
方案详情
文
中草药(CHM)的认证是一项艰巨的任务,这一事实可归因于植物和CHM中使用的制剂的高度复杂性以及各个植物的自然变异性。 通常,色谱指纹图谱被认为是最有效的方法之一。 作为分析中国传统处方的一个例子,本应用指南显示了四物汤以及四物汤中所含各个草药的全面二维液相色谱分析。 对于每种草药,选择特征成分峰,然后创建模板。 模板与在四物汤中检测到的峰相匹配,以研究检测传统中药中各个草药的特征成分的可能性。 另外,检测了从四物汤遗漏和替换一种草药后模板匹配的变化。
方案详情

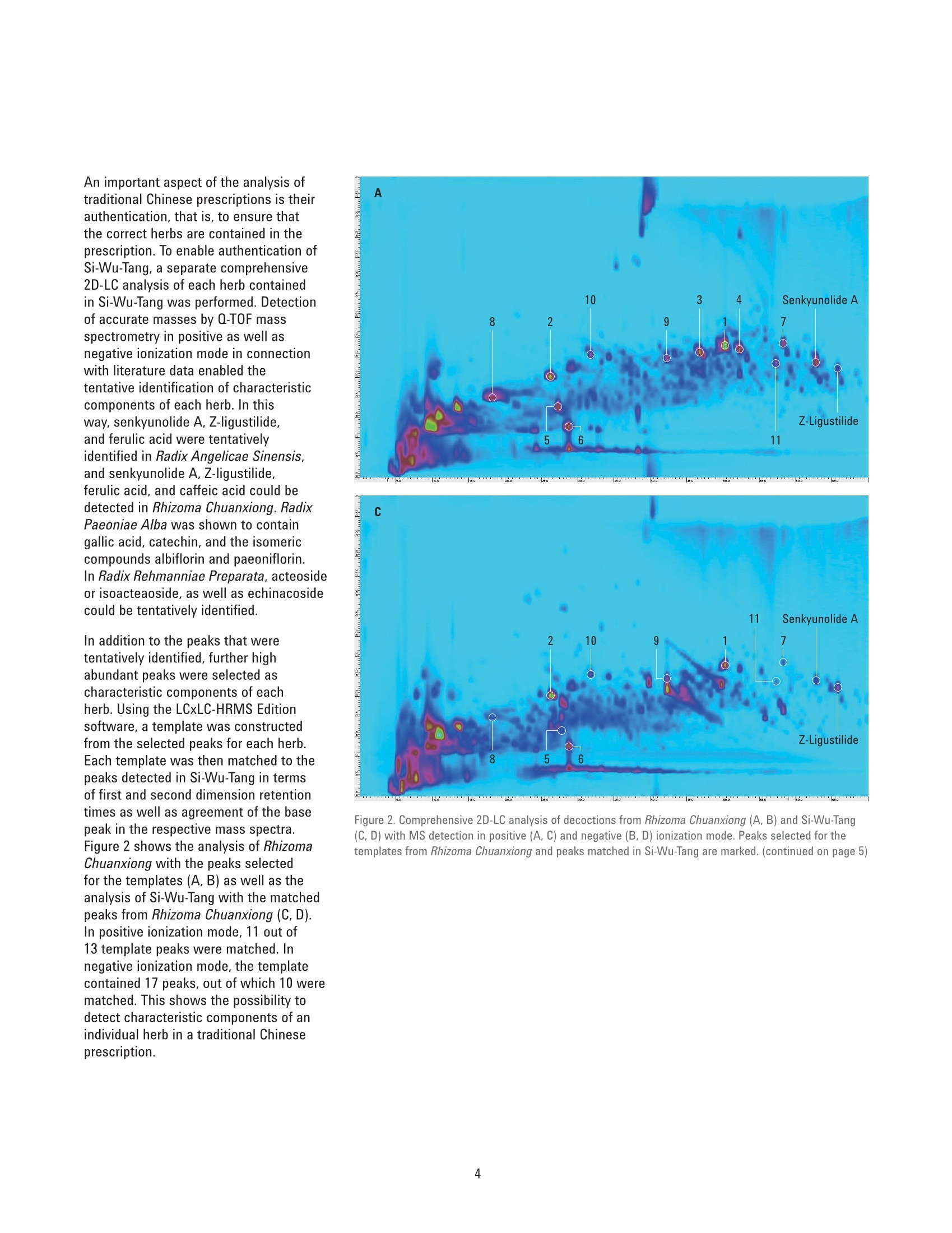
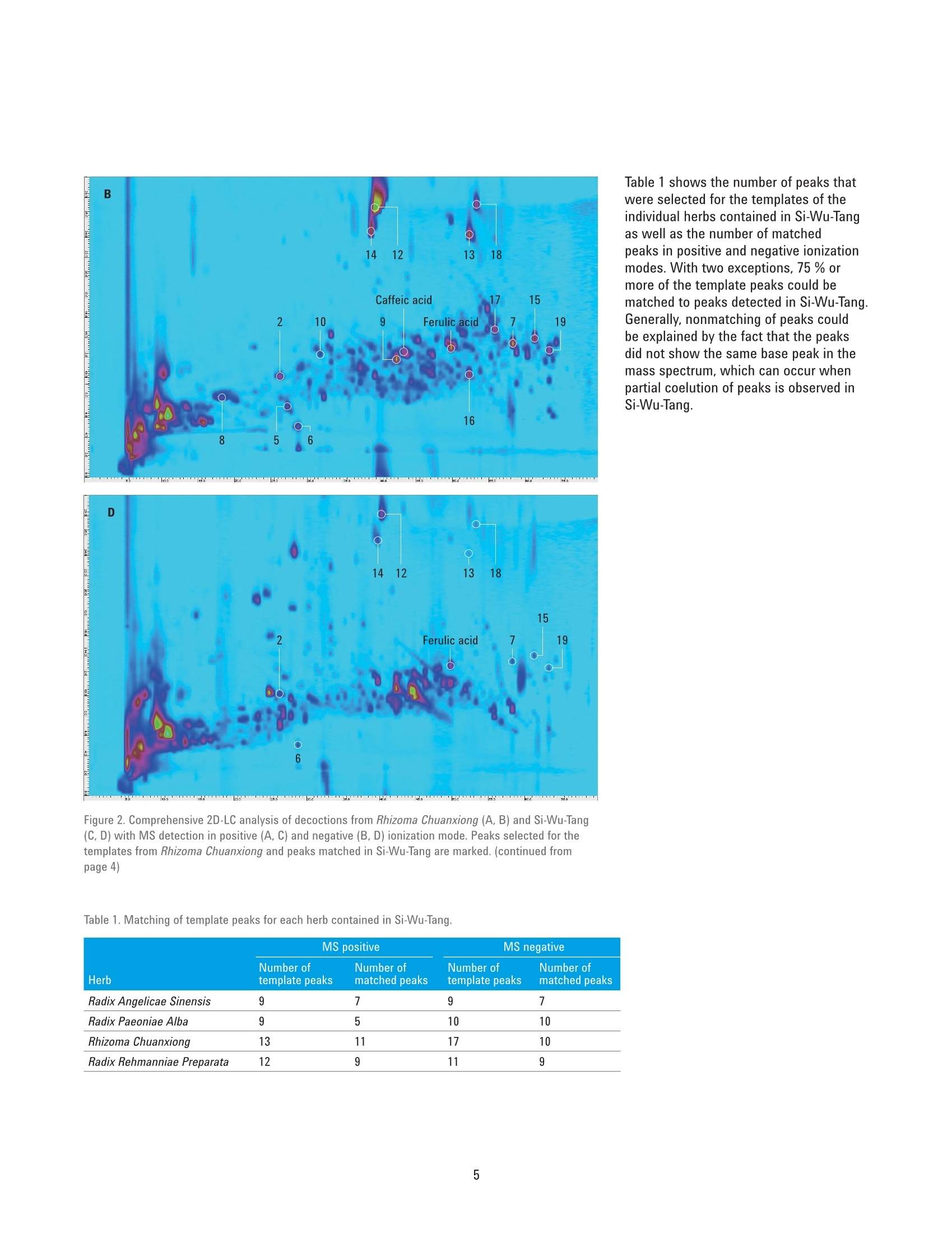
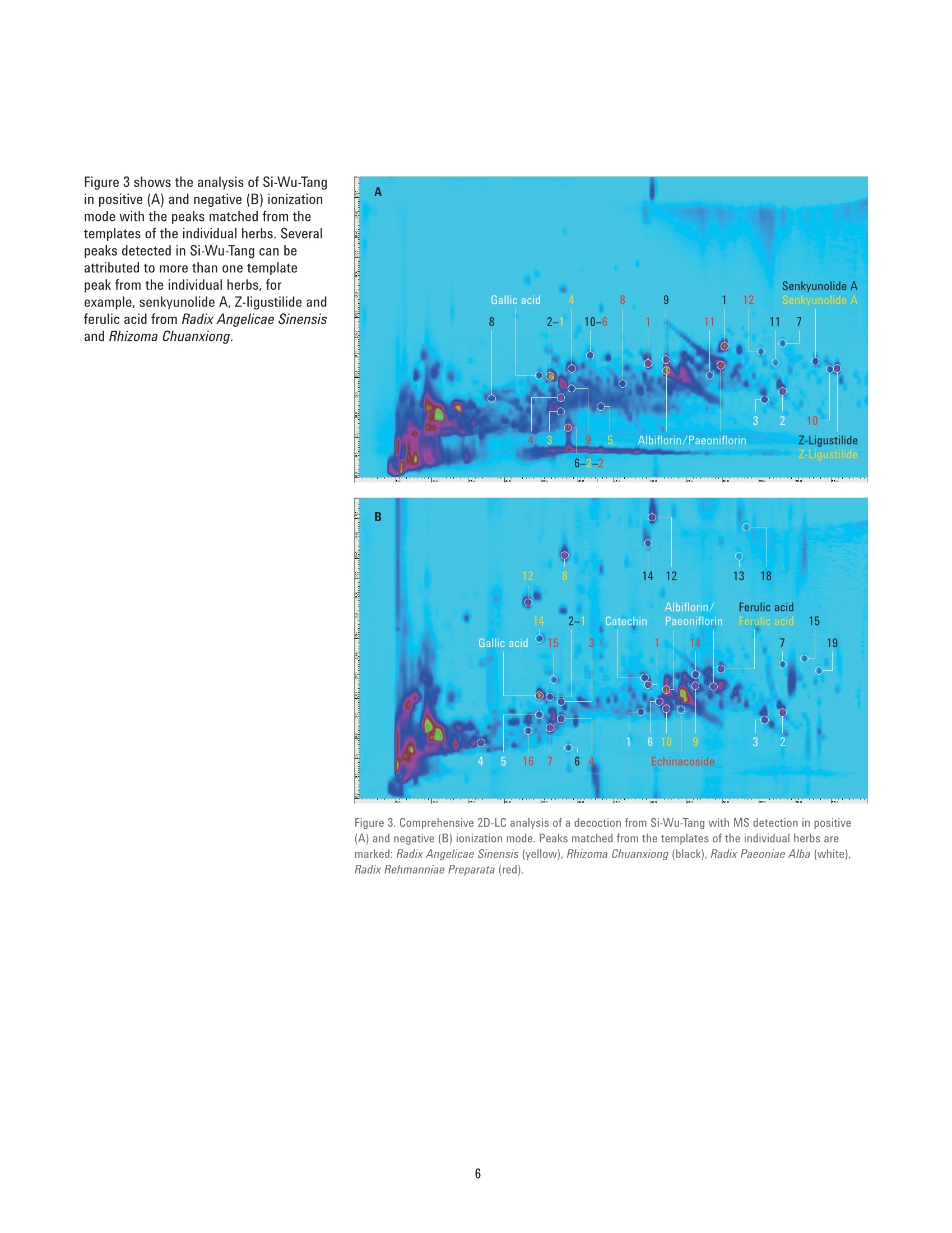
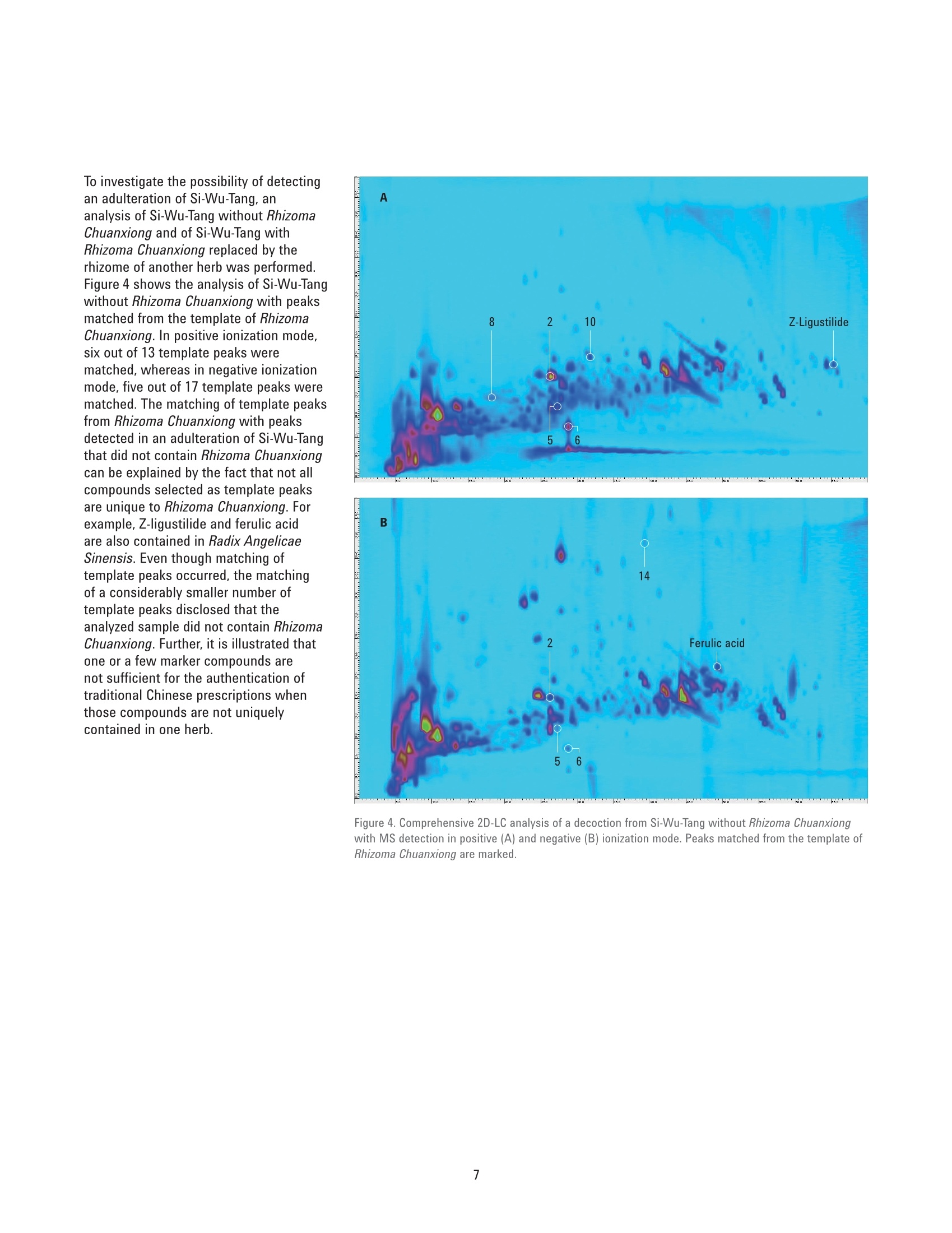
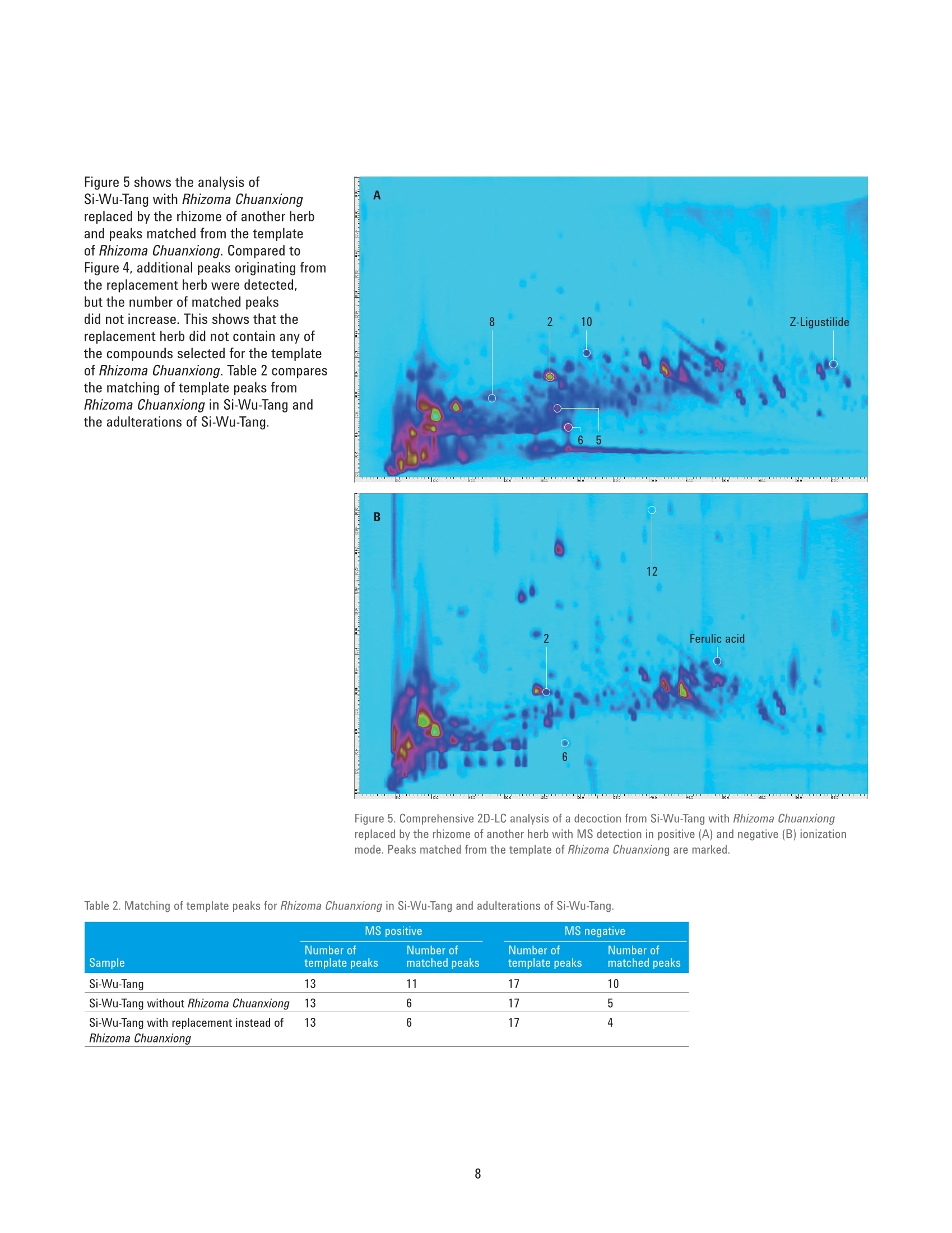
Authentication of Traditional ChinesePrescriptions Using Comprehensive2D-LC The Agilent 1290 Infinity 2D-LC Solution Application Note Small Molecule Pharmaceuticals and Generics Author Abstract Sonja Krieger Agilent Technologies, Inc. Waldbronn. Germany The authentication of Chinese herbal medicine (CHM) is a challenging task, a factthat can be attributed to the highly complex nature of plants and preparationsused in CHM and the natural variability of the individual plants. Generally,chromatographic fingerprinting is regarded as one of the most effectivemethods. As an example of the analysis of a traditional Chinese prescription, thisApplication Note shows the comprehensive 2D-LC analysis of Si-Wu-Tang as wellas of the individual herbs contained in Si-Wu-Tang. For each herb, characteristiccomponent peaks are selected, and a template is created. The templates arematched to the peaks detected in Si-Wu-Tang to investigate the possibility todetect characteristic components of the individual herbs in a traditional Chineseprescription. Additionally, changes in template matching following the omissionand replacement of one herb from Si-Wu-Tang are examined. Agilent Technologies Traditional Chinese medicine (TCM)represents a holistic healthcare systemthat focuses on the overall functionalstate of the patient and aims to restoreand maintain the dynamic balance.Chinese herbal medicine (CHM), oneaspect of TCM, uses single plants orpreparations of several plants, mainlyin form of decoctions prepared byextraction with boiling water24. Especiallyin poorer countries, such as parts ofAfrica and Asia, counterfeiting of drugsis a huge problem. Counterfeit versionsof preparations used in CHM mighthave omission or replacement of one ormore herbs in the preparation. Due tothe highly complex nature of plants andpreparations used in CHM and the naturalvariability of the individual plants, theauthentication of CHM is a challengingtask. Generally, one or a few maincompounds or pharmaceutically activecomponents are currently used as markercompounds in the analysis of CHM24. Amore