
方案详情
文
The combination of integrated ion mobility with warp-speed MS/MS acquisition on the timsTOF Pro paves the way for deep, molecular level understanding of β-cell physiology and potential new targetable pathways for diabetes.
方案详情

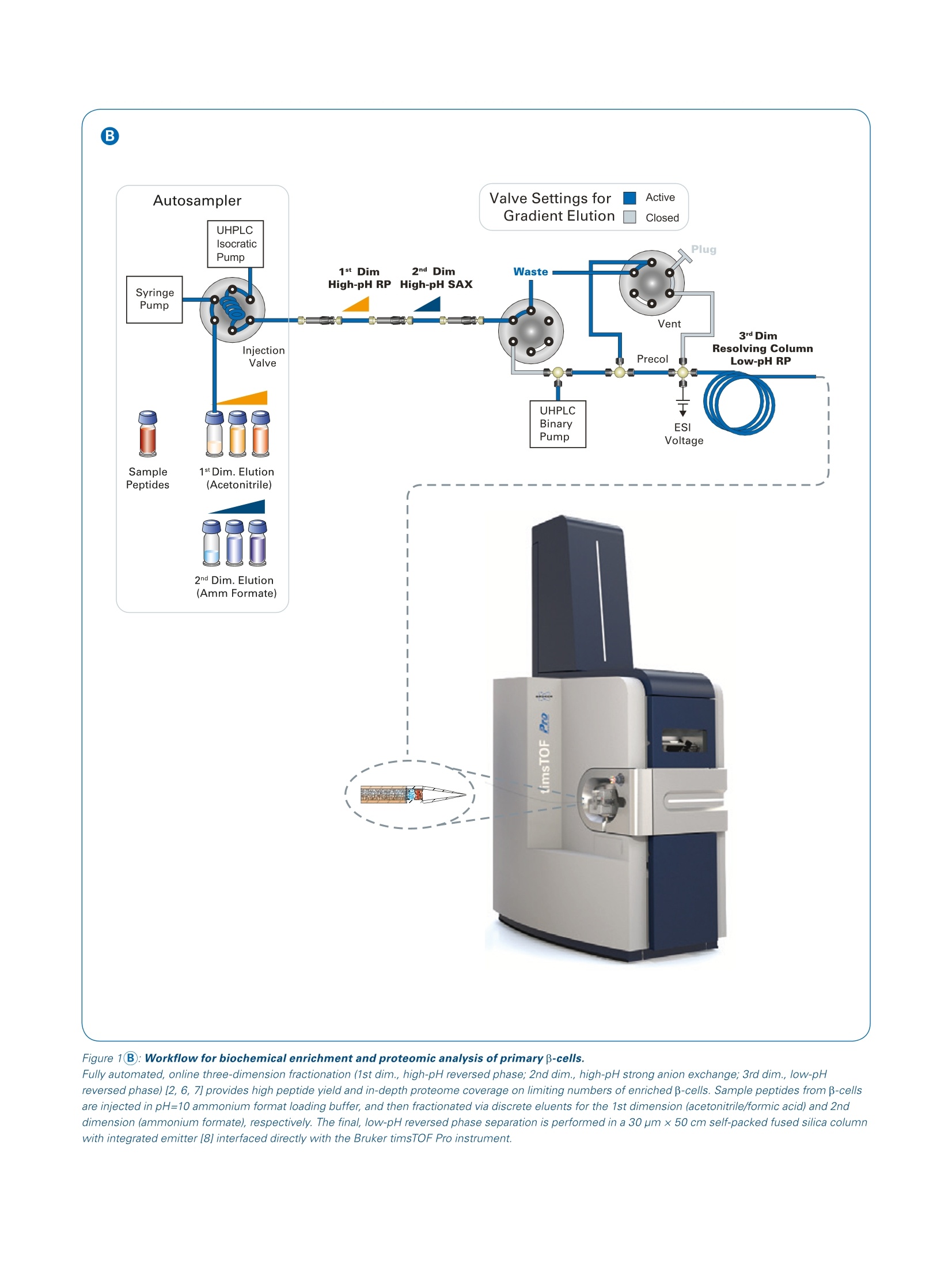
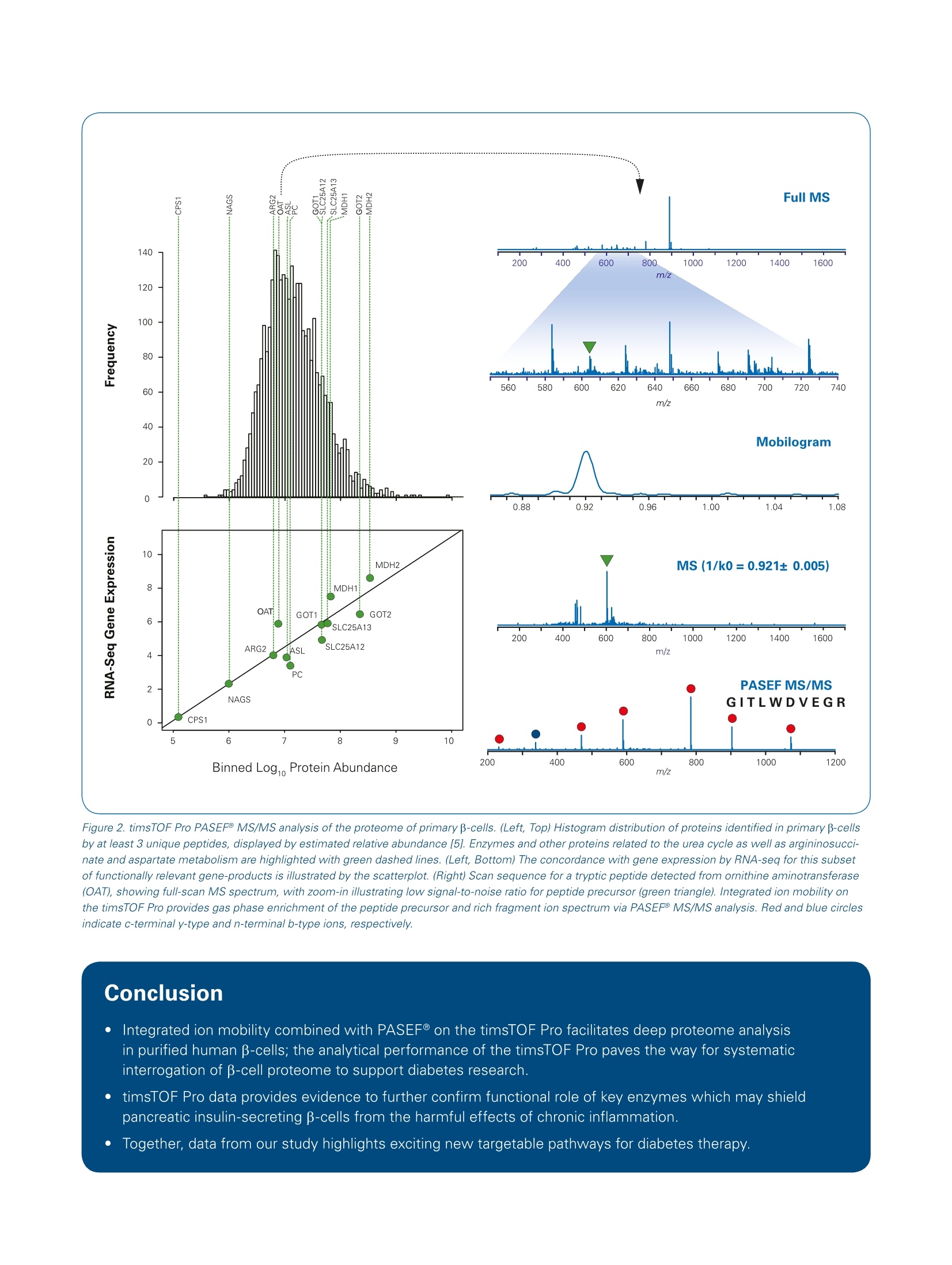
Authors: Scott B. Ficarro, Accalia Fu, Nika N. Danial, Jarrod A. MartoDana-Farber Cancer Institute, Brigham and Women’s Hospital, Harvard Medical School The combination of integrated ion mobility with warp-speed MS/MS acquisition onthe timsTOF Pro paves the way for deep, molecular level understanding of β-cellphysiology and potential new targetable pathways for diabetes. Abstract Keywords:Diabetes, timsTOF Pro,proteomics,proteinexpression In this work we leveraged theanalyticalperformance ofthetimsTOF Pro to identify expres-sion of low-level enzymes forthe first time in primary insulin- secreting beta cells (β-cells).Wefound that these proteins are crit-ical, functional components ofkey metabolic pathways whichcan support B-cell survival underinflammatory stress, an emerg-ing disease etiology of diabetes. Confirmation that these factorsare present and functional specif-ically in pancreatic B-cells pointsto new therapeutic avenues forhuman diabetes. Introduction B-cells within islets of the pancreasintegrate a myriad of endogenousand exogenous signals to properlyregulate glucoselevels, primarilythrough release of insulin. Disrup-tions in p-cell function and glucosehomeostasis arewell-establishedphysiologic hallmarks of human dia-betes. Insulin supplements can beusedtto maintainblood glucose,although insulin-resistance is a grow-ing problem among diabetes patientsand typically leads to severe, chroniccomplications which increase the clin-ical and economic burden associatedwith diabetes. More durable and lessinvasive disease-modifying therapiesrequire an improved molecular-levelunderstanding of B-cell function. Recently, the pathogenic effects ofinflammation have been recognizedas a strong, confounding factor acrossmany human diseases, including dia-betes. How inflammatory signals inter-sect the biochemical pathways thatmediate B-cell glucose metabolismand insulin secretion are unknown.Studies towards this end are compli-cated by the cellular heterogeneity ofhuman islets (B-cells comprise one ofmany functionally distinct cell typesin islets) and a lack of high-fidelitygenetic or other tractable model sys-tems for B-cells. An additional hurdleis the very low number of B-cells pres-ent in human islets, even though theysupply the entire insulin demand of ahealthy individual. As a result, directinterrogation of the p-cell proteomerequires ultra-high sensitivity massspectrometry