方案详情
文
A Vanquish Flex Quaternary UHPLC system was applied for the SEC analysis. This is a low dispersion, inert UHPLC, which can be used successfully for this type of application. For this analysis, pre-column dispersion was intentionally introduced to show the effects of dispersion in front of the column at different flow rates.
方案详情

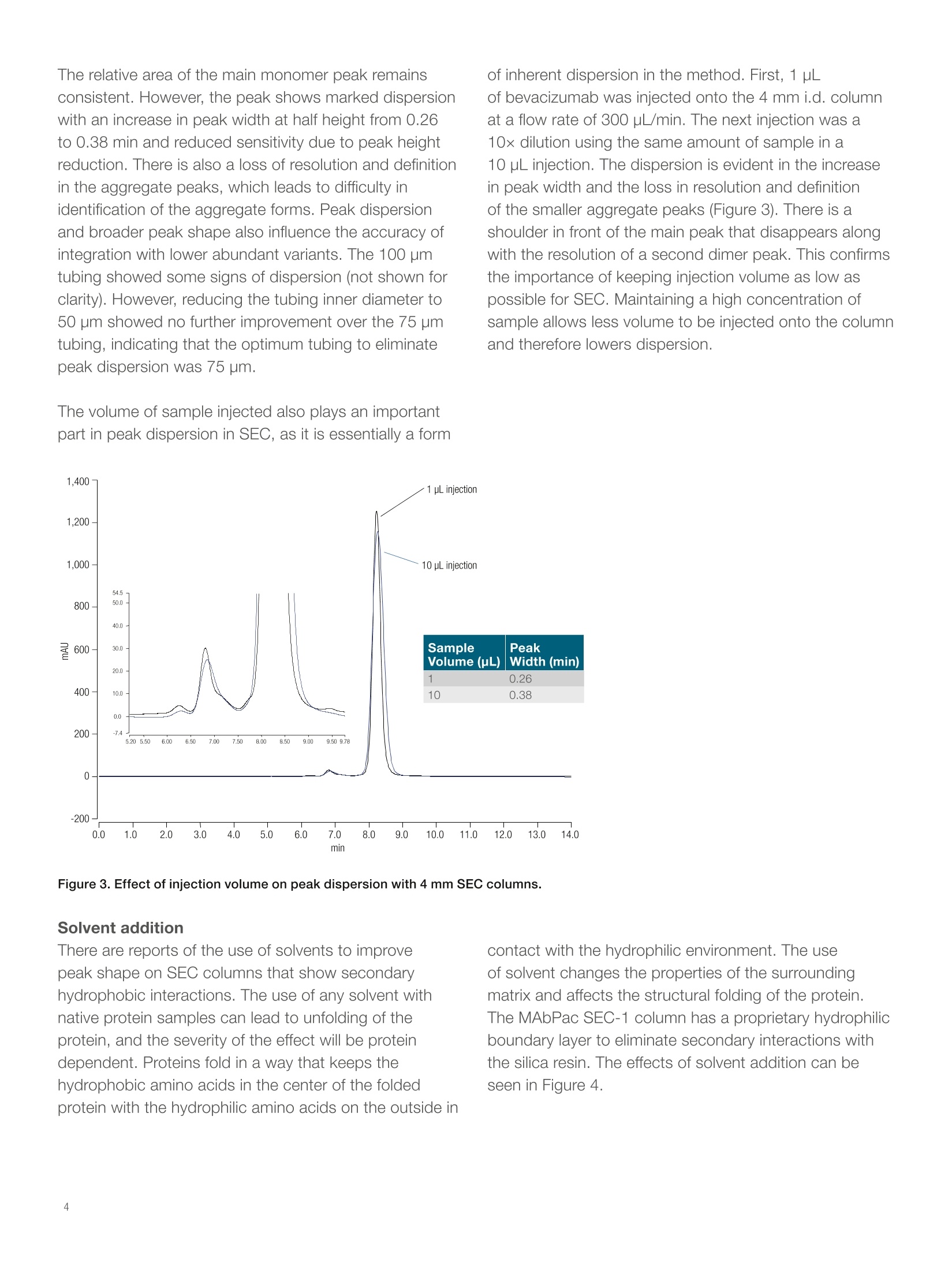
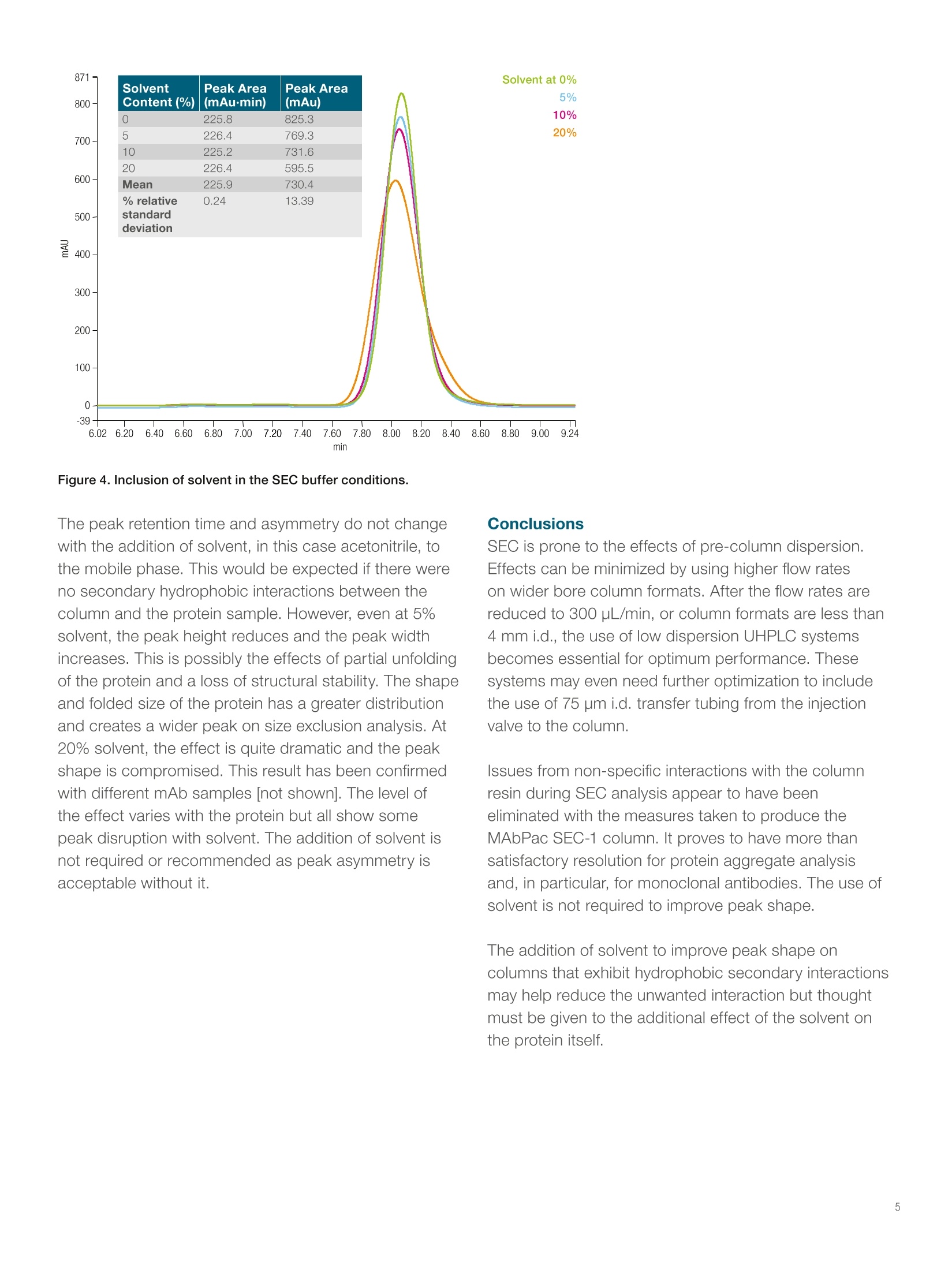
thermoscientific Results and discussionPre-column dispersion The importance of correct UHPLCinstrument setup for proteinaggregate analysis by size-exclusionchromatography Amy Farrell, Jonathan Bones, and Ken Cook2 1NIBRT, Dublin,Ireland; 2Thermo FisherScientific, Hemel Hempstead, UK Key words MAbPac SEC-1, monoclonal antibody, mAb, proteinaggregation analysis, biotherapeutics, pre-columndispersion Goal Show the applicability of Thermo ScientificMabPac" SEC-1 columns for monoclonal antibodyaggregate analysis using the Thermo ScientificVanquish Flex Quaternary UHPLC system. Introduction The industry-standard size-exclusion chromatography(SEC) column dimension for protein aggregate analysisis 7.8 mm internal diameter (i.d.), running at 1.0 mL/min.This is usually carried out using older HPLC systems,which perform well enough with these relatively highflow rates. The introduction of UHPLC, with muchlower dispersion, has allowed the use of lower flow ratecolumns and higher resolution stationary phases. It iscommonly believed that smaller dimension SEC columns are difficult to pack and this accounts for the reductionin performance seen with these columns compared tohigher flow rate 7.8 mm i.d. columns. A more likely causefor apparent reduced performance is that the UHPLCsystem used for the comparison does not have thecorrect tubing or low dispersion flow path required tomaintain peak integrity, an essential factor when usingSEC at lower flowrates.1.2 A Vanquish Flex Quaternary UHPLC system was appliedfor the SEC analysis. This is a low dispersion, inertUHPLC, which can be used successfully for this type ofapplication. For this analysis, pre-column dispersion wasintentionally introduced to show the effects of dispersionin front of the column at different flow rates. The separation was performed on MabPac SEC-1columns of differing internal diameters and flow rates. The Vanquish UHPLC system was used to showthe applicability of the MAbPac SEC-1 column formonoclonal antibody aggregate analysis. TheMAbPac SEC-1 column is silica based and has beencovalently modified with a proprietary diol hydrophiliclayer to prevent secondary interactions which can hinderthe chromatography of certain proteins.2.3 SEC is one ofthe few chromatography methods that exhibits no‘on-column’ focusing. Due to this, the pre-column dispersionon the system used is extremely important, especiallyat reduced flow rates on smaller i.d columns, as therewill be no focusing