方案详情
文
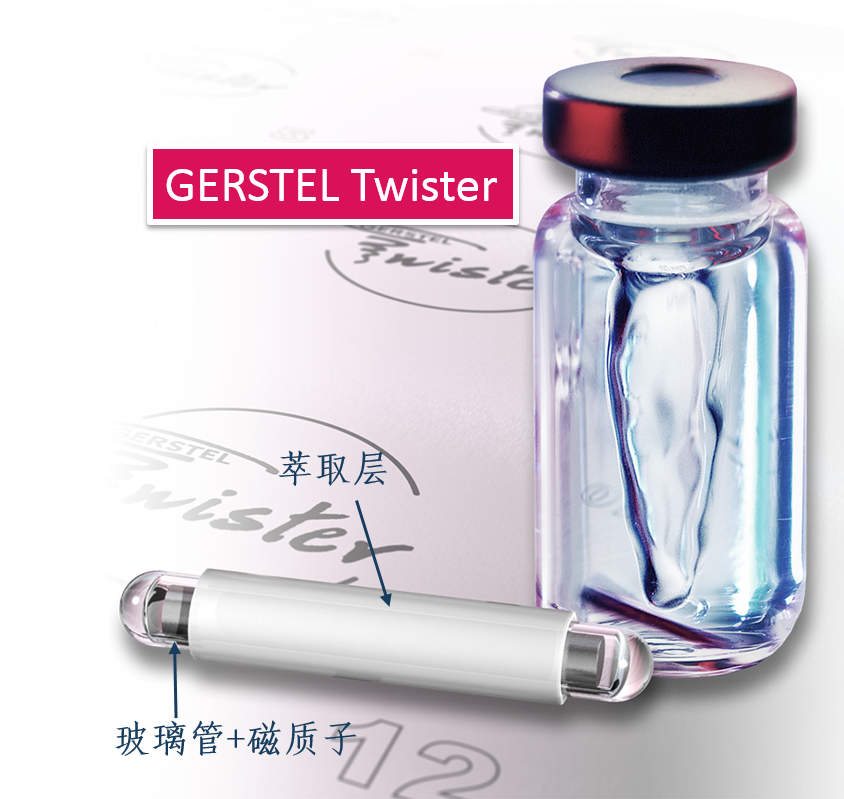
搅拌棒吸附萃取(SBSE)与热脱附-气相色谱质谱联用是一种多用途且经济有效的方法,可以有效地对不同食品中超过400种农药同时进行分析和定量。是一个简单的,无溶剂的技术,并且允许在一个步骤中实现萃取和浓缩。该方法应用于各种蔬菜(番茄,黄瓜,生菜,菠菜),水果(葡萄),饮料(茶,甘蔗汁),酒类,醋,婴儿食品中多农残的检测。检测下限在ppb到亚ppb之间。相对标准偏差低于10%,检测结果精确度高。线性范围广,并且r²平均大于0.997。
方案详情

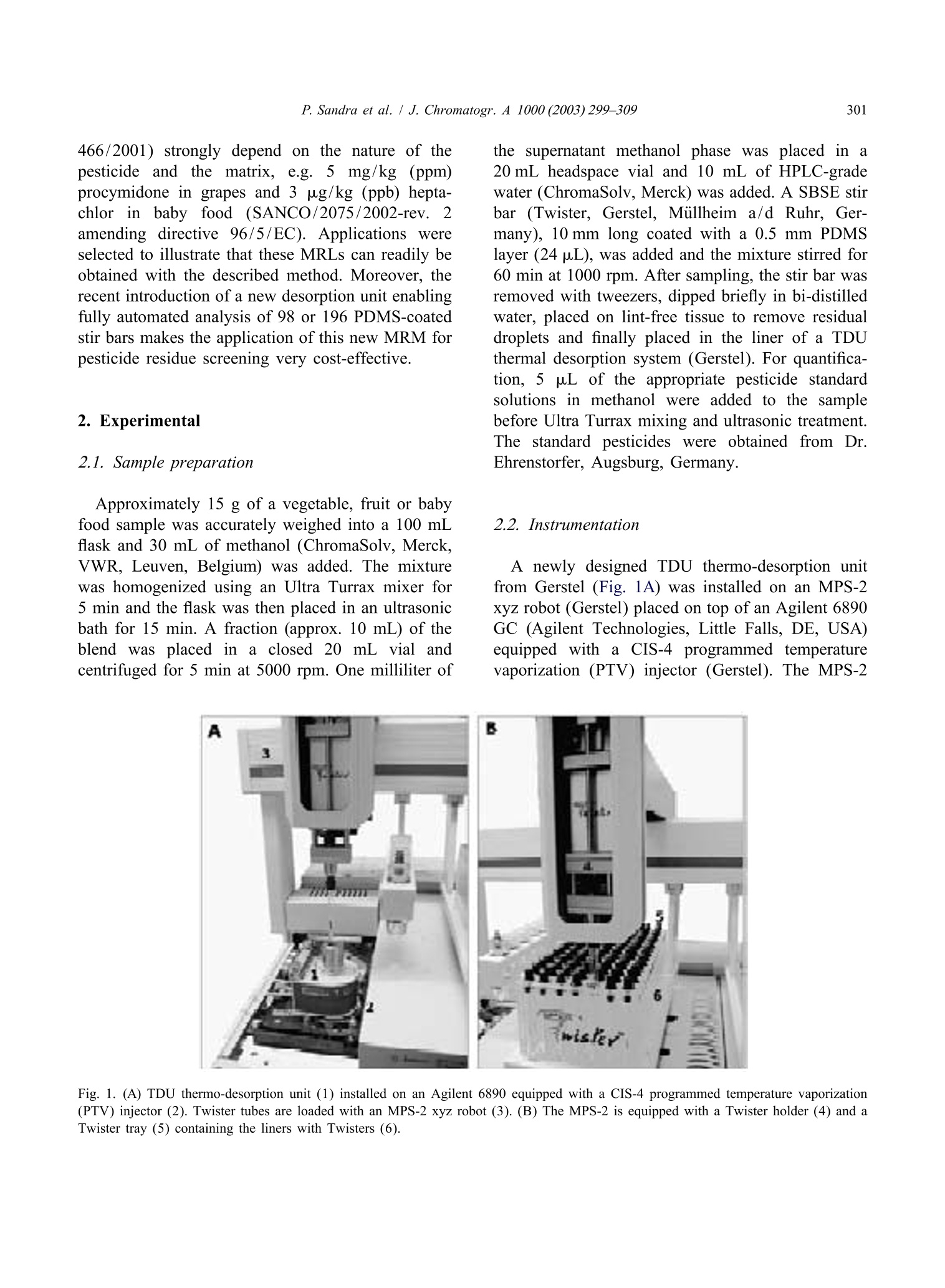
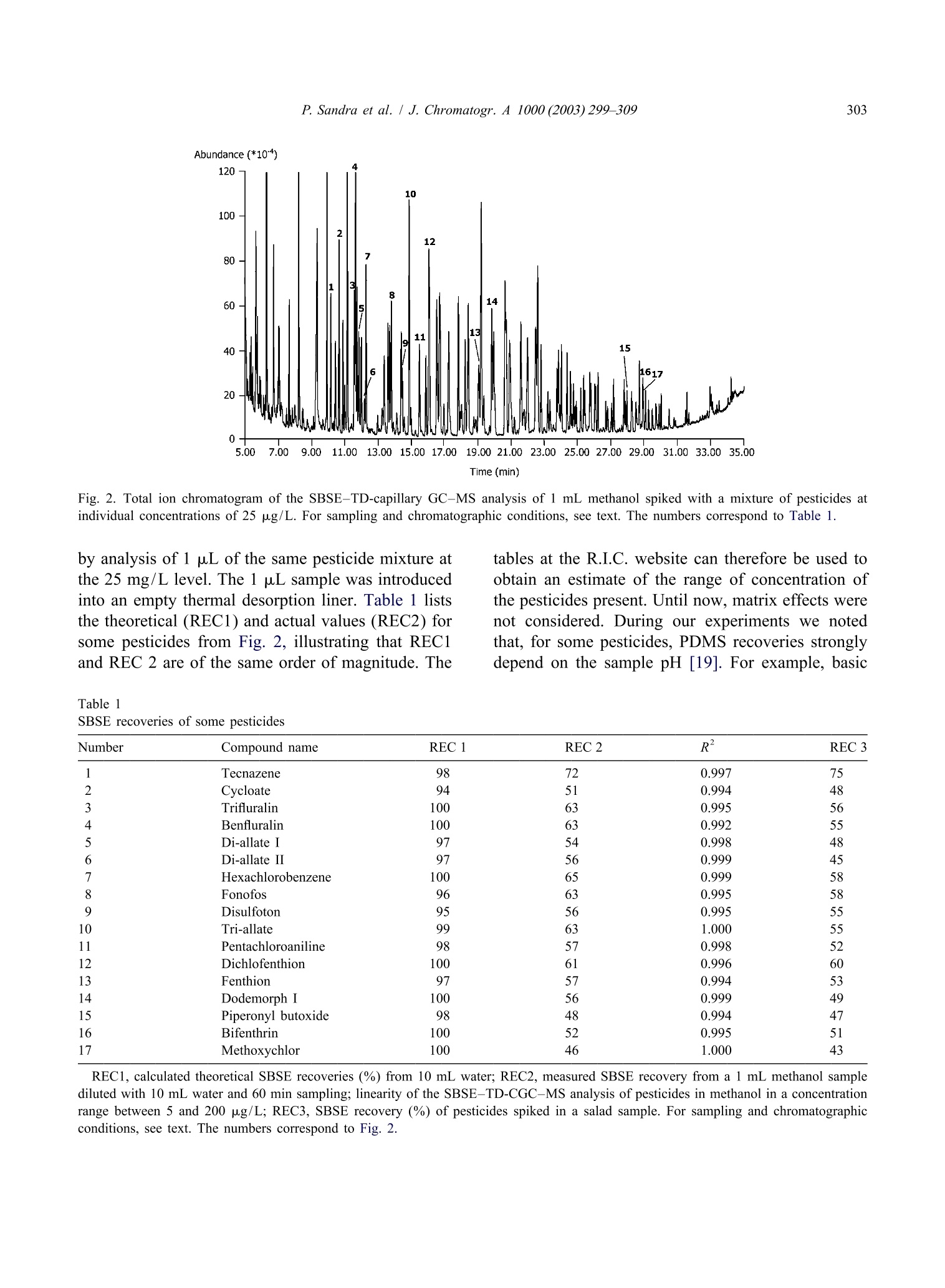
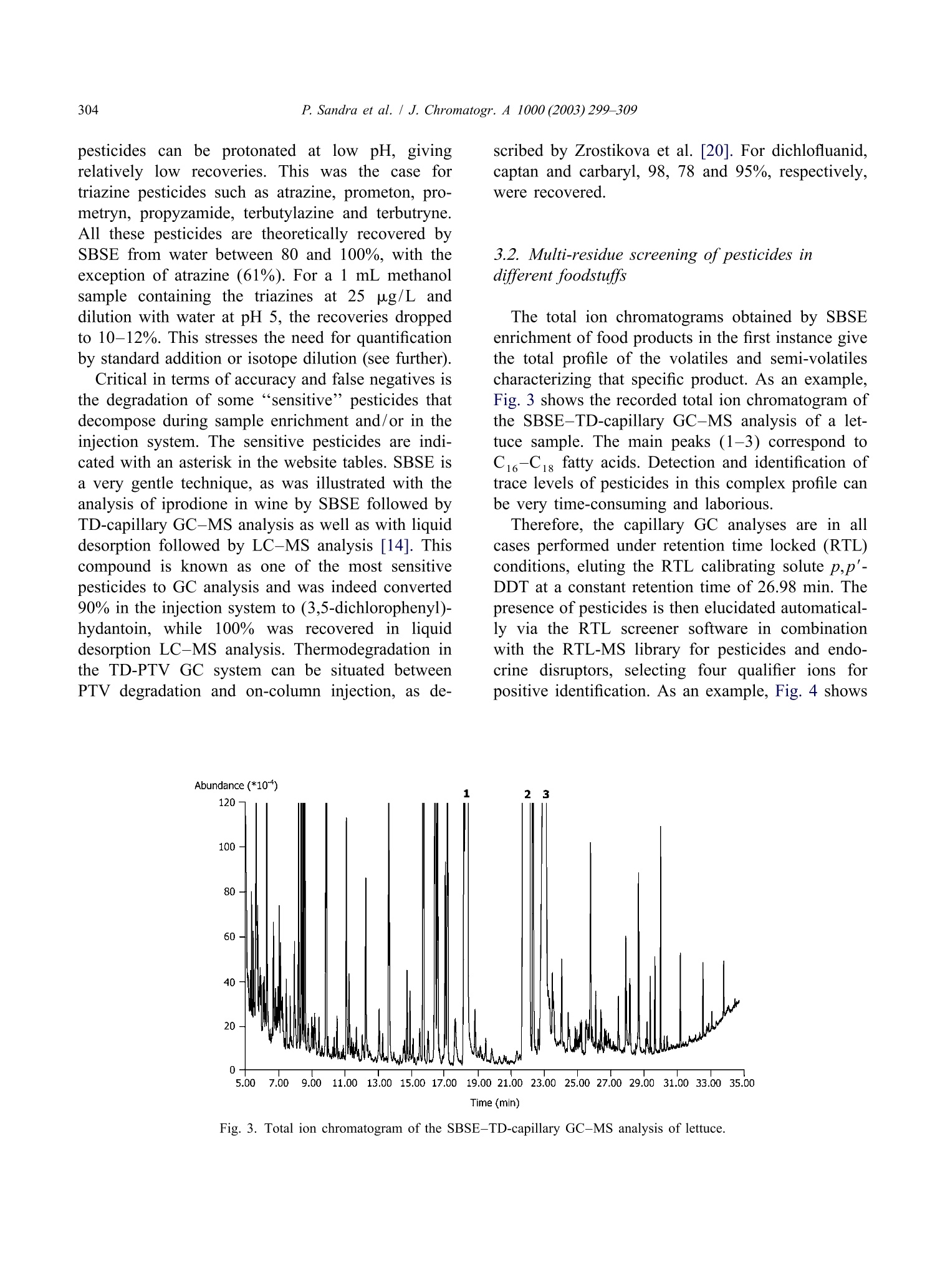
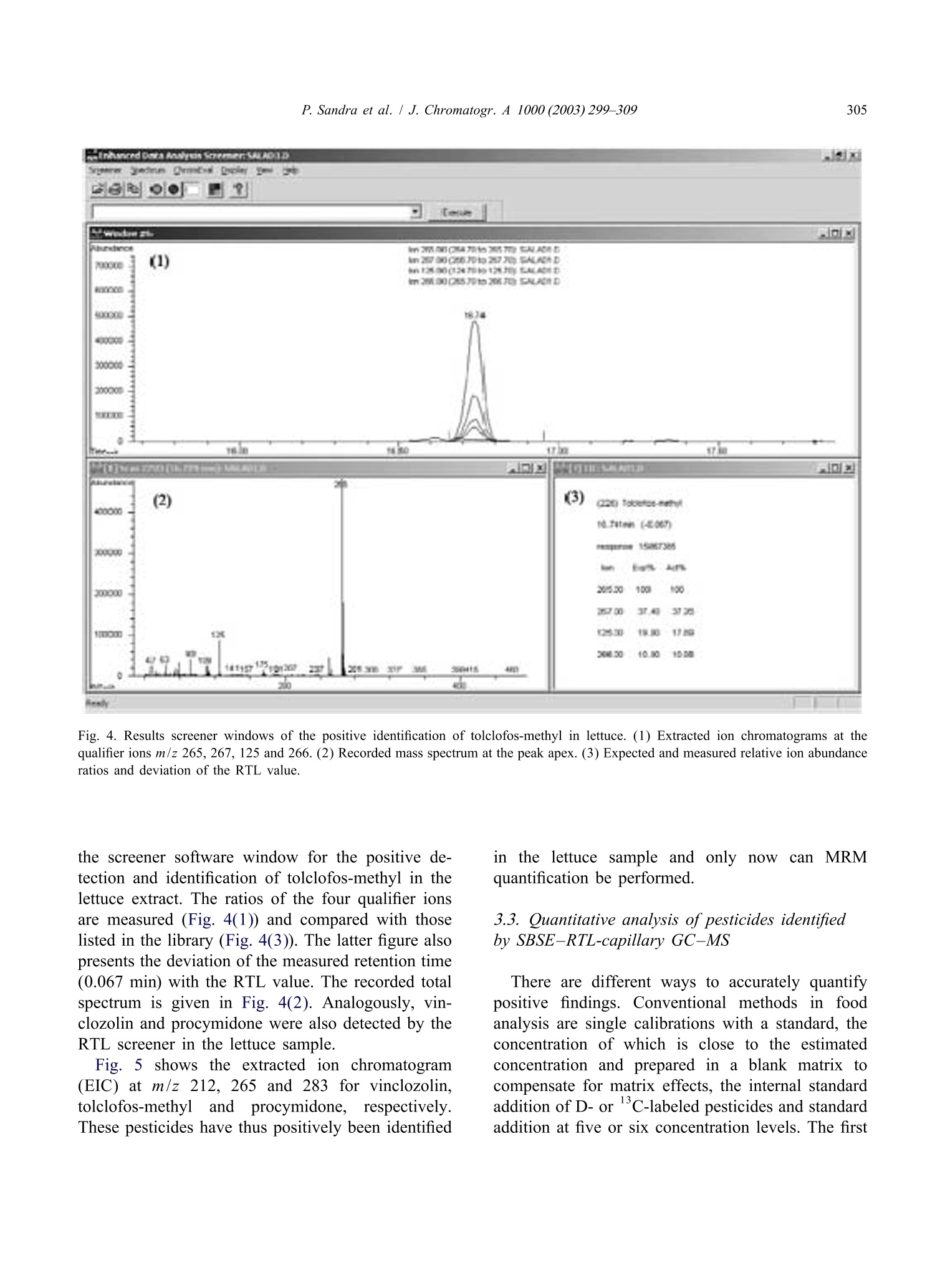
Available online at www.sciencedirect.comJOURNAL OFCHROMATOGRAPHY AJournal of Chromatography A, 1000(2003)299-309 300P. Sandra et al. / J. Chromatogr.A 1000(2003)299-309 www.elsevier.com/locate/chroma Multi-residue screening of pesticides in vegetables, fruits and babyfood by stir bar sorptive extraction-thermal desorption-capillarygas chromatography-mass spectrometry Pat Sandraa,b,c,*, Bart Tienpont, Frank David" Research Institute for Chromatography, Kennedypark 20, B-8500 Kortrijk, Belgium Laboratory of Organic Chemistry, University of Ghent, Krijgslaan 281 S4, B-9000 Ghent, Belgium Department of Chemistry, CENSSUS, University of Stellenbosch, Private Bag X1, Matieland 7602, South Africa Abstract The performance of stir bar sorptive extraction (SBSE) for the enrichment of pesticides from vegetables, fruits and babyfood samples is discussed. After extraction with methanol, an aliquot is diluted with water and SBSE is performed for60 min. By applying a new thermal desorption unit (TDU), fully automated and unattended desorption of 98 stir bars isfeasible, making SBSE very cost-effective. The presence of pesticide residues is elucidated with the retention time lockedgas chromatography-mass spectroscopy method (RTL-capillary GC-MS). With SBSE-RTL-capillary GC-MS operated inthe scan mode, more than 300 pesticides can be monitored in vegetables, fruits and baby food. The multi-residue method(MRM) described provides detectabilities from the mg/kg (ppm) to the sub-ug/kg (ppb) level, thereby complying with themaximum residue levels (MRLs) set by regulatory organizations for pesticides in different matrices. Several examples, i.e.pesticide residues in lettuce, pears,grapes and baby food, illustrate the potential of SBSE-RTL-capillary GC-MS.@ 2003 Elsevier Science B.V. All rights reserved. Keywords: Stir bar sorptive extraction; Retention time locking; Vegetables; Fruit; Baby food; Multi-residue analysis;Pesticides 1. Introduction In recent years, regulatory agencies have empha-sized more and more the need for the developmentand use of analytical methods able to determine, infood products, as many residues as possible from themany insecticides, fungicides and other compoundsapplied in agricultural practice. At present, singleresidue methods (SRMs), i.e. the determination of ( *Corresponding author. Research I n stitute for Chromatography,Kennedypark 2 0 , B - 8500 Ko r trijk, B e lgium. Tel.: + 3 2 - 56-204- 031; f ax: +32-56-204-859. ) one pesticide, e.g. chlormequat, or selective residuemethods (sMRMs), i.e. the determination of a rela-tively small number of chemically related com-pounds, e.g. N-methylcarbamate insecticid areintensively applied for pesticide residue determi-nations in a large number of samples, the pesticidetreatment history of which is known. The use ofSRM and sMRM methods will continue, but thedevelopment anduse of multi-residue methods(MRMs), i.e. the determination of as many pesticidesas possible with only one sample preparation methodand one chromatographic technique, is needed toanalyze samples with an unknown or doubtful pes-ticide treatment history. ( 0021-9673/03/$ - see front matter @ 2 00 3 Elsevier Science B.V. All rights reserved. doi:10.1016/S0021-9673(03)00508-9 ) Asingle chromatographicCttechnique cannotmonitor the currently used 800 and almost 600superceded pesticides (herbicides, fungicides, insec-ticides, araricides, nematicides, growth regulators,synergists, etc.) as listed in