platforms. Our study [1]took a combined genetic, pharmaco-logic, and multi-omic approach to inter-rogate enriched β-cells isolated fromprimary human islets. Importantly,the protective metabolic enzymes weidentified enhance human B-cell func-tion and survival following transplan-tation and reverse diabetes in mice. This application note is focused onour use of the Bruker timsTOF Proand PASEF@ MS/MS acquisition toconfirm the expression of key meta-bolic enzymes in primary B-cells aspart of our larger effort to understandthe interplay of inflammation and glu-cose metabolism in normal physiol-ogy and diabetes. Primary human islets freshly isolatedfrom deceased donors were obtainedfrom the Alberta Islet DistributionProgram (UniversitvyOT)fAlberta)and by the Integrated Islet Distribu-tion Program (IIDP) at City of Hope(http://iidp.coh.org/) and Prodo Labs(https://prodolabs.com/human-islets-for-research/). All donor material wasobtained under strict adherence toIRB guidelines. Human islets were dispersed usingAccutase (MT25058Cl. ThermoFisher Scientific) and infected withan adenovirus expressing the brightgreen fluorescent protein, ZsGreen(Clontech) under control of the ratinsulin-1 promoter (RIP1) and a mini-CMV enhancer. Cells were collected96 h after infection and loaded ontoan Aria ll Cell Sorter (BD Biosciences),to sort and isolate the live ZsGreen+cell (B-cells) FITC-positive. The B-cellfraction was confirmed to be >92%pure by immunostaining of sortedcells with insulin, and independently,by qRT-PCR and by RNA-seq. Thisprocedurewasrepeated severaltimes using islets from n=3 donors. Enriched p-cells were processed forlysis, protein reduction and alkyla-tion, trypsin digestion, and peptideclean-up. LC-MS/MS was performedby integration of our DEEP SEQfully automated3D ifractionationplatform [2] (NanoAcquity, Waters,Milford, MA) with the timsTOF Proion mobility mass spectrometer (Bruker Daltonics.,BBillerica,1,NMA).The mass spectrometer collectedion mobility MS spectra over a massrange of m/z 100-1700 and 1/k0 of0.6 to 1.6, and then performed 10cycles of PASEF@ MS/MS with atarget intensity of 20k and a thresholdof 250. Active exclusion was enabledwith a release time of 0.4 minutes.MS/MS spectra were matched topeptide sequences from human pro-teins in the Uniprot database usingPEAKS Studio 10.0 software (Bioin-formatics Solutions). Search parame-ters specified precursor and production tolerances of 20 ppm and 50mmu, respectively, as well as fixedcarbamidomethylation and variableoxidation of methionine. After map-ping peptide sequences to uniquegene identifiers using the Pep2genetool [3], multiplierz scripts [4] wereused to provide an estimate of rela-tive protein expression [5] based onthe three most abundant