方案详情
文
针对包装材料的释放量的检测,包装中可迁移化合物的快速筛查,以及材料和食品中的异味溯源,哲斯泰有着十分成熟的解决方案。
使用热脱附系统TDS,可以高效,无歧视的对包装材料(包括吸管)进行VOC-SVOC的检测。样品的前处理非常简单,也无需溶剂萃取,直接通过热脱附法结合GC-MS气质联用即可。对可迁移化合物的检测,也可以同样使用这套设备和检测技术,来对产品进行快速的筛查。通过热萃取直接快速获得可疑的“可迁移化合物”的名单,并进行定性分析。分析灵敏度为ng/mg。
方案详情

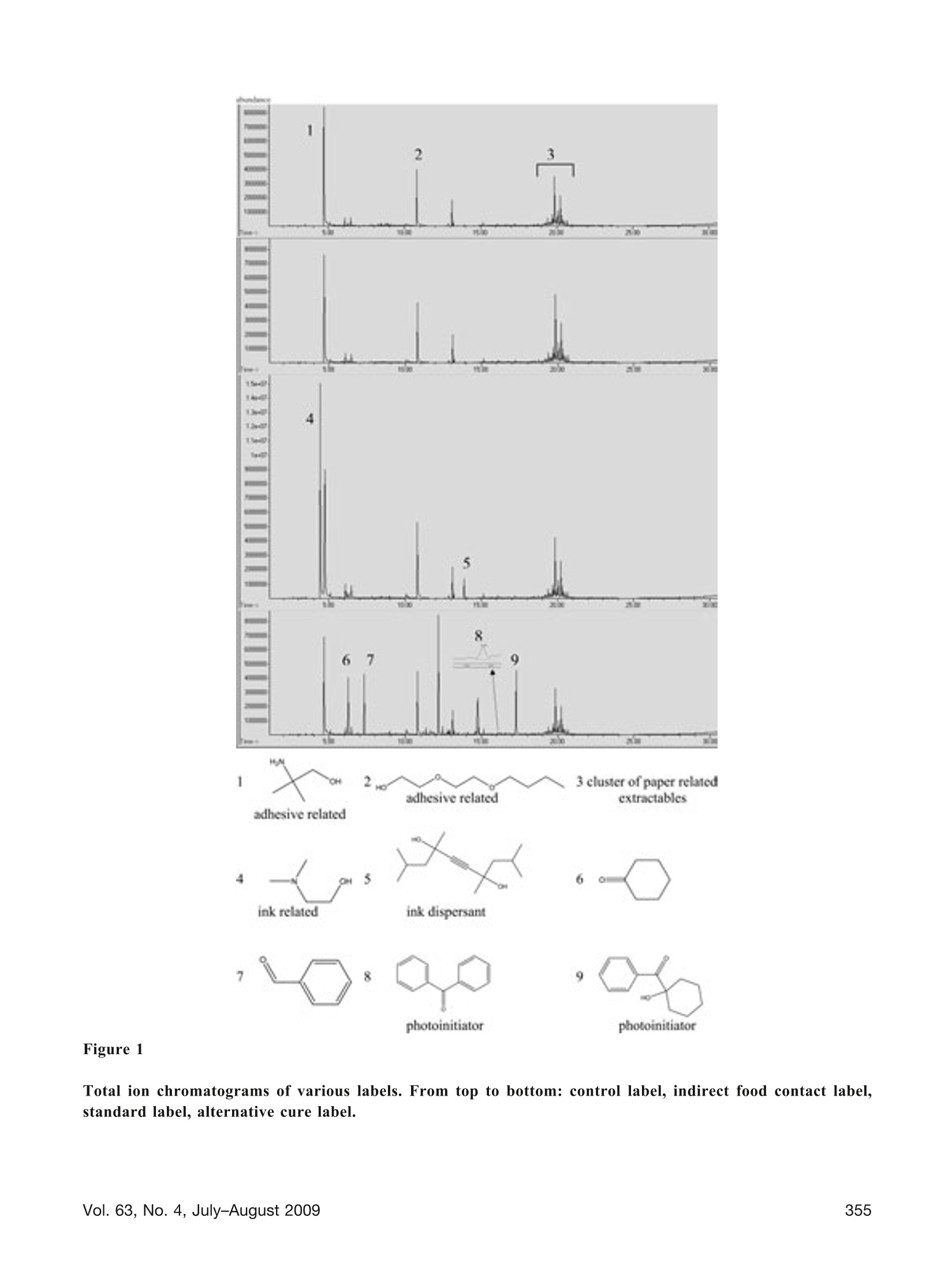
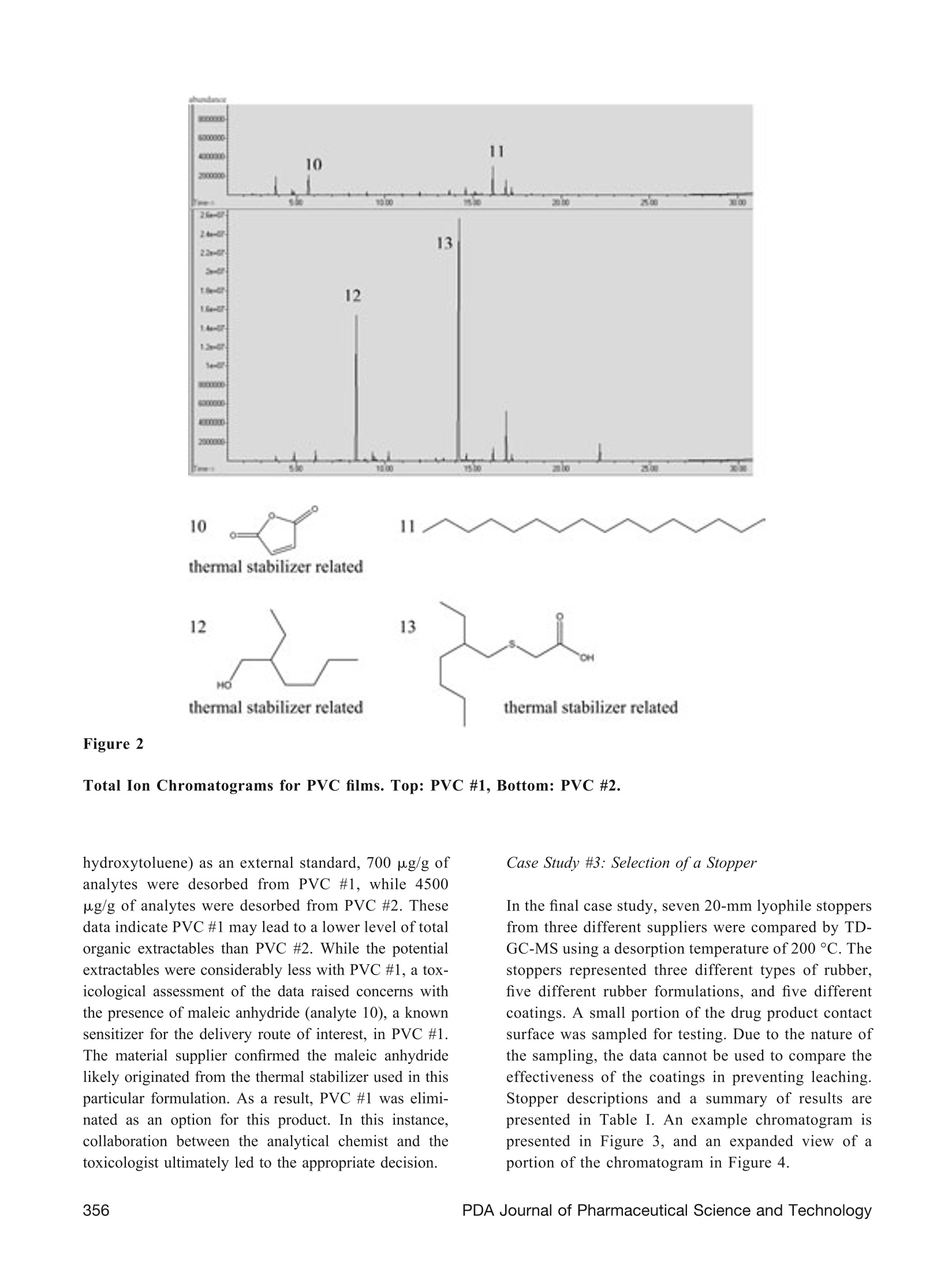
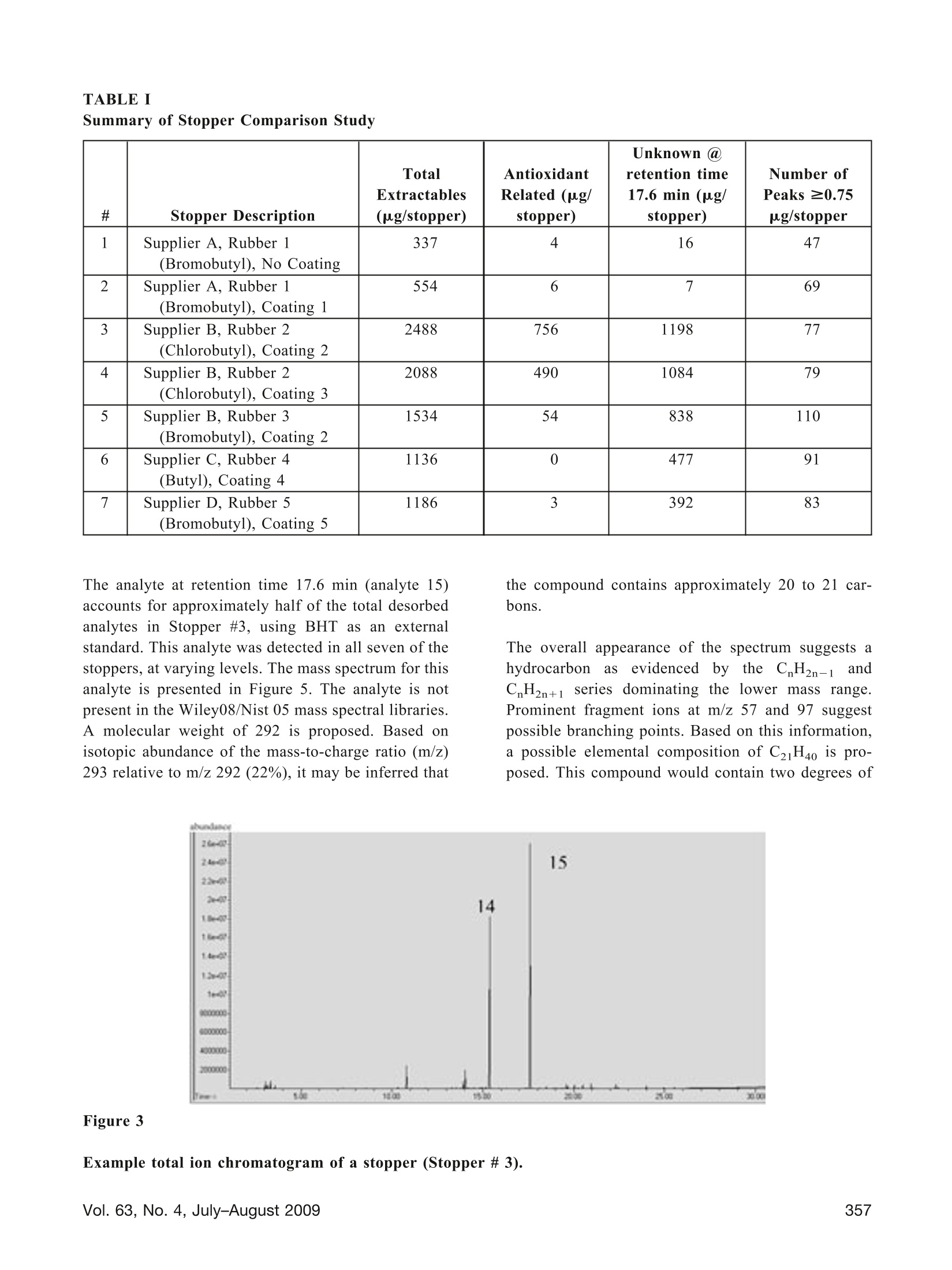
TECHNOLOGY/APPLICATION Use of Thermal Desorption GC-MS to CharacterizePackaging Materials for Potential Extractables CINDY ZWEIBEN* and ARTHUR J. SHAW Pfizer Global Research & Development, Groton/New London Laboratories, Pfizer, Inc., Groton, CT 063400OPDA.Inc. 2009 ABSTRACT: This article presents case studies involving the use of thermal desorption gas chromatography-massspectrometry to compositionally characterize pharmaceutical packaging materials for potential extractables. Knowl-edge of potential extractables and leachables early in the product development program allows the project team tomake informed decisions, potentially minimizing redevelopment efforts and reducing cost. Case studies includeselection of a label for use on a polyethylene bottle, selection of a drug contact surface of a blister packaging system,and selection of a stopper. KEYWORDS: Thermal desorption, Gas chromatography, Mass spectrometry, Extractables, Labels, PVC, Lyophilestoppers Introduction Due to the potential impact of extractables and leach-ables on product quality, materials used in containerclosure systems and device components must be care-fully evaluated for suitability of use. Knowledge ofpotential extractables and leachables early in the prod-uct development program allows the project team tomake informed decisions, potentially minimizing re-development efforts and reducing cost. It is often difficult to obtain detailed ingredient infor-mation from the packaging supplier, where intellectualproperty is generally protected through trade secrets.Therefore, it is desirable to have an analytical tech-nique that will predict possible extractables at thematerial selection phase. Since multiple materials andvendors may be under consideration, minimizing sam-ple preparation and analysis time is