电子鼻技术作为一项早期诊断的新方法,呼吸气体中VOCs的检测比较其他的方法,显然具备许多独特的优势。首先,对患者而言,它是一种无创的、安全的、相对价廉且可接受性较高的检查;其次,来自疾病发病机理方面的优势,如CYP促进致癌物质的产生,伴随着呼吸气体中的成分变化,使它能发现处于早期和可治疗阶段的肿瘤,从而潜在地可能降低了肺癌的死亡率。当然我们还需要更多的临床研究,以确定呼吸气体试验在肿瘤患者中干预和改善生存率方面是否具有远期的效果,从而真正评判它的价值。
方案详情

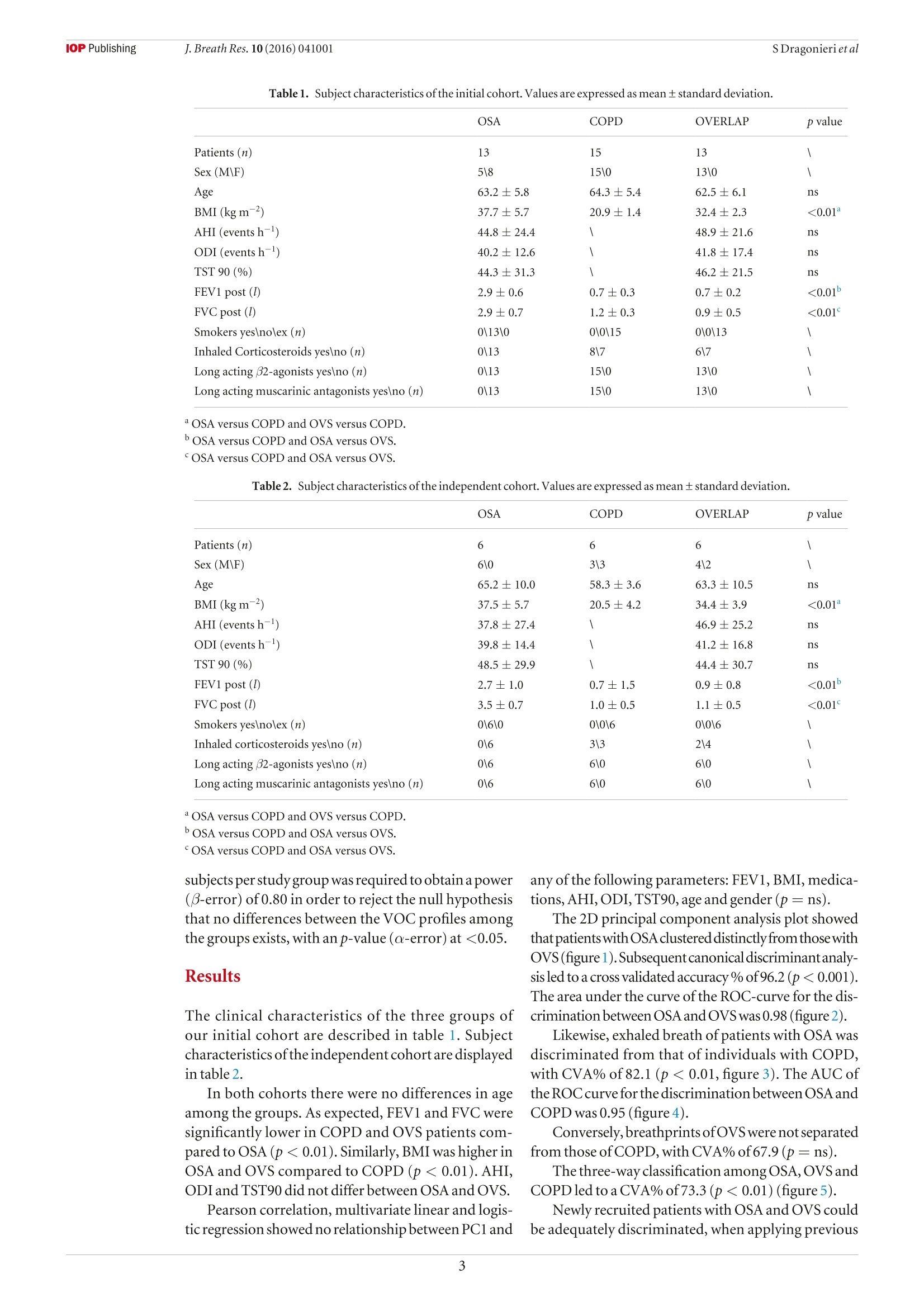
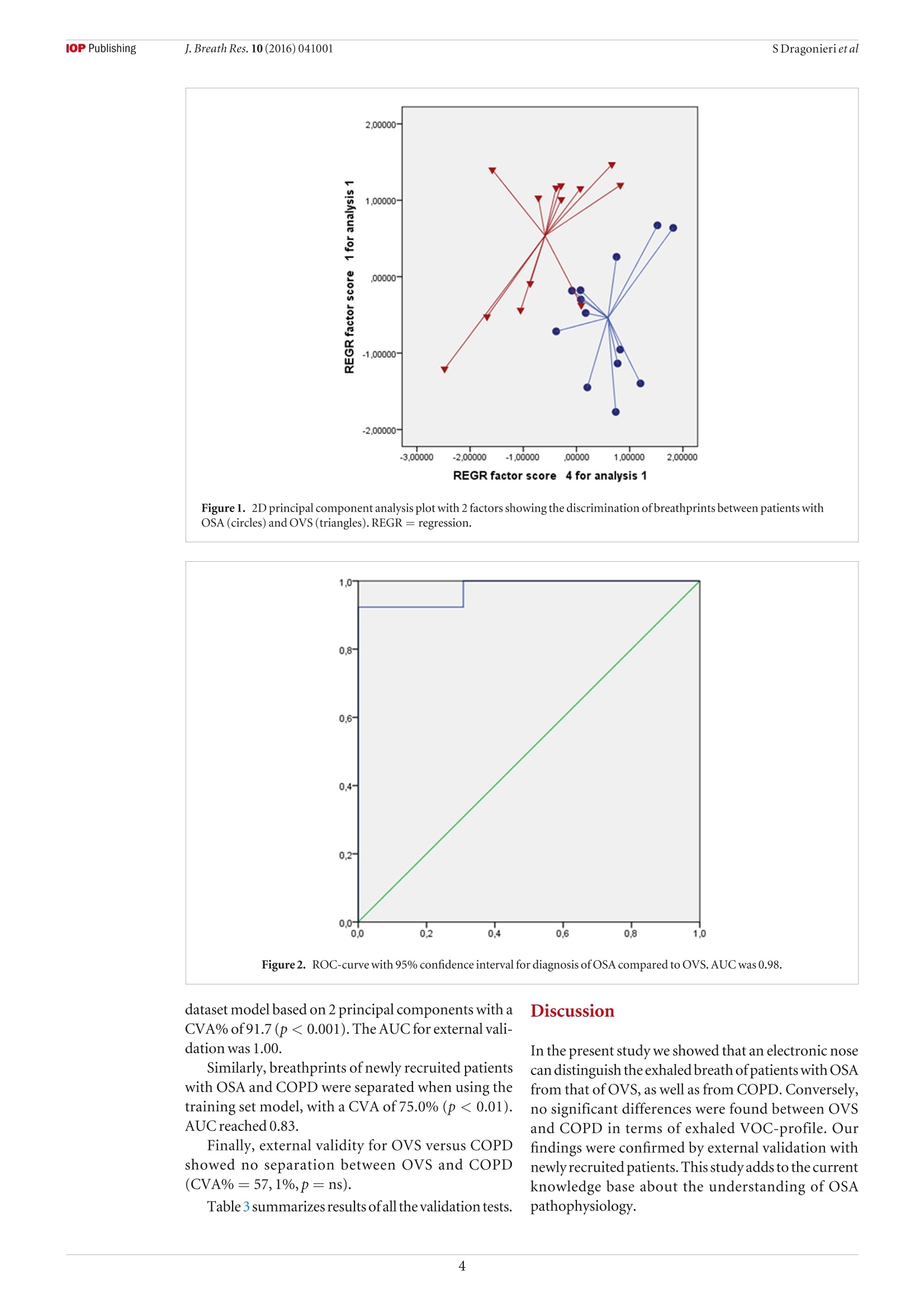
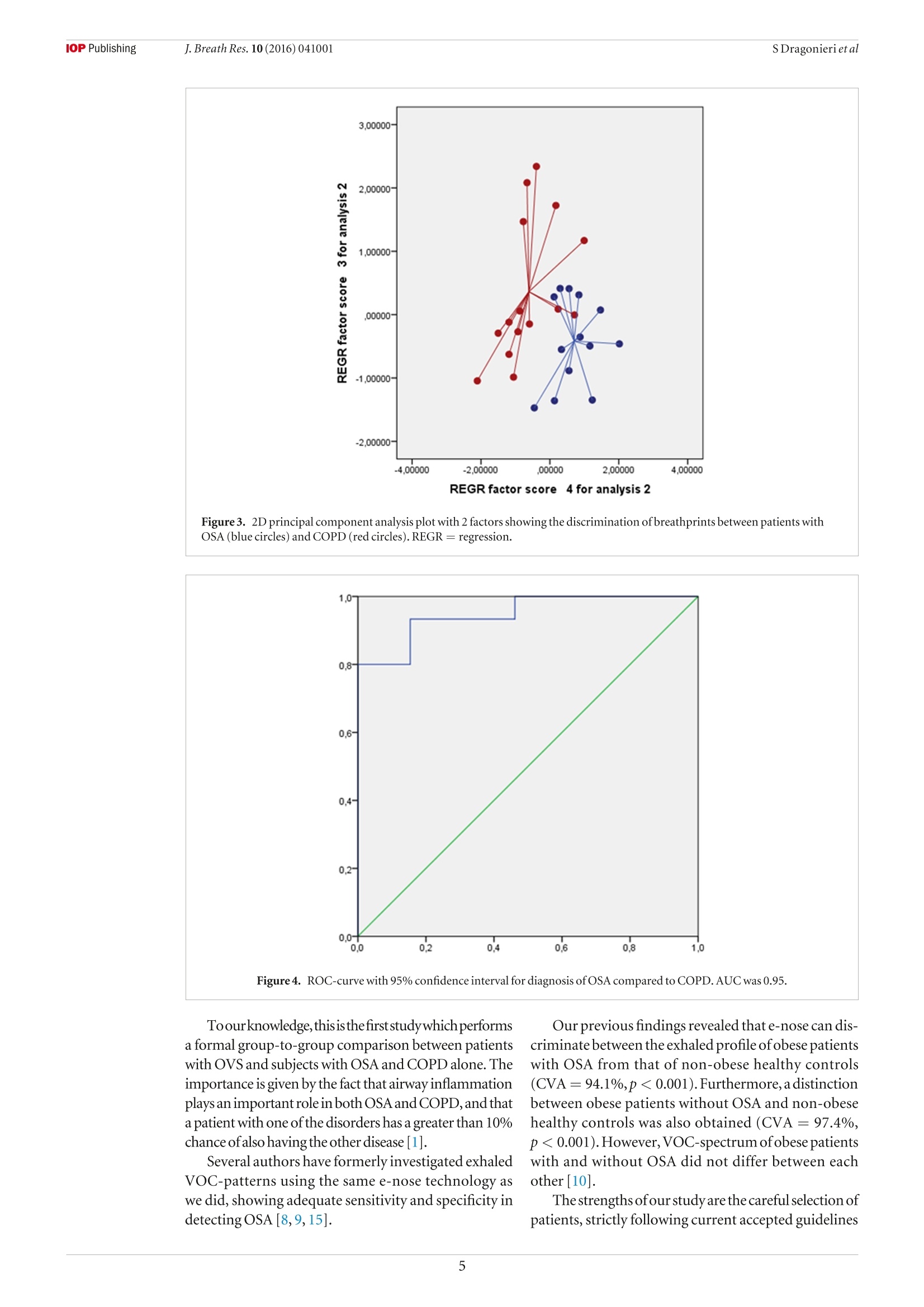
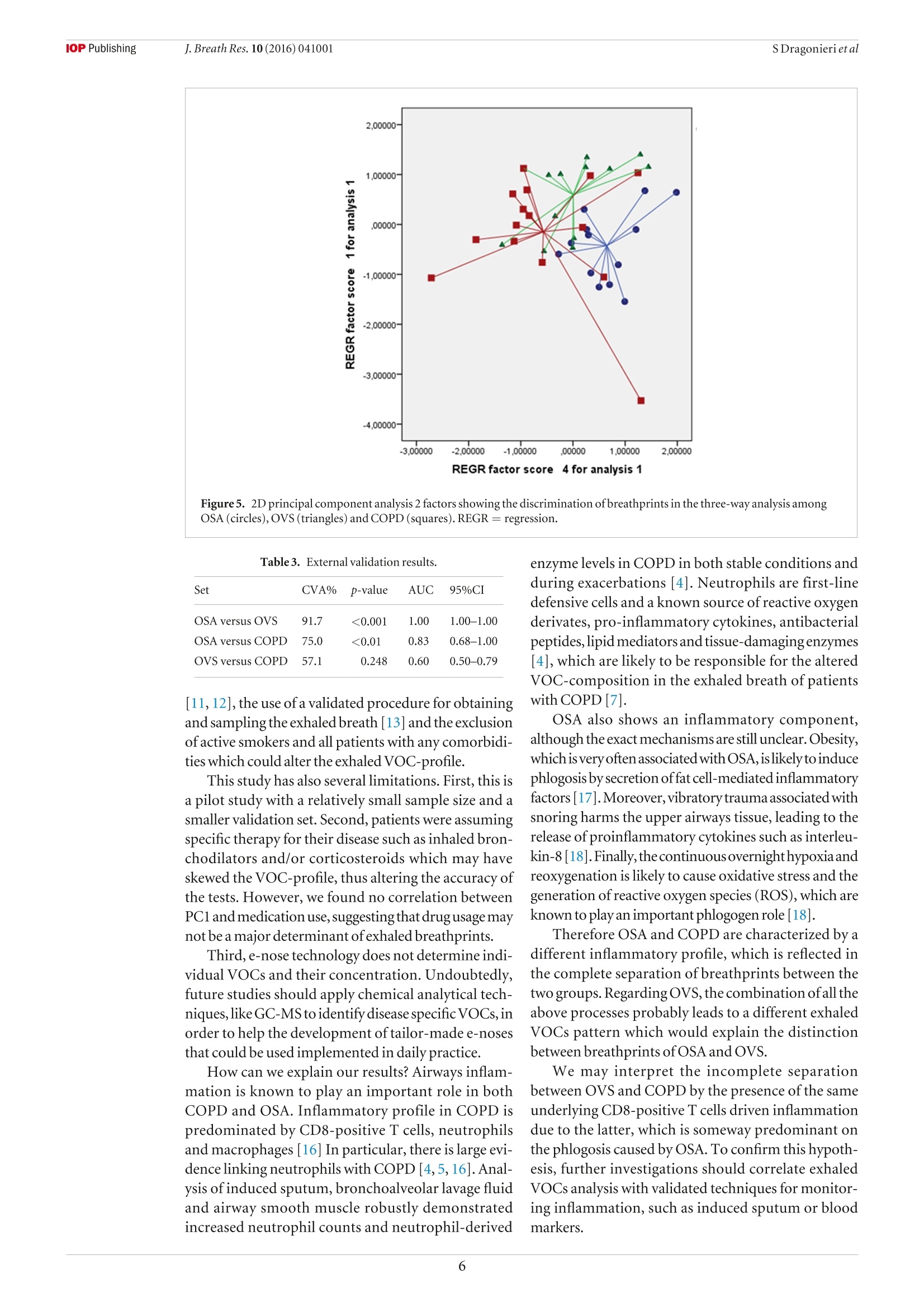
iopscience.iop.org doi:10.1088/1752-7155/10/4/041001IOP Publishing J. Breath Res. 10 (2016)041001 Home Search Collections Journals AbouttContact usMy lOPscience Exhaled breath profiling in patients with COPD and OSA overlap syndrome: a pilot study This content has been downloaded from IOPscience. Please scroll down to see the full text.2016 J. Breath Res. 10 041001 (http://iopscience.iop.org/1752-7163/10/4/041001) View the table of contents for this issue, or go to the journal homepage for more Download details: |P Address:128.227.24.141 This content was downloaded on01/12/2016 at 09:38 Please note that terms and conditions apply. You may also be interested in: An electronic nose in the discrimination of obese patients with and without obstructive sleepapnoea Silvano Dragonieri, Francesca Porcelli, Francesco Longobardi et al. integration of electronic nose technology with spirometry: validation of a new approach for exhaledbreath analysis R de Vries, P Brinkman, M P van der Schee et al. Diagnosing viral and bacterial respiratory infections in acute COPD exacerbations by an electronicnose: a pilot study Wouter H van Geffen, Marcel Bruins and Huib A M Kerstjens Established methodological issues in electronic nose research: how far are we from using theseinstruments in clinical settings of breath analysis? Andras Bikov, Zsofia Lazar and Ildiko Horvath Within-day and between-day repeatability of measurements with an electronic nose in patients with COPD Maria Bofan, Nadia Mores, Marco Baron et al. Biomarkers of inflammation in persons with chronic tetraplegia Miroslav Radulovic, William A Bauman, Jill M Wecht et al. Lack of heritability of exhaled volatile compound pattern: an electronic nose twin study David Laszlo Tarnoki, Andras Bikov, Adam Domonkos Tarnoki et al. Biomarkers in exhaled breath condensate N M Grob, M Aytekin and R A Dweik Journal of Breath Research NOTE Exhaled breath profiling in patients with COPD and OSA overlapsyndrome: a pilot study 10 October 2016 Silvano Dragonieri, Vitaliano N Quaranta,Pierluigi Carratu, Teresa Ranieriand Onofrio Resta ACCEPTED FOR PUBLICATION18 October 2016 Department of Respiratory Diseases, University of Bari, Piazza Giulio Cesare 11, 70124 Bari, Italy E-mail: silvano.dragonieri@uniba.it 3 November 2016 Keywords: exhaled breath, volatile organic compounds, electronic nose, OSA, COPD, overlap syndrome Abstract The analysis of volatile organic compounds (VOCs) by an electronic nose(e-nose) is agroundbreaking method that provides distinct exhaled molecular patterns in several respiratoryand systemic diseases. It has been shown that an e-nose can