限制尘埃沉积在调节表层海水中必需微量营养素铁浓度方面的作用,需要了解气溶胶中海水可溶性铁的通量和透光带中溶解铁(DFe)的替代时间。在这里,我们使用百慕大-大西洋时间序列研究区域的季节性分解DFe数据以及2019年百慕大气溶胶和降雨中铁的每周尺度测量来估计这些数量。
方案详情

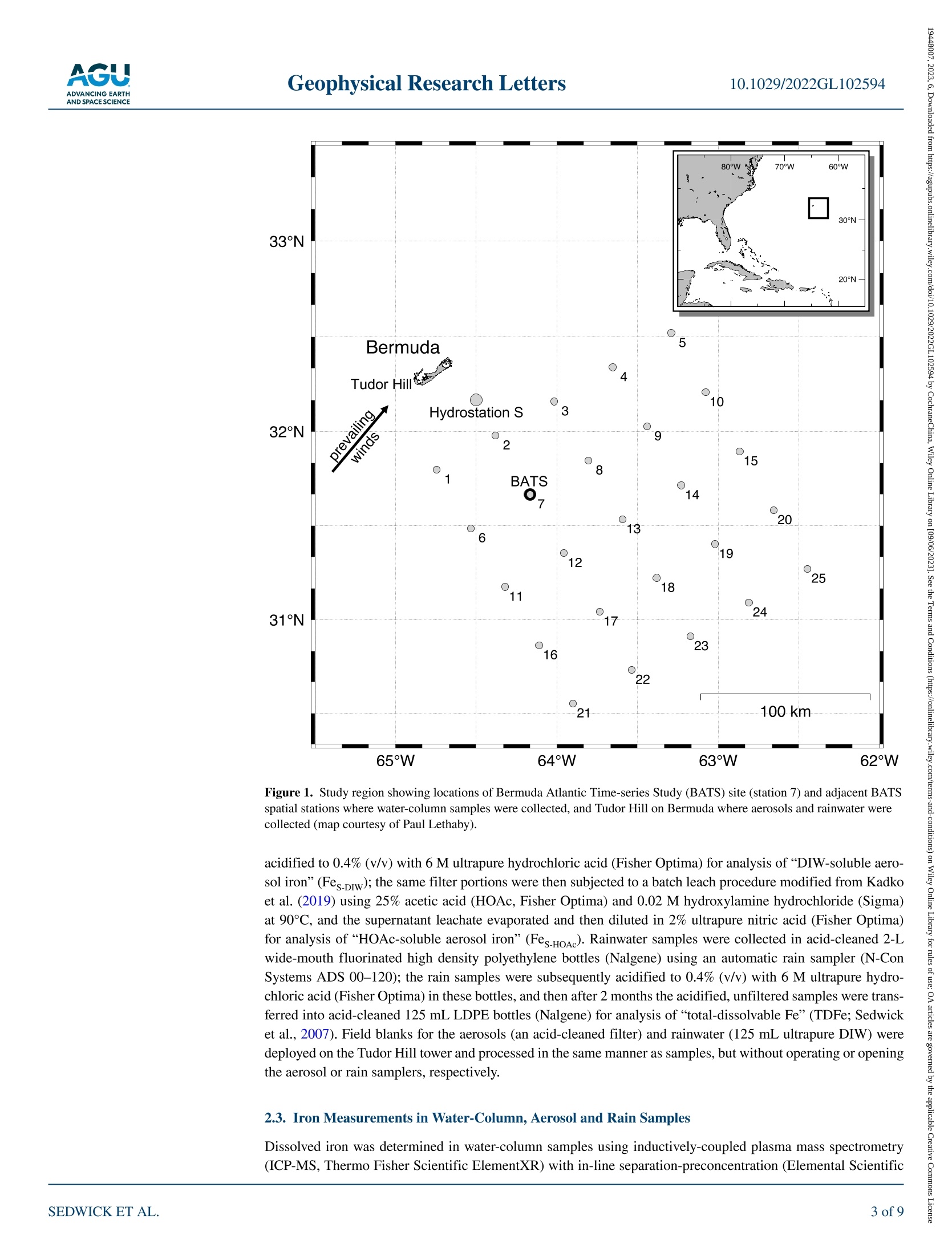
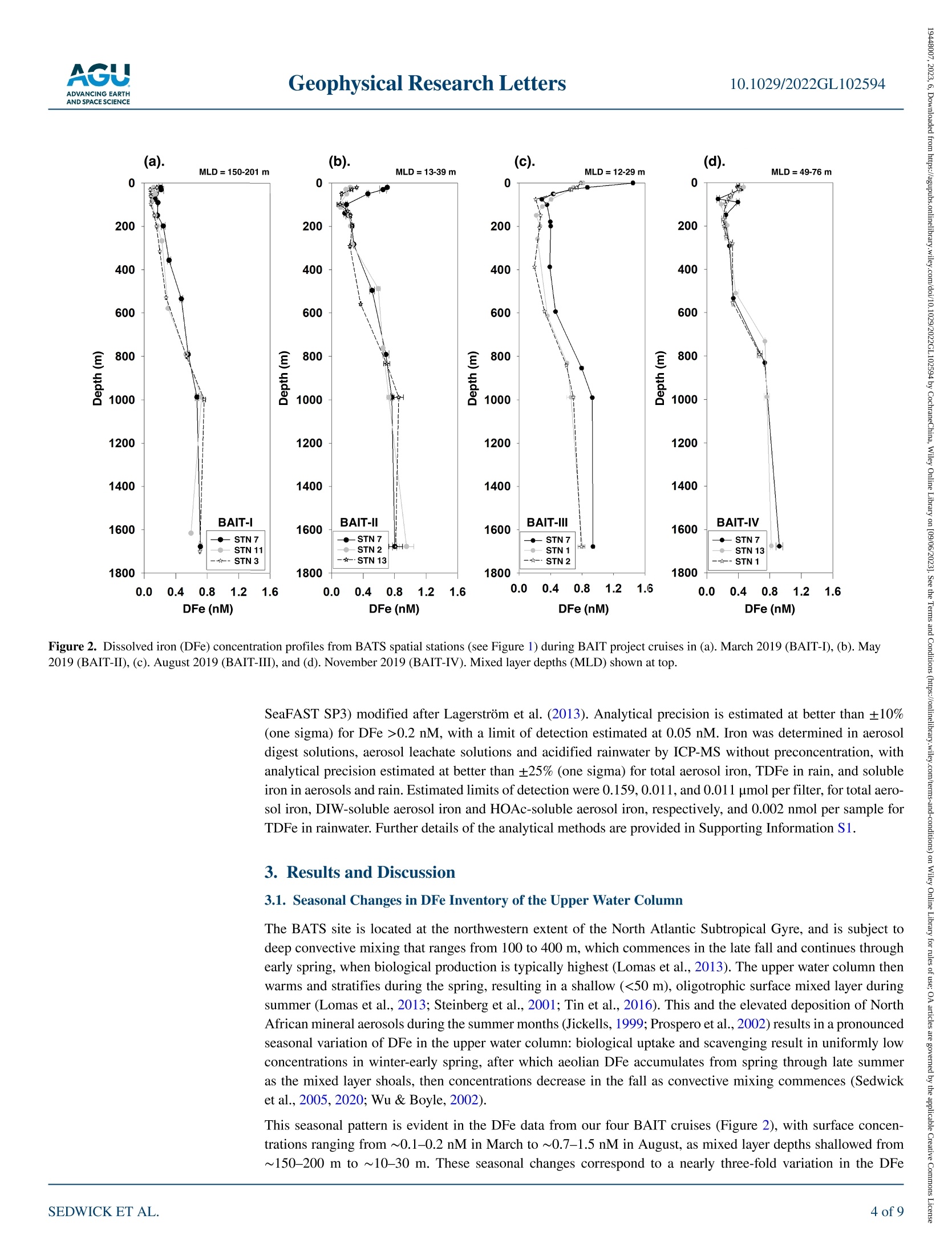
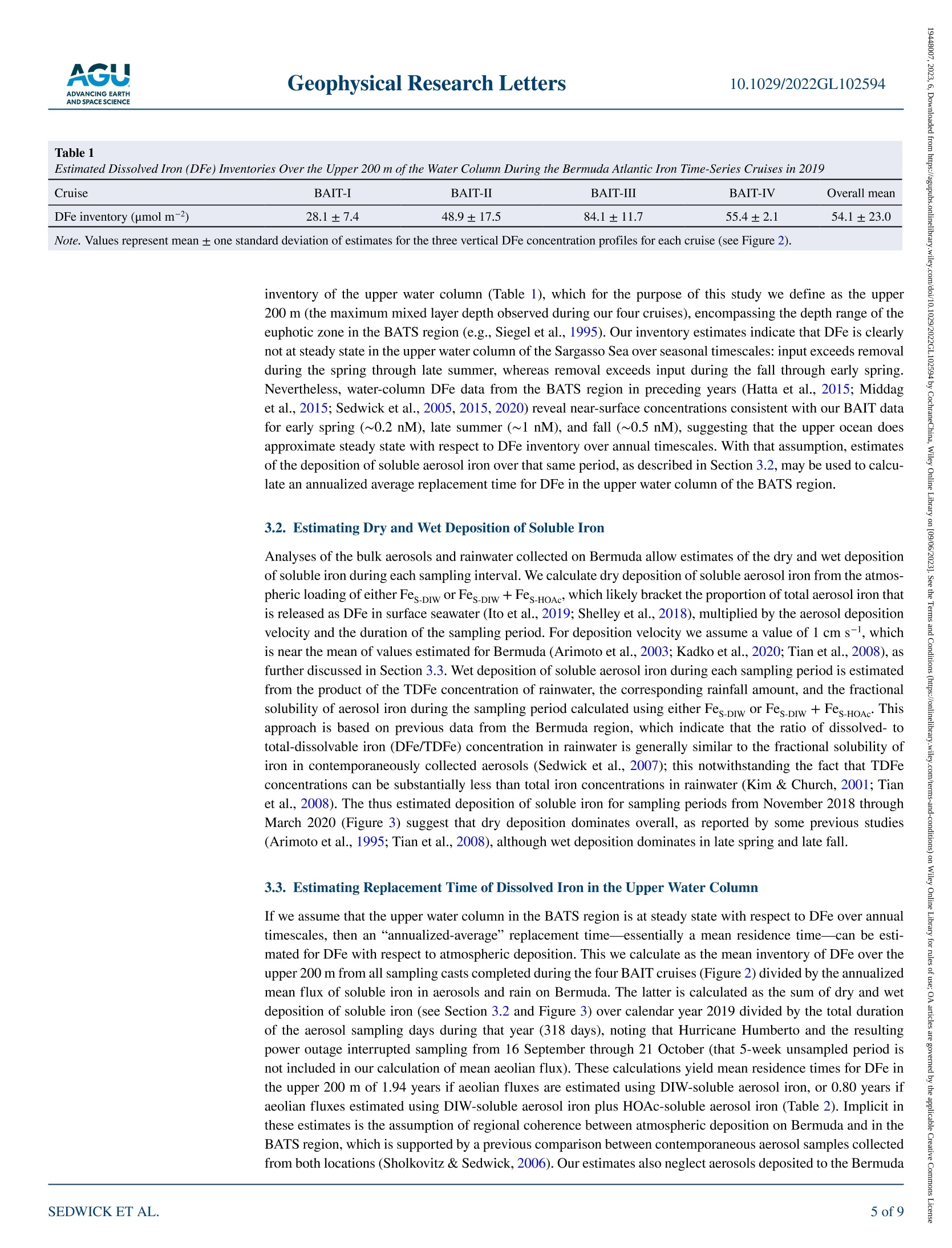
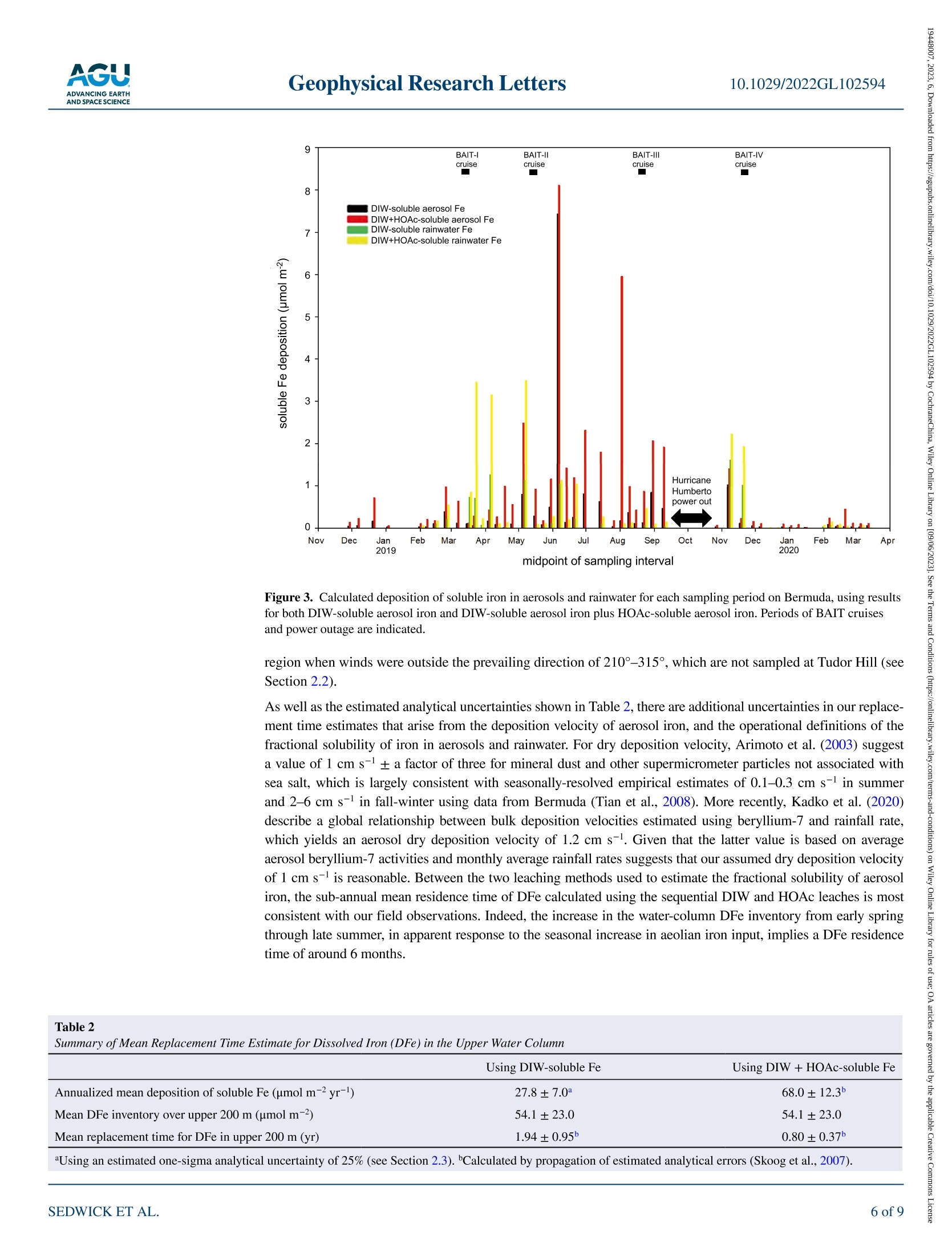
限制尘埃沉积在调节表层海水中必需微量营养素铁浓度方面的作用,需要了解气溶胶中海水可溶性铁的通量和透光带中溶解铁(DFe)的替代时间。在这里,我们使用百慕大-大西洋时间序列研究区域的季节性分解DFe数据以及2019年百慕大气溶胶和降雨中铁的每周尺度测量来估计这些数量。为了应对垂直混合、初级生产和灰尘沉积的季节性变化,地表DFe浓度从早春的~0.2 nM到夏末的>1 nM不等,在上层200 m,DFe含量分别从~30到~80μmol/m2不等。假设上层海洋每年的DFe接近稳定状态,我们的气溶胶和雨水数据要求DFe相对于风成输入的平均透光带停留时间为~0.8–1.9年。 海洋表层水中浮游植物的初级生产是海洋生态系统的基础,在维持二氧化碳的海洋-大气平衡方面发挥着关键作用,而二氧化碳调节着全球气候。铁是浮游植物所需的一种重要微量营养素,溶解铁(DFe)的可用性被认为限制了浮游植物在大面积海洋中的生长。在这种情况下,重要的是限制DFe在表层海水中的来源和持久性,这控制了可用于支持浮游植物生长的DFe的数量。这项研究的重点是北大西洋的百慕大地区,那里空气中的土壤灰尘沉积是地表水DFe的主要来源。通过将百慕大上空土壤尘埃中铁的大气负荷和溶解度的测量值与邻近海域全年的DFe测量值相结合,我们能够估计该地区尘埃沉积产生的DFe的供应率。水柱、气溶胶和雨水样品中的铁测量使用电感耦合等离子体质谱法和在线分离预浓缩法(Elemental Scientific SeaFAST SP3)Lagerström等人(2013)之后进行了修改。当DFe>0.2 nM时,分析精度估计优于±10%(一西格玛),检测极限估计为0.05 nM。在没有预浓缩的情况下,通过ICP-MS测定气溶胶消解液、气溶胶渗滤液和酸化雨水中的铁,气溶胶总铁、雨水中的TDFe以及气溶胶和雨水中的可溶性铁的分析精度估计优于±25%(一西格玛)。总气溶胶铁、DIW可溶性气溶胶铁和HOAc可溶性气溶胶铁的估计检测限分别为0.159、0.011和0.011μmol/过滤器,雨水中TDFe的估计检测极限为0.002nmol/样品。在支持信息S1中提供了分析方法的进一步细节。Check for updates Geophysical Research Letters10.1029/2022GL102594 RESEARCHLETTER 10.1029/2022GL102594 Key Points: ●An imbalance between i nput and removal produces an ~3-fold seasonal increase in the euphotic -zone inventory of dis s olved i ron (DFe) near Bermuda ●Analyses of i ron in seasonal-scale aerosol ,rain and water-column samples allow direct estimates of the replacement time of DFe ●We derive a mean residence time of ~0.8-1.9 year s for DFe in the euphotic zone (<200 m) of the Sargasso Sea near Bermuda Support i ng Information may be found i n the onl i ne version of this article . psedwick@odu.edu Cit a tion: ◎ 2023. T h e A ut ho r s .This i s an op e n a c c e ss a r tic l e un d e r th e t erms o f th e Creative Commons Attr i bution-NonCommercial -NoDeriys Li ce n se, whi ch pe r m i ts u se a nd dist r ib u tio n i n an y med i u m, p r o vid e d t he or i g i na l wor k is pr o p er l y cited , th e u s e is n o n-c o mm e r cial a n d no mo d if i c a t i ons o r a da ptation s are m ad e . Atmospheric Input and Seasonal Inventory of Dissolved Iron in the Sargasso Sea: Implications for Iron Dynamics in Surfac e Waters of the Subtropical Ocean P.N. Sedwick D, B. M. Sohstl iD ,K. N. Buck23, S. Caprara, R. J. Johnson 4D ,D. C. Ohnemus5L. E. Sofen i D ,A. Tagliabue’ ,B. S. Twinin g g 6iD ,and T. E. Williams 1i 面 D 'D e partment of Ocea n and Earth Sciences, O l d D ominio n U niversity, No r fo lk , VA , USA, 2C o l l ege of Ma r ine Science,U n i versity o f South Flo r i da , St . Peters b u rg, FL, U SA, C ollege of Ea r t h , Ocean, and Atmospheric Sciences, Oregon State U nivers it y, Corval l i s, O R , USA , 4B erm u da Insti t ute o f Ocean Science s , St. Geo r ges, B erm u d a , 5Sk i da w ay In st i t u t e of O c ea n ograp h y, Un i ve r sity of Geo r gia, Savannah, GA, USA, B i gelow L aborator y for Ocean Sciences, Eas t B ooth b ay ,ME ,U SA , 7Sc h ool of Environmental Scien c es, Un i versity of Liverpoo l, Li ve r pool, U K Abstract Constraining the role of dus t deposition in regulating the concentration of the essential micronutrient iron in surface ocean waters requires knowledge of the flux of seawater-soluble iron in aerosols and the replacement time of dissolved iron (DFe ) in the euphotic zone. Here we est i mate these quantit i es using seasonally resolved DFe data from the Bermuda Atlantic Time-series Study region and weekly-scale measurements of iron i n aerosol s and rain from Bermuda dur i ng 2019. In response to seasonal changes in vertical mixing, primary production and dust deposition, surface DFe concentrations vary from ~0.2 nM i n early spring to >1 nM in late summer, with DFe inventories ranging from ~30 to ~80 umol/m², respectively,over the upper 200 m. Assuming the upper ocean approximates steady state for DFe on an annual basis, our aerosol and rainwater data require a mean euphotic-zone residence time of ~0.8-1.9 years for DFe with respect to aeolian input. Plain La ng uage Summa ry Primary production by phytoplankton in ocean surface waters is the foundation of the mar i ne ecosystem, and plays a key role in maintaining the ocean-atmosphere balance of carbon dioxide, which regulates global climate. Iron is an essential micronutrient that i s required by phytoplankton, and t he availabil i ty of dissolved iron (DFe) is thought to limit phytoplankton growth over large areas of the ocean. In t his context, i t is important to constrain the sources and persistence of DFe in surface ocean waters, which control the amount of DFe tha t is available to support phytoplankton growth. This study focuses on the Bermuda region of the North Atlantic Ocean, where deposition of airborne soi l dust i s the major source of DFe to surface waters. By combining measurements of t he atmospheric loading and solubility of i ron in soil dust over Bermuda with measurements of DFe in adjacent ocean waters over a full year, we are able to estimate the rate of supply of DFe from dust deposition in this region, as well as the average time that this DFe persist s in the surface ocean. The latter, t ermed the DFe replacement time, i s around 1 year, which agrees well wi t h recent estimates from comparable ocean regions. 