essential. In thermal desorption gas chromatography-mass spec-trometry (TD-GC-MS), the inlet of a standard GC-MSis modified to allow for direct analysis of solid sam-ples. A solid sample is placed into a sample tube and ( * Corresponding author: Cindy Zweiben, Pfizer, Inc., MS4086 Eastern Point Road, Groton, CT 06340. Telephone:(860) 686-2160. Email: Cindy.Zweiben@Pfizer.com ) then heated while the carrier gas sweeps over thesample. As analytes volatilize, they are swept awayfrom the sample by the carrier gas and retained in acold trap. The trap is then rapidly heated to introducethe analytes onto the GC column. TD-GC-MS requires minimal sample preparation; thereduction in sample preparation steps avoids potentialcontamination from solvents and glassware (1). It isalso a rapid and highly sensitive technique with rou-tine detection levels at less than 1 ppm, capable ofpredicting volatile and semi-volatile extractables in asingle analysis. The technique is orthogonal to solventextraction because the desorption process is dependenton temperature and not on solvent polarity; both polarand non-polar extractables can be detected in the sameanalysis. The efficiency of the technique permits rapidscreening of multiple packaging options, allowing ex-tractables data to be considered in the packaging se-lection process. Information from packaging supplierscan be combined with TD-GC-MS data and subse-quent toxicological assessments to make an informeddecision (2). The high cost and laboriousness of con-ventional controlled extractables testing often prohib-its consideration of extractables during the earlierphases of product development. One drawback to TD-GC-MS is the inability to detect non-volatile extract-ables. However, in most cases the volatile and semi-volatile analytes are the most relevant for extractablesstudies due to their relatively high mobility. This article presents case studies in which variousmaterials used in pharmaceutical packaging systemsare characterized for extractables via TD-GC-MS. Experimental Design Instrumentation: Agilent 6890N/5975BB((AgilentTechnologies, Inc., Santa Clara,CA) equipped withaGerstelTwister Desorption Unit (TDU),Cooled Injection System (CIS4),and MultiPurposesSampler(MPS2) (Gerstel, Baltimore,MD) Sample size: 2-10 mg TDU: 30°C, ramp 60°C/min to finaltemperature (sample-dependent)hold 5 min Desorption Flow(Helium): 50 mL/minCIS (liquid N,): -120°℃, ramp 12C/s to finaltemperature ((sample-dependent),hold 5 minColumn: HP-5MS30) rmn> 0.25 mm0.25-um film thicknessOven Program: 40°C (hold 5 min)Ramp 10 °C/min to 325 °C (hold 5 min) Column Flow (Helium): 1.2 mL/min (constant flow) Mode: Solvent vent with 20:1 split Mass Range: 30-650 amu Ionization: Electron Ionization Results and Discussion Case Study #1: Selection ofa Label for Use on aPolyethylene Bottle Given the semi-permeable nature of many primarypackaging components, it is desirable to consider pos-sible extractables from labels and secondary packag-ing because such extractables could migrate through the primary packaging and contaminate the containeddrug product (3). In this study, a label stock wasselected and three different ink/varnish systems wereevaluated by TD-GC-MS. Four samples were ana-lyzed: (1) a control label containing only paper andadhesive, (2) a label containing ink appropriate forindirect food contact, (3) a label using what the sup-plier considered to be standard ink, and (4) a labelusing an alternative cure system with a benzophenone-free varnish as reported by the vendor. Since papercannot withstand high temperatures without degrad-ing, a lower desorption temperature of 125 °C wasused for this comparison. The total ion chromatogramsare presented in Figure 1. The differences in the extractables profiles betweenthe labels are readily apparent from the chromato-grams-there are no significant extractables in theindirect food contact label that may be attributed to theink and varnish portion of the label. The standard labelhas two significant extractables attributed to the ink/varnish, and the alternative cure label has several. Forpurposes of this study, a significant extractable is onethat would exceed 0.75 pg/day if the total amountdetected as an extractable leached into the drug prod-uct. Interestingly, the alternative cure label containstrace amounts of benzophenone (analyte 