complete characterization of CHMcan be achieved by chromatographicfingerprinting. The construction ofa chromatographic fingerprint andsubsequent comparison with thefingerprint of a clinically proven referenceproduct is regarded as an effectivemethod for authentication of CHM1-4 One example for a preparation of severalplants used in CHM is Si-Wu-Tang.Si-Wu-Tang is composed of the fourherbs Radix Angelicae Sinensis, RhizomaChuanxiong, Radix Paeoniae Alba, andRadix Rehmanniae Preparata, and iswidely used for the treatment of femaledisease6.. According to the literature,Radix Angelicae Sinensis contains arange of phthalides, such as Z-ligustilideand senkyunolide A. amongst others,as well as several organic acids suchas ferulic acid, phthalic acid, andvanillic acid8-10. Several components ofRadix Angelicae Sinensis can also befound in Rhizoma Chuanxiong as it alsocontains phthalides and organic acids1112.Compounds identified in Radix Paeoniaeinclude gallic acid, catechin, albiflorin, paeoniflorin, and paeonol13, andRadix Rehmanniae contains acteoside,isoacteoside,echinacoside, leonuride,catalpol, aucubin, and melittoside14. This Application Note shows theanalysis of a decoction from Si-Wu-Tangusing comprehensive two-dimensionalliquid chromatography (comprehensive2D-LC). Because of its inherent highpeak capacity, comprehensive 2D-LC isideally suited for the analysis of complexsamples such as CHM. The separate analysis of the herbscontained in Si-Wu-Tang and detectionby Q-TOF mass spectrometry enablesthe selection and tentative identificationof characteristic components of eachherb. Those characteristic componentsare then detected in Si-Wu-Tang as ameans of authentication of traditionalChinese prescriptions. In addition,changes following the omission andreplacement of one herb from Si-Wu-Tangare investigated. Experimental Equipment The Agilent 1290 Infinity 2D-LC Solutionwas comprised of the following modules: Two Agilent 1290 Infinity BinaryPumps (G4220A) Agilent 1290 Infinity Autosampler(G4226A) with 1290 InfinityThermostat (G1330B) Agilent 1290 Infinity ThermostattedColumn Compartment (G1316C) Agilent 1290 Infinity valve drive(G1170A) with 2-position/4-portduo-valve (2D-LC valve head,1,200 bar (p/n 5067-4214) equippedwith two 40-uL loops Agilent 1290 Infinity DiodeArray Detector (G4212A) with10-mm Max-Light cartridge cell(G4212-60008) MS measurements were done usingan Agilent 6530 Accurate-Mass Q-TOFLC/MS system with Jet Stream ESIsource (G1958-65538). Software Agilent OpenLAB CDS ChemStationEdition, revision