1. Introduction Iron is an essential micronutrient for marine phytoplankton, and dissolved iron (DFe) supply regulates primary production and nitrogen fixation over l arge areas of the surface ocean (Boyd & Ellwood, 2010; Kustka et al., 2002; Martin et al., 1991; Moore et al., 2013; Wen et al., 2022). A primary source of DFe to open-ocean surface waters is deposition and partial dissolution of mineral aerosols (dust), and considerable effort has been directed at quantifying the air-to-sea fluxes and seawater solubility of aerosol i ron (Hamilton et al., 2022; Jickells et al., 2005; Mahowald et al ., 2018; Sholkovitz et al ., 2012; Tagliabue et al., 2017). Recent work has also focused on estimating the replacement time of DFe in surface ocean waters with respect to the aeolian input; that i s, the surface-ocean inventory of DFe divided by the atmospheric input of seawater-soluble iron. If calculated over timescales for which a system approaches steady state , and assuming atmospheric i nput is the major DFe source,then this replacement time is equivalent to the residence time of DFe in the surface ocean (Black et al., 2020;Hayes et al ., 2015, 2018; Kadko et al ., 2019). Importantly, the replacement time of DFe modulates the concen tration of this micronutrient in t he euphotic zone, and thus the propensity for i ron availability to regulate primary production in ocean ecosystems. Moreover, empirical estimates of DFe replacement time provide a robust constraint on the veracity of numerical models of the ocean i ron cycle (Tagliabue et al ., 2016). One strategy for deriving such information i s to combine estimates of atmospheric deposition of i ron and othe input fluxes with water-column inventories of DFe and other tracers in specific oceanic locations, which has yielded sur f ace-ocean DFe replacement t imes ranging from months to decades (e.g., Boyle et al., 2005; Croot et al., 2004; Hayes et al., 2015; Jickells, 1999; Kadko et al., 2019; Ussher et al., 2013). However, t hese studies have thus far lacked direct estimates of soluble i ron deposition over periods comparable to the replacement time of DFe, which should provide a stronger constraint on the relationship between aeolian input and water-column inventory. We apply this approach using seasonally resolved measurements of DFe in water column samples col l ected from the Bermuda Atlantic Time-series Study (BATS) region during 2019 along with measurements of iron i n aerosols and rainwater collected on Bermuda over the same time period , to probe the dynamics of DFe in the western subtropical North Atlantic Ocean (Sargasso Sea). Our data reveal substantial seasonal variations in the DFe inventory of the euphotic zone, consistent with previously reported data from this ocean region (Hatta et al., 2015; Middag et al ., 2015; Sedwick et al ., 2005, 2020; Wu & Boyle, 2002), i ndicating that the system is not in steady state over sub-annual t i mescales. However, those data also reveal that the seasonal progression of DFe concentrations in surface waters of the BATS region is relatively consistent, i mplying that the upper-ocean DFe inventory is close to steady state from year to year. If so, then our data can be used to estimate an“annualized-average” replacement time, or mean residence time, for DFe in the upper water column of the BATS region. Our result s agree with severa l recent studies that have estimated a replacement time on the order of 1 year for DFe in surface waters of the subtropical oceans. 2. Methods 2.1. Collection and Processing of Water-Column Samples As part of the Bermuda Atlantic Iron Time-series (BAIT) project, GEOTRACES Process Study GApr13,water-column samples for trace metal measurements were col l ected from the BATS site (31°40'N, 64°10'W)and adjacent BATS spatial stations (Figure 1) during cruises in March (spring), May (early summer), August (late summer) and November (fall) 2019 aboard RV Atlantic Explorer and RV Endeavor. Seawater samples and hydrographic data were collected using a trace-metal clean conductivity-temperature-depth sensor (SBE 19 plus,SeaBird Electronics ) mounted on a custom-built trace-metal clean carousel (SeaBird Electronics) fitted with custom-modified 5-L Teflon-lined external-closure Niskin-X samplers (General Oceanics) and deployed on an Amstee l non-metall i c line (Sedwick et al ., 2022). On the August cruise, we also collected near-surface samples (~1 m depth) in a Niskin-X sampler deployed from an inflatable dinghy ~500 m upwind of the research vessel to avoid contamination from the ship. After recovery, the seawater samples were filtered through pre-cleaned 0.2-um pore AcroPak Supor filter capsules (Pall) using f iltered nitrogen gas inside a shipboard clean laboratory (Sedwick et al., 2020,2022). For DFe analysis, filtrate was collected in acid-cleaned 125 mL low-density poly ethylene (LDPE) bottles (Nalgene) and acidified to pH 1.7 post-cruise by addition of 6 M ultrapure hydrochloric acid (Fisher Optima). 