peptidesdetected for each gene product. We performed biochemical studiesdemonstrating that different activa-tors of glucokinase, which regulatesthe initial step in glucose metabo-lism, resulted in divergent cell sur-vival scenarios, depending on thepresence (i otfinflammation-associ-ated cytokines [1]. Using unbiasedmetabolomic analysis performed onprimary human islets, we observedthat the balance between cell sur-vival and cell death correlated withthe shunt of arginine to production ofeither protective (urea) or damaging(nitric oxide)) nitrogen metabolites.How inflammatory cytokines interactwith glucose metabolism to favor oroppose β-cell survival is unknown,in part because cellular heterogene-ity of human islets makes it difficultto deconvolute the functional contri-bution of β-cells independently fromthe bulk tissue. We reasoned that interrogating pro-tein expression directlyy in p-cellswould provide important molecularinsight for the intrinsic pathways bywhich these cells balance urea versusnitricoxide production.Towardsthis end we undertook the challeng-ing task of enriching β-cells frombulk islets obtained from deceasedhuman donors (Figure 1A). We col-lected approximately 30,000 viableB-cells from each of three individualdonor islets. To maximize the depthof proteome coverage we integratedour fully automated DEEP SEQ pep-tide fractionation platform [2] withthe timsTOF Pro mass spectrometer(Figure 1B). Using this approach, wedetected more than 5000 proteins from the combined samples basedon peptides that map uniquely intothe genome [3, 4]. These data sup-ported relative quantification [5] ofmore than 3000 proteins, includingseveral enzymes that regulate themetabolic axis responsible for bal-ancing the output of the urea cycle(Fig. 2,Left). To our knowledge,systematicproteomic analysisOfenriched p-cells has not been pre-viously reported.Interestinglywefound good concordance betweenprotein and gene expression data forthese enzymes (Fig. 2, Left). Thesedata provided important guidance foran intensive series of follow-up func-tional assays in primary human isletsand mouse models [1]. Collectively our data suggested that in the faceof inflammatory cytokines, pyruvatecarboxylase (PC, confirmed in β-cellsby our timsTOF Pro data) integratesglucokinase (GK) signaling to shuntarginine metabolism to ureagenesis,thereby suppressing generation ofdeleterious nitric oxide. These obser-vations nominate the GK-PC axis asan important pro-survival pathwayfor B-cells which are actively metab-. olizing glucose under conditions ofinflammatory stress. Our study moti-vates future efforts to credential thelink between glucose and argininemetabolism as a new therapeutictarget for diabetes. Figure 1 A:Workflow