detect obstructive sleep apnea(OSA) aswell as chronic obstructive pulmonary disease (COPD).OSA and COPD are sometimes associatedinto the so-called overlap syndrome (OVS). In this pilot study we hypothesized that an e-nose coulddiscriminate the exhaled breath of patients with OVS from that of subjects with OSA and COPD alone. Thirteen patients with OSA, 15 patients with COPD and 13 with OVS participated in a cross-sectional study. Exhaled breath was collected by a formerly validated method and sampled by usingan electronic nose (Cyranose 320). Raw data were analyzed by canonical discriminant analysis onprincipal component reduction. Cross-validation accuracy (CVA) and ROC-curves were calculatedExternal validation in newly recruited patients (6OSA, 6OVS and 6 COPD) was tested using theprevious training set. Breathprints of patients with OSA clustered distinctly from those with OVS (CVA= 96.2%) aswell as those with COPD (CVA=82.1%). Breathprints from OVS were not significantly separatedfrom those ofCOPD (CVA= 67.9%). External validation confirmed the above findings. The e-nose can distinguish with high accuracy the exhaled VOC-profile of patients with OSA from.those with OVS as well as those with COPD. This warrants future studies to confirm the potential ofthis technique in the non-invasive detection of sleep apnea. Introduction Obstructive sleep apnea (OSA) occasionally is associatedwith chronic obstructive pulmonary disease creating acondition generallyknownas overlap syndrome'(OVS)[1].OVS incidence is around 0.5-1% of the populationover 40 years of age [1]. Detecting this conditionis important, because OVS patients develop moreenhanced nocturnal oxygen desaturation than COPD orOSA [2].Moreover,patients with OVS have ahigher riskof pulmonary hypertension and cardiovascular diseasescompared to patients with COPD or OSA alone[3].MPar Currently, diagnosis of OSA, as well as OVS, stillrequires polysomnography, which is technically chal-lenging, cost-demanding and needs dedicated well-trained personnel. Over the past decades, several studies have shownthat airway and systemic inflammation play an impor-tant role in the pathogenesis of both diseases [2,4,5]. The search of non-invasive biomarkers of airwaysinflammation has nowadays become a major focus ofinterest in the field of respiratory medicine. A breathtest would be ideal to fulfill these requirements. Exhaled breath contains hundreds of volatileorganiccompounds(VOCs)originating fromlocal andsystemic metabolic pathways in the body [6]. Electronic nose (e-nose) technology allows high-throughput assessment and pattern metabolomicrecognition of molecular mixtures. Recent studiesapplying this technology showed promising results indetecting OSA, as well as COPD [7-10]. To the best of our knowledge, no studies to datehave investigated exhaled breath profiling by e-nose inpatients with both diseases. Therefore, the aim of our study was to assesswhether an e-nose could discriminate the exhaledbreath of patients with OVS from that of