8), more thanwas detected in the other labels (extracted ions chro-matograms were necessary to detect benzophenone inthe indirect food contact label and the standard label).This is an excellent example of how thermal desorp-tion data may be used to supplement and verify sup-plier information. Case Study #2: Selection ofa Drug Contact Surface ofa Blister Packaging System Polyvinyl chloride (PVC) is commonly used in blisterpackaging container closure systems, but PVC addi-tives vary widely from one formulation to anotherdepending on the manufacturing process. In this study,two PVC films were analyzed by TD-GC-MS in orderto select the most appropriate drug contact surface forblister packaging. PVC #1 is 30-um blown film, whilePVC #2 is 60-um extruded PVC. Both samples wereanalyzed by TD-GC-MS using a desorption tempera-ture of 180°C. The total ion chromatograms are pre-sented in Figure 2. It is immediately apparent in this case that there is alarge difference in the total amount of extractablesdesorbed from the samples. Using BHT (butylated Figure 1 Total ion chromatograms of various labels. From top to bottom: control label, indirect food contact label,standard label, alternative cure label. 10 11 thermal stabilizer related 13 ℃H thermal stabilizer related hydroxytoluene) as an external standard, 700 ug/g ofanalytes were desorbed from PVC #1, while 4500ug/g of analytes were desorbed from PVC #2. Thesedata indicate PVC #1 may lead to a lower level of totalorganic extractables than PVC #2. While the potentialextractables were considerably less with PVC #1, a tox-icological assessment of the data raised concerns withthe presence of maleic anhydride (analyte 10), a knownsensitizer for the delivery route of interest, in PVC #1.The material supplier confirmed the maleic anhydridelikely originated from the thermal stabilizer used in thisparticular formulation. As a result, PVC #1 was elimi-nated as an option for this product. In this instance,collaboration between the analytical chemist and thetoxicologist ultimately led to the appropriate decision. Case Study #3: Selection ofa Stopper In the final case study, seven 20-mm lyophile stoppersfrom three