2.2. Collection and Processing of Aerosol and Rain Samples As part of the BAIT project , we also collected composite samples of bulk aerosol and rainwater atop the 23-m height sampling tower at Tudor Hil l , Bermuda (32°15.9'N 64°52.6'W, Figure 1), on an approximately weekly basis from November 2018 through March 2020, bracketing the four project cruises. Aerosols were collected with a high-volume sampler on acid-cleaned Whatman-41 cellulose fi l ters (20 um nominal pore size), which collect particles as small as 1 um with >90% efficiency (e.g., Stafford & Ettinger, 1972). The coastal Tudor Hi l l site faces i nto the prevailing southwesterly winds (Sorooshian et al., 2020), so aerosols were only collected during winds >1 m s-l from 210° to 315° in order to avoid local sources. Sample f il ters were stored i n zip-lock polyethylene bags in a vacuum desiccator. For analysis of “total aerosol i ron”, portions of the aerosol sample fi l ters were digested with a mixture of ultrapure concentrated nit r ic and hydrofluoric acids and hydrogen perox-ide (Fisher Optima) in Teflon vessels (Morton et al., 2013), using a microwave heating system (CEM MARS 6),then evaporated on a hot plate and diluted to volume with 2% (v/v) ultrapure nitric acid. Replicate portions of the aerosol filters were also subjected to a flow-t hrough leaching procedure modified from Buck et al . (2006) using 250 mL of high-pur i ty deionized water (DIW, Barnstead Nanopure, >18.2 MQ-cm resistivity), and the leachate 65°W 62°W Figure 1. Study region showing locations of Bermuda Atlantic Time-series Study (BATS) si t e (station 7) and adjacent BATS spatial stations where water-column samples were collected, and Tudor Hil l on Bermuda where aerosols and rainwater were col l ected (map courtesy of Pau l Lethaby). acidified to 0.4% (v/v) with 6 M ultrapure hydrochloric acid (Fisher Optima) for analysis of"DIW-soluble aero-sol iron"(Fes-DIw); the same filter portions were then subjected to a batch l each procedure modified from Kadko et al. (2019) using 25% acetic acid (HOAc , Fisher Optima ) and 0.02 M hydroxylamine hydrochloride (Sigma)at 90°C , and the supernatant leachate evaporated and then di l uted in 2% ultrapure nitric acid (Fisher Optima)for analysis of "HOAc-soluble aerosol i ron" (Fes.HOA.). Rainwater samples were collected in acid-cleaned 2-L wide-mouth f l uorinated high density polyethylene bottles (Nalgene) using an automatic rain sampler (N-Con Systems ADS 00-120); the rain samples were subsequently ac i dif i ed to 0.4% (v/v) with 6 M ultrapure hydro-chloric acid (Fisher Optima) in these bottles, and then after 2 months the acidified, unfiltered samples were trans-ferred into acid-cleaned 125 mL LDPE bottles (Nalgene) for analysis of “total-dissolvable Fe” (TDFe; Sedwick et al., 2007). Field blanks for the aerosols (an acid-cleaned fi l ter) and rainwater (125 mL ultrapure DIW) were deployed on the Tudor Hil l tower and processed in the same manner as samples, but without operating or opening the aerosol or rain samplers, respectively. 2.3. Iron Measurements in Water-Column, Aerosol and Rain Samples Dissolved iron was determined i n water-column samples using inductively-coupled plasma mass spectrometry (ICP-MS, Thermo Fisher Scientific ElementXR) wi t h in-l ine separation-preconcentration (Elemental Scient i fic Figure 2. Dissolved i ron (DFe) concentration profi l es from BATS spat i al stations (see Figure 1) dur i ng BAIT project cruises i n (a). March 2019 (BAIT-I ), (b). May 2019 (BAIT-II ), (C). August 2019 (BAIT-III ), and (d). November 2019 (BAIT-IV). Mixed l ayer depths (MLD) shown at top. SeaFAST SP3) modi f ied after Lagerstrom et al. (2013). Analytical precision is estimated at better than ±10%(one sigma) for DFe >0.2 nM, with a limit of detection estimated at 0.05 nM. Iron was determined in aerosol digest solutions, aerosol leachate solutions and acidified rainwater by ICP-MS without preconcentration, with analytical precision estimated at better than ±25% (one sigma) for total aerosol i ron, TDFe in rain, and soluble iron in aerosols and rain. Estimated l imit s of detection were 0.159,0.011, and 0.011 umol per f i lter, for total aero-sol iron, DIW-soluble aerosol i ron and HOAc-soluble aerosol i ron, respectively, and 0.002 nmol per sample for TDFe in rainwater. Further details of the analytical methods are provided i n Supporting Information S1. 