方案详情
文
A dry cell battery is an electrochemical cell in which the electron transfer of a chemical reaction is conducted outside the battery through an external circuit in a load. The reactions in the battery result in a decrease in the energy content of the substances in the battery, It is this energy decrease which can be used to power a device such as a ?ashlight. It is however not possible to utilize the entire energy because of internal energy losses within the battery. The energy that cannot be used outside the battery is converted to heat within the battery. By measuring this heat the efficiency of the battery may be investigated.
方案详情

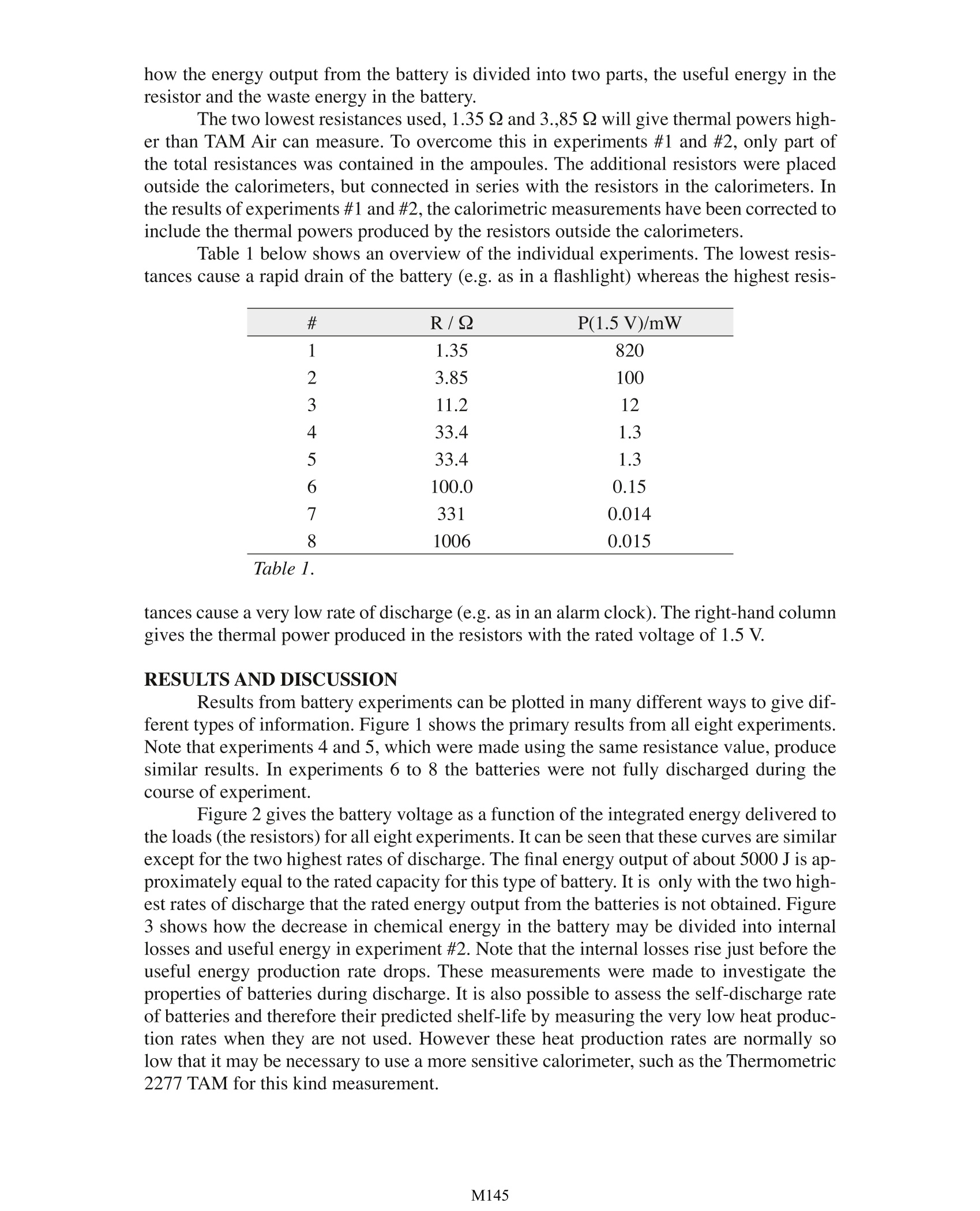
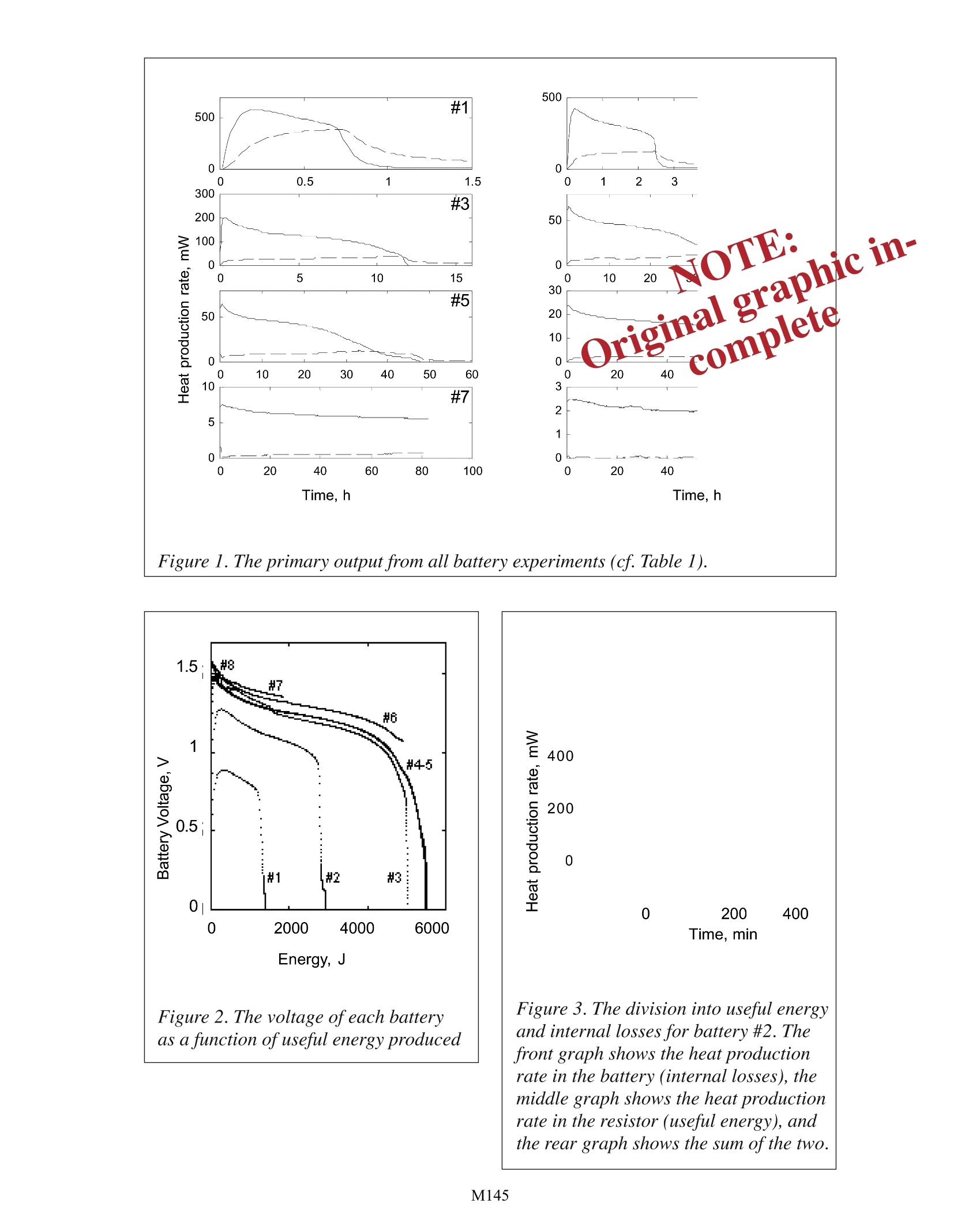
Investigations into Dry Cell Battery Discharge Rates Using TAM Air Lars Wadso Building Materials, Lund University, Sweden TA Instruments, 109 Lukens Drive, New Castle, DE 19720, USA INTRODUCTION A dry cell baltery is an electrochemical cellin which the electron transfer of a chemical reac-tion is conducted outside the battery through anexternal circuit in a load. The reactions in the bat-tery result in a decrease in the energy content ofthe substances in the battery, It is this energy de-crease which can be used to power a device suchas a flashlight. It is however not possible to utilisethe entire energy because of internal energy losseswithin the battery. The energy that cannot be usedoutside the battery is converted to heat within thebattery. By measuring this heat the efficiency ofthe battery may be investigated. MATERIALS AND METHODS Of the eight channels in TAM Air, four werecharged with 1.5 V alkaline batteries, size AA A.Four resistors of different values were placed in the other four channels for connection to the batteries. The experiment was started whenthe circuit between each battery and its resitstor was completed. The experiment was per-formed twice using four batteries in each experiment. Each battery and each resistor wasplaced within a 20 ml glass ampoule with I ml of paraffin oil on the bottom of the ampouleto increase the thermal contact. Measurements were made at 22℃, with one sample