方案详情
文
巴西胡椒科(Piperaceae)已被广泛用于巴西民间药物,并以其强大的抗氧化特性而闻名。但其主要活性成分4-橙烯二苯二酚(4-NC)对紫外光和可见光敏感,限制了中草药和中草药制剂的使用。为了提高4-NC的稳定性,本研究获得了包衣多颗粒固体剂型伞形草。以伞形草提取物为湿剂,采用挤出滚圆法制备微丸
方案详情

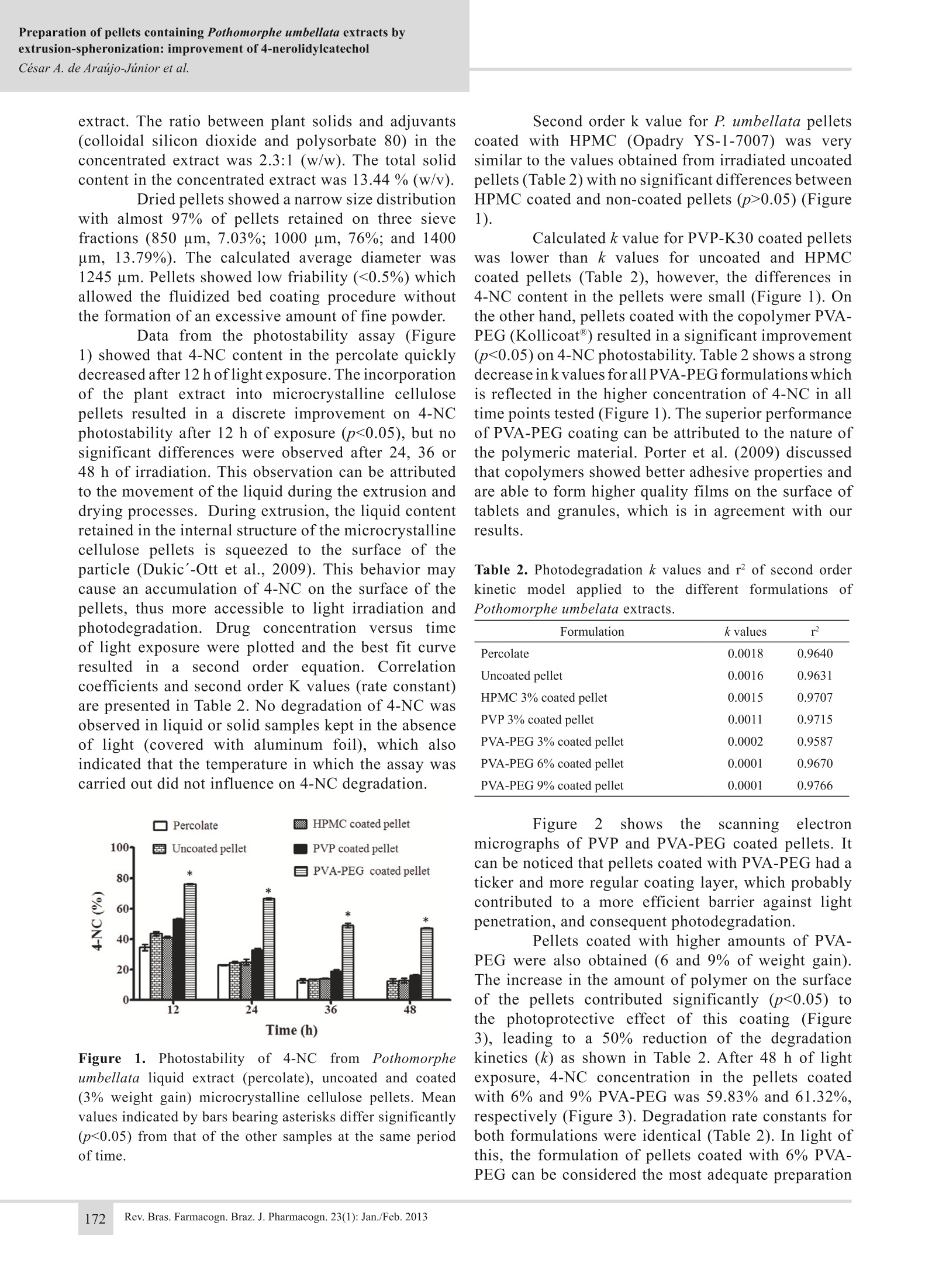
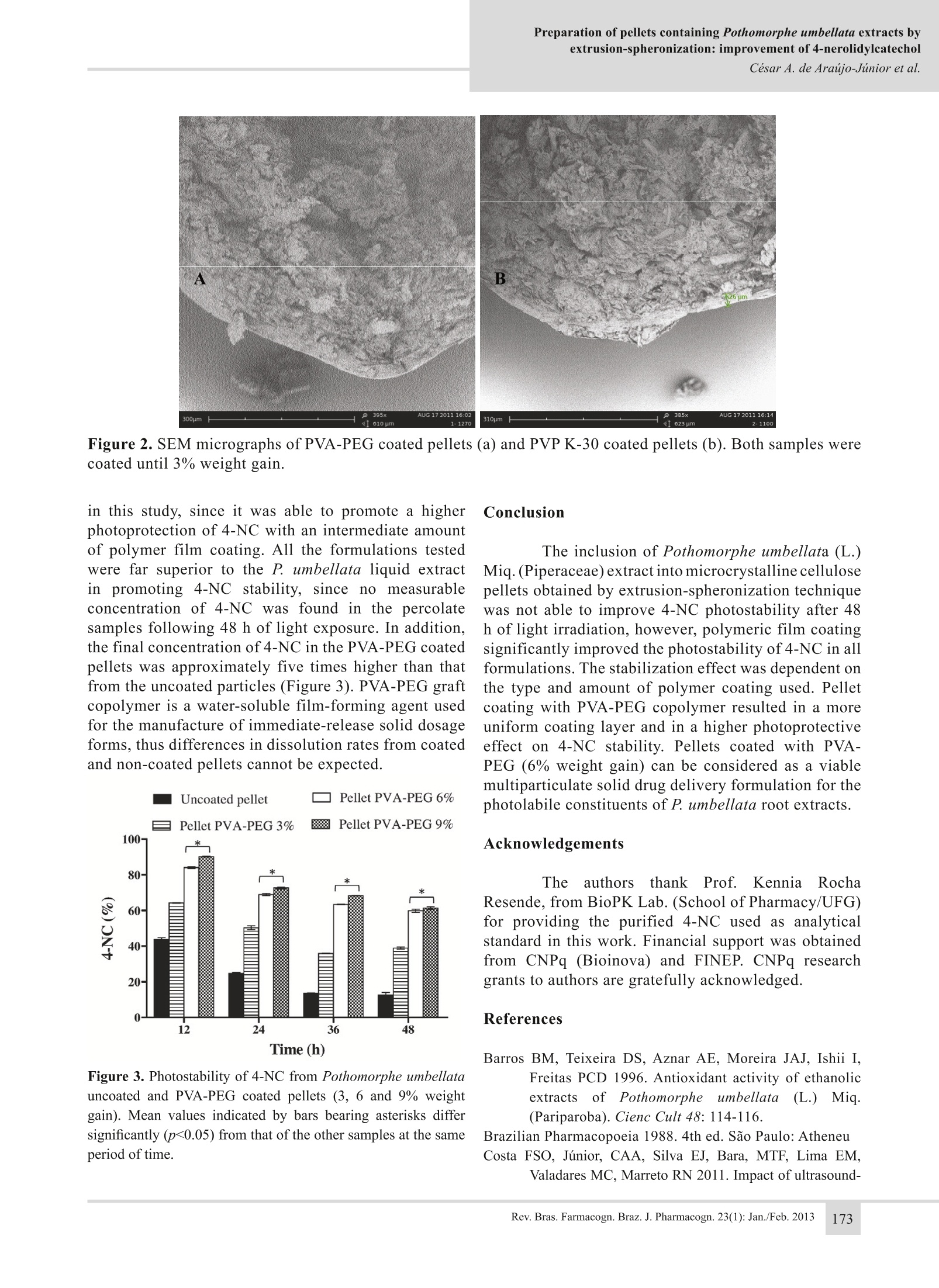
Preparation of pellets containing Pothomorphe umbellata extracts byextrusion-spheronization: improvement of 4-nerolidylcatecholCesar A. de Araujo-Junior et al. Preparation of pellets containingPothomorphe umbellata extracts byextrusion-spheronization: improvement of4-nerolidylcatechol photostability Cesar A. de Araijo-Júnior, Fernanda S. de Oliveira Costa,Stephania F. Taveira,RicardoN. Marreto,NMarize C.Valadares, Eliana M. Lima*, Laboratorio de Tecnologia Farmaceutica, Faculdade de Farmacia, UniversidadeFederal de Goias, Brazil,Laboratorio de Farmacologia e Toxicologia Celular, Faculdade de Farmacia,Universidade Federal de Goias, Brazil. Abstract: Pothomorphe umbellata (L.) Miq., Piperaceae, has been extensively usedin Brazilian folk medicine and it is well known for its strong antioxidant properties.However, its main active constituent, 4-nerolydilcatechol (4-NC), is sensitive toultraviolet and visible light, which can limit the use of intermediate and final herbalpreparations of this species. In the present work, coated multiparticulate solid dosageforms of P. umbellata were obtained with the purpose of increasing the stabilityof 4-NC. P. umbellata extract was used as a wetting liquid for the preparation ofpellets by extrusion-spheronization. Pellets were coated in a fluidized bed by threedifferent polymers (hydroxypropylmethylcellulose (HPMC), polyvynilpirrolidoneK-30 (PVP-K30), and polyvinyl alcohol-polyethylene glycol graft-copolymer (PVA-PEG)). 4-NC photostability was evaluated by an accelerated photostability protocol.Pellets showed a narrow size distribution and low friability. 4-NC photodegradationfollowed a second order degradation kinetics with similar k values for the percolate,uncoated pellets and HPMC coated pellets. Photoprotection was higher in pelletscoated with PVP-K30 and PVA-PEG. PVA-PEG coated pellets with 6 and 9% weightgain resulted in a final concentration of 4-NC approximately cinco times higher thanuncoated pellets or liquid extracts, suggesting the potential of this formulation as amultiparticulate solid dosage form for P. umbellata extracts. Revista Brasileira de FarmacognosiaBrazilian Journal of Pharmacognosy23(1):169-174,Jan./Feb.2013 Article Received 14 May 2012 Accepted 25 Aug 2012 Available online 6 Nov 2012 Keywords: extrusion-spheronizationfilm coatingherbal extractsmultiparticulate solid dosage formsnerolidylcatechol photostability ISSN 0102-695X DOI: 10.1590/S0102-695X2012005000125 Pothomorphe umbellata (L.) Miq., Piperaceae[syn. Heckeria umbellata (L.) Kunth., Piper umbellataL., Piper hilarianum Stend.] is known in Brazil as“pariparoba”and is widely employed in folk medicineas an analgesic, diuretic