C.01.07 [27]with Agilent 1290 Infinity 2D-LCAcquisition Software, revisionA.01.02 [24] Agilent MassHunter WorkstationSoftware, LC/MS data acquisitionfor Agilent 6200 series TOF/6500series Q-TOF, revision B.05.01,qualitative analysis, revisionB.06.00. GC Image LCxLC-HRMS Editionsoftware, revision 2.5b0 for 2D-LCdata analysis from GC Image LLC.,Lincoln, NE, USA. Columns First dimension Agilent ZORBAX RRHT SB-Aq 2.1×100 mm, 1.8 um (p/n 828700-914) Second dimension Agilent Poroshell 120 Bonus-RP3.0×50 mm,2.7 um (p/n699968-301) Chemicals All solvents were LC grade. Acetonitrileand methanol were purchased fromMerck, Darmstadt, Germany.Freshultrapure water was obtained from aMilli-Q Integral system equipped with a0.22-um membrane point-of-use cartridge(Millipak, EMD Millipore, Billerica, MA,USA). Formic acid was purchased fromAgilent (p/n G2453-85060). Samples Samples of the traditional Chineseprescription Si-Wu-Tang, which iscomposed of four herbs, as well as ofindividual herbs used in CHM,were kindlyprovided by Patrick Kwik from CongressPharmacy in Karlsruhe, Germany. Samples were prepared as decoctions, inthe same manner as they are prepared forpharmaceutical use. The samples wereweighed and a tenfold amount of waterwas added. The samples were allowed tosoak in the cold water for 60 minutes andwere then boiled for 30 minutes. Aftercooling, aliquots of the decoctions werecentrifuged at 10,000 rpm for 15 minutes.Aliquots of the supernatant phases werefiltered using a 1-mL plastic syringewith Captiva Premium Syringe FiltersRegenerated Cellulose, 15 mm, 0.45 pm(p/n 5190-5109) before injection into theHPLC system. Thermostatted columncompartment First dimension column on right sideat 30 °C Second dimension column on leftside at 50 °C 2-position/4-port duo-valve The 2-position/4-port duo-valve wasswitched automatically after eachsecond dimension modulation cycle of30 seconds. The loops were used in acocurrent manner (filling and elution ofthe loops in the same flow direction). Autosampler Parameter Value Injection volume 5pL Sample temperature 6°C Needle wash 10 seconds in methanol/water (50/50;v/v) Diode array detector Before detection, the effluent from thesecond dimension column was splitapproximately 4:1 between the DAD andthe MS using a T-piece. The connectionfrom the T-piece to the MS was madeusing a 0.075-mm id capillary (340-mmlength) to minimize peak broadening. Parameter Value Wavelength 254 nm/4 nm, Ref. 380 nm/40 nm Data rate 80 Hz Comprehensive 2D-LC method First dimension pump Solvent A