takenevery minute, The resistors act as the load of the battery replacing an electrical lamp or motor Theheal produced in a resistor may be considered as potentially useful energy. The battery volt-age U (V) may be calculated from the measured heat production rate in the resistor P (W)by the following equation: Here R () is the resistance of the load resistor. The thermal power produced in the resistor can also be calculated by measuring thevoltage and using Eq. 1. The setup used here has the advantage of clearly demonstrating how the energy output from the battery is divided into two parts, the useful energy in theresistor and the waste energy in the battery. The two lowest resistances used, 1.35 Ω and 3.,85 Q will give thermal powers high-er than TAM Air can measure. To overcome this in experiments #1 and #2, only part ofthe total resistances was contained in the ampoules. The additional resistors were placedoutside the calorimeters, but connected in series with the resistors in the calorimeters. Inthe results of experiments #1 and #2, the calorimetric measurements have been corrected toinclude the thermal powers produced by the resistors outside the calorimeters. Table 1 below shows an overview of the individual experiments. The lowest resis-tances cause a rapid drain of the battery (e.g. as in a flashlight) whereas the highest resis- # R/Q P(1.5V)/mW 1 1.35 820 2 3.85 100 3 11.2 12 4 33.4 1.3 5 33.4 1.3 6 100.0 0.15 7 331 0.014 8 1006 0.015 Table 1. tances cause a very low rate of discharge (e.g. as in an alarm clock).The right-hand columngives the thermal power produced in the resistors with the rated voltage of 1.5V. RESULTS AND DISCUSSION Results from battery experiments can be plotted in many different ways to give dif-ferent types of information. Figure 1 shows the primary results from all eight experiments.Note that experiments 4 and 5, which were made using the same resistance value, producesimilar results. In experiments 6 to 8 the batteries were not fully discharged during thecourse of experiment. Figure 2 gives the battery voltage as a function of the integrated energy delivered tothe loads (the resistors) for