and anti-spasmodic agent, aswell as for the treatment of inflammatory disorders,malaria, asthma, liver diseases and gastrointestinaldisorders (Perazzo et al., 2005; Ropke et al., 2005). Pothomorphe umbellata was included in thefirst edition of the Brazilian Pharmacopoeia (Silva,1926) and its antioxidant activity has been reportedin different experimental models (Barros et al., 1996;Desmarchelier et al., 1997; Ropke et al., 2005). Thebiological activity of P. umbellata is mainly attributedto its most abundant metabolite, 4-nerolidylcatechol(4-NC), a lipophilic compound found in the roots and leaves (Kijjoa et al., 1980). Previousresultsftrom our researchhgroupdemonstrated that the P. umbellata root extract andthe isolated 4-NC did not have mutagenic effect onmouse bone marrow cells, but conversely, a protectiveeffect against cyclophosphamide induced genotoxicitywas observed (Valadares et al., 2007). In addition,the root extractwas proven more effective thanthe isolated compound. In spite of their potential,4-NC and .umbellataeextractspresented highphotosensitivity to UVA and visible light radiation(Costa et al., 2011), which can limit their use. Costaet al. (2011) also reported that P. umbellata extractsexhibited a more potent antioxidant activity, but weremore photosensitive compared to isolated 4-NC. Toimprove the usefulness of P. umbellata preparations itis necessary to overcome these photoinstability issues.Aviable technological strategy towards improving photostability of compoundsin solution is theirincorporation into solid substrates, since the rates ofphotolytic reactions are lower in solid state. Therefore,the incorporation of P. umbellata liquid extracts intosolid dosage forms might be an interesting approach inorder to stabilize the photolabile 4-NC. In the present wok, microcrystalline cellulosepellets containinggp. umbellataeextracts wereprepared by extrusion-spheronization technique andsubsequently coated with different types of polymers.Pellets characteristics and photostability profiles of4-NC were determined and compared. Materials and Methods Materials The roots of Pothomorphe umbellata (L.)Miq., Piperaceae, were purchased from Centroflora(Botucatu, SP, Brazil, batch number 204090080). Thematerial was ground in an industrial mill to obtainan average particle diameter of 250 um. A sample ofthe dried and ground commercial sample has beendeposited in the Herboteca da Faculdade de Farmaciada Universidade Federal de Goias (registry numberFF001). Ethanol used in the extraction procedure andglacial acetic acid used in the HPLC mobile phase wereof analytical reagent grade. Acetonitrile and methanolwere HPLC grade and were purchased from JT Baker(USA). Isolated 4-NC used as reference standard wasobtained from the hydroethanolic root extract accordingto Gustalfson et al., (1992) by Prof. Kennia R. Resende,from the School of Pharmacy, Federal University ofGoias, Brazil. For