different suppliers were compared by TD-GC-MS using a desorption temperature of 200 °C. Thestoppers represented three different types of rubber,five different rubber formulations, and five differentcoatings. A small portion of the drug product contactsurface was sampled for testing. Due to the nature ofthe sampling, the data cannot be used to compare theeffectiveness of the coatings in preventing leaching.Stopper descriptions and a summary of results arepresented in Table I. An example chromatogram ispresented in Figure 3, and an expanded view of aportion of the chromatogram in Figure 4. # Stopper Description Total Extractables(ug/stopper) AntioxidantRelated (ug/ stopper) Unknown @ retention time17.6 min (ug/stopper) Number ofPeaks ≥0.75ug/stopper 1 Supplier A, Rubber 1 (Bromobutyl), No Coating 337 4 16 47 2 Supplier A,Rubber 1 (Bromobutyl), Coating 1 554 6 7 69 3 Supplier B, Rubber 2(Chlorobutyl), Coating 2 2488 756 1198 77 4 Supplier B, Rubber 2(Chlorobutyl), Coating 3 2088 490 1084 79 5 Supplier B, Rubber 3(Bromobutyl), Coating 2 1534 54 838 110 6 Supplier C, Rubber 4(Butyl), Coating 4 1136 0 477 91 7 Supplier D, Rubber 5 (Bromobutyl), Coating 5 1186 3 392 83 The analyte at retention time 17.6 min (analyte 15)accounts for approximately half of the total desorbedanalytes in Stopper #3, using BHT as an externalstandard. This analyte was detected in all seven of thestoppers, at varying levels. The mass spectrum for thisanalyte is presented in Figure 5. The analyte is notpresent in the Wiley08/Nist 05 mass spectral libraries.A molecular weight of 292 is proposed. Based onisotopic abundance of the mass-to-charge ratio (m/z)293 relative to m/z 292 (22%), it may be inferred that the compound contains approximately 20 to 21 car-bons. The overall appearance of the spectrum suggests ahydrocarbonaas evidenced bby the(CH2n-1andC.H2n+1 series dominating the lower mass range.Prominent fragment ions at m/z 57 and 97 suggestpossible branching points. Based on this information,a possible elemental composition of C21H40 is pro-posed. This compound would contain two degrees of Figure 3 Example total ion chromatogram of a stopper (Stopper#3). 