3. Results and Discussion 3.1. Seasonal Cha ng e s in DFe Inventor y o f the Up pe r Water Column The BATS site is located at the northwestern extent of the North Atlantic Subtropical Gyre, and i s subject to deep convective mixing that ranges from 100 to 400 m, which commences in the late fall and continues through early spring, when biological produc t ion is typically highest (Lomas et al., 2013). The upper water column then warms and stratifies during t he spring, resulting in a shallow (<50 m), oligotrophic surface mixed layer dur i ng summer (Lomas et al., 2013; Steinberg et al ., 2001; Tin et al ., 2016). This and the elevated deposition of North African mineral aerosols during the summer months (Jickells, 1999; Prospero et al ., 2002) results in a pronounced seasonal variation of DFe in the upper water column: biological uptake and scavenging result i n uniformly low concentrations in winter-early spring, after which aeolian DFe accumulates from spring through late summe as the mixed l ayer shoals, then concentrations decrease in the fall as convective mixing commences (Sedwick et al., 2005,2020; Wu & Boyle, 2002). This seasonal pattern is evident in the DFe data from our four BAIT cruises (Figure 2), with surface concen-trations ranging from ~0.1-0.2 nM in March to ~0.7-1.5 nM in August, as mixed layer depths shallowed from ~150-200 m to ~10-30 m. These seasonal changes correspond to a nearly three-fold variation in the DFe Table 1 Estimated Dissolved Iron (DFe) Inventories Over t he Upper 200 m of the Water Column During the Bermuda Atlantic Iron Time-Series Cruises in 2019 Cruise BAIT-I BAIT-II BAIT-III BAIT-IV Overall mean DFe inventory (umol m-2) 28.1±7.4 48.9±17.5 84.1±11.7 55.4±2.1 54.1±23.0 Note. Values represent mean ± one standard deviation of estimates for the three vertical DFe concentration profiles for each cruise (see Figure 2). inventory of the upper water column (Table 1), which for the purpose of this study we define as the upper 200 m (the maximum mixed layer depth observed during our four cruises), encompassing the depth range of the euphotic zone i n the BATS region (e.g., Siegel et al., 1995). Our inventory estimates i ndicate that DFe is clearly not at steady state in the upper water column of the Sargasso Sea over seasonal timescales: input exceeds removal during the spring through late summer, whereas removal exceeds input during the fall through early spring.Nevertheless, water-column DFe data from the BATS region in preceding years (Hatta et al., 2015; Middag et al., 2015; Sedwick et al., 2005, 2015, 2020) reveal near-surface concentrations consistent with our BAIT data for early spring (~0.2 nM), l ate summer (~1 nM), and fall (~0.5 nM), suggesting that the upper ocean does approximate steady state with respect to DFe i nventory over annual timescales. With that assumption, estimates of t he deposition of soluble aerosol iron over that same period, as desc r ibed in Section 3.2, may be used to calcu-late an annualized average replacement time for DFe in the upper water column of the BATS region 3.2. Estimating Dry and Wet Deposition of Soluble Iron Analyses of the bulk aerosols and rainwater collected on Bermuda allow estimates of the dry and wet deposition of soluble i ron during each sampling interval . We calculate dry deposition of soluble aerosol i ron f rom the atmos-pheric loading of either Fes -piw or Fes.DIw +Fes-HOAc, which likely bracket the proportion of total aerosol iron that is released as DFe i n surface seawater (Ito et al., 2019; Shelley et al., 2018), multipl i ed by the aerosol deposition velocity and the duration of the sampling period. For deposition velocity we assume a value of 1 cm s-, which is near the mean of values est i mated for Bermuda (Arimoto et al., 2003; Kadko et al ., 2020; Tian et al., 2008),as further discussed in Section 3.3. Wet deposition of soluble aerosol iron during each sampling period is estimated from the product of the TDFe concentration of rainwater, t he corresponding rainfall amount, and the fractional solubility of aerosol i ron during the sampling period calculated using either Fes-pw or Fes.pIw + Fes .HoAc. This approach is based on previous data from the Bermuda region, which indicate that the ratio of dissolved- to total-dissolvable i ron (DFe/TDFe) concentration in rainwater is generally similar to t he fractional solubility of iron i n contemporaneously collected aerosols (Sedwick et al., 2007); this notwithstanding the fact that TDFe concentrations can be substantially less than total iron concentrations in rainwater (Kim & Church, 2001; Tian et al ., 2008). The thus estimated deposition of soluble i ron for sampling per i ods from November 2018 through March 2020 (Figure 3) suggest that dry deposition dominates overall, as reported by some previous studies (Arimoto et al., 1995; Tian et al., 2008), although wet deposition dominates in late spring and late fal l . 