the preparation and coating of the pelletsmaterials used were microcrystalline cellulose PH 101(Blanver, Sao Paulo, Brazil), lactose monohydrate(Milkaut, Santa Fe, Argentina), polyvinylpyrrolidoneK30 (ISP, Sao Paulo, Brazil), Kollicoat((Basf.Ludwigshafen, Germany), Opadry YS1-7007(Colorcon, Sao Paulo, Brazil), polyethylene glycol6,000 (Polioles, Ciudade de Mexico,Mexico), titaniumdioxide (Quimica Brasil LTDA, Sao Paulo,Brazil). Methods Percolation procedure The percolation was performedaccordingto Brazilian Pharmacopoeia (Silva, 1926) with 1000g of P. umbellata roots. The ethanol/water mixture(3:1,v/v) was added to the percolator until the plantmaterial was entirely exhausted. The fractions obtainedwere combined and the solvent was evaporated underreduced pressure in a rotary vacuum evaporator (IKA, RV 10, Germany) at 40 °C to a final volume of 4000mL. The ethanol and total solid content of the percolatewere determined by standard pharmacopeial procedures(Brazilian Pharmacopeia,1988). Colloidalsilicon dioxide nd polysorbate80 were added to the percolate (0.25 and 0.75% w/v,respectively) and the mixture was submitted to anadditional concentration step in a rotary evaporator(IKA, RV 10, Germany) at 40 °C. The concentratedextract was used as the wetting liquid in the preparationof P. umbellata pellets. Preparation of pellets by extrusion-spheronization Pellet characterization Pellet size was determined by sieve analysisusing a Sonic Sifter Separator L3P-26 (AdvantechMFG, Wisconsin,USA) equipped with a series of sieveswith apertures ranging between 300-2,000 um. Themean diameter of pellets was calculated by weightedmean. For friability evaluation, an aliquot of uncoatedpellets was put into a Nova Etica friabilator (NovaEtica, Brazil) working at 20 rpm for 5 min. Friabilityand size analyses were performed in triplicate. Scanning electron micrographs were obtainedin a Phenom Scanning Electron Microscope (FEICompany,) following the deposition of a 20 nm goldcoating using a sputter coater EM SCD 050 (Leica,Germany). Pellet coating Thirty five grams of pellets (800-1400 um indiameter) were fluidized in a fluid bed unit (Mycrolab,Huttlin, Germany) and then coated with a polymerdispersion (Table 1) under the following conditions:atomization air pressure of 11 psi; fluidization air Table 1. Composition of the polymer dispersions used for pellet coating. Formulation Hydroxypropyl Polyvinyl alcohol-polyethylene Polyvinyl Polyethylene TiO. methylcellulose glycol graft-copolymer pyrrolidone glycol 6000 HPMC coated pellet 3% -- -- 3% 3% PVP coated pellet -- -- 3% 3% 3% PVA-PEG coated pellet -- 3% -- 3% 3% weight gain 3%;bweight gain 3, 6 and 9%. flow of 1.8 m’/h; spray rate 1.0 g/min and inlet airtemperature of 40°C. Polyethylene glycol 6,000 wasadded as plasticizer and titanium dioxide was used asopacifier in all formulations (Table 1). Following theapplication of the polymer dispersion, pellets