subjects withOSAand COPDalone. The secondary aim was to verify whether theseclassification are replicated in a set of newly recruitedpatients as externalvalidation. Materials and methods Patients The second group consisted of 15 subjects withouta history of sleep disturbances and with a well-defineddiagnosis ofCOPD. Inclusion criteria were: symptomsofchronic cough and dyspnea, historyofsmoking (>15packyr-), post-32 FEV1<80% of predicted and post32 FEV1/FVCratio <0.7012]. The third group included 13 individuals with aproven diagnosis ofOVS. Inclusion criteria were: pres-ence of symptoms of snoring and daily sleepiness,AHI> 5 events h-l, symptoms of chronic cough anddyspnea, history of smoking (>15 pack yr-),post-32 FEV1 <80% of predicted and post 32 FEV1/FVCratio <0.70. All participants were recruited among those vis-iting the outpatient clinic of our respiratory diseasesunit. Patients were visited in stable conditions and wereexcluded from the study in case of other pulmonaryor systemic diseases than OVS, OSA or COPD, includ-ing asthma, allergy, diabetes mellitus, renal or hepaticdisease and/or ifthey had any respiratory or systemicinfection in the 4 weeks before the study.Current smok-ers were also ruled out from the study. Eighteen newly recruited patients were used for val-idation sets using exactly the same inclusion and exclu-sion criteria as above. Therefore, we obtained threeadditional groups as follows: (a) 6 patients with OSA;(b) 6 subjects with OVS; (c) 6 individuals with COPD. The studywas approved by thelocal medicalethicalcommittee (protocol approval number 4283-06) and allparticipants gave their written informed consent. The initial patients group of 41 patients wasrecruited from December 2014 to January 2016. Theindependent cohort of 18 patients was recruited from19.7.16 until 13.08.16. Study design The current study had a cross-sectional design. Afterscreening for inclusion and exclusion criteria weperformed all measurements in one visit. Before e-nosesampling, subjects did not eat, drink and performedphysical exercise for at least the 3 precedent hours.Patients were also asked not to assume their inhaled(corticosteroids,long-acting 6-2 agonists and long-acting muscarinic antagonists) and/or oral therapy (ACE inhibitors, angiotensin II receptor antagonists,HMG-CoA reductase inhibitors and allopurinol) in the12 h before the test. Exhaled breath was collected fromall participants and sampled by the e-nose within 5 min. Exhaled breath collection and sampling Exhaled breath was collected by a previously validatedmethod [13]. Subjects breathed VOC-filtered air for5 min with a specifically designed equipment. Then,a vital capacity volume was exhaled into an inert bag,connected to the e-nose (Cyranose 320,IOS,Pasadena,CA, USA). Samples were collected during morningtime, always by the same operators and in the sameroom,with stable ambient conditions. Lung function Spirometry (MasterscreenPneumo, Jaeger, Germany)was executed by trained nurses strictly followingthe latest