Water+ 0.1 % formic acid Solvent B Methanol+ 0.1 % formic acid Flow rate 0.05 mL/min Gradient 0 minutes 0 %B; 10 minutes 0 %B; 70 minutes 95 %B; 80 minutes 95%B Stop time 80 minutes Post time 30 minutes Second dimension pump Solvent A Water +0.1 % formic acid Solvent B Acetonitrile+0.1 % formic acid Flow rate 2.5 mL/min Gradient and gradient 0.00 minutes 2 %B; 30 minutes 2 %B; 52 minutes 2 %B;70 minutes 40 %B modulation 0.40 minutes 35 %B; 30 minutes 35 %B;52 minutes 60 %B; 70 minutes 95 %B 2D Gradient stop time 0.40 minutes Modulation time 0.50 minutes Mass spectrometer The Agilent 6530 Accurate-Mass Q-TOFLC/MS system was operated in positiveand negative ionization mode with anacquisition rate of 10 spectra/secondand the following conditions for the JetStream ESI source (G1958-65538). Parameter Value Gas temperature 300 °C Gas flow 8 L/min Nebulizer 50 psig Sheath gas temperature 300°C Sheath gas flow 9 L/min Capillary 3,000 V Nozzle 500 V Results and Discussion Based on the comprehensive 2D-LCanalysis of decoctions from plants usedin CHM shown in a previous ApplicationNote15, a method for comprehensive2D-LC analysis of a decoction fromSi-Wu-Tang was developed. Figure 1shows the resulting separation withUV detection at 254 nm. Especiallyin the first dimension retention timeranges from 25 to 30 minutes and 40 to50 minutes, peaks that would coelute in aone-dimensional analysis are separated inthe second dimension. Figure 1. Comprehensive 2D-LC analysis of a decoction from Si-Wu-Tang with UV detection at 254 nm. An important aspect of the analysis oftraditional Chinese prescriptions is theirauthentication, that is, to ensure thatthe correct herbs are contained in theprescription. To enable authentication ofSi-Wu-Tang, a separate comprehensive2D-LC analysis of each herb containedin Si-Wu-Tang was performed. Detectionof accurate masses by Q-TOF massspectrometry in positive as well asnegative ionization mode in connectionwith literature data enabled thetentative identification of characteristiccomponents of each herb. In thisway, senkyunolide A, Z-ligustilide,and ferulic acid were tentativelyidentified in Radix Angelicae Sinensisand senkyunolide A, Z-ligustilide,ferulic acid, and caffeic acid could bedetected in Rhizoma Chuanxiong. RadixPaeoniae Alba was shown to contain,gallic acid, catechin, and the isomericcompounds albiflorin and paeoniflorin.In Radix Rehmanniae Preparata, acteosideor isoacteaoside, as well as echinacosidecould be tentatively identified. In addition to the peaks that weretentatively