The Pesticide Manual[1], and the application of both GC and HPLC ismandatory. Half of the currently used pesticides are,however, amenable to capillary GC analysis and byreplacing the classical selective detection methods bythe universal and specific mass spectrometer, manyclasses of pesticides can be analyzed in a single run.Moreover, the need for confirmation of positivesamples by a secondary technique becomes obsoleteand the MS has the sensitivity required for residueanalysis. A variety of capillary GC-MS-based multi-residuemethods have been developed. For example, workinggroup 4 of the Technical Committee (2TC 2175) of theEuropean Committee for Standardization (CEN)provides information on five multi-residue methodsfor non-fatty foods (EN 1528:1996) [2]. All methodsrequire extraction with organic solvents such asacetone [3-5] acetonitrile [6] and ethylacetate [7];with the exception of Ref. [7], they all requirepartitioning into a solvent mixture, and further clean-up by column chromatography or gel permeationchromatography (GPC) is advised. The multi-residuecapillary GC-MS method that was applied in ourlaboratory until the application of the Twister de-scribed in this contribution is a method used by thelaboratories of the Dutch Inspectorate for HealthProtection [7]. This method is similar to the Lukemethod [3], but the extraction procedures have beenminiaturized to reduce solvent consumption. Recent-ly, Fillion described the analysis of 191 GC-amen-able pesticides in fruit and vegetables by capillaryGC-MS. The sample preparation comprises extrac-tion with 150 mL acetonitrile, a salting-out step,clean-up by solid-phase extraction on octadecyl andon aminopropyl silica and a concentration step [8]. In the present era of “green chemistry”, extractionwith large quantities of toxic solvents is difficult tojustify for multi-residue determinations of pesticidesin 1foodstuffs and solventless sample preparationtechniques should be favored. Solventless sample preparation techniques basedon sorptive extraction have been demonstrated to begood and environmentally friendly alternatives to liquid extraction. The principles and applications ofsorptive extraction have recently been reviewed [9].Solid-phase microextraction (SPME) [10] and stirbar sorptive extraction [11] on polydimethylsiloxane(PDMS) as extraction medium have been applied forthe determination of pesticides in aqueous foodsamples such as drinking water, fruit juices, bever-ages, etc. Yang et al. [12] applied SPME for thedetermination of pesticide residues in fruit juice andBoyd-Boland et al. [13] used SPME for the analysisof pesticide residues in water samples. In both cases,PDMS was selected as being the best sorbent. SBSEfollowed by thermal desorption or liquid desorptionwas used by Sandra et al. [14] for the analysis ofdicarboximide fungicides in wines. The main differ-ence between SPME and SBSE is the much largerquantity of PDMS used in the latter, resulting in veryhigh recoveries. For multi-residue analysis by capillary GC-MS,important improvements have been made in recentyears. Through the features of electronic pneumaticcontrol (EPC), retention time locked libraries (RTLs)for GC-amenable pesticides and endocrine disrupterscan be constructed, andtby linking the lockedretention times to the mass spectral data, hardly anypesticide that is in the library can escape detectionand elucidation [15,16]. The Agilent RTL-MS librarypresently comprises 556677 s:ubstances. We recentlyevaluated SBSE as a sample preparation techniquefor the enrichment of pesticides from aqueous ma-trices (water and beverages) and came to the conclu-sion that more than 400 pesticides in the RTL-MSlibrary can be enriched with recoveries complyingwith the required limits of quantification (LOQs) setby regulatory organizations, e.g. the 0.1 ug/1 (ppb)norm for drinking water. The list of pesticidesamenable to SBSE