weremaintained in the fluidized bed unit for an additionaldrying time of 10 min at 45 °C. The amount of polymerdispersion used for the coating of the pellets allowedfor a weight gain of either 3, 6 or 9%, as presented inTable 1. aluminum foil and immediately analyzed by HPLC. Aparallel experiment was run in the dark as a negativecontrol for the effects of light on the degradation.For this assay, percolated samples were diluted withethanol (1:1 ratio) and put into a Petri dish. Pelletsamples (1 g) were spread across the Petri dish to givea unique solid layer. After light exposure, pellets weretriturated with a mortar and pestle and 200 mg wereappropriately extracted and diluted with ethanol andanalyzed by HPLC for the quantitative determinationof 4-NC content. Results and Discussion 4-Nerolidylcatechol content in the percolate,concentrated percolate and pellets was determined usinga Varian HPLC system (PS410,Varian,USA) equippedwith an UV-visible absorbance detector (PS325) and aquaternary pump (PS240). Separations were performedon a Varian ChromSpher 5 C18 reverse phase column(15 cm, 4.6 mm, 5 um). The mobile phase consistedof acidified methanol (0.1% glacial acetic acid), water(0.1% glacial acetic acid) and acetonitrile (90:9:1).The flow rate was 1 mL/min and the injection volumewas 10 uL. The detection wavelength was 282 nm, andthe column temperature was 25 °C. All samples werefiltered through a 0.45 um filter prior to injection intothe chromatographer. Dilutions ofisolated 4-NC (usedas external standard) in ethanol were used to obtain theconcentration analytical curve of the 4-NC. Photostability assay Percolate extract and pellets containing P.umbellata (coated and uncoated)were submittedto an accelerated photostability assay in a 424 CFPhotostability Chamber (Nova Etica, Brazil) equippedwith a near-UV fluorescent lamp (15 W) with a spectraldistribution from 320 to 400 nm and several cool whitefluorescent lamps (15 W). Samples were exposed toradiation, which provided integrated UVA energies of98.4, 196.8,295.2 and 393.6 W/m2 corresponding toexposure periods of 12, 24, 36 and 48 h, respectively.The experiment was performed according to ICH(1996). After light exposure, samples were covered with The therapeutic usefulness of Pothomorpheumbellata (L.) Miq., Piperaceae, extracts may becompromised by the high photosensitivity of its mainactive constituent, the 4-NC (Soares et al., 2009). Silvaet al. (2005) showed that P.umbellata liquid extractswere not degraded by UVB radiation. On the otherhand, Costa et al. (2011) showed that different kindsof liquid P. umbellata preparations (percolate andultrasound extracts) were strongly degraded by UVAand visible light. Solid state photostability, in general,is higher than that in liquid state (Tonnesen, 2001) andseveral variables may affect light penetration in a solidmaterial, such as particle size, type of excipients,colorand crystalline