方案详情
文
本应用说明报告了对一个核酸样品的熔化温度和热力学参数的评价。使用V-700系列紫外可见分光光度计和PAC-743自动6/8位样品池更换器测量260 nm处的吸光度,该计可以同时测量8个样品。
关键词:Oligonucleotide therapeutic, oligonucleotide, nucleic acid, thermodynamic parameter, enthalpy, entropy, PCR, Southern blot, intracellular environment, molecular crowding
方案详情

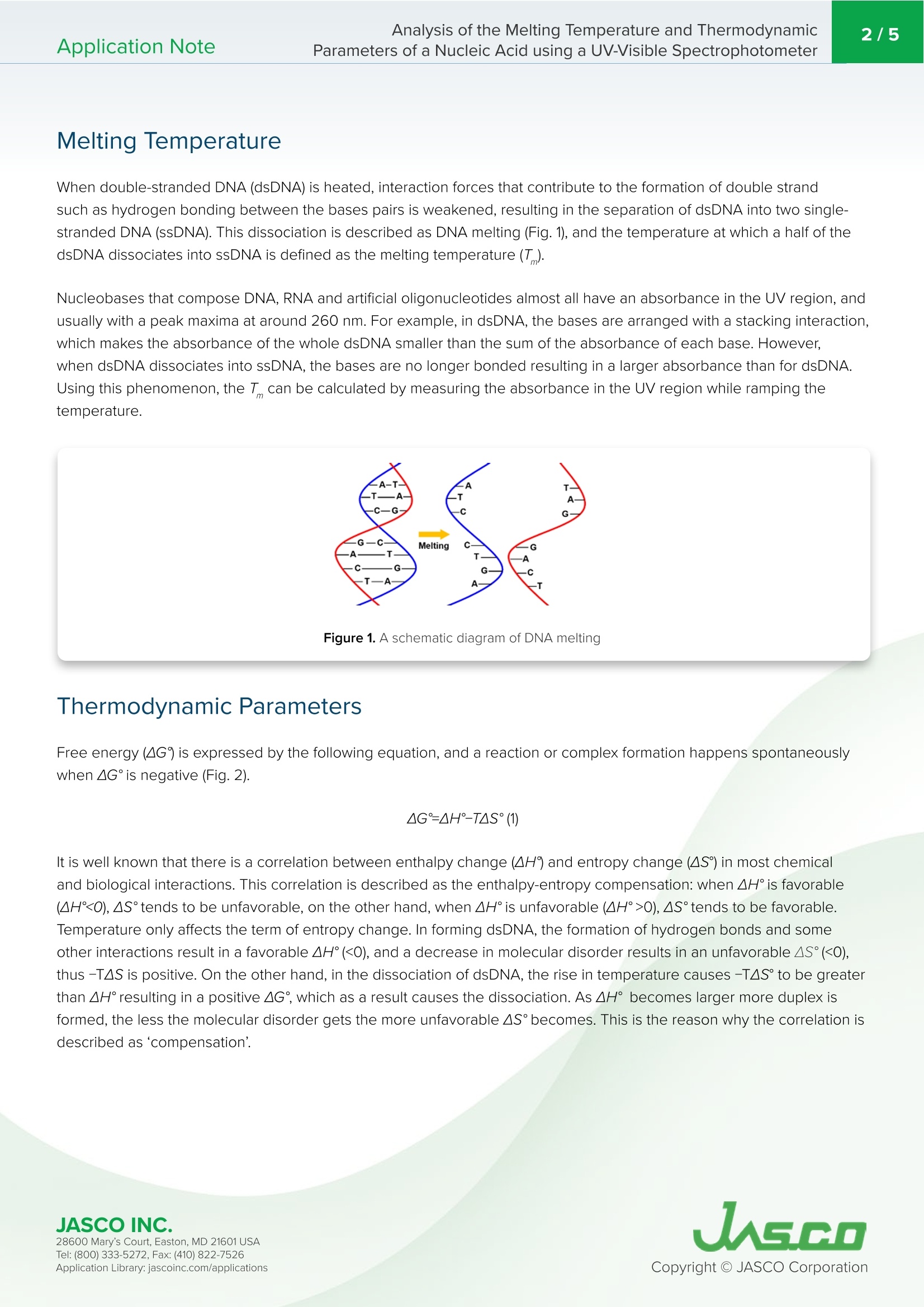
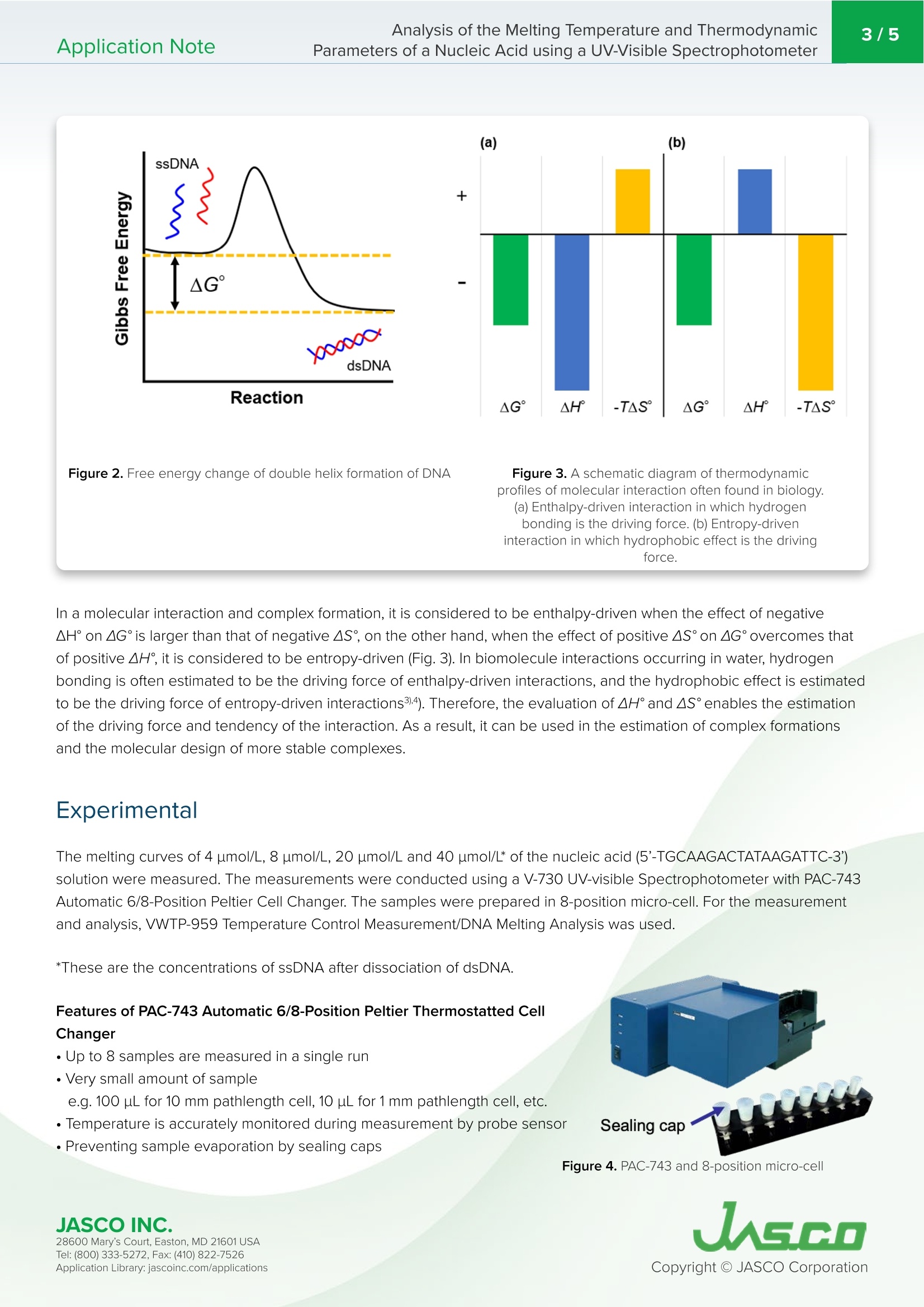
近年来,衍生自核酸如DNA或RNA的基于 oligonucleotide的治疗方法的使用显著增加。由于两个重要因素,它们被认为是下一代药物:首先,它们不仅作用于传统小分子或抗体基础药物靶向的蛋白质,还作用于mRNA和miRNA等功能性RNA;其次,它们可以通过化学合成制造,这意味着它们可以很容易地大规模生产。 oligonucleotide疗法主要基于人工寡核苷酸,人工 oligonucleotide是化学修饰的DNA或RNA,可以提高其在人体内的结构稳定性和对靶分子的亲和力。评估它们和靶分子的相互作用特性并用作药物,优化它们的碱基数量和序列是至关重要的。因此,检查和评估方法对优化这些性能的重要性已经得到了很好的定义。热力学性质的重要性已经得到强调,因此需要一种有效的方法来分析这些特性。本应用说明显示,PAC-743可同时测量多个样本,同时准确监测温度。此外,可以通过使用DNA熔融分析程序计算Tm、∆H°和∆S°来评估核酸的热稳定性和复合物的稳定性。不用说,Tm的评估在PCR(聚合酶链式反应)和Southern印迹的使用中也很重要,这是复制或检测DNA的常用方法。此外,Tm和热参数的评估是估计分子拥挤环境下复合物形成的关键,分子拥挤环境是细胞内环境的伪环境,也一直受到关注。Application Note Analysis of the Melting Temperature and Thermodynamic2/5Application NoteParameters of a Nucleic Acid using a UV-Visible Spectrophotometer Analysis of the Melting Temperatur e and Thermodynamic Parameters of a Nucleic Acid using a UV-Visible Spectrophotometer Introduction In recent years the use of oligonucleotide based therapeutics derived from nucleic acids such as DNA or RNA, has increased significantly. They are considered to be next-generation drugs due to two important factors: firstly, they act not only on proteins that have traditionally been targeted by conventional small-molecule or antibod y base drugs, but also on functional RNA such as mRNA and miRNA, and secondly they can be manufactured by chemical synthesis, which means that they can be easily mass produced. Oligonucleotide therapeutics are mainly based on artificial oligonucleotides that are chemically modified DNA or RNA, which improves their structural stability in the human body and affinity to their target molecules ). The evaluation of their i nteraction characteristics with target molecules and use as drugs, optimization of their number of the bases and sequence are critical. Thus, the i mportance of the examination and evaluation methods fo r the optimization of these properties has been