15 Sce Figure 5 16 analyte present at reporting threshold(0.75pg/stopper) unsaturation based upon a ring/double bond calcula-tion. Analysis of the data using MassWorks Clipssoftware (Cerno Bioscience, Danbury, CT), whichuses a calibration scheme involving both mass andspectral accuracy (5), also resulted in a proposed mo-lecular formula of C21H40. Given the non-polar natureof the analyte, it is unlikely to be a significant leach-able in aqueous drug product formulations. As noted in Table I, there were large differencesobserved in the total amounts of analytes desorbed,the total amounts of antioxidant related analytes,and the total number of peaks. For this comparison,0.75 pg/stopper was used as a cut-off for reportingpeaks. This is derived from a safety concern thresh-old of 1.5 pg/day, assuming a drug is dosed at 1 vialper day, and includes a 50% adjustment for uncer-tainty in analyte response (4). Analytes are quanti- fied against BHT as an external standard. Becausenon-volatile compounds are not detected by thismethodology, additionale ractables maybepresent in the stopper. The data for total extractables (micrograms/stopper)and unknown at retention time 17.6 min (ug/stopper)are presented graphically in Figure 6. Stopper 6 contained early eluting (more volatile) polaranalytes that may become significant leachables in anaqueous formulation. Stopper 3 contained the highestlevel of total analytes desorbed; however, it did notcontain any early-eluting polar analytes. Stoppers land 2 contained the lowest levels of total desorbedanalytes, and are expected to lead to the lowest levelof organic extractables and leachables relative to theother stoppers. Mass spectrum of analyte at retention time 17.6 min in rubber stoppers. Total Extractables Unknown at retention time 17.6 min Total micrograms/stopper and total unknown at retention time 17.6 min. Thermal desorption GC-MS can provide detailed in-formation on the composition of packaging materialsand may be a useful tool for determining extractablesassociated with such materials. Acknowledgements The authors acknowledge the following individualsfrom Pfizer, Inc.: Doug Ball, Bill Beierschmitt, andBill McConnell