structure (Tonnesen,2001). In the present work, P. umbellata extracts wereincorporated into microcrystalline cellulose (MCC)pellets and the photostability of the active compound,4-NC, was evaluated. P. umbellata hydroetanolic extractshowed a total solid content of 2.36±0.16% (w/v); theethanol amount in the preparation was 32.72±0.14%(w/w). The concentration of 4-NC in the percolate was1.053 mg/mL. In order to incorporate higher amountsof 4-NC in the pellets without compromising theirpreparation and physical characteristics, the percolatewas concentrated in a rotary evaporator and used asthe agglomeration liquid in the pellet preparation. Theconcentrated extract had 6.03 mg/mL of 4-NC. Due tothe high hydrophobicity of 4-NC and it's tendency toprecipitate in the concentrated extract upon removal ofethanol, colloidal silicon dioxide and polysorbate 80were added to the percolate before the evaporation stepin order to maintain the homogeneity ofthe concentrated extract. The ratio between plant solids and adjuvants(colloidal silicon dioxide and polysorbate 80) in theconcentrated extract was 2.3:1 (w/w). The total solidcontent in the concentrated extract was 13.44%(w/v). Dried pellets showed a narrow size distributionwith almost 97% of pellets retained on three sievefractions (850 um, 7.03%;1000 um, 76%; and 1400um, 13.79%). The calculated average diameter was1245 um. Pellets showed low friability (<0.5%) whichallowed the fluidized bed coating procedure withoutthe formation of an excessive amount of fine powder. Data from the photostability assay (Figure1) showed that 4-NC content in the percolate quicklydecreased after 12 hoflight exposure. The incorporationof the plant extract into microcrystalline cellulosepellets resulted in a discrete improvement on 4-NCphotostability after 12 h of exposure (p<0.05), but nosignificant differences were observed after 24, 36 or48 h of irradiation. This obseryation can be attributedto the movement of the liquid during the extrusion anddrying processes. During extrusion, the liquid contentretained in the internal structure of the microcrystallinecellulose pellets is squeezed to the surface of theparticle (Dukic'-Ott et al., 2009). This behavior maycause an accumulation of 4-NC on the surface of thepellets, thus more accessible to light irradiation andphotodegradation. Drug concentration versus timeof light exposure were plotted and the best fit curveresulted in a second order equation. Correlationcoefficients and second order K values (rate constant)are presented in Table 2. No degradation of 4-NC wasobserved in liquid or solid samples kept in the absenceof light (covered with aluminum foil), which alsoindicated that the temperature in which the assay wascarried out did not influence on 4-NC degradation. t Figure 1. Photostability of 4-NC from Pothomorpheumbellata liquid extract (percolate), uncoated and coated(3% weight gain) microcrystalline