of broad peak volumes at the headof the column. In adsorption chromatography, evenunder isocratic elution conditions, one would expectsome focusing of the injection volume at the head ofthe column. In SEC, the volume in which the sample ispresented to the column will only get larger in volume asit moves through the column. Therefore, many columnsdo not attain their expected resolution when using olderHPLC systems with inherent dispersion on-injection.The effect of pre-column dispersion has been the subjectof several reviews and can easily lead to up to 50%increase in peak widths on dispersive HPLC systems.The low dispersion Vanquish UHPLC system was appliedfor the SEC analysis to control and study the effects ofdispersion. The separation was performed on MabPacSEC-1 columns with a commonly used high salt buffer atpH 6.8. Experimental Consumables · Fisher ScientificHPLC grade water (P/N 10449380) · Deionized water, 18.2 MQ.cm resistivity · Fisher Scientific Sodium phosphate dibasic anhydrous(P/N 10440481) · Fisher Scientific Sodium phosphate monobasicanhydrous (P/N 10751135) · Fisher Scientific Sodium chloride (P/N 11964051) · Thermo ScientificVirtuosoVial Identification System(P/N 60180-VT100) · Virtuoso 9 mm Wide Opening SureStop Screw ThreadVial Convenience Kit (P/N 60180-VT405) Sample preparation Bevacizumab was diluted to a 25 mg/mL solution withmobile phase containing 0, 5, 10, or 20% solvent asappropriate (see Figure 4). Separation conditions Instrumentation Vanquish Flex Quaternary UHPLC system equipped with: ·System Base Vanquish Flex (VF-S01-A) ·Quaternary Pump F (P/N VF-P20-A) ·Split Sampler FT (P/N VF-A10-A) ·Column Compartment (P/N VH-C10-A) ·Diode Array Detector HL (P/N VH-D10-A) ·Thermo Scientific LightPipeFlow Cell, Standard,10 mm (P/N 6083.0100) Columns: MAbPac SEC-1, 7.8x 300 mm(P/N 088460)MAbPac SEC-1, 4x300 mm(P/N 074696) SEC buffer Mobile phase: 0.2 M NaCl in 100 mMphosphate buffer pH 6.8Flow rate: 1.0 mL/min for 7.8 mm i.d.column and 0.3 mL/min for4 mm i.d. columnColumn temperature: 30°CInjection volume: 1 pL, unless stated UV: 214 nm Data processing The Thermo ScientificChromeleon7.2 SR2Chromatography Data System was used for dataacquisition and analysis. Columns of 4.0 and 7.8 mm i.d. were used to test theeffects of pre-column dispersion. The optimal flow ofthese columns is 0.3 and 1.0 mL/min, respectively. Theflow rate used has a profound effect on the setup of thesystem. Lower flow rates were much more prone to theeffects of any pre-column dispersion. At the higher flowrate of 1.0 mL/min using the 7.8 mm i.d. column, therewas no discernible difference when the pre-column tubingwas changed from the standard 100 pm i.d. tubing to75 pm. Peak width, asymmetry, and resolution were allthe same in both analyses, as can be seen in the overlayin Figure 1. This column was flowing at 1.0 mL/min,which generated 30 bar