well defined. The i mportance of the thermodynamic properties has been highlighted, and therefore an efficient method to analyze t hese characteristics is required. This application note reports the evaluation of the melting temperature and t hermodynamic parameters of a nucleic acid sample. T he absorbance at 260 nm was measured for different sample concentrations while ramping the temperature)using a V-700 Series UV-visible spectrophotometer with PAC-743 Automatic 6/8-position Peltier cell changer, which can measure up to eight samples simultaneously. Keyword s Oligonucleotide therapeutic, ol i gonucleotide, nucleic acid, thermodynamic parameter, enthalpy, entropy, PCR, Southern blot, i ntracellular env i ronment, molecular crowding Mel t ing Temperature When double-stranded DNA (dsDNA) i s heated, interaction forces that contribute to the format i on of double strand such as hydrogen bonding between the bases pairs is weakened, resulting i n the separation of dsDNA into two single-stranded DNA (ssDNA). This dissociation is described as DNA melting (Fig.1), and the temperature at which a half of the dsDNA dissociates into ssDNA is defined as the melting temperature (T). Nucleobases that compose DNA, RNA and ar t ificial oligonucleotides almost all have an absorbance i n t he UV region, and usually with a peak maxima at around 260 nm. For example, i n dsDNA, the bases are arranged with a stacking interact i on,which makes the absorbance of the whole dsDNA smalle r than the sum of the absorbance of each base. However.when dsDNA dissociates into ssDNA, the bases are no longer bonded resulting in a larger absorbance than for dsDNA.Using this phenomenon, the T can be calculated by measuring the absorbance i n the UV region while ramping the temperature. Figure 1. A sch em a t ic diagra m o f DNA m el t i n g Thermodynamic Parameter s Free energy (AG) i s expressed by the following equation, and a reaction or complex formation happens spontaneously when AG° is negative (Fig. 2). It is wel l known that t here is a correlat i on between enthalpy change (AH) and entropy change (AS) in most chemical and biological interactions. This correlation is described as the enthalpy-entropy