ERS guidelines [14]. FEV1 and FVC weremeasured before and after administering 400 ug ofSalbutamol. Cardiorespiratory nocturnal monitoring r Overnight cardiorespiratory monitoring wasperformed in room air and spontaneous breathingusing a portable 8-channel device (Somnea, Italy). Werecorded the following parameters: snoring sounds,heart rate, body posture, oxyhaemoglobinic saturation,oro-nasalairflow, thoracicand abdominal movementswere recorded. We calculated AHI, oxygen desaturationindex (ODI) and total sleep time with oxyhemoglobinsaturation below 90% (TST90). Statistical analysis SPSS software version 18.0 (SPSS Inc.,Chicago, IL,USA) was used for data analysis. E-nose raw data werereduced by principal component analysis (PCA) intoa set of principal components. Principal component 1(PC1), which captured the 92.1% ofthe total variance,was selected for correlation with clinical variables.Pearson correlation, multivariate linear and logisticregression were used to assess relations between PC1and clinical parameters. The best discriminatingcomponents were chosen by univariate ANOVAanalysis. Then, the selected principal components wereincluded into a linear canonical discriminant analysis(CDA), to classify cases in a categorical way. We usedthe ‘leave-one-out method'to determine the crossvalidatedaccuracy percentage (CVA,%). Subsequently,the discriminants resulting from the CDA were usedto build ROC curves with 95% confidence limits. Weconsidered significant a p-value of <0.05. Similarly to the above, the principal componentswhich discriminated among the study groups were usedfor pattern recognition analysis in the validation groups(external validity). The sample size was based on the results ofour for-mer studies on exhaled VOCanalysis [10,13].Wecon-cluded that per selected time-interval a minimal often OSA COPD OVERLAP pvalue Patients (n) 13 15 13 \ Sex (M\F) 5\8 15\0 13\0 \ Age 63.2±5.8 64.3±5.4 62.5±6.1 ns BMI (kg m-2) 37.7±5.7 20.9±1.4 32.4±2.3 <0.01 AHI (events h-) 44.8±24.4 48.9±21.6 ns ODI (events h) 40.2 ±12.6 41.8±17.4 ns TST 90(%) 44.3±31.3 46.2±21.5 ns FEV1 post () 2.9±0.6 0.7±0.3 0.7±0.2 <0.01 FVC post (l) 2.9±0.7 1.2±0.3 0.9±0.5 <0.01 Smokers yes\no\ex (n) 0\13\0 0\0\15 0\0\13 \ Inhaled Corticosteroids yes\no (n) 0\13 8\7 6\7 \ Long acting B2-agonists yes\no (n) 0\13 15\0 13\0 \ Long acting muscarinic antagonists yes\no (n) 0\13 15\0 13\0 \ OSA versus COPD and OVS versus COPD. POSA versus COPD and OSA versus OVS. OSA versus COPD and OSA versus OVS. subjects per study group was requiredto obtaina power(B-error) of 0.80 in order to reject the null hypothesisthat no differences between the VOC profiles amongthe groups exists, with anp-value(a-error) at <0.05. Results The clinical characteristics of the three groups ofour initial cohort are described in table1. Subjectcharacteristics ofthe independent cohort are displayedin table 2. In both cohorts there were no differences in ageamong the groups. As expected, FEV1 and FVC weresignificantly lower in COPD and OVS patients