enrichment and RTL-capillaryGC-MS analysis for solid food samples is somewhatsmaller (i.e. ca. 350 pesticides) because of matrixeffects in solid samples. The complete pesticide listsffor both aqueous (Tables 1-4) and food samples(Tables 3 and 4) can be found on the websitewww.richrom.com/html/ric_appnotes.html. The listscontain the locked retention times, four qualifier ionsfor MS confirmation, the log P values and thetheoretical SBSE recoveries on Twisters of 24 and116 uL. The maximum residue levels (MRLs) set bythe European Community (Directives 645/2000 and 466/2001) strongly depend on the nature of thepesticide and the matrix., e.g.5 mg/kg (ppm)procymidone in grapes and 3 ug/kg (ppb) hepta-baby food (SANCO/2075/2002-reV. 2amending directive 96/5/EC). Applications wereselected to illustrate that these MRLs can readily beobtained with the described method. Moreover, therecent introduction of a new desorption unit enablingfully automated analysis of 98 or 196 PDMS-coatedstir bars makes the application of this new MRM forpesticide residue screening very cost-effective. 2. Experimental 2.1. Sample preparation Approximately 15 g of a vegetable, fruit or babyfood sample was accurately weighed into a 100 mLflask and 30 mL of methanol (ChromaSolv, Merck,VWR, Leuven, Belgium) was added. The mixturewas homogenized using an Ultra Turrax mixer for5 min and the flask was then placed in an ultrasonicbath for 15 min. A fraction (approx. 10 mL) of theblend was placed in a closed 20 mL vial andcentrifuged for 5 min at 5000 rpm. One milliliter of the supernatant methanol phase was placed in a20 mL headspace vial and 10 mL of HPLC-gradewater (ChromaSolv, Merck) was added. A SBSE stirbar (Twister, Gerstel, Millheim a/d Ruhr, Ger-many), 10 mm long coated with a 0.5 mm PDMSlayer (24 pL), was added and the mixture stirred for60 min at 1000 rpm. After sampling, the stir bar wasremoved with tweezers, dipped briefly in bi-distilledwater, placed on lint-free tissue to remove residualdroplets and finally placed in the liner ofa TDUthermal desorption system (Gerstel). For quantifica-tion, 55 pL of the appropriate pesticide standardsolutions in methanol were added to the samplebefore Ultra Turrax mixing and ultrasonic treatment.The standard pesticides were obtained from Dr.Ehrenstorfer, Augsburg, Germany. 2.2. Instrumentation A newly designed TDU thermo-desorption unitfrom Gerstel (Fig. 1A) was installed on an MPS-2xyz robot (Gerstel) placed on top of an Agilent 6890GC (Agilent Technologies, Little Falls, DE, USA)eequipped with a CIS-4 programmed te turevaporization (PTV) injector (Gerstel). The MPS-2 Fig. 1. (A) TDU thermo-desorption unit (1) installed on an Agilent 6890 equipped with a CIS-4 programmed temperature vaporization(PTV) injector (2). Twister tubes are loaded with an MPS-2 xyz robot (3). (B) The MPS-2 is equipped with a Twister holder (4) and aTwister tray (5) containing the liners with Twisters (6). was equipped with a Twister tray (Fig.1B) allowingautomated and unattended desorption of 98 Twisters. Splitless thermal desorption was performed byprogramming the TDU from 40 to 280 ℃ (5 min) ata rate of 60 ℃/min. The analytes were cryo-focusedin the PTV at -150 ℃ with liquid nitrogen prior toinjection. An empty baffled liner was used in thePTV injector. For splitless injection (2 min) the PTVwas ramped from -150 to 280 ℃ (2 min) at a rateof 600C/min. Capillary GC analysiwsas per-fc ed on a 30 m×250 um I.D., 0.25um deHP-5MS column (Agilent Technologies). The ovenwas programmed from 70℃ (2 min) at 25 ℃/minto 150 ℃, at 3 C/min to 200℃ and finally at8℃/min to 300℃. This is the temperature programrequired for the RTL screener option (Agilent Tech-nologies). Helium was used as carrier gas. The headpressure was calculated using the retention timelocking (RTL) software so that p,p'-DDT. waseluting at a constant retention time of 26.98 min. AnAgilent 5973 mass