanocrystals: Resolving pharmaceutical formulation issues associated with poorly y water-soluble compounds. In Particles. Marcel Dekker, 2002. 6. Loftsson, T.; Brewster, M. E. Pharmaceutical applications of cyclodextrins: basicscience and product development.J. Pharm.Pharmacol. 2010, 62 (11), 1607-1621. DOI: 10.1111/j.2042-7158.2010.01030.X. 7. Mora, C. P .; Martinez, F. Solubi li ty of naproxen i n severa l organic solvents at different temperatures. Fluid Phase Equilib. 2007,255(1), 70-77. DOI: 10.1016/j.fluid.2007.03.029. 8. Pezzini, B. R.; Beringhs, A. O.; Ferraz, H. G.;Silva, M. A. S.; Stulzer,H. K.; Sonaglio, D. Liquisolid technology applied to pellets:evaluation of the feasibility and dissolution performance using felodipine as a model drug. Chem. Eng. Res. Des. 2016, 110,62-69. DOI: 10.1016/j.cherd.2016.01.037. 9. Spireas, S.; Bolton, S. M. Liquisolid systems and methods of preparing same. U.S. Patent US5800834A, September 1, 1998. https://patentimages.storage.googleapis.com/fb/ed/5a/7bc00f214c6de1/US5800834.pdf (accessed April 13,2022). 10. Lam, M.; Commandeur, D.; Maniruzzaman, M.; Tan, D. K.;Nokhodchi, A. The crucial effect of water and co -solvent on Liqui-Pellet pharmaceutical performance.Adv. Powder Technol.2020,31(5), 1903-1914. DOI: 10.1016/j.apt.2020.02.025. 11.S Sir i sha, V. R. K.; Suresh, K.; Vijayasree, K.; Devanna, N.; Murthy,P. N. Recent advances i n pelletization techniques-A review.Int.J. Pharm. Sci. Rev.Res.2014,27,217-223. 12. Clarke, G.; Newton, J .; Short, M. Comparative gastrointestinal transit of pellet systems of varying density. Int. J. Pharm. 1995,114(1),1-11. DOI: 10.1016/0378-5173(94)00200-0. 13.B Bechgaard, H.; Nielsen, G. H. Controlled-release multiple-units and single-unit doses a literature review. Drug Dev. Ind. Pharm.1978,4(1),53-67. DOI : 10.3109/03639047809055639. 14.Eskilson, C. Controlled release by microencapsulation. Manuf.Chem. 1985, 56,33-39. 15.Dhandapani, N. V.; Shrestha, A. Pel l etization by extrusion-spheronization: A detailed review. All Results J. Biol. 2012,3,10-23.DOI? 16.Muley, S.; Nandgude, T .; Poddar, S. Extrusion-spheronization a promising pelletizationtechnique::In-depth r review.Asian J. Pharm. Sci. 2016, 11 (6), 684-699. DOI: 10.1016/j.ajps.2016.08.001 17. Sonaglio, D.; Bataille, B.;Ortigosa, C.; Jacob, M. Factorial design in the feasibility of producing Microcel MC 101 pellets by extrusion/spheronization . Int. J. Pharm. 1995, 115 (1), 53-60.DOI: 10.1016/0378-5173(94)00246-2. 18.Fielden, K. E.; Newton, J. M.; O'Brien, P.; Rowe, R. C. Thermal studies on the interaction of water and microcrystalline cellulose. J. Pharm. Pharmacol. 1988, 40 (10), 674-678. DOI:10.1111/j.2042-7158.1988.tb06993.x. 19. Soh,J.L.; Yang, L.; Liew, C. V.; Cui, F. D.; Heng, P. W. Importance of small pores in microcrystal li ne cellulose for controlling water distribution during extrusion-spheronization. AAPS PharmSciTech.2008,9(3),972-981. DOI : 10.1208/s12249-008-9134-9. 20. Newton, J. M. The preparation of spherical granules by extrusion/spheronisation. STP pharma. 1990, 6,396-398. 21.Chamsai, B.: Sriamornsak, P. Novel disintegrating microcrystallinecellulos s e ep pel l ets with improved drug dissolution performance. Powder Technol. 2013,233,278-285.DOI : 10.1016/j.powtec.2012.08.019. 22. Kleinebudde, P. Shrinking and swelling properties of pellets containing microcrystalline cellulose and low substituted hydroxypropylcellulose: I. Shrinking properties. Int. J. Pharm.1994,109 (3), 209-219. DOI: 10.1016/0378-5173(94)90383-2. 23. Kleinebudde. P. The crystallite-gel-model for microcrystalline cellulose e i i r n wet-granulation,6extrusion,and spheronization. Pharm. Res. 1997, 14 (6), 804-809. DOI:10.1023/A:1012166809583. 24. Dukic-Ott, A.; Thommes, M.; Remon, J. P.; Kleinebudde, P.;Vervaet, C. Production of pellets via extrusion-spheronisation without the incorporation of microcrystalline cel l ulose: A cri t ical review. Eur. J. Pharm. Biopharm. 2009,71(1), 38-46.DOI: 10.1016/j.ejpb.2008.08.005. 25.Vervaet, C.; Baert, L.; Remon, J. P. Extrusion-spheronisation a literature review. Int. J. Pharm. 