com-pared to OSA (p<0.01). Similarly, BMI was higher inOSA and OVS compared to COPD (p<0.01). AHI,ODI and TST90 did not differ between OSAand OVS. Pearson correlation, multivariate linear and logis-tic regression showed no relationship between PCl and any ofthe following parameters: FEV1, BMI, medica-tions,AHI, ODI,TST90, age and gender(p=ns). The 2D principal component analysis plot showedthat patients with OSAclustered distinctly from thosewithOVS(figure1). Subsequent canonical discriminantanaly-sis led to a cross validated accuracy %of 96.2(p<0.001).The area under the curve of the ROC-curve for the dis-crimination between OSAand OVS was 0.98 (figure2). Likewise,exhaled breath of patients with OSA wasdiscriminated from that of individuals with COPD,with CVA% of 82.1 (p< 0.01, figure 3). The AUC oftheROC curve for the discrimination between OSAandCOPD was 0.95 (figure4). Conversely,breathprints ofOVS were not separatedfrom those of COPD, with CVA% of67.9 (p=ns). The three-way classification among OSA,OVS andCOPD led to a CVA% of 73.3 (p <0.01) (figure5). Newly recruited patients with OSA and OVS couldbe adequately discriminated, when applying previous Figure 1. 2D principal component analysis plot with 2 factors showing the discrimination of breathprints between patients withOSA (circles)andOVS (triangles). REGR=regression. Figure2. ROC-curve with 95% confidence interval for diagnosis ofOSA compared to OVS. AUC was 0.98. dataset model based on 2 principal components with aCVA% of91.7(p<0.001).The AUC for external vali-dation was 1.00. Discussion Similarly, breathprints of newly recruited patientswith OSA and COPD were separated when using thetraining set model, with a CVA of 75.0% (p<0.01).AUC reached0.83. Finally, external validity for OVS versus COPDshowed no separation between OVS and COPD(CVA%=57,1%,p=ns). Table 3 summarizes results ofall the validation tests. In the present study we showed that an electronic nosecan distinguishthe exhaled breath ofpatients with OSAfrom that of OVS, as well as from COPD.Conversely,no significant differences were found between OVSand COPD in terms of exhaled VOC-profile. Ourfindings were confirmed by external validation withnewly recruited patients.This study adds to the currentknowledge base about the understanding of OSApathophysiology. Figure 3. 2D principal component analysis plot with 2 factors showing the discrimination of breathprints between patients withOSA (blue circles) and COPD (red circles). REGR regression. Figure4. ROC-curve with 95% confidence interval for diagnosis ofOSA compared to COPD. AUC was 0.95. Toourknowledge,thisisthefirststudywhich performsa formal group-to-group comparison between patientswith OVS and subjects with OSA and COPD alone. Theimportance is given by the fact that airway inflammationplays an importantrole in both OSA andCOPD,and thata patient with one ofthe disorders has a greater than 10%chance ofalso having the other disease [1]. Several authors have formerly investigated exhaledVOC-patterns using