spectrometric detector (MSD)was used in the scan mode (m/z 40-500) for allsamples. Screening of pesticideswasssperformedusing the automatic RTL screener software in combi-nation with the Agilent RTL pesticide library. Forthe baby food sample, quantitation of piperonylbutoxide: was performed using the MSD in theselected-ion monitoring (SIM) mode at m/z 176(quantitation ion) and m/z 177, 144 and 178 (con-firmation ions). The dwell time was set to 100 ms. 3. Results and discussion 3.1. SBSE-TD-capillary GC-MS analysis ofpesticides Solid samples cannot be extracted directly usingstir bar sorptive extraction and a pre-extraction ofpesticides in vegetables, fruitss or baby foodistherefore performed. Acetonitrile, methanol and ace-tone were evaluated as extraction media and ex-traction efficiencies for acetonitrile and methanolwere very similar, both being more efficient thanacetone. Methanol was preferred because it is en-vironmentally more friendly than acetonitrile. Veget-ables and fruit samples were homogenized in anUltra Turrax and 30 mL methanol was added to 15 g of the homogenized sample. Baby food samples weregenerally pastes and were extracted as such. Onemilliliter of the methanol extract was then dilutedwith1 water to obtain an aqueous matrix beforeextraction. To the best of our knowledge this is thefirst enrichment technique described in which dilu-tion isinvolved. Recoveries of pesticides fromaqueous samples by SBSE can be estimated from theoctanol-water distribution coefficient (K。/w) and thesample-PDMS phase ratio, B [11]. The lists atwww.richrom.com/html/ric_appnotes.htmll containthe Ko/w or log P values, calculated with a dedicatedSRC-KOWWIN software package (Syracuse Re-search, Syracuse, NY, USA) according to a fragmentconstant estimation methodology [17] for a widevariety of pesticides. The theoretical SBSE re-coveries were calculated for a 10 mL water sampleusing Twisters containing 24 pL (10 mm L×0.5 mmd.) and 116 uL PDMS (20 mm L×1.0 mm d). Alarger PDMS phase volume affects the sorptiveenrichment and recoveries are higher for the largerTwister. For variations inPDMS and/or watervolume the Twister calculator present on the samewebsite may be applied. For the pesticides listed inTables 3 and 4 on the website, the stir bar with24 pL PDMS performs very well and this Twisterwas applied throughout this work. The theoretical recoveries represent only indica-tive values because (i) equilibrium of the solutesbetween the PDMS coating and the sample is not yetattained after 60 min sampling, (ii) methanol consti-tutes 10% of the sample and (iii) matrix effects arenot taken into consideration. Reaching equilibriumconditions is impractical (several hours) and notstringent as long as sampling conditions are keptconstant for calibration. For solutes with log P > 2.5it has been shown that 10%methanol has noinfluence onnrecovery [18]. To compensate formatrix effects, quantitation is performed by standardaddition (see further). Recovery values from water-methanol (90:10) at 60 min sampling time for somepesticides were calculated by analyzing a sample bySBSE-TD-capillary GC-MS composed of 1 mLmethanol spiked with a mixture of pesticides toindividual concentrations of 25 ug/L (ppb) anddSBailuted with10 mL water (Fig. 2). The SBSErecoveries were measured by comparison of the peakareas of the SBSE experiments with those obtained Time (min) Fig. 2. Total ion chromatogram of the SBSE-TD-capillary GC-MS analysis of 1 mL methanol spiked with a mixture of pesticides atindividual concentrations of 25 ug/L. For sampling and chromatographic conditions, see text. The numbers correspond to Table 1. by analysis of 1 uL of the same pesticide mixture atthe 25 mg/L level. The 1 uL sample was introducedinto an empty thermal desorption liner. Table 1 liststhe theoretical (REC1) and actual values (REC2) forsome pesticides from Fig. 2, illustrating that REC1and REC 