方案详情
文
Monoclonal antibodies (mAbs) are currently the dominant class of protein therapeutics in the biopharmaceutical industry due to their high specificity to target antigens, long serum half-life in humans, and capabilities for use in the treatment of a wide range of ailments such as inflammatory diseases and cancer. During product expression and purification from host cells, formulation, and storage, mAbs may undergo various degradation processes, which may alter the safety, efficacy, and quality profile of the drug product. Consequently, a number of critical quality attributes (CQAs) must be monitored throughout drug development and production to ensure biotherapeutic substances are suitable for clinical use.
方案详情

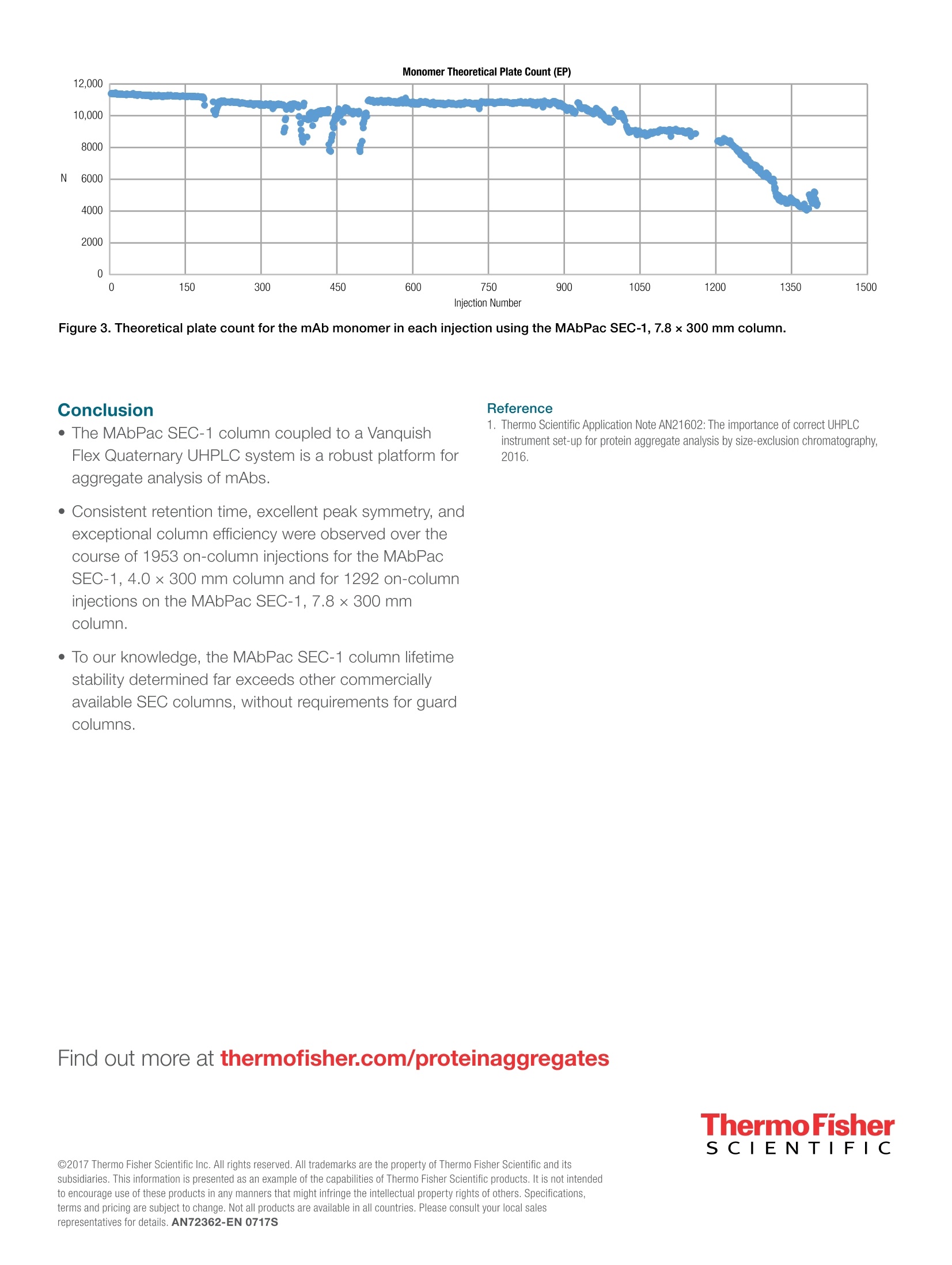
Monomer Theoretical Plate Count (EP)ThermoFisherS CIENTIFI C Lifetime stability of size exclusion chromatoaraphycolumns for protein aggregate analysis thermoscientific APPLICATION NOTE 72362 AuthorsAmy Farrell1, Craig Jakes1,Alexander Ley2, Mauro De Pra²,Frank Steiner2, and JonathanBones 1The National Institute for Bioprocessing Research andTraining (NIBRT), Dublin, Ireland2Thermo Fisher Scientific. Germering, Germany KeywordsMAbPac SEC-1, MonoclonalAntibody, mAb, Protein AggregationAnalysis, Size ExclusionChromatography, Biotherapeutics,Critical Quality Attributes, VanquishFlex UHPLC ·Columns for analysis of mAb aggregates with exceptionally long lifetime · A biocompatible UHPLC system capable of operating with high salt contentand potentially corrosive mobile phases · A UHPLC solution for mAb aggregate analysis that operates continuouslyfor several weeks Goal To demonstrate the long-term stability of the Thermo ScientificMAbPac"SEC-1 column for monoclonal antibody aggregate analysis using theThermo ScientificVanquish Flex Quaternary UHPLC system. Introduction Monoclonal antibodies (mAbs) are currently the dominant class of proteintherapeutics in the biopharmaceutical industry due to their high specificityto target antigens, long serum half-life in humans, and capabilities foruse in the treatment of a wide range of ailments such as inflammatorydiseases and cancer. During product expression and purification from host cells, formulation, and storage, mAbs may undergovarious degradation processes, which may alter thesafety, efficacy, and quality profile of the drug product.Consequently, a number of critical quality attributes(CQAs) must be monitored throughout drug developmentand production to ensure biotherapeutic substances aresuitable for clinical use. Aggregation is a common degradation process fortherapeutic proteins that can result from partial unfoldingor other types of conformational changes in proteinstructure to form dimers, trimers, and other higher orderstructures. Protein aggregation is considered a CQA asaggregates may reduce product efficiency by loweringthe effective concentration of the product and have beenfound to result in immunogenic effects in patients. Hence,it is a regulatory requirement to monitor