the same e-nose technology aswe did, showing adequate sensitivity and specificity indetecting OSA [8,9,15]. Our previous findings revealed that e-nose can dis-criminate between the exhaled profile ofobese patientswith OSA from that of non-obese healthy controls(CVA =94.1%,p<0.001).Furthermore, adistinctionbetween obese patients without OSA and non-obesehealthy controls was also obtained (CVA=97.4%,p<0.001). However,VOC-spectrum ofobese patientswith and without OSA did not differ between eachother [10]. Thestrengths ofour study are the careful selection ofpatients, strictly following current accepted guidelines Figure 5. 2D principal component analysis 2 factors showing the discrimination of breathprints in the three-way analysis amongOSA (circles),OVS (triangles) and COPD (squares). REGR= regression. Table 3. External validation results. Set CVA% p-value AUC 95%CI OSA versus OVS 91.7 <0.001 1.00 1.00-1.00 OSA versus COPD 75.0 <0.01 0.83 0.68-1.00 OVS versus COPD 57.1 0.248 0.60 0.50-0.79 [11, 12], the use of a validated procedure for obtainingand sampling the exhaled breath [13] and the exclusionofactive smokers and all patients with any comorbidi-ties which could alter the exhaled VOC-profile. This study has also several limitations. First, this isa pilot study with a relatively small sample size and asmaller validation set. Second, patients were assumingspecific therapy for their disease such as inhaled bron-chodilators and/or corticosteroids which may haveskewed the VOC-profile, thus altering the accuracy ofthe tests. However, we found no correlation betweenPC1 and medication use, suggesting that drug usage maynot be a major determinant ofexhaled breathprints. Third, e-nose technology does not determine indi-vidualVOCs and their concentration. Undoubtedly,future studies should apply chemical analytical tech-niques, like GC-MSto identify disease specific VOCs, inorder to help the development oftailor-made e-nosesthat could be used implemented in daily practice. How can we explain our results? Airways inflam-mation is known to play an important role in bothCOPD and OSA. Inflammatory profile in COPD ispredominated by CD8-positive T cells, neutrophilsand macrophages [16] In particular, there is large evi-dencelinking neutrophils with COPD [4, 5,16].Anal-ysis of induced sputum, bronchoalveolar lavage fluidand airway smooth muscle robustly demonstratedincreased neutrophil counts and neutrophil-derived enzyme levels in COPD in both stable conditions andduring exacerbations [4]. Neutrophils are first-linedefensive cells and a known source of reactive oxygenderivates, pro-inflammatory cytokines, antibacterialpeptides, lipid mediators and tissue-damagingenzymes[4], which are likely to be responsible for the alteredVOC-composition in the exhaled breath of patientswith COPD [71. OSA also shows an inflammatory component,although the exact mechanisms are still unclear. Obesity,which is very often associated with OSA, islikelyto inducephlogosis by secretion offat cell-mediated