for biochemical enrichment and proteomic analysis of primary p-cells.Human islets, comprising multiple cell types, obtained from deceased donors are processed to facilitate enrichment of insulin-secreting β-cells (red),followed by lysis, cysteine alkylation, digestion with trypsin,and peptide de-salting ( F i g u r e 1 B: W orkflow for biochemical enrichment and p r oteomic analys i s of primary β-cell s. ) Fully automated, online three-dimension fractionation (1st dim., high-pH reversed phase; 2nd dim., high-pH strong anion exchange; 3rd dim., low-pHreversed phase) [2, 6, 7] provides high peptide yield and in-depth proteome coverage on limiting numbers of enriched β-cells. Sample peptides from B-cellsare injected in pH=10 ammonium format loading buffer, and then fractionated via discrete eluents for the 1st dimension (acetonitrile/formic acid) and 2nddimension (ammonium formate), respectively. The final, low-pH reversed phase separation is performed in a 30 pmx50 cm self-packed fused silica columnwith integrated emitter [8] interfaced directly with the Bruker timsTOF Pro instrument. Figure 2. timsTOF Pro PASEFMS/MS analysis of the proteome of primary B-cells. (Left, Top) Histogram distribution of proteins identified in primary B-cellsby at least 3 unique peptides, displayed by estimated relative abundance [5]. Enzymes and other proteins related to the urea cycle as well as argininosucci-nate and aspartate metabolism are highlighted with green dashed lines. (Left, Bottom) The concordance with gene expression by RNA-seq for this subsetof functionally relevant gene-products is illustrated by the scatterplot. (Right) Scan sequence for a tryptic peptide detected from ornithine aminotransferase(OAT), showing full-scan MS spectrum, with zoom-in illustrating low signal-to-noise ratio for peptide precursor (green triangle). Integrated ion mobility onthe timsTOF Pro provides gas phase enrichment of the peptide precursor and rich fragment ion spectrum via PASEF@ MS/MS analysis. Red and blue circlesindicate c-terminal y-type and n-terminal b-type ions, respectively. Conclusion Integrated ion mobility combined with PASEF on the timsTOF Pro facilitates deep proteome analysisin purified human β-cells; the analytical performance of the timsTOF Pro paves the way for systematicinterrogation of β-cell proteome to support diabetes research. timsTOF Pro data provides evidence to further confirm functional role of key enzymes which may shieldpancreatic insulin-secreting B-cells from the harmful effects of chronic inflammation. Together, data from our study highlights exciting new targetable pathways for diabetes therapy. Learn More You are looking for further Information? Check out the link or scan the QR code