the aggregationprofile of therapeutic proteins. Size exclusion chromatography (SEC) is the mostcommonly applied method for protein aggregate analysis.In SEC, sample molecules are passed through a columncontaining porous polymer or silica beads to facilitateseparation of species based on their size. For proteintherapeutics, a pore size of 300 A is used, which allowssmaller species to penetrate into the porous beads(e.g. fragments, monomers) while larger molecules (e.g.dimers,trimers) are more excluded from the pores andtherefore elute more quickly from the column. Mostfrequently, SEC columns with widths of 7.8 mm are usedfor aggregate analysis in industry. However, use of lowdispersion, biocompatible UHPLC systems and narrowinternal diameter pre-column tubing has been shownto improve peak shape in chromatograms generatedusing columns of reduced widths. Narrow columns haveadvantages such as small sample volume requirementsand reduced mobile phase consumption. Due to high costs associated with drug development andproduction and the potential for mAb-based biosimilarsresulting from patent expiry for some of the top-sellingmAb therapeutics, reliable, long-use consumables andequipment for CQA evaluation is needed. Suppliers ofSEC columns have illustrated column stability lifetimesof approximately 550 injections (without a column guard) and up to 902 injections (with a column guard). In thisstudy, a MAbPac SEC-1 column (4×300 mm, 5 pm,300 A) and a Vanquish Flex Quaternary UHPLC systemwere applied to assess the long-term stability of thecolumn based on protein aggregation monitoring of thecommercial drug product bevacizumab, a humanizedmonoclonal IgG1 antibody produced from a Chinesehamster ovary mammalian cell expression system. Useof pre-column tubing with internal diameter of 75 pmensured that excellent peak shape was achieved using aSEC column with dimensions of 4 × 300 mm. However,as SEC columns with widths of 7.8 mm are mostcommonly utilized in the biopharmaceutical industry,the column lifetime stability of an MAbPac SEC-1 column(7.8×300 mm, 5 um, 300 A) was also evaluated. Experimental Recommended consumables ·Deionized water, 18.2 MQ.cm resistivity · Thermo Scientific MAbPac SEC-1, 5 um, 300 A,4.0×300 mm (P/N 074696) Thermo Scientific MAbPac SEC-1, 5 pm, 300 A,7.8×300 mm (P/N 0088460) · Fisher Scientificsodium phosphate dibasic anhydrous(P/N 010440481) · Fisher Scientific" sodium phosphate monobasicanhydrous (P/N 010751135) · Fisher Scientificsodium chloride (P/N 011964051) ·Thermo ScientificVirtuosoVial Identification System(P/N 060180-VT100) · Virtuoso 9 mm Wide Opening SureStop Screw ThreadVial Convenience Kit (P/N 060180-VT405) Sample preparation Bevacizumab (25 mg/mL) was diluted 1:10 in 100 mMsodium phosphate, pH 6.8, 300 mM NaCl (for MAbPacSEC-1 4.0×300 mm column) or 50 mM sodiumphosphate, pH 6.8, 300 mM NaCl (for MAbPac SEC-17.8×300 mm column). Diluted samples were aliquotedand stored at -20 ℃. On each day of analysis, a freshaliquot of diluted bevacizumab sample was