backpressure on the pre-column100 pm tubing alone. The backpressure generated bythe 75 pm tubing was 90 bar, but the change showed noimprovement, indicating the chromatography was alreadyoptimum with the standard configuration on the system. The change when using the 4 mm id. column at0.3 mL/min is quite dramatic. To mimic the configurationof a standard HPLC system, 180 pm tubing was usedas well as 100 pm and 75 pm. These configurations arecommonly used in SEC separations. In the overlay shownin Figure 2 there is a marked reduction in performanceusing 180 um tubing in front of the column. This is whatcan be expected using a standard HPLC system atthis flow rate on 4 mm i.d. SEC columns. This effect iscompounded by the addition of the dispersion in theinjection valves of older HPLC systems. Figure 2. Overlay of bevacizumab using 180 pm and 75 pm i.d. tubing in front of the column. Column i.d. was 4.0 mm. Figure 1.Overlay of bevacizumab SEC analysis with 100 pm and75 pm i.d. tubing placed between the injection valve and the frontof the column.Column i.d. was 7.8 mm. of inherent dispersion in the method. First, 1 pLof bevacizumab was injected onto the 4 mm i.d. columnat a flow rate of 300 uL/min. The next injection was a10x dilution using the same amount of sample in a10 pL injection. The dispersion is evident in the increasein peak width and the loss in resolution and definitionof the smaller aggregate peaks (Figure 3). There is ashoulder in front of the main peak that disappears alongwith the resolution of a second dimer peak. This confirmsthe importance of keeping injection volume as low aspossible for SEC. Maintaining a high concentration ofsample allows less volume to be injected onto the columnand therefore lowers dispersion. There are reports of the use of solvents to improvepeak shape on SEC columns that show secondaryhydrophobic interactions. The use of any solvent withnative protein samples can lead to unfolding of theprotein, and the severity of the effect will be proteindependent. Proteins fold in a way that keeps thehydrophobic amino acids in the center of the foldedprotein with the hydrophilic amino acids on the outside in contact with the hydrophilic environment. The useof solvent changes the properties of the surroundingmatrix and affects the structural folding of the protein.The MAbPac SEC-1 column has a proprietary hydrophilicboundary layer to eliminate secondary interactions withthe silica resin. The effects of solvent addition can beseen in Figure 4. The peak retention time and asymmetry do not changewith the addition of solvent, in this case acetonitrile, tothe mobile phase. This would be expected if there wereno secondary hydrophobic interactions between thecolumn and the protein sample. However, even at 5%solvent, the peak height reduces and the peak widthincreases. This is possibly the effects of partial unfoldingof the