inflammatoryfactors [17].Moreover, vibratory trauma associated withsnoring harms the upper airways tissue, leading to therelease of proinflammatory cytokines such as interleu-kin-8[18]. Finally, thecontinuous overnighthypoxia andreoxygenation is likely to cause oxidative stress and thegeneration of reactive oxygen species (ROS), which areknown to play an important phlogogen role [18]. Therefore OSA and COPD are characterized by adifferent inflammatory profile,which is reflected inthe complete separation of breathprints between thetwo groups. Regarding OVS, the combination ofall theabove processes probably leads to a different exhaledVOCs pattern which would explain the distinctionbetween breathprints ofOSA andOVS. We may interpret the incomplete separationbetween OVS and COPD by the presence ofthe sameunderlying CD8-positive T cells driven inflammationdue to the latter, which is someway predominant onthe phlogosis caused by OSA. To confirm this hypoth-esis, further investigations should correlate exhaledVOCs analysis with validated techniques for monitor-ing inflammation, such as induced sputum or bloodmarkers. It must be stressed that obesity per se may causesystemic inflammation due several inflammatory adi-pokines, such as leptin and adiponectin produced byadipocytes, especially during hypoxia [4].The connec-tion between obesity and airway inflammation is stillunclear. We previously showed that the exhaled VOC-profile differed between obese patients without OSAand healthy non obese controls [10]. This warrantsfurther studies to evaluate whether obesity alone caninduce pulmonary inflammation and what is the linkbetween the above conditions. In conclusion, our findings indicate that VOC-profilingcan differentiate the exhaled profile ofpatientswith OSA from those with OVS as well as those withCOPD, supporting the view of using breath to betterunderstand the pathophysiology of sleep disorderedbreathing. Future studies, strictly following the STARD state-ment for the validation of new tests [19], are compulsoryto compare e-noses diagnostic potential with conven-tional procedures,such as sleep questionnaires andpoly-somnography.Moreover,larger cohorts of patients withdifferentcomorbidities should also be included. Conflict ofinterest statement None of the authors have any potential conflict ofinterest related to this manuscript. References ( [ 1] Weitzenblum E e t al 2008 Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease P roc.Am.Thorac. Soc. 1 5 23 7 - 4 2 ) ( [2 N ] u ral S et al2013 Inflammatory processes and effects ofcontinuous positive airway pressure ( CPAP) in overlapsyndrome Inflammation 3 6 66- 7 4 ) ( [3 ] C h aouat A et al 1 997 Sleep-related O2 desaturation and daytime pulmonary haemodynamics in COPD patients with mildhypoxaemia Eur. Respir. J. 1 0 173 0 -5 ) ( [4 ] Lacedonia D et al 2011 Airway cell patterns in patients suffering from COPD and OSAS (overlap syndrome) R espir. Med. 105 30 3 - 9 ) ( [5] M cNicholas WT 2 00 9 Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology,systemic