compensation: when AH° i s favorable (AH<0),AS°tends to be unfavorable, on the other hand, when AH° i s unfavorable (AH°>0), AS°tends to be favorable.Temperature only affects the term of entropy change. In forming dsDNA, the formation of hydrogen bonds and some other i nteractions result in a favorable AH°(<0), and a decrease in molecu l ar disorder results in an unfavorable AS°(<0),thus -TAS i s posi t ive. On the other hand, i n the dissociation of dsDNA, the rise in temperature causes -TAS° to be greatel than AH° result i ng in a positive AG°, which as a resu l t causes the dissociation. As AH° becomes l arger more duplex i s formed , the less the molecular disorder gets the more unfavorable AS°becomes. This is the reason why the correlation is described as ‘compensat i on. JASCO IN C. JAGGO 28600 Mary's Cour t , E asto n, MD 21601US A T el : (800) 333-5272, Fa x : (410) 822-7526A ppli c a tio n Lib r ary: jasc o i n c.com /appli c ati o ns Figure 2. Fr e e en er gy ch a nge o f do ub le h e l ix for ma tion of DNA Figure 3. A sch ema tic diagra m of t hermod y na m ic pro fi les o f molecu l ar i n tera cti on o ften f ound in biolog y .(a) E nth al py -dr i v en int e ra cti on i n whi c h hy drogen b on ding is t h e dr i vi n g forc e. (b) En t ropy-d r iven i n tera ctio n i n whi c h hy dr oph ob ic e f fect i s t h e d r i v ing force. In a molecular interaction and complex formation, it is considered to be enthalpy-driven when the effect of negative AH° on AG°is larger than that of negative AS°, on t he other hand, when the effect of positive AS°on AG°overcomes that of positive AH°, it i s considered to be entropy-driven (Fig. 3). I n biomolecule interac t ions occurring in water, hydroger bonding is often estimated to be t he driving force of enthalpy-dr i ven interactions, and the hydrophobic effect is estimated to be the driving force of entropy-driven interactions3)4). Therefore, the evaluat i on of AH°and AS°enables the estimation of the driving force and tendency of the i nteraction. As a result, i t can be used in the estimation of complex formations and the molecular design of more stable complexes. Experimental The melting curves of 4 umol/L, 8 