identified, further highabundant peaks were selected ascharacteristic components of eachherb. Using the LCxLC-HRMS Editionsoftware, a template was constructedfrom the selected peaks for each herb.Each template was then matched to thepeaks detected in Si-Wu-Tang in termsof first and second dimension retentiontimes as well as agreement of the basepeak in the respective mass spectra.Figure 2 shows the analysis of RhizomaChuanxiong with the peaks selectedfor the templates (A, B) as well as theanalysis of Si-Wu-Tang with the matchedpeaks from Rhizoma Chuanxiong (C, D).In positive ionization mode, 11 out of13 template peaks were matched. Innegative ionization mode, the templatecontained 17 peaks, out of which 10 werematched. This shows the possibility todetect characteristic components of anindividual herb in a traditional Chineseprescription. Figure 2. Comprehensive 2D-LC analysis of decoctions from Rhizoma Chuanxiong (A, B) and Si-Wu-Tang(C, D) with MS detection in positive (A. C) and negative (B, D) ionization mode. Peaks selected for thetemplates from Rhizoma Chuanxiong and peaks matched in Si-Wu-Tang are marked. (continued on page 5) Figure 2. Comprehensive 2D-LC analysis of decoctions from Rhizoma Chuanxiong (A, B) and Si-Wu-Tang(C, D) with MS detection in positive (A, C) and negative (B, D) ionization mode. Peaks selected for thetemplates from Rhizoma Chuanxiong and peaks matched in Si-Wu-Tang are marked. (continued frompage 4) Table 1. Matching of template peaks for each herb contained in Si-Wu-Tang. Herb MS positive MS negative Number of Number of Number of Number of template peaks matched peaks template peaks matched peaks Radix Angelicae Sinensis 9 7 9 7 Radix Paeoniae Alba 9 5 10 10 Rhizoma Chuanxiong 13 11 17 10 Radix Rehmanniae Preparata 12 9 11 9 Table 1 shows the number of peaks thatwere selected for the templates of theindividual herbs contained in Si-Wu-Tangas well as the number of matchedpeaks in positive and negative ionizationmodes. With two exceptions, 75 % ormore of the template peaks could bematched to peaks detected in Si-Wu-Tang.Generally, nonmatching of peaks couldbe explained by the fact that the peaksdid not show the same base peak in themass spectrum, which can occur whenpartial coelution of peaks is observed inSi-Wu-Tang. Figure 3 shows the analysis of Si-Wu-Tangin positive (A) and negative (B) ionizationmode with the peaks matched from thetemplates of the individual herbs. Severalpeaks detected in Si-Wu-Tang can beattributed to more than one templatepeak from the