protein and a loss of structural stability. The shapeand folded size of the protein has a greater distributionand creates a wider peak on size exclusion analysis. At20% solvent, the effect is quite dramatic and the peakshape is compromised. This result has been confirmedwith different mAb samples [not shown]. The level ofthe effect varies with the protein but all show somepeak disruption with solvent. The addition of solvent isnot required or recommended as peak asymmetry isacceptable without it. Conclusions SEC is prone to the effects of pre-column dispersion.Effects can be minimized by using higher flow rateson wider bore column formats. After the flow rates arereduced to 300 uL/min, or column formats are less than4 mm i.d., the use of low dispersion UHPLC systemsbecomes essential for optimum performance. Thesesystems may even need further optimization to includethe use of 75 pm i.d. transfer tubing from the injectionvalve to the column. Issues from non-specific interactions with the columnresin during SEC analysis appear to have beeneliminated with the measures taken to produce theMAbPac SEC-1 column. It proves to have more thansatisfactory resolution for protein aggregate analysisand, in particular, for monoclonal antibodies. The use ofsolvent is not required to improve peak shape. The addition of solvent to improve peak shape oncolumns that exhibit hydrophobic secondary interactionsmay help reduce the unwanted interaction but thoughtmust be given to the additional effect of the solvent onthe protein itself. thermoscientific References 1. Grznarovaa, G.; Polakovica, M.; Acaia, P.; Gornerb,T. Extra-column dispersion of macromolecular solutesin aqueous-phase size-exclusion chromatography,Journal of Chromatography A, 2004, 1040(1), 33-43. 2. Hong, P.; Koza, S.; Bouvier, E.S.P. Size exclusionchromatography for the analysis of proteinbiotherapeutics and their aggregates, Journal of LiquidChromatography & Related Technologies, 2012Nov; 35(20),2923-2950. 3. Arakawa, T.; Philo, J. S.; Ejima,D.; Tsumoto, K.;Arisaka, F. Aggregation analysis of therapeuticproteins, BioProcess International, 2006,4(10), 42-43. Find out more at thermofisher.com/chromatography For Research Use Only. Not for use in diagnostic procedures. 2016 Thermo Fisher Scientific Inc. A Vanquish Flex Quaternary UHPLC system was applied for the SEC analysis. This is a low dispersion, inert UHPLC, which can be used successfully for this type of application. For this analysis, pre-column dispersion was intentionally introduced to show the effects of dispersion in front of the column at different flow rates.
确定


还剩4页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《蛋白质(protein)中聚合凝胶排阻层析分析(aggregate analysis)检测方案(液相色谱仪)》,该方案主要用于其他中聚合凝胶排阻层析分析(aggregate analysis)检测,参考标准--,《蛋白质(protein)中聚合凝胶排阻层析分析(aggregate analysis)检测方案(液相色谱仪)》用到的仪器有赛默飞 Vanquish Flex UHPLC系统
推荐专场
相关方案
更多
该厂商其他方案
更多