removed fromthe freezer for analysis. Buffer preparation MAbPac SEC-1 4.0×300 mm column: 100 mM sodiumphosphate, pH 6.8 in 300 mM NaCl was used as mobilephase. Buffers were filtered through a 0.2 um filtermembrane before use. MAbPac SEC-1 7.8×300 mm column: 50 mM sodiumphosphate, pH6.8 in 300 mM NaCl was used as mobilephase. Buffers were filtered through a 0.2 um filtermembrane before use. Separation conditions Instrumentation Vanquish Flex UHPLC system, including: · Quaternary Pump (P/N VF-P20-A) ·Column Compartment H (P/N VH-C10-A) · Split Sampler FT (P/NVF-A10-A) with 25 pLSample Loop ·Diode Array Detector HL (P/N VH-D10-A) with LightPipe10 mm Standard Flow Cell (P/N6083.0100) ·System Base Vanquish Flex (P/N VF-S01-A) Flow rate · MAbPac SEC-1 4.0×300 mm column: 0.250mL/min ·MAbPac SEC-1 7.8×300 mm column: 0.8 mL/minColumn temperature · 30°℃ Injection details ·MAbPac SEC-1 4.0×300 mm column: 2.0 uL of2.5 pg/pL bevacizumab, diluted in mobile phase. e MAbPac SEC-1 7.8×300 mm column: 0.5 uL of2.5 pg/pLbevacizumab, diluted in mobile phase Detector settings · MAbPac SEC-1 4.0×300 mm column: Data wascollected at 280 nm and 214 nm on a DAD detector. ●MAbPac SEC-1 7.8×300 mm column: Data wascollected at 280 nm on a DAD detector. Data processing The Thermo ScientificChromeleon7.2 SR4Chromatography Data System was used for dataacquisition and analysis. Results and discussion To evaluate the stability of a MAbPac SEC-1 analyticalcolumn for aggregate analysis of therapeutic monoclonalantibodies, repeated injections of bevacizumab drugsubstance were performed using a Vanquish Flex UHPLCsystem with Chromeleon software version 7.2. TheMAbPac SEC-1 column is a silica-based SEC columncovalently modified with a proprietary diol hydrophiliclayer to prevent secondary interactions of analytesto the stationary phase. A new and unused columnwas employed for the study and was conditionedby performing ten injections of mAb until a constantpeak area and peak height were observed as per themanufacturer’s guidelines. Each day of analysis andfor each new buffer preparation, blank injections wereperformed prior to mAb determination to ensure columnequilibration. The industry-standard SEC column dimension for proteinaggregate analysis is 7.8 mm internal diameter (i.d.) usingflow rates of 1 mL/min. It has previously been shown thatpre-column dispersion often associated with reduced i.d.SEC columns (4 mm) and low flow rates (<0.3 mL/min)may be overcome using a low-dispersion, biocompatibleUHPLC, such as the Vanquish Flex UHPLC, with smallerinjection volumes and fitted with narrow i.d. pre-column.transfer tubing (75 pm i.d. tubing). Using 75 pmpre-column transfer tubing, a 4 mm i.d. MAbPacSEC-1 column was evaluated. Over the course of thestudy excellent resolution of aggregates and fragmentpeaks from the main mAb monomer peak was observedas shown in Figure 1. 