individual herbs, forexample, senkyunolide A, Z-ligustilide andferulic acid from Radix Angelicae Sinensisand Rhizoma Chuanxiong. Figure 3. Comprehensive 2D-LC analysis of a decoction from Si-Wu-Tang with MS detection in positive(A) and negative (B) ionization mode. Peaks matched from the templates of the individual herbs aremarked: Radix Angelicae Sinensis (yellow), Rhizoma Chuanxiong (black), Radix Paeoniae Alba (white),Radix Rehmanniae Preparata (red). To investigate the possibility of detectingan adulteration of Si-Wu-Tang, ananalysis of Si-Wu-Tang without RhizomaChuanxiong and of Si-Wu-Tang withRhizoma Chuanxiong replaced by therhizome of another herb was performed.Figure 4 shows the analysis of Si-Wu-Tangwithout Rhizoma Chuanxiong with peaksmatched from the template of RhizomaChuanxiong. In positive ionization mode,six out of 13 template peaks werematched, whereas in negative ionizationmode, five out of 17 template peaks werematched. The matching of template peaksfrom Rhizoma Chuanxiong with peaksdetected in an adulteration of Si-Wu-Tangthat did not contain Rhizoma Chuanxiongcan be explained by the fact that not allcompounds selected as template peaksare unique to Rhizoma Chuanxiong. Forexample,Z-ligustilide and ferulic acidare also contained in Radix AngelicaeSinensis. Even though matching oftemplate peaks occurred, the matchingof a considerably smaller number oftemplate peaks disclosed that theanalyzed sample did not contain RhizomaChuanxiong. Further, it is illustrated thatone or a few marker compounds arenot sufficient for the authentication oftraditional Chinese prescriptions whenthose compounds are not uniquelycontained in one herb. Figure 4. Comprehensive 2D-LC analysis of a decoction from Si-Wu-Tang without Rhizoma Chuanxiongwith MS detection in positive (A) and negative (B) ionization mode. Peaks matched from the template ofRhizoma Chuanxiong are marked. Figure 5 shows the analysis ofSi-Wu-Tang with Rhizoma Chuanxiongreplaced by the rhizome of another herband peaks matched from the templateof Rhizoma Chuanxiong. Compared toFigure 4, additional peaks originating fromthe replacement herb were detected,but the number of matched peaksdid not increase. This shows that thereplacement herb did not contain any ofthe compounds selected for the templateof Rhizoma Chuanxiong. Table 2 comparesthe matching of template peaks fromRhizoma Chuanxiong in Si-Wu-Tang andthe adulterations of Si-Wu-Tang. Figure 5. Comprehensive 2D-LC analysis of a decoction from Si-Wu-Tang with Rhizoma Chuanxiongreplaced by