美国加利福尼亚大学伯克利分校的科学家,利用单细胞Western技术在期刊SCIENCE ADVANCES(IF:12.804)发表文章:Assessing heterogeneity among single embryos and single blastomeres using open microfluidic design, Sci. Adv. 2020; 6: eaay1751 22 April 2020. 本文利用单细胞Western技术与现有的工作流程整合在一起,为评估植入前胚胎发育固有的细胞间分子异质性开辟了新途径。
方案详情

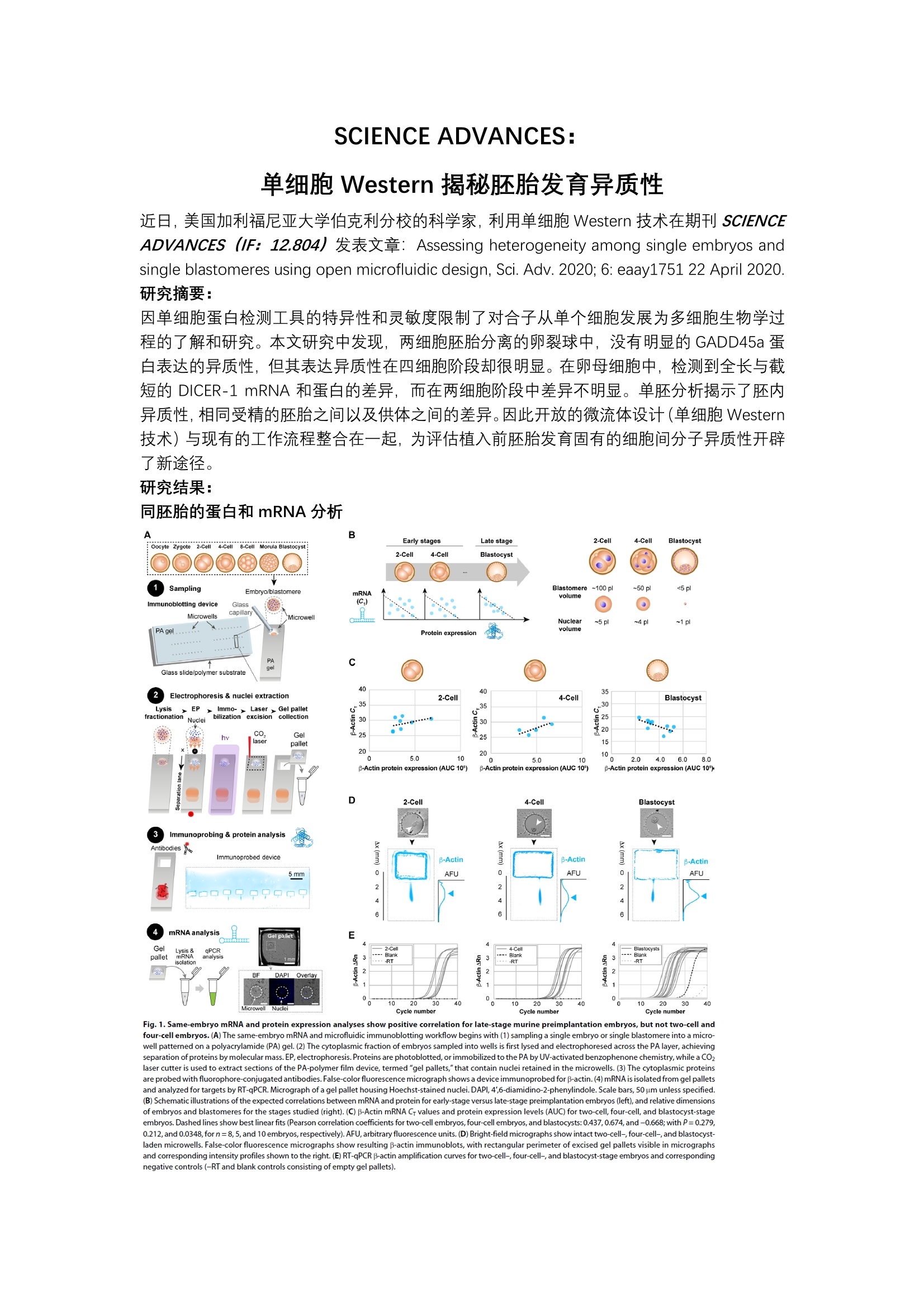
SCIENCE ADVANCES:单细胞 Western 揭秘胚胎发育异质性 近日,美国加利福尼亚大学伯克利分校的科学家,利用单细胞 Western 技术在期刊 SCIENCEADVANCES (IF: 12.804)发表文章: Assessing heterogeneity among single embryos andsingle blastomeres using open microfluidic design, Sci. Adv. 2020; 6:eaay1751 22 April 2020. 研究摘要: 因单细胞蛋白检测工具的特异性和灵敏度限制了对合子从单个细胞发展为多细胞生物学过程的了解和研究。本文研究中发现,两细胞胚胎分离的卵裂球中,没有明显的 GADD45a 蛋白表达的异质性,但其表达异质性在四细胞阶段却很明显。在卵母细胞中,检测到全长与截短的DICER-1 mRNA 和蛋白的差异,而在两细胞阶段中差异不明显。单胚分析揭示了胚内异质性,相同受精的胚胎之间以及供体供间的差异。因此开放的微流体设计(单细胞 Western技术)与现有的工作流程整合在一起,为评估植入前胚胎发育固有的细胞间分子异质性开辟了新途径。 研究结果: 同胚胎的蛋白和mRNA分析 A B Early stages 2-Cell 4-Cell Blastocyst Late s8-celtage Morulale: Oocyte Zygote 2-Cell stocyst2-Cell 4-Cell BlastocystSamplingEmbryo/blastomereBlastomere~100 pl ~50 pl <5plmRNAvolumeImmunoblotting device Glass(C) ..capillaryMicrowellsMMicrowellon Nuclear ~5 pl ~4pl ~1 plPA gel v Protein expressionvaolumeGlass slide/polymer substrate4040 35Electrophoresis & nuclei extraction 2-Cell 4-Cell BlastocystLysis >EP>.Immo-r. Laser> Gel palletx-30mr hiifractionationNucleibilization excision collection新.*.a.. 30s 25..CO. ...520Geel2:laseipallet1520 205.0 1010纷 5.0 10 2.0 4.0 6.08.0B-Actin protein expression (AUC 10】 B-Actin protein expression (AUC 10') p-Actin protein expression (AUC 10)D 2-Cell 4-Cell BlastocystImmunoprobing & protein analysisAntibodies A 货Immunoprobed device 3 B-ActinB-Actin3 3 3 B-Actin m 0 AFU 0AFU 0 AFU 2 2oo999oorCmRNAanalysisGel pallet EmRGel 2-Ce 4-Cellnt -ysis& PCR ink Blankpallet3-RT cDAPI Ove i 2110 20 30 40 10 :20 30 Microwell Nuclei Cycle numbt Cycle number Cycle number Fig.1. Same-embryo mRNA and protein expression analyses show positive correlation for late-stage murine preimplantation embryos, but not two-cell andfour-cell embryos.(A) The same-embryo mRNA and microfluidic immunoblotting workflow begins with (1) sampling a single embryo or single blastomere into a micro-well patterned on a polyacrylamide (PA) gel. (2) The cytoplasmic fraction of embryos sampled into wells is first lysed and electrophoresed across the PA layer,achievingseparation of proteins by molecular mass.EP,electrophoresis. Proteins are photoblotted,or immobilized to the PA by UV-activated benzophenone chemistry, while a CO2laser cutter is used to extract sections of the PA-polymer film device, termed "gel pallets,"that contain nuclei retained in the microwells.