for more details. www.bruker.com/timstofpro References [1] Fu A, Alvarez-Perez J,Avizonis D, Kin T, Ficarro SB, Choi DW, Karakose E, Badur MG, Evans L, Rosselot C, Bridon G, Bird GH, Seo HS,Dhe-Paganon S, Kamphorst JJ, Stewart AF, Shapiro J, Marto JA Walensky LD, Jones RG, Garcia-Ocana A, Danial NN. Glucose-dependent partitioning to urea cycle spares p-cells from inflammation. Nature Metabolism 2020;2:432-446. [2] Zhou F LuY, Ficarro SB, Adelmant G, Jiang W, Luckey CJ, Marto JA. DEEP SEQ mass spectrometry: a platform for genome-scale proteome quantification.Nat Comm 2013;4:2171. [3] Askenazi M, Marto JA, Linial M. The complete peptide dictionary- a meta-proteomics resource. Proteomics 2010;10:4306-10. [4] Alexander WM, Ficarro SB, Adelmant G, Marto JA. Multiplierz v2.0: a python-based ecosystem for shared access and analysis of nativemass spectrometry data. Proteomics 2017;17:1700091 [5]Silva, J. C., Gorenstein, M. V., Li, G.-Z., Vissers, J. P C. & Geromanos,S. J. Absolute quantification of proteins byLCMSE.Mol Cell Proteom 2006;5:144-156. [6] ]Zhou F, Sikorski TW, Ficarro SB, Webber JT, Marto JA. An online nanoflow rp-sax-rp Ic-ms/ms platform for efficient and in-depth proteome sequenceanalysis of complex organisms. Anal Chem 2011;83:6996-7005. [7] Ficarro SB, Zhang Y, Alfonso MC, Adelmant GO, Garg B, Webber JT, Luckey CJ, Marto JA. Online nanoflow mult-dimensional fractionation strategiesfor high efficiency phosphopeptide analysis. Mol Cell Proteomics 2011;10:M111.011064, 1-19. [8]l Ficarro SB, Zhang Y, LuY, Moghimi AR, Askenazi M, Hyatt E, Smith ED, Boyer L, Schlaeger TM, Luckey CJ, Marto JA. Improved electrospray ionizationefficiency compensates for diminished chromatographic resolution and enables proteomics analysis of tyrosine signaling in embryonic stem cells. AnaChem 2009;81:3440-7. For Research Use Only. Not for Use in Clinical Diagnostic Procedures. Bruker Daltonik GmbH Bruker Scientific LLC Bremen· GermanyPhone +49 (0)421-2205-0 Billerica, MA · USA Phone +1 (978) 663-3660 ms.sales.bdal@bruker.com-www.bruker.com In this work we leveraged the analytical performance of the timsTOF Pro to identify expres-sion of low-level enzymes for the first time in primary insulin-secreting beta cells (β-cells). We found that these proteins are critical, functional components of key metabolic pathways whichcan support β-cell survival under inflammatory stress, an emerging disease etiology of diabetes. Confirmation that these factors are present and functional specifically in pancreatic β-cells points to new therapeutic avenues for human diabetes.
确定



还剩4页未读,是否继续阅读?
布鲁克·道尔顿(Bruker Daltonics)为您提供《分泌胰岛B细胞分泌蛋白质中质谱检测方案(液质联用仪)》,该方案主要用于其他中质谱检测,参考标准--,《分泌胰岛B细胞分泌蛋白质中质谱检测方案(液质联用仪)》用到的仪器有
相关方案
更多