all eight experiments. It can be seen that these curves are similarexcept for the two highest rates of discharge. The final energy output of about 5000 J is ap-proximately equal to the rated capacity for this type of battery. It is only with the two high-est rates of discharge that the rated energy output from the batteries is not obtained. Figure3 shows how the decrease in chemical energy in the battery may be divided into internallosses and useful energy in experiment #2. Note that the internal losses rise just before theuseful energy production rate drops. These measurements were made to investigate theproperties of batteries during discharge. It is also possible to assess the self-discharge rateof batteries and therefore their predicted shelf-life by measuring the very low heat produc-tion rates when they are not used. However these heat production rates are normally solow that it may be necessary to use a more sensitive calorimeter, such as the Thermometric2277 TAM for this kind measurement. Figure 1. The primary output from all battery experiments (cf. Table 1). Time, min Figure 3. The division into useful energyand internal losses for battery #2. Thefront graph shows the heat productionrate in the battery (internal losses), themiddle graph shows the heat productionrate in the resistor (useful energy), andthe rear graph shows the sum ofthe two. M A dry cell battery is an electrochemical cell in which the electron transfer of a chemical reaction is conducted outside the battery through an external circuit in a load. The reactions in the battery result in a decrease in the energy content of the substances in the battery, It is this energy decrease which can be used to power a device such as a flashlight. It is however not possible to utilize the entire energy because of internal energy losses within the battery. The energy that cannot be used outside the battery is converted to heat within the battery. By measuring this heat the efficiency of the battery may be investigated.
确定

还剩1页未读,是否继续阅读?
TA仪器为您提供《干电池中放电速率检测方案(差示扫描量热)》,该方案主要用于一次电池中放电速率检测,参考标准--,《干电池中放电速率检测方案(差示扫描量热)》用到的仪器有TA仪器+等温量热仪+TAM Air
推荐专场
