inflammation, and cardiovascular disease Am. Respir. Crit. Care Med. 180 69 2 - 7 00 ) ( [6] P auling Letal 1971 Quantitative analysis ofurine vapor andbreath by gas-liquid partition chromatography Proc. NatlAcad. Sci. USA 68 23 74 - 6 ) ( [7] FensNetal2011 Exhaled air molecular profiling in relation to inflammatory subtype and activity in COPD Eur. Respir. J. 381301 - 9 ) ( [8]Greulich T etal 2013 Detection of obstructive sleep apnoea byan electronic nose Eur. Respir. J . 4 2 1 4 5- 5 5 ) ( [9 ] Kunos L et al 2015 Evening and morning exhaled volatile compound patterns are different in obstructive sleep apnoeaassessed with electronic nose Sleep Breath 1 9 247- 5 3 ) ( [ 1 0] D r agonieri S et al 2015 An electronic nose in the discrimination ofobese patients with and without obstructive sleep apnoeaJ. Breath Res. 9 026005 ) [11] Epstein L Jet al 2009 Clinical guideline for the evaluation,management and long-term care ofobstructive sleep apnoeain adultsJ. Clin. Sleep Med. 5 263-76 ( [ 12] R abe K Fet al 2 0 07 Global strategy for the diagnosis, management,and prevention of chronic obstructive pulmonary disease: GOLD executive summary Am.J. Respir. Crit. Care Med . 17 6 53 2- 5 5 ) [13] Dragonieri S et al 2007 An electronic nose in the discrimination ofpatients with asthma and controls J. AllergyClin. Immunol. 120 856-62 ( [14] Miller M R et al 2005 Standardization of spirometryEur. Respir.J . 2 6 319 - 38 ) ( [15] BenedekP et al 2013 E xhaled biomarker pattern is alteredin children w ith obstructive sleep apnoea syndrome Int. J. Pediatr. Otorhinolaryngol. 77 1 2 4 4- 7 ) ( [ 1 6] Chung K F et al 2008 Multifaceted mechanisms in COPD: i nflammation, immunity, and tissue repair and destruction E ur. R espir. J .3 133 4 - 5 6 ) ( [17] Carpagnano GEetal2008 Exhaled pH, nitric oxide andinduced sputum cellularity in obese patients with obstructive s leep apnoea syndrome Transl. Res. 151 4 5- 5 0 ) ( [ 1 8] Calvin A D et al 2009Obstructive sleep apnea,inflammation,andt h e metabolic syndrome Metab. Syndr. Relat. Disord. 7271 - 8 ) ( [19] Bossuyt P M et al 2003 The STARD statement for reportingstudies of diagnostic accuracy: explanation and elaboration Ann. Intern. Med . 1 381 - 1 2 ) @IOP Publishing Ltd 背景用电子鼻 (e-nose) 分析挥发性有机化合物 (VOCs) 是一种突破性的方法, 提供了独特的呼气分子模式在几个呼吸和全身性疾病。研究表明, 电子鼻可以检测到阻塞性睡眠呼吸暂停 (OSA)以及慢性阻塞性肺病 (COPD)。OSA 和慢性阻塞性肺病有时与入所谓的重叠综合症 (OVS)。在这项试点研究中, 我们假设电子鼻可以将 OVS 患者呼出的呼吸与仅 OSA 和 COPD 患者的呼气进行鉴别。13例 OSA 患者、15例慢性阻塞性肺病患者和13例 OVS 患者参加横断面治疗研究。呼气是通过以前经过验证的方法收集的, 并通过使用电子鼻子 (Cyranose 320)。通过规范判别分析对原始数据进行分析。主要成分减少。计算了交叉验证精度 (CVA) 和 roc 曲线。对新招募的患者 (6 例 OSA、6例 OVS 和6例 COPD) 进行了外部验证测试。以前的训练集。OSA 患者的呼吸图明显聚集在 OVS 患者中 (CVA = 96.2%)作为以及与慢性阻塞性肺病 (CVA = 82.1)。OVS 的呼吸图没有明显分离慢性阻塞性肺病 (CVA = 67.9%)。外部验证证实了上述发现。电子鼻可以很高的准确性区分 osa 患者呼出的 voc 剖面。有 OVS 的人以及患有慢性阻塞性肺病的人这就需要今后的研究来证实该技术在睡眠呼吸暂停的无创检测中的应用。
确定




还剩6页未读,是否继续阅读?
图拉扬科技有限公司为您提供《呼出气体中VOCs检测方案(感官智能分析)》,该方案主要用于其他中VOCs检测,参考标准--,《呼出气体中VOCs检测方案(感官智能分析)》用到的仪器有Cyranose 320 电子鼻
推荐专场
感官智能分析系统(电子鼻/电子舌)
相关方案
更多
该厂商其他方案
更多
