3.3. Estimat ing Replacement Time of Dissolved Iron in the Upper Water Column If we assume that the upper water column i n the BATS region is at steady state with respect to DFe over annual timescales, then an “annualized-average”replacement time-essentially a mean residence t ime-can be esti-mated for DFe with respect to atmospheric deposition. This we calculate as the mean inventory of DFe over the upper 200 m from all sampling casts completed during the four BAIT cruises (Figure 2) divided by the annualized mean flux of soluble i ron i n aerosols and rain on Bermuda. The l atter is calculated as the sum of dry and wet deposition of soluble i ron (see Section 3.2 and Figure 3) over calendar year 2019 divided by the total duration of the aerosol sampling days during that year (318 days), noting that Hurricane Humberto and the resulting power outage interrupted sampling from 16 September through 21 October (that 5-week unsampled period is not included in our calculation of mean aeolian flux ). These calculations yield mean residence t imes for DFe in the upper 200 m of 1.94 years if aeolian f l uxes are est i mated using DIW-soluble aerosol i ron, or 0.80 years i f aeolian fluxes estimated using DIW-soluble aerosol i ron plus HOAc-soluble aerosol i ron (Table 2). Implicit i n these estimates is the assumption of regional coherence between atmospheric deposition on Bermuda and in the BATS region, which i s supported by a previous comparison between contemporaneous aerosol samples collected from both locations (Sholkovitz & Sedwick, 2006). Our estimates also neglect aerosols deposited to the Bermuda Figure 3. Calculated deposition of soluble i ron in aerosols and rainwater for each sampling period on Bermuda, using results for both DIW-soluble aerosol iron and DIW-soluble aerosol iron plus HOAc-soluble aerosol iron. Periods of BAIT cruises and power outage are indicated. region when winds were outside the prevailing direction of 210°-315°, which are not sampled at Tudor Hill (see Section 2.2). As wel l as the estimated analyt i cal uncertainties shown in Table 2, t here are additional uncertainties in our replace-ment t ime estimates that arise from the deposition veloc i ty of aerosol iron, and t he operational definitions of the frac t ional solubility of i ron in aerosols and rainwater. For dry deposition velocity, Arimoto et al. (2003) suggest a value of 1 cm s-± a factor of three for mineral dust and other supermicrometer particles not associated with sea salt, which is largely consistent with seasonally-resolved empirical est i mates of 0.1-0.3 cm s- in summer and 2-6 cm s-in f all -winter using data from Bermuda (Tian et al., 2008). More recently, Kadko et al. (2020)describe a global relationship between bulk deposition velocities estimated using beryllium-7 and rainfall rate,which yields an aerosol dry deposition velocity of 1.2 cm s-l . Given that the l atter value is based on average aerosol beryllium-7 activities and monthly average rainfal l rates suggests t hat our assumed dry deposition velocity of 1 cm s-i s reasonable. Between the two leaching methods used to est i mate the fractional solubi l ity of aeroso]iron, the sub-annual mean residence time of DFe calculated using the sequential DIW and HOAc leaches is most consistent with our field observations. Indeed, the increase in the water-column DFe i nventory from early spring through l ate summer, in apparent response to the seasonal i ncrease in aeolian iron input , implies a DFe residence time of around 6 months. Table 2Summary of Mean Replacement Time Estimate for Dissolved Iron (DFe) in the Upper Water Column Using DIW-soluble Fe Using DIW +HOAc-soluble Fe Annualized mean deposition of soluble Fe (umol m-2 yr-) 27.8±7.0a 68.0±12.35 Mean DFe inventory over upper 200 m (umol m-2) 54.1±23.0 54.1±23.0 Mean replacement time for DFe in upper 200 m (yr) 1.94±0.95 0.80±0.375 Using an estimated one-sigma analytical uncertainty of 25% (see Section 2.3). Calculated by propagation of estimated analytical errors (Skoog et al., 2007). Using an estimated one-sigma analytical uncertainty of 25% (see Section 2.3). Calculated by propagation of estimated analytical errors (Skoog et al ., 2007). Acknowledgments We gratefully acknowledge the assistance of the crews and marine technicians of RV Atlantic Explorer and RV Endeavor,the BATS program team, and Dan Dickinson, Shannon Burns, Mat t Hayden and Andrew Peters. This research was suppor t ed by U.S. National Science Foun-dation awards 1829833 to PNS,1829777to KNB, 1829844 to RJ J , 1829819 to BST and 1829686 to Andrew Peters , and U.K.N a tional Environment Research Council award NE/S013547/1 to AT. It should also be noted that there is considerable variability in near-surface DFe concentrations at the submeso-to mesoscale in the Sargasso Sea during the summer (Sedwick et al., 2020), which adds uncertainty to our DFe inventory estimates, although our sampling at the BATS station (station 7 in Figure 1) as well as adjacent spatial stations during each cruise (e.g., see concentration differences in the upper 100 m in Figures 2b and 2c)likely dampens the impact of such lateral concentration gradients on the mean DFe inventories. However,it is conceivable that mesoscale eddies could provide a net source of DFe to the upper water column of the BATS region over seasonal to annual timescales (Conway et al., 2018; Sedwick et al., 2020), in which case our estimates of DFe residence time based on aeolian i nput only (i.e., ignoring lateral advective inputs) would constitute an overestimate. For example, Conway et al. (2018) argue that cold-core eddies may supply between 3% and 75% of the aeolian i nput of DFe to the upper water column of t he North Atlantic Subtropical Gyre in which case our residence t imes would be overestimated by as much as ~4-10 months, depending on the leaching method used to def i ne seawater-soluble aerosol i ron. Any vertical resupply of DFe from below the euphotic zone would also reduce our residence time estimates, although that flux is expected to be small in the BATS region, given the generally gentle vertical gradients in DFe concentration over the 200-400 m depth range (Figure 2). 