umol/L, 20 umol/L and 40 umol/L* of the nucleic acid (5'-TGCAAGACTATAAGATTC-3)solutio n were measured. The measurements were conducted using a V-730 UV-visible Spectrophotometer with PAC-743Automatic 6/8-Position Peltier Cell Changer. The samples were prepared in 8-position micro-cell . For the measurement and analysis, VWTP-959 Temperature Control Measurement/DNA Melting Analysis was used. *These are the concentrations of ssDNA after dissoc i a t ion of dsDNA. Features of PAC-743 Automatic 6/8-Position Peltier Thermostatted Cell Changer · Up to 8 samples are measured in a single run ·Very small amou n t of sample e.g . 100 pL for 10 mm pat h length cel l , 10 pL for 1 mm pathlength cell, etc. · T emperature is accurate l y monitored during measurement by probe sensor ·Prevent in g sample evaporat i on by sealing caps Figure 4. PAC -743 and 8-pos i t ion mic ro -cell JASCO IN C. 28600 Mary's Cour t , E asto n, MD 21601US A JAGE O A ppli c a tio n Lib r ary: jasc o i n c.com /appli c ati o ns Features of DNA Melting Analysis Program ·Calculation of T,AH,AS°, and GC-content ·High-accurate calculation of thermodynamic parameters by 1/Tm vs.In(C,/4) plot** **C. stands for the molar co n centration of ssDNA · Analysis of both dsDNA composed of two sequence-identical strands and the one composed of two seque n ce-different strands ·Analysis of multiple data conducted at once Melting curves of the nucleic acid were measured under the cond i tions shown below. The T of the sample was calculated from these curves,and AH°and AS°were calculated by 1/T vs. In(C,/4) plot. Figure 5. DNA M el t i ng Ana l y sis p ro gr am 0.96 sec Wavelength 260nm Response Pathlength 10mm Sample Volume 100 uL Results The melting curves for the different concentrat i ons of nucleic acid are shown i n Figure 6. The Tm of each solution is shown in Table 1, and 1/T vs. In(C,/4) plot is shown in F i gure 7.AH° and AS° are calculated f rom t he approximate l i ne of the plot; -578.0 kJ/mol and -1.635 kJ/(mol.K), respectively. Table 1. C o n c en tra t ion and mel t i n g t em p er atur e o f nucl ei c a c i d so l u ti o n s Figure 6. M el t i n g c ur v es Figure 7.1/T vs.In(C, /4) plot Conclusion The