for toxicological consultation; BobBergeson, Mike Martowska, Doug Pearce, and LisaWilson for packaging samples and consultation; RonMorris for mass spectral interpretation; and, EileenBohler, Matt Coates, Joanne Lukaszewicz, and MarySchafer for project-specific consultation. References 1. Wahl, H. G.; Hoffmann, A.; Haring, H.; Liebich,H. M.Identification of plasticizers in medical prod-ucts by a combined direct thermodesorption-cooledinjection system and ggas chromatography-mass spectrometry. J. Chromatogr., A 1999, 847(1-2),1-7. 2. Poochikian, G. PQRI Leachables and ExtractablesWorkshop; Rockville, Maryland; December 5-6,2005, p 9-14. 3. Fang, X.; Cherico, N.; Barbacci, D.; Harmon,A. M.; Piserchio,M.; Perpall, H. Leachable studyon solid dosage form. American PharmaceuticalReview 2006,9 (7),58,60-63. 4. Safety Thresholds and Best Practices for Extract-ables and Leachables in Orally Inhaled and NasalDrug Products; submitted to the PQRI Drug Prod-uct Technical Committee, PQRI Steering Commit-tee, and U.S. Food and Drug Administration by thePQRI[ Leachables and Extractables WorkingGroup, September 8, 2006. 5. Chen, J.; Gu, M.; Wang, Y.; Kuehl, D. Improvingthe confidence of unknown compound identifica-tion by first responder mobile GC-MS laboratoriesin time-critical environmental and homeland secu-rity incidents. LC-GC 2008, 26 (9),938-945. Vol. No. , July-August PDA Journal of Pharmaceutical Science and Technology 。42食品行业将迎来“反塑料革命”随着全世界对环境保护的意识越来越强,首当其冲的就是无法降解的塑料制品。当科学家们在人体的排泄物中,海洋中的微生物,北极的冰川中,还有空气中发现微塑料的存在时,塑料突然成为了始作俑者,人人喊打。其实,在上个世纪初塑料被发明出来时,是所有人的宠儿。在Susan Freinkel撰写《塑料:一个有毒的爱情故事》中,描写了我们的生活与塑料密不可分的关系。只是经过了一个世纪后,那颗朱砂痣渐渐变成了蚊子血。2018年5月欧盟通过一项“限塑”草案,禁止包括棉花棒、吸管、餐具等10种一次性塑料制品,欧盟成员国有两年时间将其转变为国家法律,也就是说2021年欧洲将实现全面限塑。2018年印度总理莫迪提出,将全面禁止包括塑料袋在内的六种一次性塑料包装的使用。2019年1月,国家发展改革委,生态环境部公布了《关于进一步加强塑料污染治理的意见》:2020年底,我国将率先在部分地区,部分领域禁止、限制部分塑料制品的生产、销售和使用;到2022年底,一次性塑料制品的消费量将明显减少,替代产品得到推广。到今年年底,全国餐饮行业将全面禁用不可降解一次性塑料吸管。不用塑料吸管用什么?塑料吸管常见的替换方案,可以归纳为3类:纸吸管、PLA(聚乳酸)等可降解吸管、直饮杯盖。于是各大食品、饮料、包装品牌,开始了一波又一波的操作。。。2018年星巴克年宣布,在全球范围停止使用塑料吸管。2019年6月起喜茶开始在全国门店开始推行纸吸管2019年九月底开始,日本雀巢KitKat系列的部分巧克力产品舍弃塑料包装。2020年6月麦当劳中国和塑料吸管正式说“拜拜”,亮出“为了地球,我不管了”宣言2019年7月利乐则在欧洲率先推出了新的纸质吸管,将用于两款儿童饮料和乳品中。