4. Conclusions Our estimate of 0.8-1.9 years for t he mean residence t ime of DFe in the upper 200 m of the BATS region is generally consistent with several recent estimates for comparable settings and depth ranges in t he oligotrophic open-ocean. Focusing on aeolian input, Hayes et al. (2015,2018) used thorium-230 and thorium-232 to estimate replacement times of 0.5-1 year for DFe i n t he upper 250 m at the Hawaii Ocean Time-series station ALOHA in the subtropical North Pacific , and <1 year for the upper 200 m along the GEOTRACES GA03 transect, which included t he BATS station. A more recent study of i ron cycling at station ALOHA similarly used water-column measurements of thorium and DFe to calculate a replacement time of ~0.7 years for DFe i n the upper 150 m (Hawco et al., 2022). Finally, Black et al. (2020) have combined a compilation of thorium-234 and sediment trap data to quantify t he removal of DFe via export of biogenic and authigenic particles, from which they infer mean and median residence times of 2 and 0.12 years, respectively, for DFe in the upper 200 m of t he subtropical gyres.These relatively short residence times point to the dynamic nature of DFe in the surface ocean, and t hus the potential for rapid onset of iron l imitation of phytoplankton growth due to seasonal imbalances between the input and removal of DFe from the euphotic zone-even in high-dust regimes such as the subtropical North Atlantic. Data Availability Statement Data presented and discussed here, and associated metadata, are available from the U.S. National Science Foundation's Biological and Chemical Oceanography Data Management Of f ice at : https://www.bco-dmo.org/project/822807. References Arimoto, R., Duce, R. A., Ray, B. J., Ellis, W. G., Jr ., Cullen, J . D., & Merrill, J . T . (1995). Trace elements i n the atmosphere over the North Atlantic . Journal of Geophy s ical Research, 100(D1), 1199-1213. https://doi.org/10.1029/94j d02618 Arimoto,R., Duce, R. A., Ray, B. J ., & Tomza, U. (2003). Dry deposition of trace elements to t he western North Atlantic. Global Biogeochemical Cycles, 17(1). https://doi.or g/10.1029/2001gb 001406 Black, E. E., Kienast, S. S ., Lemaitre, N., Lam, P. J., Anderson, R. F., Planquette, H., et al. (2020). Ironing out Fe residence t i me i n the dynamic upper ocean. Global Biogeochemical Cycles,34(9),e2020GB006592. https://doi.or g/10.1029/2020gb006592 Boyd, P. W., & Ellwood,M.J.(2010). The biogeochemical cycle of iron in t he ocean. Nature Geoscience,3(10),675-682. https://doi .org/10.1038/ngeo964 Boyle, E. A., Bergquist , B. A., Kayser, R. A., & Mahowald, N. (2005). Iron , manganese, and l ead at Hawaii Ocean Time -ser i es station ALOHA:Temporal variabil i ty and an intermediate water hydrothermal plume . Geochimica et Cosmochimica Acta, 69(4), 933-952. https://doi.org/10.1016/j.gca.2004.07.034 Buck, C. S., Landing, W. M., Resing, J. A., & Lebon, G. T . (2006). Aerosol i ron and aluminum solubil i ty in the northwest Pacific Ocean: Results from the 2002 IOC cruise. Geochemistry, Geophysics , Geosystems, 7(4), Q04M07. https://doi.org/10.1029/2005gc000977 Conway , T. M., Palter, J. B., & de Souza,G. F. (2018). Gulf Stream rings as a source of iron to the North At l antic subtropical gyre. Nature Geosc i -ence ,11(8),594-598. https://doi.org/10.1038/s41561-018-0162-0 Croot, P. L., Streu, P., & Baker , A. R. (2004). Short residence time for i ron in surface seawater impacted by atmospheric dry depos i tion from Saharan dust events. Geophysical Research Le t ters , 31(23), L23S08. https://doi .org/10.1029/2004gl020153 Hamil t on, D. S., Per r on, M. M., Bond, T. C., Bowie , A. R., Buchholz, R. R., Guieu, C., et al. (2022). Ear t h, wind, fire, and pollution : Aero-sol nutrient sources and i mpacts on ocean biogeochemistry. Annual Review of Marine Science , 14(1), 303-330. https://doi .org/10.1146/annurev-marine-031921-013612 Hatta, M., Measures, C. I ., Wu, J ., Roshan, S., Fitzsimmons, J. N ., Sedwick, P., & Morton, P. (2015). An overview of dissolved Fe and Mn di s tri-butions dur i ng the 2010-2011 US GEOTRACES North Atlantic cruises: GEOTRACES GA03. Deep Sea Research Part II: Topical Studies in Oceanography,116, 117-129. https://doi .org/10.1016/j.dsr2.2014.07.005 Hawco,N. J., Yang, S. C., Pinedo-Gonzalez, P., Black, E . E., Kenyon,J., Ferron, S., et al . (2022). Recycling of dissolved i ron in the North Pacific subtropical gyre . Limnology & Oceanography,67(11), 2448-2465. https://doi .org/10.1002/lno.12212 Hayes, C. T., Anderson,R. F., Cheng, H., Conway,T.M.,Edwards,R. L., Fleisher, M. Q.,et al . (2018). Replacement t i mes o f a s pec t rum of elements in the North Atlantic