the rhizome of another herb with MS detection in positive (A) and negative (B) ionizationmode. Peaks matched from the template of Rhizoma Chuanxiong are marked. Table 2. Matching of template peaks for Rhizoma Chuanxiong in Si-Wu-Tang and adulterations of Si-Wu-Tang. MS positive MS negative Number of Number of Number of Number of Sample template peaks matched peaks template peaks matched peaks Si-Wu-Tang 13 11 17 10 Si-Wu-Tang without Rhizoma Chuanxiong 13 6 17 5 Si-Wu-Tang with replacement instead of 13 6 17 4 Rhizoma Chuanxiong Conclusion This Application Note shows thecomprehensive 2D-LC analysis ofthe traditional Chinese prescriptionSi-Wu-Tang, which is composed offour herbs, as well as the analysis ofthe individual herbs. For each herb,characteristic component peaks wereselected and a template was created. Thetemplates were matched to the peaksdetected in Si-Wu-Tang and, with twoexceptions, at least 75 % of templatepeaks could be matched. This showsthat it is possible to detect characteristiccomponents of the individual herbsin a traditional Chinese prescription.Additionally, adulteration throughomission and replacement of one herbfrom Si-Wu-Tang could be detected bythe matching of a considerably reducednumber of template peaks, whichprovides a means of authentication ofSi-Wu-Tang. References 1. Xie, P. S., Leung, A. Y., Understandingthe traditional aspect of Chinesemedicine in order to achievemeaningful quality control ofChinese materia medica, Journa/of Chromatography A, 2009, 1216,1933-1940 2. LLiang, X. M., et al., Qualitative andquantitative analysis in quality controlof traditional Chinese medicines.Journal of Chromatography A, 2009,1216,2033-2044 3. YYang, D. Z., et al., MultidimensionalInformation-Based HPLC Technologiesto Evaluate Traditional ChineseMedicine, Journal of ChromatographicScience, 2013,51,716-725 4. LLiang, Y. Z., et al., Quality controlof herbal medicines, Journal ofChromatography B-AnalyticalTechnologies in the Biomedical andLife Sciences, 2004, 812, 53-70 5. CCockburn, R., et al., The global threatof counterfeit drugs: why industry andgovernments must communicate thedangers, PLoS Med,2005,2,e100 6.CChen, X. P., et al., Identification andCharacterization of Constituents inSi-Wu-Tang by Liquid ChromatographyConnected with Time of Flight MassSpectrometry and lon Trap MassSpectrometry, Asian Journal ofChemistry, 2013, 25, 6263-6266 7. Wang, Z. J., et al., Simultaneousquantification of active componentsin the herbs and products ofSi-Wu-Tang by high performance liquidchromatography-mass