120 Figure 1. Aggregate analysis of bevacizumab using a MAbPac SEC-1 column. As Table 1 shows, consistent retention times wereobserved for the monomer peak over the course of morethan 1950 injections, with a retention time differenceof just 0.043 minutes between injection numbers 25and 1953; this illustrates the absence of chemical orelectrostatic interactions with the column stationaryphase following extensive use. Similarly, excellent peaksymmetry was detected throughout the column lifetimestability study (asymmetry range 0.88 to 0.93), furtherdemonstrating a lack of secondary interactions with the column packing material and hardware across the lifetimeof the column. Column efficiency, based on EuropeanPharmacopoeia theoretical plate count, was found tobe greater than 85% of initial efficiency following 1953injections on the column (equivalent to 1866 injectionsof mAb). After 1866 injections of bevacizumab, a lossin column performance was observed as shown inFigure 2. The loss in column performance (<85%theoretical plate count) did not appear to result in achange to the aggregation profile of bevacizumab. On Column Retention Monomer Relative Monomer Peak Width Asymmetry Theoretical Time Peak Area @50% Height Plates (EP) Injection # (min (%) (min) (EP) 25 9.554 96.96 0.237 0.92 9032 404 9.558 97.21 0.240 0.92 8797 665 9.558 96.96 0.241 0.93 8736 1213 9.567 97.27 0.243 0.89 8604 1551 9.544 96.99 0.244 0.88 8460 1953 9.524 96.30 0.255 0.89 7737 Bevacizumale Injection Number Figure 2. Theoretical plate count for the mAb monomer in each injection using the MAbPac SEC-1, 4.0×300 mm column. Greater than 85%of initial column efficiency was preserved following 1866 injections of bevacizumab (corresponding to 1953 on column injections). A loss of columnperformance was observed after 1866 injections of bevacizumab. Because SEC columns with internal diameters of7.8 mm are most often applied for aggregate analysisin the biopharmaceutical industry, the columnifetime stability of a MAbPac SEC-1, 5 pm, 300 A,7.8×300 mm column was also evaluated. In this case.a new and unused column was also employed forthe study and was conditioned prior to use followingrecommended guidelines. During analysis the columnwas injected ten times with bevacizumab, followed bytwo injections of a protein check standard. This injectioncycle was repeated until column degradation wasobserved. Table 2 shows highlighted chromatography data forbevacizumab analyzed using a MAbPac SEC-1,7.8 ×300 mm column. The final line on the tabledisplays information for the final injection that wasabove the column efficiency specification for monoclonalantibodies, namely injection number 1292 (>6300theoretical plates (EP) for the monomer peak). Likethe MAbPac SEC-1, 4.0×300 mm column, the MAbPac SEC-1, 7.8 ×300 mm column displayed relativelyconsistent retention time and peak symmetry andexcellent column efficiency over the lifetime ofthe column (Figure 3). Despite exhibiting reducedcolumn lifetime stability in comparison to the MAbPacSEC-1, 4.0×300 mm column, the MAbPac SEC-1,7.8 ×300 mm column showed extended column lifetimestability when compared to the lifetime stability