cellulose pellets. Meanvalues indicated by bars bearing asterisks differ significantly(p<0.05) from that of the other samples at the same periodof time. Second order k value for P. umbellata pelletscoated with HPMC (Opadry YS-1-7007) was verysimilar to the values obtained from irradiated uncoatedpellets (Table 2) with no significant differences betweenHPMC coated and non-coated pellets (p>0.05) (Figure1). Calculated k value for PVP-K30 coated pelletswas lower than k values for uncoated and HPMCcoated pellets (Table 2), however, the differences in4-NC content in the pellets were small (Figure 1). Onthe other hand, pellets coated with the copolymer PVA-PEG(Kollicoat@) resulted in a significant improvement(p<0.05) on 4-NC photostability. Table 2 shows a strongdecrease in k values for all PVA-PEG formulations whichis reflected in the higher concentration of 4-NC in alltime points tested (Figure 1). The superior performanceof PVA-PEG coating can be attributed to the nature ofthe polymeric material. Porter et al. (2009) discussedthat copolymers showed better adhesive properties andare able to form higher quality films on the surface oftablets and granules, which is in agreement with ourresults. Table 2. Photodegradation k values and rof second orderkinetic model applied to the different formulations ofPothomorphe umbelata extracts. Formulation k values r² Percolate 0.0018 0.9640 Uncoated pellet 0.0016 0.9631 HPMC 3% coated pellet 0.0015 0.9707 PVP 3% coated pellet 0.0011 0.9715 PVA-PEG 3% coated pellet 0.0002 0.9587 PVA-PEG 6% coated pellet 0.0001 0.9670 PVA-PEG 9% coated pellet 0.0001 0.9766 Figure 2showsthescanningelectronmicrographs of PVP and PVA-PEG coated pellets. Itcan be noticed that pellets coated with PVA-PEG had aticker and more regular coating layer, which probablycontributed to a more efficient barrier against lightpenetration, and consequent photodegradation. Pellets coated with higher amounts of PVA-PEG were also obtained (6 and 9% of weight gain).The increase in the amount of polymer on the surfaceof the pellets contributed significantly (p<0.05) tothe photoprotective effect of this coating (Figure3),leading to a 50% reduction of the degradationkinetics (k) as shown in Table 2. After 48 h of lightexposure, 4-NC concentration in the pellets coatedwith 6% and 9% PVA-PEG was 59.83% and 61.32%.respectively (Figure 3). Degradation rate constants forboth formulations were identical (Table 2). In light ofthis, the formulation of pellets coated with 6% PVA-PEG can be considered the most adequate preparation Figure 2. SEM micrographs of PVA-PEG coated pellets (a) and PVP K-30 coated pellets (b). Both samples werecoated until 3% weight gain. in this study, since it was able to promote a higherphotoprotection of 4-NC with an intermediate amountof polymer film coating. All the formulations testedwere far superior to the P. umbellata liquid extract1npromoting 4-NC stability, since no