(3) The cytoplasmic proteinsare probed with fluorophore-conjugated antibodies.False-color fluorescence micrograph shows a device immunoprobed for p-actin. (4)mRNA is isolated from gel palletsand analyzed for targets by RT-qPCR. Micrograph of a gel pallet housing Hoechst-stained nuclei. DAPI, 4',6-diamidino-2-phenylindole. Scale bars, 50 um unless specified.(B) Schematic illustrations of the expected correlations between mRNA and protein for early-stage versus late-stage preimplantation embryos (left), and relative dimensionsof embryos and blastomeres for the stages studied (right). (C) B-Actin mRNA Cr values and protein expression levels (AUC) for two-cell, four-cell, and blastocyst-stageembryos.Dashed lines show best linear fits (Pearson correlation coefficients for two-cell embryos, four-cell embryos,and blastocysts: 0.437, 0.674, and-0.668;with P=0.279,0.212,and 0.0348,forn=8,5,and 10 embryos, respectively). AFU,arbitrary fluorescence units. (D) Bright-field micrographs show intact two-cell-, four-cell-,and blastocyst-laden microwells. False-color fluorescence micrographs show resulting B-actin immunoblots, with rectangular perimeter of excised gel pallets visible in micrographsand corresponding intensity profiles shown to the right.(E)RT-qPCR B-actin amplification curves for two-cell-, four-cell-, and blastocyst-stage embryos and correspondingnegative controls (-RT and blank controls consisting of empty gel pallets). 结果显示,后期植入前胚胎的 mRNA 和蛋白表达分析呈正相关,但两细胞和四细胞胚胎阶段并未呈现这种相关性。鉴于转录产物和蛋白质在胚胎非常早期和不稳定的发育阶段之间存在不可预测的关系,因此用相同胚胎的蛋白质分析来补充转录分析。 单细胞 Western 技术检测两细胞和四细胞胚胎阶段分离的卵裂球中 GADD45a 蛋白表达 Fig. 2. Microfluidic immunoblotting measures intraembryonic heterogeneity in GADD45a expression in murine four-cell and two-cell embryos.(A) GADD45atranscript levels have been shown to exhibit bimodality at the two-and four-cell stages.(B) Four-cell embryos are dissociated into individual blastomeres and immuno-blotted for protein expression of f-tubulin,B-actin, and GADD45a, as shown in false-color fluorescence micrographs.