2 are of the same order of magnitude. The Table 1SBSE recoveries of some pesticides Number Compound name REC 1 REC 2 R2 REC3 Tecnazene 0.997 75 2 Cycloate 0.994 Trifluralin 100 0.995 Benfluralin 100 0.992 5 Di-allate I 0.998 6 Di-allate II 0.999 Hexachlorobenzene 0.999 Fonofos 0.995 9 Disulfoton 0.995 Tri-allate 1.000 Pentachloroaniline 0.998 Dichlofenthion 100 0.996 Fenthion 97 0.994 Dodemorph I 100 0.999 Piperonyl butoxide 98 0.994 Bifenthrin 100 0.995 Methoxychlor 100 1.000 REC1, calculated theoretical SBSE recoveries (%) from 10 mL water; REC2, measured SBSE recovery from a 1 mL methanol samplediluted with 10 mL water and 60 min sampling; linearity of the SBSE-TD-CGC-MS analysis of pesticides in methanol in a concentrationrange between 5 and 200 pg/L; REC3, SBSE recovery (%) of pesticides spiked in a salad sample. For sampling and chromatographicconditions, see text. The numbers correspond to Fig. 2. pesticides can1be protonated at low pH, givingrelatively low recoveries.s. This was the case fortriazine pesticides such as atrazine, prometon, pro-metryn, propyzamide, terbutylazine and terbutryne.All these pesticides are theoretically recovered bySBSE from water between 80 and 100%, with theexception of atrazine (61%). For a1 mL methanolsample containing the triazines at 25pg/L anddilution with water at pH 5, the recoveries droppedto 10-12%. This stresses the need for quantificationby standard addition or isotope dilution (see further). Critical in terms of accuracy and false negatives isthe degradation of some“sensitive” pesticides thatdecompose during sample enrichment and/or in theinjection system. The sensitive pesticides are indi-cated with an asterisk in the website tables. SBSE isa very gentle technique, as was illustrated with theanalysis of iprodione in wine by SBSE followed byTD-capillary GC-MS analysis as well as with liquiddesorption followed by LC-MS analysis [14]. Thiscompound is known as one of the most sensitivepesticides to GC analysis and was indeed converted90% in the injection system to (3,5-dichlorophenyl)-hydantoin, while 100%6wass recovered in liquiddesorption LC-MS analysis. Thermodegradation inthe TD-PTV GC system can be situated betweenPTV degradation and on-column injection, as de- scribed by Zrostikova et al. [20]. For dichlofluanid,captan and carbaryl, 98, 78 and 95%, respectively,were recovered. 3.2. Multi-residue screening of pesticides indifferent foodstuffs The total ion chromatograms obtained by SBSEenrichment of food products in the first instance givethe total profile of the volatiles and semi-volatilescharacterizing that specific product. As an example,Fig.3 shows the recorded total ion chromatogram ofthe SBSE-TD-capillary GC-MS analysis of a let-tuce sample. The main peaks (1-3) correspond toC16-C18 fatty acids. Detection and identification oftrace levels of pesticides in this complex profile canbe very time-consuming and laborious. Therefore, the capillary GC analyses are in allcases performed under retention time locked (RTL)conditions, eluting the RTL calibrating solute p,p'-DDT at a constant retention time of 26.98 min. Thepresence of pesticides is then elucidated automatical-ly via the RTL screener software in combinationwith the RTL-MS library for pesticides and endo-crine disruptors, selecting four qualifier ions forpositive identification. As an example, Fig. 4 shows Fig. 3. Total ion chromatogram of theSBSE-TD-capillary GC-MS analysis of lettuce. Fig. 4. Results screener windows of the positive identification of tolclofos-methyl in lettuce. (1) Extracted ion chromatograms at thequalifier ions m/z 265,267,125 and 266. (2) Recorded mass spectrum at the peak apex. (3) Expected and measured relative ion abundanceratios and deviation of the RTL value. the screener software window for the positive de-tection and identification of tolclofos-methyl in thelettuce extract. The ratios of the four