measurableconcentration of 4-NC was found in the percolatesamples following 48 h of light exposure. In addition,the final concentration of 4-NC in the PVA-PEG coatedpellets was approximately five times higher than thatfrom the uncoated particles (Figure 3). PVA-PEG graftcopolymer is a water-soluble film-forming agent usedfor the manufacture of immediate-release solid dosageforms, thus differences in dissolution rates from coatedand non-coated pellets cannot be expected. Time (h) Figure 3. Photostability of 4-NC from Pothomorphe umbellatauncoated and PVA-PEG coated pellets (3, 6 and 9% weightgain). Mean values indicated by bars bearing asterisks differsignificantly (p<0.05) from that of the other samples at the sameperiod of time. Conclusion The inclusion of Pothomorphe umbellata (L.)Miq. (Piperaceae)extract into microcrystalline cellulosepellets obtained by extrusion-spheronization techniquewas not able to improve 4-NC photostability after 48h of light irradiation, however, polymeric film coatingsignificantly improved the photostability of 4-NC in allformulations. The stabilization effect was dependent onthe type and amount of polymer coating used. Pelletcoating with PVA-PEG copolymer resulted in a moreuniform coating layer and in a higher photoprotectiveeffect on 4-NC stability. Pellets coated with PVA-PEG (6% weight gain) can be considered as a viablemultiparticulate solid drug delivery formulation for thephotolabile constituents of P. umbellata root extracts. Acknowledgements TheeaauthorsthankkFProf.t.KenniaRochaResende, from BioPK Lab. (School of Pharmacy/UFG)for providing the purified 4-NC used as analyticalstandard in this work. Financial support was obtainedfrom CNPq (Bioinova) and FINEP. CNPq researchgrants to authors are gratefully acknowledged. References ( Barros BM, Teixeira D S, A znar AE, M o reira J A J, I s hii I ,Freitas PC D 1996. A n tioxidant ac t ivity of ethanolic extractsts Oof P othomorphe 2 1 umbellata a ( (L.) Miq 1 (Pariparoba). Cienc Cult 48: 114-1 1 6. ) Brazilian Pharmacopoeia 1988. 4th ed. Sao Paulo: AtheneuCosta FSO, Júnior, CAA, Silva EJ, Bara, MTF, Lima EM,Valadares MC, Marreto RN 2011. Impact of ultrasound- ( assisted extraction on quality and photostability of thePothomorphe umbellata extracts. Ultrason Sonochem 18: 1002-1007. ) Desmarchelier C, Barros S, Repetto M, Latorre LR, Kato M,Coussio J, Ciccia G 1997. 4-nerolidylcatechol fromPothomorphe spp. scavenges peroxyl radicals inhibitsFe (II)-dependent DNA damage. Planta Med 63: 561-563. ( Dukic'-Ott A, Thommes M , R e mon JP , Kleinebudde P,Vervaet C 2 009. Production o f pellets v ia extrusion- spheronisation without t the incorporation of microcrystalline c ellulose: A critical r eview. Eur J Pharm Biopharm. 