spectrometry,J. Pharm. Biomed. Anal.,2009,50,232-244 8. Peng, Z., et al., LC-DAD-MSdetermination of the majorconstituents in Radix Angelicaesinensis, Chromatographia, 2008, 67973-978 9.YYi, L. Z., et al., The analysis of RadixAngelicae Sinensis (Danggui), Journalof Chromatography A, 2009, 1216,1991-2001 10.Bai, Y. J., et al., Effect of differentdrying methods on the quality ofAngelicae Sinensis Radix evaluatedthrough simultaneously determiningfour types of major bioactivecomponents by high performanceliquid chromatography photodiodearray detector and ultra-highperformance liquid chromatographyquadrupole time-of-flight massspectrometry, J. Pharm. Biomed. Anal.,2014,94,77-83 11.Zhang, X. L., et al., A high performanceliquid chromatography fingerprintingand ultra high performance liquidchromatography coupled withquadrupole time-of-flight massspectrometry chemical profilingapproach to rapidly find characteristicchemical markers for qualityevaluation of dispensing granules, acase study on Chuanxiong Rhizoma,J.Pharm. Biomed. Anal., 2014, 88,391-400 12. Chen, X. G., et al., Separationand identification of compoundsin Rhizoma chuanxiong bycomprehensive two-dimensionalliquid chromatography coupledto mass spectrometry, Journal ofChromatography A, 2004, 1040,169-178 13. Li, S. L., et al., Chemical profilingof Radix Paeoniae evaluatedby ultra-performance liquidchromatography/photo-diode-array/quadrupole time-of-flight massspectrometry, J. Pharm. Biomed.Anal.,2009,49,253-266 14. Xu, J., et al., Simultaneousdetermination of iridoid glycosides,phenethylalcohol glycosides andfurfural derivatives in RehmanniaeRadix by high performance liquidchromatography coupled withtriple-quadrupole mass spectrometry,Food Chemistry, 2012,135,2277-2286 15. Krieger, S., Comprehensive 2D-LCAnalysis of Chinese Herbal Medicine,Agilent Technologies Application Note,publication number 5991-5028EN,2014 www.agilent.com/chem This information is subject to change without notice. C Agilent Technologies,Inc., 2015 Published in the USA, April 1, 2015 5991-5555EN Agilent Technologies 中草药(CHM)的认证是一项艰巨的任务,这一事实可归因于植物和CHM中使用的制剂的高度复杂性以及各个植物的自然变异性。 通常,色谱指纹图谱被认为是最有效的方法之一。 作为分析中国传统处方的一个例子,本应用指南显示了四物汤以及四物汤中所含各个草药的全面二维液相色谱分析。 对于每种草药,选择特征成分峰,然后创建模板。 模板与在四物汤中检测到的峰相匹配,以研究检测传统中药中各个草药的特征成分的可能性。 另外,检测了从四物汤遗漏和替换一种草药后模板匹配的变化。
确定







还剩10页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《中药中四物汤检测方案(液相色谱仪)》,该方案主要用于中药材和饮片中四物汤检测,参考标准--,《中药中四物汤检测方案(液相色谱仪)》用到的仪器有安捷伦 1290 Infinity 二元液相色谱系统(1290 LC)、OpenLAB 软件
推荐专场
相关方案
更多
该厂商其他方案
更多
