qualifier ionsare measured (Fig. 4(1)) and compared with thoselisted in the library (Fig. 4(3)). The latter figure alsopresents the deviation of the measured retention time(0.067 min) with the RTL value. The recorded totalspectrum is given in Fig. 4(2). Analogously, vin-clozolin and procymidone were also detected by theRTL screener in the lettuce sample. Fig. 5shows the extracted ion chromatogram(EIC) at m/z 212, 265 and 283 for vinclozolin,tolclofos-methyl and procymidone, respectively.These pesticides have thus positively been identified in the lettuce sample and only nowcan1W:MRMquantification be performed. 3.3. Quantitative analysis of pesticides identifiedby SBSE-RTL-capillary GC-MS There are different ways to accurately quantifypositive findings. Conventional methodss in foodanalysis are single calibrations with a standard, theconcentration of which is close to the estimatedconcentration and prepared in a blank matrix tocompensate for matrix effects, the internal standardaddition of D-or 1c-labeled pesticides and standardaddition at five or six concentration levels. The first method requires a blank sample to compensate formatrix effects, but as strange as this may appear,such samples are not readily available. The secondapproach cannot be applied inaaMRM becauselabeled standardsasof only a few pesticidesarecommercially available. The last method is by far theeasiest to use in a routine environment and has beenapplied for the determination of dicarboximide fun-gicides in wines by SBSE-capillary GC-MS [14].However, this method is time-consuming and thuscostly. Precise quantification, in fact, is only neededwhen the detected quantity is expected to exceed themaximum allowable level. Maximum residue levels(MRLs) in foodstuffs, with the exception of babyfood, are relatively high and, with semi-quantitativemethods, elucidation of negative, i.e. far below theMRL, and positive samples,i.e. concentration aroundthe MRL values, can easily be made. Only accuratequantification is needed for positive samples. For the lettuce sample (Fig. 5) the methanolextraction efficiency and matrix effects were mea-sured indirectly by SBSE recovery calculation ofsome pesticides spiked in 15 g lettuce at the in-dividual level of 5 ug/kg. The spiked sample wasextracted with 30 mL methanol and 1 mL of theextract was analyzed as described above. The total methanol liquid extraction-SBSE recoveries (REC3)are listed in Table 1 and are very similar to those ofREC2. This indicates a nearly quantitative extractionof the pesticides by methanol from the salad sample.This implies that semi-quantitation can be performedby constructing a calibration line in methanol andrecalculation of the concentration to the sampleamount. A pesticide mixture containing the identifiedpesticides was prepared and spiked in 1 mL ofmethanol to concentrations of5, 10, 25, 50,100 and200 pg/L, corresponding to approximate levels of10, 20, 50,100,200 and 400 pg/kg sample. Thecorrelation coefficients are listed in Table 1 and areall greater than 0.99. The main qualifier ion was usedto construct the calibration graphs. Vinclozolin,tolclofos-methyl and procymidone in1the lettucesample (Fig. 5) were quantified at 175, 17 and 249ug/kg, respectively. These are mean values of sixcomplete analyses (n=6) and the RSDs % were 5.4,8.8 and 4.6, respectively. All these values are farbelow the MRL of the European Community, whichare 5 mg/kg for vinclozolin and procymidone and0.5 mg/kg for tolclofos-methyl in lettuce. Accuratequantitation is thus not required because the lettucesample can be considered negative. In the same way,pear and grape samples were analyzed by the SBSE- Time (min) Fig. 6. Extracted ion chromatograms at m/z 137, 272 and 341 for tolylfluanid (peak 1), endosulfan-sulfate (peak 2) and bromopropylate(peak 3, out of scale), respectively, of a pear sample analysed by SBSE-TD-capillary GC-MS. For