7 1: 38-46. ) ( Gustafson KR, Cardellina JH, McMahon JB, Pannell L K,Cragg GM, Boyd MR 1992. The peltatolls novel HIV- inhibitory catechol d erivates f rom P. p eltata. J Org Chem 57:2809-2811. ) ( ICH 1996. International Conference of Harmonization. Photostability testin g of new drug substances a ndproducts. Guideline Q1B. ) ( Kijjoa A, Giesbrecht AM, Akisue MK, Gottlieb OR, GottliebHE 1 9 80. T he chemistry of Br a zilian Piperaceae:2,4-nerolidylcatecho l from Pothomorphe umbellate. Planta Med 39: 85-87. ) ( Perazzo FF, Souza GHB, Lopes W, Cardoso LGV, Carvalho JCT, N anayakkara l N PD, B astos J K 2005. Anti-inflammatory andanalgesic p roperties o f water- ethanolic e xtract from Pothomorphe umbellata (Piperaceae) a erial parts. J Ethnopharmacol 9 9 : 2 1 5- 220. ) ( Porter S, Sackett G, Liu L 2009. D evelopment,optimization,and s cale-up of process p a rameters: p a n coating. In:Developing Solid Oral Forms: Pharmaceutical Theoryand Practice.New York: Elsevier Inc, p. 761-804 ) ( R opke CD, S a wada TC, da Si l va VV, Michalany NS, BarrosSBM 2005. P h otoprotective effect of Pothomorpheumbellata r o ot extract against ultraviolet radiationinduced chronic skin damage in t he hairless mouse.Clin Exp Dermatol 30:272-276. ) ( Silva VV, Ropke CD, De Almenida RL , M i randa D V , Kera CZ, Rivell i DP , Sawad a TCH, Barros SBM 2005. C hemical stability and S PF determination ofPothomorphe umbellata ex t ract gel and photostability of 4-nerolidylcatechol. Int J Pharm 303: 125-131. ) Silva RAD 1926. Pharmacopeia dos Estados Unidos doBrasil. Sao Paulo: Cia.,Ed. Nacional 1926. ( Soares LA, L eal AFVB, Fraceto LF, Maia ER, R e sck I S ,Kato MS, G il ES, Souza AR, Cunha L C, R esendeKR 2009. H o st-guest s y stem of 4-nerolidylcatechol in 2-hydroxylpropyl-β-cyclodextrin: preparation, characterizatio n an d molecular modeling. J Incl Phenom Macro 64: 2 3-35. ) ( Tonnesen H H 2001. Fo r mulation an d st a bility testing ofphotolabile drugs. Int J Pharm 225: 1-14. ) ( Valadares MC, Rezende KR. Pereira ERT. Sous a MCB 2007. Protective effects of 4 -nerolidylcatechol a gainst genotoxicit y induce d by cyclophosphamide. Food Chem Toxicol 40: 1975-1978. ) *Correspondence Eliana Martins Lima Laboratorio de Tecnologia Farmaceutica, Faculdadee(deFarmacia, Universidade Federal de GoiasPraca Universitaria, Praca Universitaria c/ 1. Avenida, Qd.62,74.605-220 Goiania-GO Brazilemlima@farmacia.ufg.br Tel:+55 62 3209 6039 Rev. Bras.Farmacogn. Braz. J. Pharmacogn. : Jan./Feb. 巴西胡椒科(Piperaceae)已被广泛用于巴西民间药物,并以其强大的抗氧化特性而闻名。但其主要活性成分4-橙烯二苯二酚(4-NC)对紫外光和可见光敏感,限制了中草药和中草药制剂的使用。为了提高4-NC的稳定性,本研究获得了包衣多颗粒固体剂型伞形草。以伞形草提取物为湿剂,采用挤出滚圆法制备微丸将三种不同的聚合物(羟丙基甲基纤维素(HPMC)、聚乙烯基吡咯烷酮K-30 (PVP-K30)和聚乙烯醇-聚乙二醇接枝共聚物(PVA-PEG))包覆在流化床中。4-NC光稳定性通过加速光稳定性协议进行评估。球团粒度分布窄,脆性低。4-NC光降解的k值与滤过液、未包被微球和HPMC包被微球相似,均为二级降解动力学。pva - k30和PVA-PEG涂层的微丸具有更高的光保护效果。PVA-PEG包衣微丸增重6和9%,其4-NC的最终浓度大约是未包衣微丸或液体提取物的5 / 5倍,这表明该配方作为伞菇提取物的多颗粒固体剂型的潜力。
确定




还剩4页未读,是否继续阅读?
产品配置单
嘉盛(香港)科技有限公司为您提供《微丸、胡椒、中草药中4-橙烯二苯二酚检测方案(喷雾干燥机)》,该方案主要用于植物油脂和提取物中理化性质检测,参考标准--,《微丸、胡椒、中草药中4-橙烯二苯二酚检测方案(喷雾干燥机)》用到的仪器有实验室挤出滚圆机、实验室挤出滚圆机、实验室挤出滚圆机、湿法制粒挤出滚圆一体机
推荐专场
相关方案
更多
该厂商其他方案
更多