previouslydemonstrated for alternative SEC columns. Greater than55% of initial column efficiency was preserved following1077 injections of bevacizumab (corresponding to1292 on column injections). A loss of columnperformance was observed after 1077 injections ofbevacizumab. The efficiency drops observed between300 and 500 injections were not attributed to columndegradation. It was observed that sample agingnegatively affected peak width. When sample wasreplaced, high efficiency values were restored. The rootcause for this behaviour could not be found. When thecolumn degraded, the replacement of aged sample withfresh did not have any beneficial effect on peak width. Table 2. Monomer peak information acquired following aggregate analysis of bevacizumab using a MAbPac SEC-1, 7.8×300 mmanalytical column. On Column Time Peak Area @ 50% Height Asymmetry Plates Injection # (min) (%) (min) (EP) (EP) 30 11.192 97.56 0.247 1.08 11349 236 11.183 97.30 0.252 1.11 10870 555 11.192 97.74 0.252 1.11 10918 769 11.200 97.95 0.253 1.06 10876 975 11.225 97.92 0.262 0.93 10158 1292 11.225 97.40 0.333 1.08 6301 Figure 3. Theoretical plate count for the mAb monomer in each injection using the MAbPac SEC-1, 7.8×300 mm column. Conclusion · The MAbPac SEC-1 column coupled to a VanquishFlex Quaternary UHPLC system is a robust platform foraggregate analysis of mAbs. ( ·C ons i ste n t reten t i o n ti m e , e x c e lle nt peak s y m m et r y , and e x c e pti on a l colu m n e f f ic ie nc y w ere obse r v ed over t he co ur s e of 1 953 o n- col u m n in j ec t i on s for th e MA bPacSE C - 1, 4 . 0× 30 0 mm colum n a n d fo r 12 92 on-columnin jec t ions o n th e MA bPac S E C - 1, 7. 8×300 m m c o lu m n. ) · To our knowledge, the MAbPac SEC-1 column lifetimestability determined far exceeds other commerciallyavailable SEC columns, without requirements for guardcolumns. Reference 1. Thermo Scientific Application Note AN21602: The importance of correct UHPLCinstrument set-up for protein aggregate analysis by size-exclusion chromatography,2016. Monoclonal antibodies (mAbs) are currently the dominant class of protein therapeutics in the biopharmaceutical industry due to their high specificity to target antigens, long serum half-life in humans, and capabilities for use in the treatment of a wide range of ailments such as inflammatory diseases and cancer. During product expression and purification from host cells, formulation, and storage, mAbs may undergo various degradation processes, which may alter the safety, efficacy, and quality profile of the drug product. Consequently, a number of critical quality attributes (CQAs) must be monitored throughout drug development and production to ensure biotherapeutic substances are suitable for clinical use.
确定





还剩4页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《蛋白质(protein)中总量分析(aggregate analysis)检测方案(液相色谱仪)》,该方案主要用于其他中总量分析(aggregate analysis)检测,参考标准--,《蛋白质(protein)中总量分析(aggregate analysis)检测方案(液相色谱仪)》用到的仪器有赛默飞 Vanquish™ UHPLC超高效液相色谱系统
推荐专场
相关方案
更多
该厂商其他方案
更多