(C) Dot plot of expression of β-tubulin (blue), B-actin(cyan), and GADD45a (red) normalized to total expression by individual blastomeres from two representative four-cell embryos (top). Dot plot of intraembryonic coeffi-cient of variation (CV) in protein expression for B-tubulin, p-actin, and GADD45a (bottom, Mann-Whitney U test, P=0.0012 for CVGADD45a versus CVp-tub and CVGADD45aversus CVp-actin, and P=0.805 CVp-tub versus CVp-actin, for n=6 dissociated embryos). **p<0.01.(D) Two-cell embryos are dissociated into individual blastomeres and as-sayed for protein expression of p-tubulin, B-actin,and GADD45a, as shown in false-color fluorescence micrographs.(E) Dot plots of p-tubulin, B-actin,and GADD45a ex-pression by sister blastomeres, normalized to sum of expression of sister blastomeres, for six representative two-cell embryos (top). Dot plot of interblastomeric CV% inexpression of β-tubulin, B-actin, and GADD45a (bottom, Mann-Whitney Utest,P=0.0323 for CVGADD45a versus CVptubulin P=0.130 for CVGADD45a versus CVpactin, and P=0.598for CVB.tubulin versus CVp-actin, for n=11 dissociated two-cell embryos). Same marker for a given embryo in (C) and (E) indicates same blastomere. Horizontal bars in (C) and(E) indicate mean±SD. Scale bars,200 um. 结果显示,尽管有报道两细胞和四细胞卵裂球之间的 Gadd45a 转录水平具有异质性,但利用单细胞 Western 技术发现: GADD45a 蛋白表达水平,在两细胞胚胎分离的卵裂球中没有明显差异,而其表达异质性在四细胞阶段却很明显。预示着每个胚胎期卵裂球中与细胞命运相关的蛋白质在早期胚胎中存在谱系偏倚。 单细胞 Western 技术检测卵母细胞和两细胞胚胎阶段的 DICER-1 表达 Oocyte Somatic cell Fig. 3. Higher DICER-1 isoform expression in murine oocytes than in two-cell embryos correlates with mRNA levels. (A) DICER, a truncated isoform of DICER-1,appears at the oocyte stage and is a product of alternative promoter usage. aa, amino acid.Bright-field micrographs of (B) a settled oocyte, **p<0.01 and (C) a settledtwo-cell embryo, with corresponding overlaid false-color fluorescence micrographs and intensity profiles showing protein bands for loading controls (a-actinin andp-tubulin) and DICER-1. Oocyte immunoblot demonstrates presence of a full-length DICER-1 (top arrow) and a lower molecular mass isoform (bottom arrow). Underthese, dot plots of DICER isoform mRNA levels normalized by endogenous control Rfx1 (top) and protein expression (AUC, bottom). Expression of the truncated isoformis higher than the full-length DICER-1 for both mRNA and protein in oocytes (mRNA/Rfx1DICER-1 versus mRNA/tDICER:Mann-Whitney Utest, P=0.0052 for n= 18; for AUCDICER-1versus AUCDICER:Mann-Whitney U test, P=0.0079 forn=5), but not in two-cell embryos (for mRNA/Rfx1DICER-1 versus mRNA/Rfx1DICER:Mann-Whitney U test, P=0.9551forn=7 for DICER-1 and n=8 for DICER ; for AUCDICER-1 versus AUCDICER : Mann-Whitney U test, P=0.20 for n=4). Oocytes show higher mRNA and protein expressionthan two cells for the truncated isoform (mRNA/Rfx1DICER: Mann Whitney Utest,P=0.0004 for n=18 oocytes and 8 two cells; AUCDICER : Mann Whitney Utest,P=0.0159for n=5 oocytes and 4 two-cell embryos), but not the full-length DICER-1 (mRNA/Rfx1DICER-1: Mann Whitney U test, P=0.084 for n=18 oocytes and 7 two-cell embryos;AUCDICER-1: Mann Whitney U test,P=0.9048 for n=5 oocytes and 4 two-cell embryos). Horizontal bars indicate mean ± SD. Scale bars, 200 um.(D) Fluorescence micro-graph of a single oocyte immunoblotted for DICER-1 with corresponding fluorescence intensity profiles and Gaussian curve fits to the DICER°isoform (low molecular mass,R=0.83, solid black line) and DICER-1 full-length isoform (R=0.89, dotted black line). To the right, slab-gel Western blot analysis of pooled oocytes (n=85 oocytes)andcorresponding grayscale value profile show two DICER-1 protein bands. Oocytes were pooled, lysed, and assayed by nonreducing SDS-PAGE followed by immunoprobing.Buffers and immunoprobing reagents matched the single-cell protocol. Black arrowheads mark the position of the DICER-1 bands. 利用单细胞 Western 技术检测结果显示,卵母细胞的全长 DICER-1 和截短蛋白 DICER°的表达存在显著差异,但到两细胞胚胎阶段时这种差异消失了。揭示了 DICER在受精前在卵母细胞成熟和纺锤体组装中起着关键作用。受精后DICER'的清除率提示该作用仅限于卵母细胞内但不延伸到植入前的胚胎细胞。 单细胞 Western 技术+全细胞成像分析单胚胎和卵裂球表型 Fig.4. Whole-cell imaging adds phenotypic content to protein analysis of single embryos and blastomeres.(A) Cell type-graded expression of CDX-2 and SOX-2 inblastomeres from dissociated blastocysts.Bright-field and fluorescence micrographs of dissociated blastocysts after incubation with fluorescent microspheres and corre-sponding micrographs of blastocysts immunoblotted for B-tubulin,CDX-2, and SOX-2. Scale bars, 50 um. (B) Detection of membrane proteins in morula and blastocysts.Bright-field micrographs showing embryos settled into microwells before lysis, with resulting false-color micrographs immunoblots for B-tubulin, E-cadherin,and FGFR-1,with corresponding fluorescence intensity profiles shown to the right. Scale bars, 100 um. Dot plots of E-cadherin (left) and FGFR-1 (right) expression for morula andblastocysts (forE-cadherin: n=3 and 3 morula and blastocysts, for FGFR-1:n=6 and 5 morula and blastocysts). Horizontal bars represent mean ±SD. (C) Microscopyandsingle-blastomere immunoblotting identifies correlations