results show that the enthalpy change greatly overcomes the entropy change in dsDNA formation of the nucleic acid solution. This indicates that dsDNA format i on of the sample is enthalpy -dr i ven, which suggests that the dr i ving force of the formation is mainly hydroge n bonding and stacking interactio n . This observatio n agrees with the mecha n ism of dsDNA formation d i scovered by Watson and C r ick. This application note shows that the PAC-743 provides simultaneous measurements of multiple samples, while accurately monitoring the temperature. Also, evaluation of thermal stability of nucleic acid and stability of complex can be done by calculating T,AH°and AS ° using the DNA Melting Analysis program. Needless to say, the evaluation of Tm is also important in the use of PCR (polymerase chain reaction) and Southern blot, which are well-used methods to duplicate or detect DNAs. Moreover, the evaluation of T. and thermal parameters is key for the estimation of complex f ormat i on under a molecular -crowding environment , a pseudo environment o f intracel l ular environment tha t has also been gathering attention. We bel i eve that the combination of PAC-743 and DNA Melting Analysis program i s suitable for the ef f icient acquisition of useful i nformation for the development of ol i gonucleotide therapeutics and for research methods based on the nature of DNA and double-helix formation. References 1.) S. Obika, Y. Kasahara, Folia Pharmacal. Jpn.,2016, 148,100-104 2.)N. Sugimoto, Gene and Biotechnology, Tokyo, Maruzen Publishi n g Co., Ltd., 1999 3.) Y. Kitaou, T . Ohnuma, Kagaku to Seibutsu, 2015,53(12),834-842 4.) K. Tsumoto,M. Ui , Yakugaku Zasshi, 2009, 129(11), 1311-1317 This application was developed under the guidance of Prof. Takehiko Wada of Institute of Multidisciplinary Research for Advanced Materials, Tohoku Univers i ty. JASCO IN C. 28600 Mary's Cour t , E asto n, MD 21601US A T el : (800) 333-5272, Fa x : (410) 822-7526 A ppli c a tio n Lib r ary: jasc o i n c.com /appli c ati o ns
确定



还剩3页未读,是否继续阅读?
佳士科商贸有限公司为您提供《用紫外可见分光光度计分析核酸的熔化温度和热力学参数》,该方案主要用于天然高分子材料中热力学参数、融化温度检测,参考标准--,《用紫外可见分光光度计分析核酸的熔化温度和热力学参数》用到的仪器有jasco紫外可见分光光度计 V-700
推荐专场
相关方案
更多
该厂商其他方案
更多