based on thorium supply. Global Biogeochemical Cycles,32(9), 1294-1311. https://doi .org/10.1029/2017gb005839 Hayes, C. T ., Fitzsimmons, J . N., Boyle, E. A., McGee, D., Anderson, R. F., Weisend, R., & Morton, P. L. (2015). Thor i um isotopes tracing the iron cycle at the Hawaii Ocean Time-series Station ALOHA. Geochimica et Cosmochimica Acta , 169, 1-16. https://doi.org/10.1016/j.gca.2015.07.019 I t o, A., My r iokefalitakis, S., Kanakidou, M., Mahowald, N. M., Scanza, R. A., Hamilton, D. S., et al. (2019). Pyrogenic i ron: T he missing link to high iron solubi l ity in aerosols. Science Advances,5(5),eaau7671.https://doi .org/10.1126/sciadv .aau7671 J i ckells, T. D. (1999). The inputs of dus t derived elements to t he Sargasso Sea: A synthesis. Marine Chemistry , 68(1-2), 5-14. https://doi.org/10.1016/s0304-4203(99)00061-4 Jickells, T. D., An, Z. S ., Andersen, K. K., Baker, A. R ., Bergamett i , G ., Brooks, N., et al. (2005). Global iron connections between desert dust ,ocean biogeochemistry, and cl i mate. Sc i ence , 308(5718),67-71. ht t ps://doi .org /10.1126/science.1105959 Kadko, D., Aguilar-Isl a s, A., Bolt, C., Buck , C. S., Fitzsimmons, J . N., Jensen, L. T., et al. (2019). The residence t imes of t race elements determined in the surface Arc t ic Ocean dur i ng the 2015 US Arctic GEOTRACES expedition. Marine Chemistry, 208, 56-69. https://doi.org/10.1016/j .marchem.2018.10.011 Kadko, D., Landing, W. M., & Buck, C. S. (2020). Quantifying atmospheric trace element depos i tion over the ocean on a globa l scale wi t h satel -lite rainfall products. Geophysical Research Letters , 47(7), e2019GL086357. https://doi .org/10.1029/2019gl086357 Kim, G., & Church, T. M. (2001). Sea s onal biogeochemical f luxes of 234Th and 210Po i n t he Upper Sargasso Sea: I nf l uence from atmospheric i ron deposition. Global Biogeochemical Cycles, 15(3), 651-661. https://doi.org/10.1029/2000gb001313 Kustka, A., C a rpenter, E. J ., & Safiudo-Wilhelmy , S. A. (2002). Iron and marine nitrogen fixation: Progress and future directions. Research in Microbiology,153(5), 255-262. https://doi.org/10.1016/s0923-2508(02)01325-6 Lagerstrom, M. E., Field, M. P., Seguret, M., Fischer, L., Hann, S ., & Sherrell , R. M. (2013). Automated on-line flow-injection ICP-MS deter-mination of trace me t als (Mn, Fe, Co, Ni, Cu and Zn) i n open ocean seawater : Application to the GEOTRACES program. Marine Chemistry ,155, 71-80. https ://doi.org/10.1016/j.marchem.2013.06.001 Lomas, M. W., Bates, N. R ., J ohnson, R. J ., Knap, A. H., Steinberg, D. K., & Carlson, C . A. (2013). Two decades and counting: 24-years of sustained open ocean biogeochemica l measurement s i n t he Sargasso Sea . Deep Sea Research Part II : Topical Studies in Oceanography , 93,16-32. https://doi.org/10.1016/j.dsr2.2013.01.008 Mahowald, N. M., Hamilton, D. S., Mackey , K. R., Moore, J . K., Baker, A. R., Scanza, R. A., & Zhang, Y. (2018). Aerosol trace metal leaching and i mpacts on mar i ne microorganisms. Nature Communications,9(1),2614. https://doi .org/10.1038/s41467-018-04970-7 Mar t in, J . H., Gordon, M., & Fitzwater,S. E. (1991). The case for i ron. Limnology & Oceanography , 36(8), 1793-1802. https://doi.org/10.4319/lo.1991.36.8.1793 Middag,R., Seferian, R., Conway, T. M., John, S. G., Bruland, K. W., & de Baar, H. J . (2015). Intercomparison of dissolved trace elements at the Bermuda Atlantic Time Series station. Marine Chemistry , 177, 476-489. https://doi.org/10.1016/j.marchem.2015.06.014 Moore, C. M., Mills, M. M., Arrigo, K. R ., Berman-Frank, I., Bopp, L ., Boyd, P. W.,et al . (2013). Processes and patterns of oceanic nutrient limitation . Nature Geosc i ence ,6(9),701-710. https://doi .org/10.1038/ngeo1765 Morton, P. L., Landing, W. M ., Hsu, S. C., Mi l ne, A., Aguilar-Islas, A. M., Baker , A. R.,et al. (2013). Methods for the sampling and analysis of marine aerosols: Results from the 2008 GEOTRACES aerosol intercalibration exper i ment. Limnology and Oceanography: Methods,11(2),62-78.https://doi.org/10.4319/lom.2013.11.62 Prospero, J. M., Ginoux, P., Torres, O., Nicholson, S. E., & Gill, T. E. (2002). Env i ronmental charac t erization of global sources of atmospher i c soi l dust identified with the Nimbus 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product. Reviews of Geophys i cs , 40(1),2-1.https://doi.org/10.1029/2000rg000095 Sedwick, P.N., Bowie, A. R., Church, T. M., Cullen,J . T., Johnson, R. J., Lohan , M. C., et al. (2020). Dissolved iron i n the Bermuda r egion of the subtropical North Atlant i c Ocean: Seasonal dynamics, mesoscale vari a bility, and physicochemical s peciation. Marine Chemistry , 219,103748.ht t ps://doi .o rg /10.1016/j.marchem.2019.103748 Sedwick, P. N., Church, T . M., Bowie, A. R., Marsay , C. M., Ussher , S.J., Achi l l e s, K. M.,et al.(2005). Iron in the Sargasso Sea (Bermuda At l an tic Time-ser i es Study region) during summer : Eolian i mprint, spatiotemporal variability , and ecological implications. Global Biogeochemical Cycles, 19(4), GB4006. https://doi.org/10.1029/2004gb002445 Sedwick, P. N., Sholkovitz, E . R., & Church, T . M.