between cell volume and marker expression in dissociated morula blastomeres.False-color fluorescence micro-graphs show p-tubulin, B-actin,and SOX-2 protein bands, with intensity profiles adjacent to micrographs. Arrows mark the position of protein bands. Scale bars, 100 um.To the right, bivariate plot of blastomere cell volume and loading control expression (B-tubulin and B-actin) shows significant positive linear correlation (Pearson correlation,n=8,p=0.883 and 0.908, P=0.00366 and 0.00018, respectively). Bivariate plot of cell volume versus SOX-2 expression normalized by B-tubulin and p-actin expressionshow a negative, but nonsignificant, association (Pearson correlation,n=8,p=-0.487 and -0.315,P=0.221 and 0.447,respectively). 利用单细胞 Western 技术,分析滋养外胚层 (TE)与内细胞群(ICM)表型标记蛋白 CDX-2与 SOX-2的表达水平。利用单细胞 Western 技术检测桑椹胚和囊胚中膜蛋白细胞粘附蛋白(E-cadherin)和细胞表面受体成纤维细胞生长因子受体 1 (FGFR-1)蛋白的表达差异。以及利用显微镜和单细胞 Western 技术鉴定了解离的桑椹胚卵裂球中细胞体积与标志物蛋白表达之间的相关性。 系统特点 抗体: Western Blot抗体及荧光抗体单张芯片进行约1000个单细胞Western Blot 检测单次可进行6-12个靶蛋白检测通过洗脱再杂交可进行数十个靶蛋白检测 全流程4小时左右 近日,美国加利福尼亚大学伯克利分校的科学家,利用单细胞Western技术在期刊SCIENCE ADVANCES(IF:12.804)发表文章:Assessing heterogeneity among single embryos and single blastomeres using open microfluidic design, Sci. Adv. 2020; 6: eaay1751 22 April 2020. 研究摘要:因单细胞蛋白检测工具的特异性和灵敏度限制了对合子从单个细胞发展为多细胞生物学过程的了解和研究。本文研究中发现,两细胞胚胎分离的卵裂球中,没有明显的GADD45a蛋白表达的异质性,但其表达异质性在四细胞阶段却很明显。在卵母细胞中,检测到全长与截短的DICER-1 mRNA和蛋白的差异,而在两细胞阶段中差异不明显。单胚分析揭示了胚内异质性,相同受精的胚胎之间以及供体之间的差异。因此开放的微流体设计(单细胞Western技术)与现有的工作流程整合在一起,为评估植入前胚胎发育固有的细胞间分子异质性开辟了新途径。研究结果:同胚胎的蛋白和mRNA分析结果显示,后期植入前胚胎的mRNA和蛋白表达分析呈正相关,但两细胞和四细胞胚胎阶段并未呈现这种相关性。鉴于转录产物和蛋白质在胚胎非常早期和不稳定的发育阶段之间存在不可预测的关系,因此用相同胚胎的蛋白质分析来补充转录分析。 单细胞Western技术检测两细胞和四细胞胚胎阶段分离的卵裂球中GADD45a蛋白表达结果显示,尽管有报道两细胞和四细胞卵裂球之间的Gadd45a转录水平具有异质性,但利用单细胞Western技术发现:GADD45a蛋白表达水平,在两细胞胚胎分离的卵裂球中没有明显差异,而其表达异质性在四细胞阶段却很明显。预示着每个胚胎期卵裂球中与细胞命运相关的蛋白质在早期胚胎中存在谱系偏倚。单细胞Western技术检测卵母细胞和两细胞胚胎阶段的DICER-1表达利用单细胞Western技术检测结果显示,卵母细胞的全长DICER-1和截短蛋白DICER0的表达存在显著差异,但到两细胞胚胎阶段时这种差异消失了。揭示了DICER0在受精前在卵母细胞成熟和纺锤体组装中起着关键作用。受精后DICER0的清除率提示该作用仅限于卵母细胞内但不延伸到植入前的胚胎细胞。单细胞Western技术+全细胞成像分析单胚胎和卵裂球表型利用单细胞Western技术,分析滋养外胚层(TE)与内细胞群(ICM)表型标记蛋白CDX-2与SOX-2的表达水平。利用单细胞Western技术检测桑椹胚和囊胚中膜蛋白细胞粘附蛋白(E-cadherin)和细胞表面受体成纤维细胞生长因子受体1(FGFR-1)蛋白的表达差异。以及利用显微镜和单细胞Western技术鉴定了解离的桑椹胚卵裂球中细胞体积与标志物蛋白表达之间的相关性。
确定



还剩2页未读,是否继续阅读?
ProteinSimple为您提供《单细胞,胚胎单细胞,卵裂球,卵母细胞中单细胞蛋白表达检测方案(蛋白印迹仪)》,该方案主要用于其他中单细胞蛋白表达检测,参考标准--,《单细胞,胚胎单细胞,卵裂球,卵母细胞中单细胞蛋白表达检测方案(蛋白印迹仪)》用到的仪器有Milo 单细胞蛋白质表达定量分析系统
推荐专场
相关方案
更多