1995, 116 (2), 131-146. DOI:10.1016/0378-5173(94)00311-R. 26.D Dreu, R.; Sirca, J.; Pintye-Hodi , K.; Bur j an, T.; Planinsek, O.; Srcic,S. Physicochemical properties of granulating liquids and their influence on microcrystalline cellulose pellets obtained by extrusion-spheronisation technology. Int. J. Pharm. 2005, 291(1-2), 99-111. DOI: 10.1016/j.ijpharm.2004.07.047. 27. Dukic-Ott, A.; Remon , J. P.; Foreman, P.; Vervaet, C. Immediate release of poorly soluble drugs from starch-based pellets prepared via extrusion/spheronisation. Eur. J. Pharm. Biopharm.2007,67(3), 715-724. DOI : 10.1016/j.ejpb.2007.04.014. 28.Rowe, R. C.; Sheskey, P. J .; Quinn , M. E.; Handbook of Pharmaceutical Excipients, 6th ed. Amer i can Pharmacists Association.Pharmaceutical Press, 2009. 29.N Noyes, A. A.; Whitney, W. R . The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897,19(12),930-934. 30. Razali, S.; Wong, T. W. Design of superdisintegrant- and effervescent agent-less dispers i ble fast-release melt pellets.Powder 厂 Technol.2013,2235.,289-298.DOI :10.1016/j.powtec.2012.10.028. 31. Yuksel , N.; Kanik, A. E .; Baykara T. Comparison of in vitro dissolution profiles by ANOVA-based, model-dependent and -independent methods. Int. J. Pharm. 2000, 209 (1-2), 57-67.DOI : 10.1016/S0378-5173(00)00554-8. 32.N Moore, J . W.; Flanner, H. H. Mathematical comparison of dissolution profiles. Pharm. Technol.1996,20,64-74. 33. O'Hara , T.; Dunne, A.; Butler, J.; Devane, J. A review of methods used to compare dissolution profile data. Pharm.Sci. Technol. Today. 1998, 1 (5), 214-223. DOI: 10.1016/S1461-5347(98)00053-4. 34.LDissolution Testing of Immediate Release Solid Oral Dosage Forms; Gu i dance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), U.S. Government Printing Office: Washington,DC, 1997. 35.Immediate Release Solid Oral Dosage Forms, Scale-Up and PostapprovalC Changes: Chemistry, Manufacturing,and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation; Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration,Center for Drug Evaluation andResearch (CDER),LU.S.Government Printing Office: Washington, DC, 1995 36.F Fielden, K. E.; Newton, J . M.; Rowe, R. C. The influence of moisture content on spheronization of extrudate processed by a ram extruder. Int. J. Pharm. 1993, 97(1-3), 79-92. DOI:10.1016/0378-5173(93)90128-3. 37. Harrison, P. J .; Newton, J. M.; Rowe, R. C. Convergent flow analysis in the extrusion of wet powder masses. J. Pharm.Pharmacol . 1984,36 (12),796-798. DOI: 10.1111/j.2042-7158.1984.tb04879.x. 38. Harrison, P.J.; Newton, J . M.; Rowe, R. C. Flow defects in wet powder mass extrusion.J. Pharm. Pharmacol.1985, 37(2),81-83. DOI:10.1111/j.2042-7158.1985.tb05011.x. 39.K Ku, C. C.; Joshi, Y. M.; Bergum, J. S.; J ain, N. B. Bead manufacture by extrusion/spheronization-a stat i stical design for process optimization. Drug Dev. Ind. Pharm. 1993, 19 (13),1505-1519.DOI: 10.3109/03639049309069323. 40. Pinto, J. F .; Buckton, G.; Newton, J. M. The influence of four selected processing and formulation factors on the production of spheres by extrusion and spheronisation. Int. J. Pharm. 1992,83(1-3), 187-196. DOI : 10.1016/0378-5173(82)90022-9. 41. Wan,L.S. C.;Heng, P. W.S.; Liew, C. V. Spheronization conditions on spheroid shape and size. I nt.J.Pharm. 1993,96(1-3),59-65.DOI: 10.1016/0378-5173(93)90212-X. 42.S Spireas, S.; Sadu,S. Enhancement of prednisolone dissolution properties using liquisol i d compacts. In t. J. Pharm. 1998, 166(2), 177-188. DOI: 10.1016/S0378-5173(98)00046-5. 43.A Aulton, M. E.; Taylor, K. M. G. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines. E ls evier Health Sciences;2013. 44.K Krogel, I.; Bodmeier, R. Floating or pulsatile drug delivery systems based on coated effervescent cores. Int. J. Pharm.1999, 187(2), 175-184. DOI:10.1016/S0378-5173(99)00189-1.
确定





还剩6页未读,是否继续阅读?
嘉盛(香港)科技有限公司为您提供《一种新型泡腾剂在萘普生液体微丸中的应用》,该方案主要用于原料药中前处理检测,参考标准--,《一种新型泡腾剂在萘普生液体微丸中的应用》用到的仪器有湿法制粒挤出滚圆一体机、英国Caleva湿法造粒搅拌扭矩流变仪MTR3
推荐专场
相关方案
更多
该厂商其他方案
更多
