sampling and chromatographicconditions, see text. Time (min) Fig. 7. Extracted ion chromatograms at m/z 283 for procymidone (peak 1) and m/z 183 for permethrin I (peak 2) and II (peak 3) of a grapesample analyzed by SBSE-TD-capillary GC-MS. For sampling and chromatographic conditions, see text. capillary GC-MS procedure. Fig. 6) sshows theextracted ion chromatograms at m/z 137, 272 and341 of the pear sample, indicating the presence oftolylfluamid at 59 pg/kg (peak 1), endosulfan-sul-fate at 3 ug/kg (peak2) and bromopropylate at 190pg/kg (peak 3), respectively. The EC MRL valuesfor pears are 2, 0.3 and 2 mg/kg, respectively. Fig. 7 shows the EICs at m/z 283 and 183 forprocymidone (peak 1) and permethrin I and II (peaks2 and 3) elucidated by the RTL screener in a grapesample. The concentration levels were measured at 172 ug/kg (EC norm 5 mg/kg), 20 pg/kg and83 ug/kg (EC norm for the sum 1 mg/kg), respec-tjtively. MRLs in baby food are becoming more and morestringent and ultra-trace level analysis (ug/kg andsub-ug/kg) is required. Baby food is a more com-plex matrix because, besides vegetables or fruits,small quantities of fat are also present. This alsoaffects the efficiency of the pre-extraction in metha-nol as well as the SBSE recovery. Standard additioncalibration is the only valid alternative for baby food. Abundance(*10°) Time (min) Fig. 8. Selected ion chromatograms at m/z 176 for piperonyl butoxide (peak 1) in the extract of an unspiked (A) and a spiked (2pg/kg)baby food sample (B). Standard addition curve of piperonyl butoxide in the concentration range between 2 and 50 pg/kg (C). For samplingand chromatographic conditions, see text. Ten baby food samples were analyzed and, in foursamples, pesticide traces between 0.5 and 2 ppb weredetected by the described method, namely piperonylbutoxide, pyrimethanil (two samples) and bromo-propylate. As an illustration, the analysis of a babyfood containing vegetables, complete rice and chick-en using the described method is presented. Pre-liminary screening was performed with the massspectrometric detector in the scan mode, therebyelucidating the presence of small traces of piperonylbutoxide. The pesticide shows high affinity forPDMS (log K。/w 4.29) and can be extracted from1:10 diluted methanol in water with a recovery of48% (REC3 in Table 1). For accurate quantitation,the MSD was used in the selected-ion monitoring(SIM) mode at m/z 176. Six sub-samples of the babyfood sample were spiked with a piperonyl butoxidestandard in methanol at concentrations of 0, 2, 5, 10,20 and 50 pg/kg. Fig. 8 shows the selected ionchromatogram at m/z 176 of an unspiked sample andof a sample spiked at 2 ug/kg piperonyl butoxide.The correlation coefficient of the standard additioncurve was R’>0.99 (Fig. 8C) and the concentrationin the sample was calculated at 1.1 ug/kg. 4. Conclusion Stir bar sorptive extraction (SBSE) in combinationwith thermal desorption-RTL-locked capillary GC-MS is a versatile and cost-effective method for theelucidation and quantification of over 350 pesticidesin different foodstuffs down to sub-ppb levels. Acknowledgements Gerstel GmbHand Agilent Technologiesaarethanked for supporting our SBSE pesticide research.B.T. thanks the Institute for Scientific Technology,Flanders, Belgium, for a study grant. ( [ 1] C.D.S. T omlin ( E d.), The P e sticide M a nual, 12 t h ed . , Th e B ritish Crop Protection Council, F a rnham, Su r rey, 20 0 0. ) ( [2] C. Schenzler, H.-P . Their, Foo d Addit. Contam. 18 (10)(2001) 875. ) ( [3] M.A. Luke, J.E. Froberg, G.M. Doose, H.T. Masumoto, J. Assoc. Off. Anal. Chem. 6 4 (1981) 11 8 7. ) ( [4] G. B ecker, in: Manual of Pesticide Residue Analysis, Vol. 2, VCH, Weinheim, 1 992, p.313. ) ( [5] W. Specht, i n : Manual of Pesticide Residue Analysis, Vol. 2, VCH, Weinheim,1992,p. 317. ) ( [6] P. Cunniff (Ed.), 16th ed., Officia l Methods of Analysis ofAOAC In t ernational, Vol 1 , AOAC, Arlington, V A , 1 9 95, p . 