这个吸管由FSC认证的纸材制成,可以和包装的其他部分一起回收。纸制产品真的如此完美吗?用过纸质吸管的朋友,一定有亲身体检,纸质吸管一般都:有味道和不耐泡。不耐泡是物理特性,大家都能理解。但是这个味道是哪里来的?我们很多朋友也肯定很喜欢书的味道。这些气味其实是纸质材料中被释放出来的VOC及SVOC,包括油墨挥发物等等。特别是如果使用的是再生纸的话,情况更为严重。当纸制制品的挥发量达到一定的浓度以后,特别是当一些有毒有害物质被释放出来时,对人类的健康会产生影响。此外,一些质量不过关的纸制包装材料,也会和内部的食品接触,造成对食品的污染,我们称之为包装污染物的迁移,轻则影响食物的口感,重则影响人体健康。许多包装材料是用再生纸做的。再生纸是以废纸做原料,将其打碎、去色制浆经过多种工序加工生产出来的纸张。由于其原料的80%来源于回收的废纸,因而被誉为低能耗、轻污染的环保型用纸。因为其环保性,同样也是备受推崇,但是德国联邦材料研究和测试所BAM在对再生纸和原浆纸的释放测试中发现。再生纸的SVOC排放量远远高于原生浆纸。图一: 原木浆纸的直接热萃取色谱图。1=o-Hydroxybipenyl, 2=Hexdecanoic acid, 3=Octadecene, 4=Eicosene, 5=Docosene, 6=unknown图二:再生纸的直接热萃取色谱图。1=Aectic acid, 2=Hexanal, 3=Isopropyl laurate, 4=Dissopropylnaphtaline, 5=Octadecane, 6=Octadecene, 7=Methyl oleate, 8=Eicosene, 9=Oocosene这一结果也可以从上图的色谱图中很明显的看出:图二比图一在28分钟保留时间后,峰面积更大,而且有很明显的油山。这些SVOC来自于再生纸使用的废纸原料,很有可能是废纸里的印刷油墨的关系。比如再生纸里的二异丙基萘肯定是来源于复写纸,同样是在废纸的一部分。。42哲斯泰包装材料检测解决方案针对包装材料的释放量的检测,包装中可迁移化合物的快速筛查,以及材料和食品中的异味溯源,哲斯泰有着十分成熟的解决方案。使用热脱附系统TDS,可以高效,无歧视的对包装材料(包括吸管)进行VOC-SVOC的检测。样品的前处理非常简单,也无需溶剂萃取,直接通过热脱附法结合GC-MS气质联用即可。对可迁移化合物的检测,也可以同样使用这套设备和检测技术,来对产品进行快速的筛查。通过热萃取直接快速获得可疑的“可迁移化合物”的名单,并进行定性分析。分析灵敏度为ng/mg。哲斯泰的热脱附系统TDS3对于更高的检测需求和自动化需求,我们有热脱仪TD3.5+配上大型动态顶空DHS large,如果需要对异味进行溯源,则只需再加上嗅觉检测口ODP即可。此套设备不但可以完成上述热脱附系统TDS3的热萃取任务,更是可以对大型包装材料进行整体的分析,而不用把样品剪小,结果更有代表性。哲斯泰包装材料高级解决方案:热脱附仪TD3.5+,大型动态顶空DHS Large, 多动能全自动样品前处理平台MPS roboitc大型动态顶空的样品容量最高为1L,可以装下整个包装材料,分析释放量哲斯泰的嗅觉检测口ODP4可以用于分析异味物质来源后语塑料产品并不是恶魔,纸制产品也并非对环境和人体健康绝对无害,而包装材料的利也远远大于弊。只要对产品严格把关,所有的这些消费品,都是我们生活中“无毒的情人”。我们要杜绝的,不是一种材料,而是一种“一次性”的消费和生活模式,让世界可以可持续发展。我们的客户Tetra Pak 利乐图片来源:利乐官网图片来源:搜狐利乐是全球领先的食品加工和包装解决方案提供商。利乐与客户和供应商密切合作,提供安全、创新和环保的产品,每天在全球 160 多个国家/地区满足数亿人的需求。利乐在全球拥有24000多名员工,追求负责任的行业领导和可持续的业务发展。利乐的座右铭,“保护好品质<™”,反映了利乐让安全的食品随处可得的愿景。为了保持竞争力,我们的客户需要更快、更好、更节约的研发成果来降低运营成本和提高绩效。为了响应客户、消费者和市场需求,我们不断投资开发新的技术和产品。利乐在全球拥有5个研发中心,为世界各地的众多项目提供支持。内容来源:利乐官网 https://www.tetrapak.com/cn/about/tetra-pak-in-brief我们的客户BAM德国联邦材料研究与测试所图片来源:WikipediaBAM是在欧盟指令基础上,按照国际法规和国家法律规定, 从事材料研究与产品测试和质量的管理体系认证。BAM是德国的一个联邦上级机构和部门研究机构。根据他的技术和化学安全指南,来负责公共技术安全和化学计量任务。(Wikipedia)
确定



还剩5页未读,是否继续阅读?
GERSTEL(哲斯泰)为您提供《食品包装,纸质吸管中VOC,SVOC检测方案(热解吸仪)》,该方案主要用于包装中VOC,SVOC检测,参考标准--,《食品包装,纸质吸管中VOC,SVOC检测方案(热解吸仪)》用到的仪器有GERSTEL热脱附系统TDS3 (热解吸、热解析)、GERSTEL大型动态顶空DHS large
推荐专场
相关方案
更多