(2007). Impact of anthropogenic combustion emissions on the fractional solubility of aerosol iron: Evidence from the Sargasso Sea. Geochemistry , Geophysics , Geosystems , 8(10),Q10Q06.https://doi .org/10.1029/2007gc001586 Sedwick , P. N., Sohst , B. M.,O'Hara, C ., Stammerjohn, S. E., Loose, B., Dinniman, M. S ., et al . (2022). Seasona l dynamics of dissolved i ron on the Antarct i c cont i nental shelf : Late-f all observations from the Terra Nova Bay and Ross Ice Shelf Polynyas. Journal of Geophy s ical Research:Oceans,127(10), e2022JC018999. https://doi .org/10.1029/2022jc018999 Sedwick, P . N., Sohst , B. M., Ussher, S . J., & Bowie, A. R. (2015). A zonal picture of the water column distribution of dissolved iron (I I) during the US GEOTRACES North At l antic transect cruise (GEOTRACES GA03). Deep Sea Research Par t II: Topical Studies i n Oceanography ,116,166-175. https://doi.org/10.1016/j .dsr2.2014.11.004 Shel l ey, R. U ., Landing, W. M., Ussher, S. J ., Planquette, H., & Sar t hou, G. (2018). Regional trends in the fractional solubility of Fe and other metal s f rom North Atlantic aerosols (GEOTRACES cruises GA01 and GA03) following a two-stage leach. Biogeosciences ,15(8),2271-2288. ht t ps://doi.org/10.5194/bg -15-2271-2018 Sholkovitz, E. R., & Sedwick, P. N. (2006). Open-ocean deployment of a buoy-mounted aerosol sampler on the Bermuda Testbed Mooring:Aerosol i ron and sea salt over the Sargasso Sea. Deep Sea Research Par t I: Oceanographic Research Papers, 53(3), 547-560. https://doi .org/10.1016/j.dsr.2005.12.002 Sholkovitz, E. R., Sedwick, P. N., Church, T . M., Baker , A. R., & Powell, C. F. (2012). Fractional solubility of aerosol iron: Synthesis of a global-scale data set . Geochimica et Cosmochimica Acta, 89,173-189. Siegel, D. A., Michaels, A. F ., Sorensen, J . C .,O'Brien, M. C., & Hammer , M. A. (1995). Seasonal variability of light availability and utilization in the Sargasso Sea . Journal of Geophysical Research, 100(C5), 8695-8713. https://doi .org/10.1029/95jc00447 Skoog, D. A., Hol l er , F. J., & Crouch, S. R. (2007). Princ i ples of instrumental analysis (6th ed.). Thomson Brooks/Cole. Sorooshian, A., Corral , A. F., Braun, R. A., Cai r ns, B., Crosbie, E., Ferrare, R., et al . (2020). Atmospheric research over the western North Atlan-tic Ocean region and North American East Coast : A review of past work and challenges ahead. Journal of Geophysical Research: Atmospheres,125(6),e2019JD031626.https://doi.org/10.1029/2019jd031626 Stafford, R. G., & Et t inger, H. J . (1972). Fi l ter ef fi ciency as a f unction of particle size and veloc i ty. Atmospheric Environment, 6(5), 353-362.ht t ps://doi.org/10.1016/0004-6981(72)90201-6 Steinberg, D. K., Carlson, C. A., Bates, N. R., J ohnson, R. J ., Michaels, A. F., & Knap, A. H. (2001). Overview of the US JGOFS Bermuda Atlantic t ime-series study (BATS): A decade-scale look at ocean biology and biogeochemistry. Deep Sea Research Part II: Topical Studies in Oceanography,48(8-9), 1405-1447. https://doi.or g/10.1016/s0967-0645(00)00148-x Tagliabue, A., Aumont, O., DeAth, R., Dunne, J. P., Dutkiewicz, S., Galbraith, E., et al. (2016). How well do global ocean biogeochemistry models simulate dissolved iron dist ri butions ? Global Biogeochemical Cycles, 30(2), 149-174. https://doi .org/10.1002/2015gb005289 Tagliabue, A., Bowie, A. R., Boyd,P. W., Buck, K. N., Johnson, K. S., & Saito, M. A. (2017). The integral role of i ron in ocean biogeochemistry.Nature, 543(7643), 51-59. https ://doi .org/10.1038/nature21058 Tian, Z., Ol l ivier, P., Veron, A., & Church, T. M. (2008). Atmospheric Fe deposi t ion modes at Bermuda and the adjacent Sargasso Sea. Geochem-istry , Geophysics, Geosystems, 9(8), Q08007. https://doi.org/10.1029/2007gc001868 T i n, H. C., Lomas, M. W., & Is hizaka,J .(2016). Satel l i t e-derived est i mates of primary production during the Sargasso Sea winter/spring bloom:Integration of i n-situ time-series data and ocean color remote sensing observations. Regional Studies in Marine Sc i ence, 3, 131-143. ht t ps://d o i .org/10.1016/j.rsma.2015.07.002 Ussher , S . J ., Achterberg , E. P., Powell, C ., Baker, A. R., Jickell s , T. D., Torres, R., & Worsfold, P. J . (2013). Impact of atmospheric deposition on the contrasting i ron biogeochemistry of the North and South Atlantic Ocean. Global Biogeochemical Cycles , 27(4), 1096-1107. https ://doi .org/10.1002/gbc.20056 Wen, Z ., Browning, T . J., Cai , Y ., Dai, R., Zhang, R., Du, C ., et al. (2022). Nutrient regulation of biological nitrogen fixation across the tropical western North Pacific . Science Advances,8(5), eabl7564. https://doi .org/10.1126/sciadv.abl7564 Wu , J., & Boyl e , E . (2002). Iron in the Sargasso Sea : Implications for the processes control l ing dissolved Fe distr i bution i n the ocean . Global Biogeochemical Cycles,16(4), 33-1. https://doi.org /10.1029/2001gb001453
确定





还剩7页未读,是否继续阅读?
上海凯来仪器有限公司为您提供《马尾藻海中溶解铁的大气输入和季节性库存:对亚热带海洋表层水铁动力学的影响》,该方案主要用于海水中(类)金属及其化合物检测,参考标准--,《马尾藻海中溶解铁的大气输入和季节性库存:对亚热带海洋表层水铁动力学的影响》用到的仪器有seaFAST 全自动海水预浓缩系统
推荐专场
该厂商其他方案
更多
