1. ) ( [7] P. van Zoonen (Ed.), Analytical M ethods f o r P e sticide R esidues i n F oodstuffs, P art 1, General I nspectorate f orHealth P rotection, RIVM, Bilthoven, N etherlands, 1 996. ) ( [8] J. Fullion, www.chem.a g ilent.c o m / cag/ p eak/peak1-96/ p es- t icide. h tm l . ) ( [9] E. Baltussen, C.A. C ramers, P. Sandra, Anal. Bioanal. Chem. 373 (2002) 3. ) ( [10] J. Pawliszyn, Solid Phase M icroextraction. Theory a nd Applications, Wiley-VCH, N ew Y o rk, 1 9 97. ) ( [1 1 ] E. Baltussen, P. Sandra, F. David, C.A. Cramers, J. Microcol.Sep. 11 (1999) 737. ) ( [12] K .-W. Yang, R. Eisret, H. L ord, J. Pawliszyn, in: J . Pawliszyn(Ed.), Applications of Solid Phase Microextraction , RSC, H ertfordshire, UK, 1 999, p . 435. ) ( [13] A.A. Boyd-Boland, M . Chai, Y .Z. L uo, Z. Z hang, J. Paw- zha l iszyn, T . Gorecki, Environ. Sci. Technol. 2 8 (1 9 94) 5 9 6A. ) ( [14] P. Sandra , B. Tienpont, J. Vercammen , A. Tredoux, T.Sandra, F. David,J. Chromatogr. A 9 2 8 (2001) 117 . ) ( [ 1 5] P h. Wylie, B. Quimby, Agilent Application Note 5967-5860E(2000), www.agilent.co m /chem. ) ( [19] A. Tredoux, H. L auer, T. H eideman, P . S andra, J. High R esolut. Chromatogr. 23 (2000) 644. ) ( [20] J. Zrostlikova, J. Hajslova, M. G odula, K . Ma s tovska, J.Chromatogr. A 9 37 (2001) 7 3. ) 通常农药残留分析有这几个步骤,首先用有机溶剂来进行液液萃取,然后通过柱色谱法和/或凝胶渗透色谱(GPC)来实现萃取液的净化,对净化后的萃取进行浓缩,最后使用色谱分离和质谱鉴定。在这些传统的样品制备技术中,大多数步骤繁琐、费时、劳动密集,相当复杂,而且它们消耗大量溶剂。近年来,固相萃取(SPE)和基质固相分散(MSPD)在农药残留分析中作为替代样品制备方法被引入。这些小型化方法可以大大减少溶剂消耗。然而,主要缺点是,利用这些技术的富集因子相当有限,即原始样品量与最终提取体积量有限,所以要么需要提取液浓缩到较小的体积(<1毫升)或是对原提取液进行大体积注射,以补偿较低的整体灵敏度。出于这个原因,搅拌棒吸附萃取(SBSE),这是一个简单的,无溶剂的技术,允许在一个步骤中萃取和浓缩。搅拌棒吸附萃取SBSE所使用的磁力搅拌棒TwisterSBSE的萃取原理与SPME相同,是利用萃取层来萃取样品中或是顶空中的分析物。区别在于,1. SBSE使用的萃取层更大,是SPME的50-250倍,故更灵敏。目前市面上类似萃取技术中萃取层最大的,也是最灵敏的。2. SBSE使用搅拌棒浸入式萃取,对液体样品的萃取效率最高, 特别是对挥发性低的化合物有更高的回收率。3. 样品无需加热,室温下萃取即可,因此更适合现场采样。4. 离线平行采样,不占用分析仪器,高通量,更灵活。SBSE的采样是离线平行采样, 在一个萃取时间内可以同时完成若干个样品使用SBSE搅拌棒吸附萃取来检测食品中的农残的操作非常简单,比如只要将取15克食品样品[1](蔬菜,水果,婴儿食品等)加入30毫升的甲醇,然后使用实验分散器搅拌均匀,再放入超声波中15分钟萃取,取1毫升萃取液,加入10毫升水,最后放入Twister(带有吸附层的磁力搅拌棒)搅拌60分钟即可。搅拌完取出Twister, 这时各种各样的农残已经被吸附在吸附层中,然后使用热脱附-质谱联用进样分析即可。对其他的液体样品如茶[2,3],酒[4],甘蔗汁[5],醋[6]就更简单,只要将Twister直接放入一定量的样品中,搅拌一段时间即可完成萃取。搅拌棒吸附萃取(SBSE)与热脱附-气相色谱质谱联用是一种多用途且经济有效的方法,可以有效地对不同食品中超过400种农药同时进行分析和定量。是一个简单的,无溶剂的技术,并且允许在一个步骤中实现萃取和浓缩。该方法应用于各种蔬菜(番茄,黄瓜,生菜,菠菜),水果(葡萄),饮料(茶,甘蔗汁),酒类,醋,婴儿食品中多农残的检测。检测下限在ppb到亚ppb之间。相对标准偏差低于10%,检测结果精确度高。线性范围广,并且r²平均大于0.997。400多种农残部分列表参考文献[1] Multi-residue screening of pesticides in vegetables, fruits and babyfood by stir bar sorptive extraction–thermal desorption–capillarygas chromatography–mass spectrometry. Journal of Chromatography A, 1000 (2003) 299–309[2]Multi-Residue Method for the Determination of Five Groups of Pesticides in Non-Fatty Food Samples by Dual Stir Bar Sorptive Extraction (Dual SBSE) and Thermal Desorption GC-MS. Gerstel AppNote 3/2005[3]SBSE-GC-ECD/FPD in the Analysis of Pesticide Residues inPassiflora alata Dryander Herbal Teas. J. Agric. Food Chem. 2003, 51, 27−33 27.[4]Stir bar sorptive extraction applied to the determination ofdicarboximide fungicides in wine. Journal of Chromatography A, 928 (2001) 117–126[5]Comparison of stir bar sorptive extraction and membrane-assisted solventextraction as enrichment techniques for the determination of pesticideand benzo[a]pyrene residues in Brazilian sugarcane juice. Journal of Chromatography A, 1114 (2006) 180–187[6]Optimization of stir bar sorptive extraction applied tothe determination of pesticides in vinegars. Journal of Chromatography A, 1165 (2007) 144–150
确定



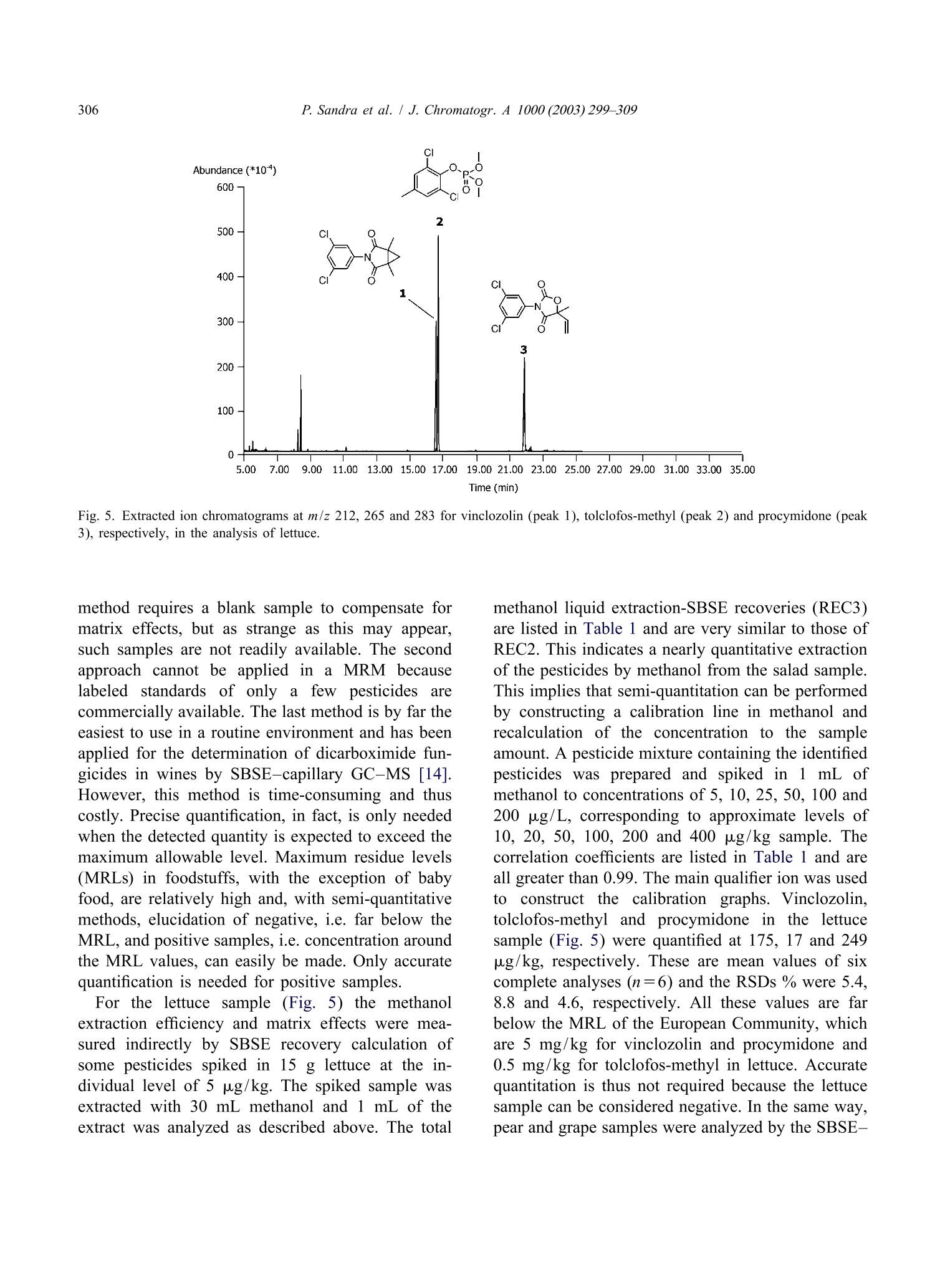
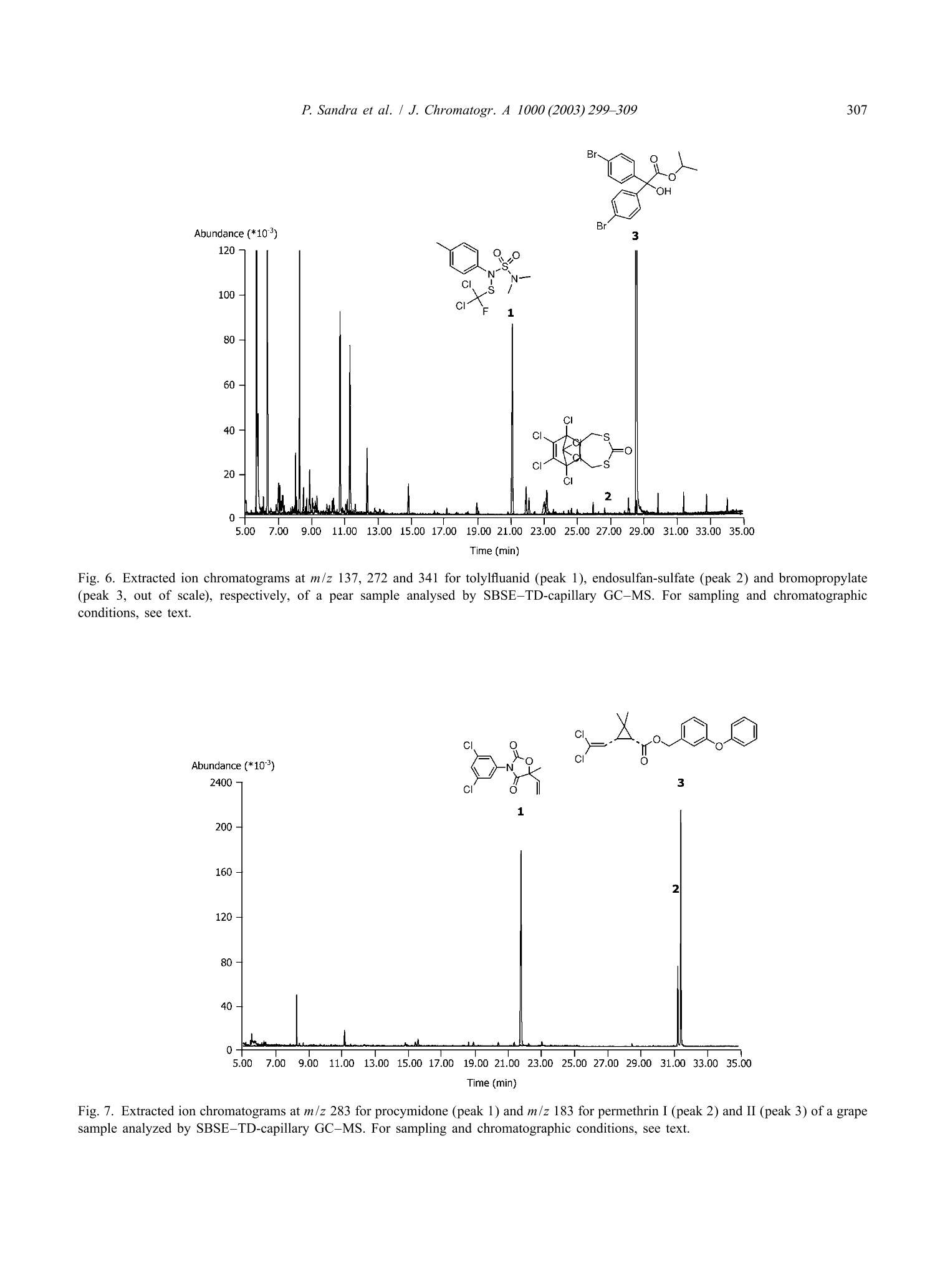
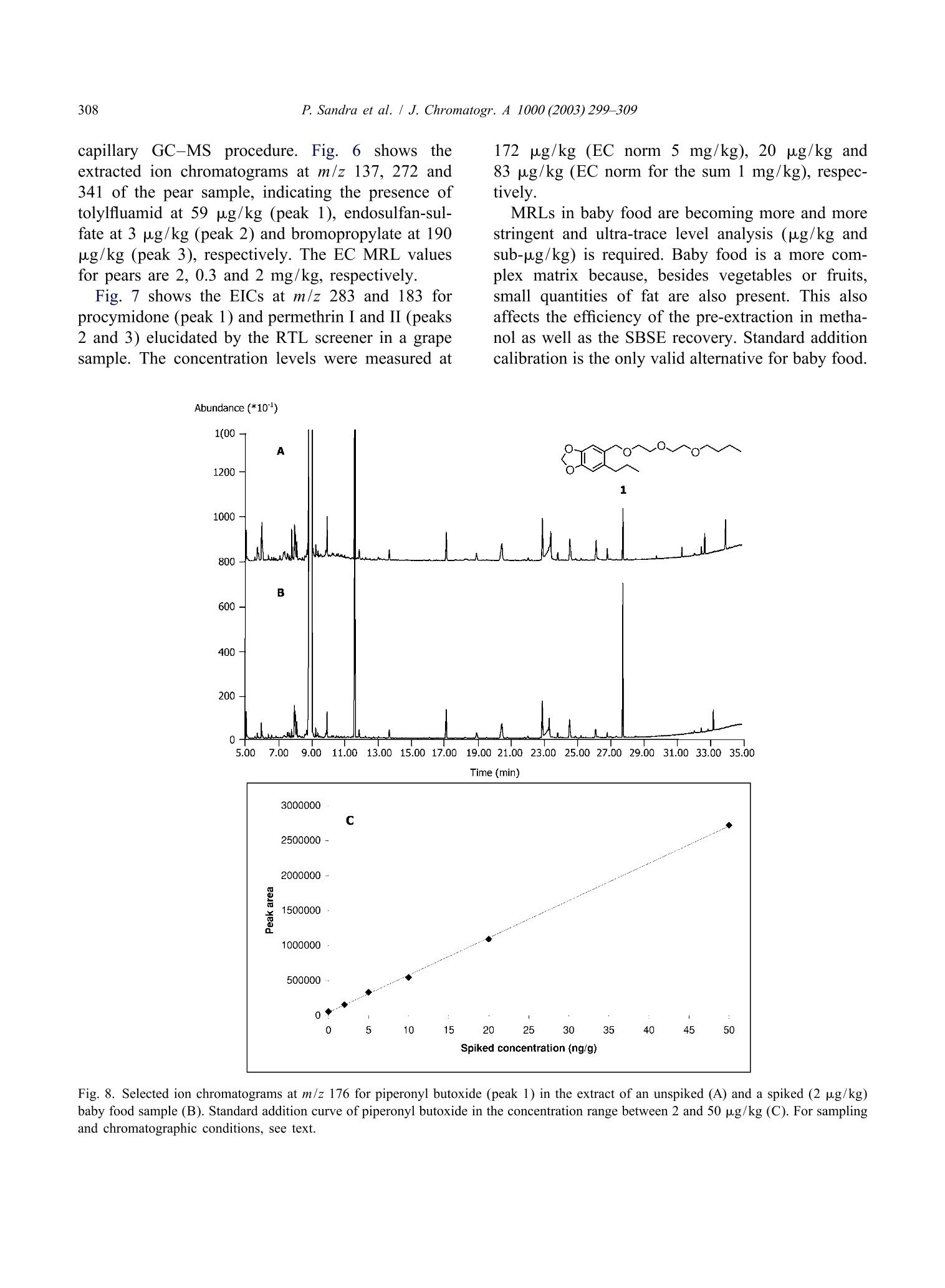

还剩9页未读,是否继续阅读?
GERSTEL(哲斯泰)为您提供《蔬菜, 水果中农残检测方案(其它萃取设备)》,该方案主要用于蔬菜中农药残留检测,参考标准--,《蔬菜, 水果中农残检测方案(其它萃取设备)》用到的仪器有GERSTEL 搅拌棒Twister (萃取、固相微萃取)、GERSTEL热脱附单元TDU2 (热解吸,热解析)
推荐专场
固相微萃取仪、固相微萃取装置
更多
相关方案
更多
该厂商其他方案
更多