方案详情
文
The biopharmaceutical industry continues to develop mAb-based biotherapeutics in increasing numbers. Due to the complexity of these biotherapeutics, there are several key quality attributes (CQAs) that need to be measured and controlled to guarantee their safety and efficacy. The presence of aggregates in a formulated drug product must be assessed to avoid potential issues with immunogenicity.
方案详情

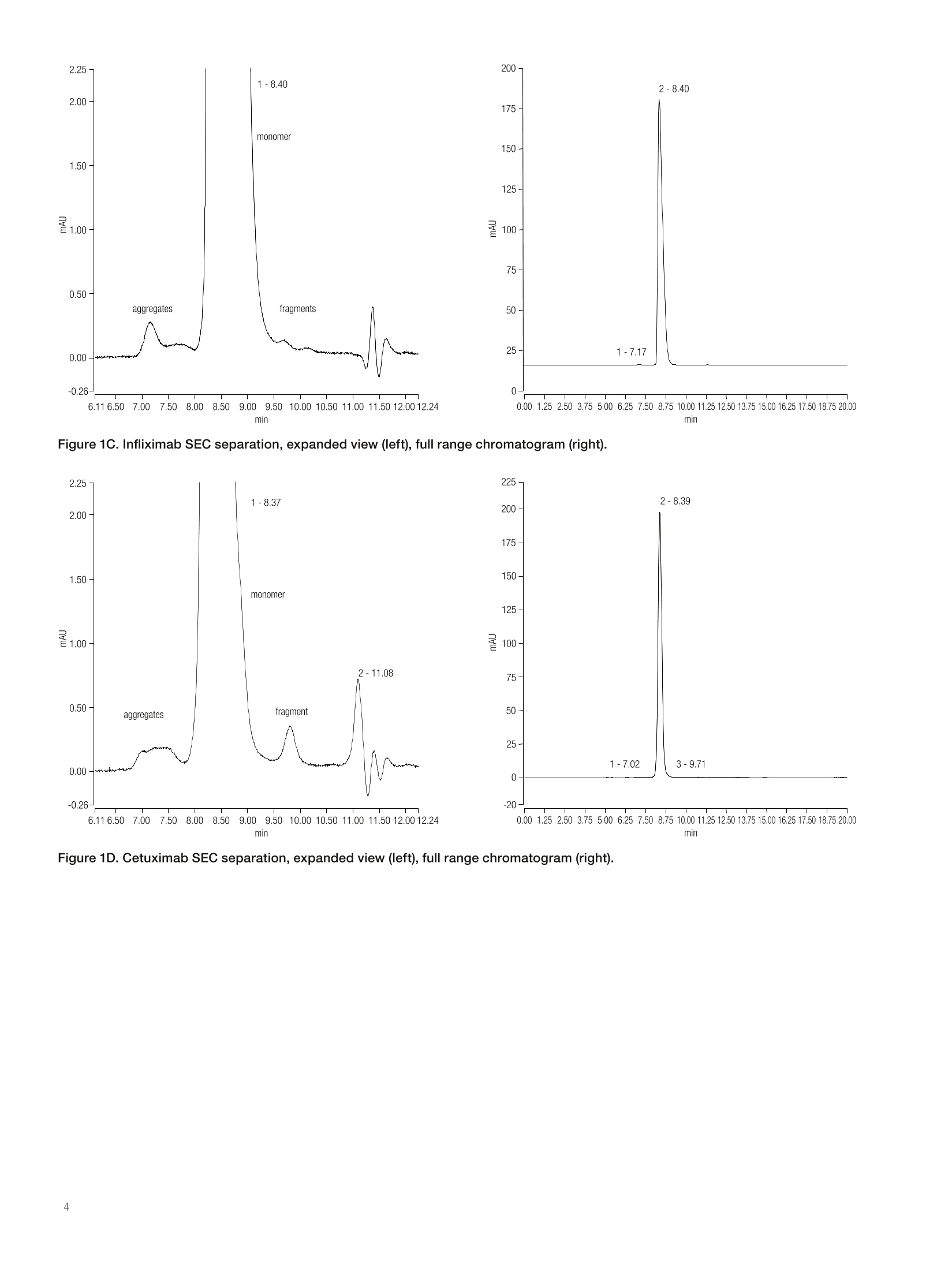
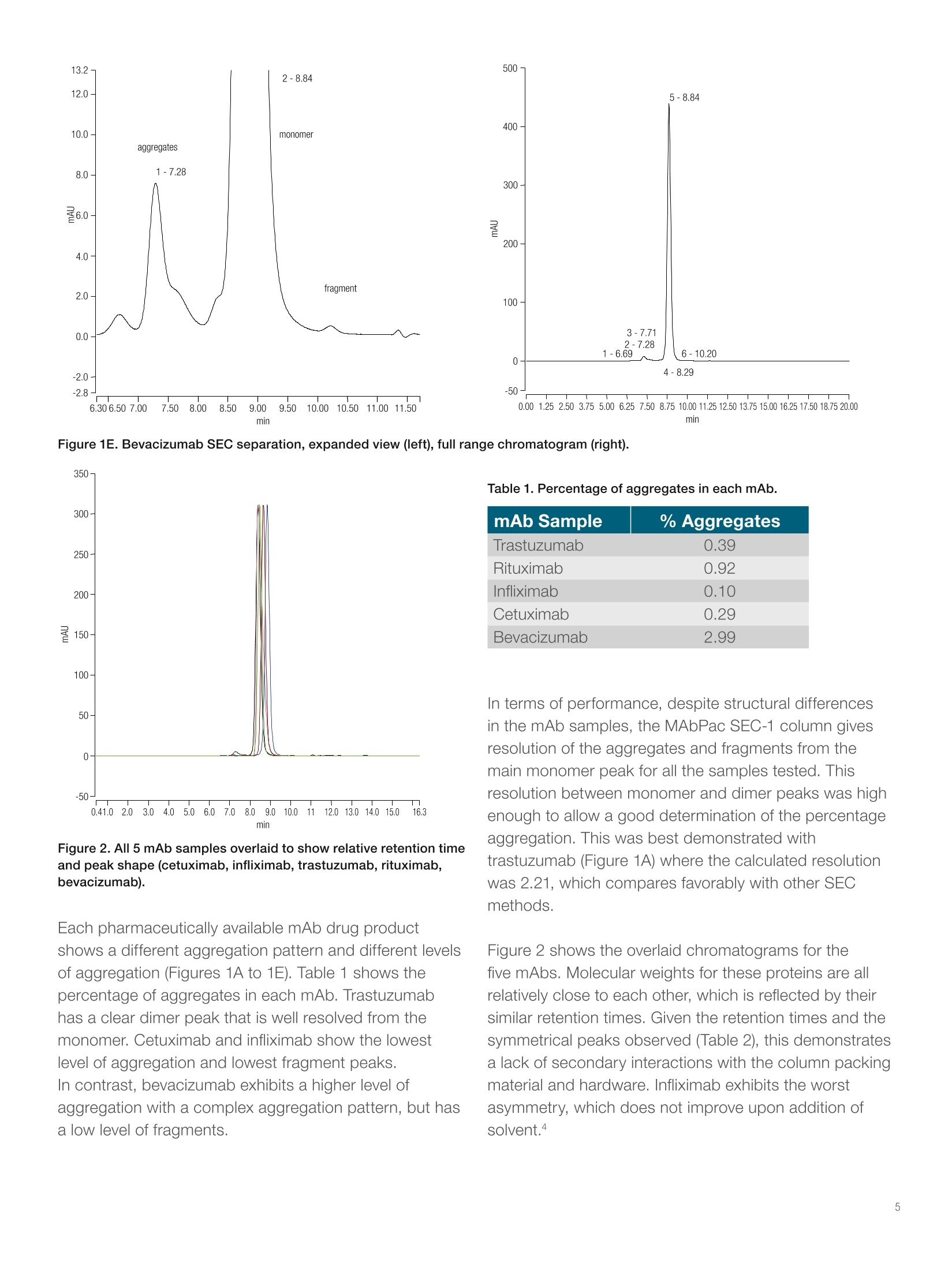
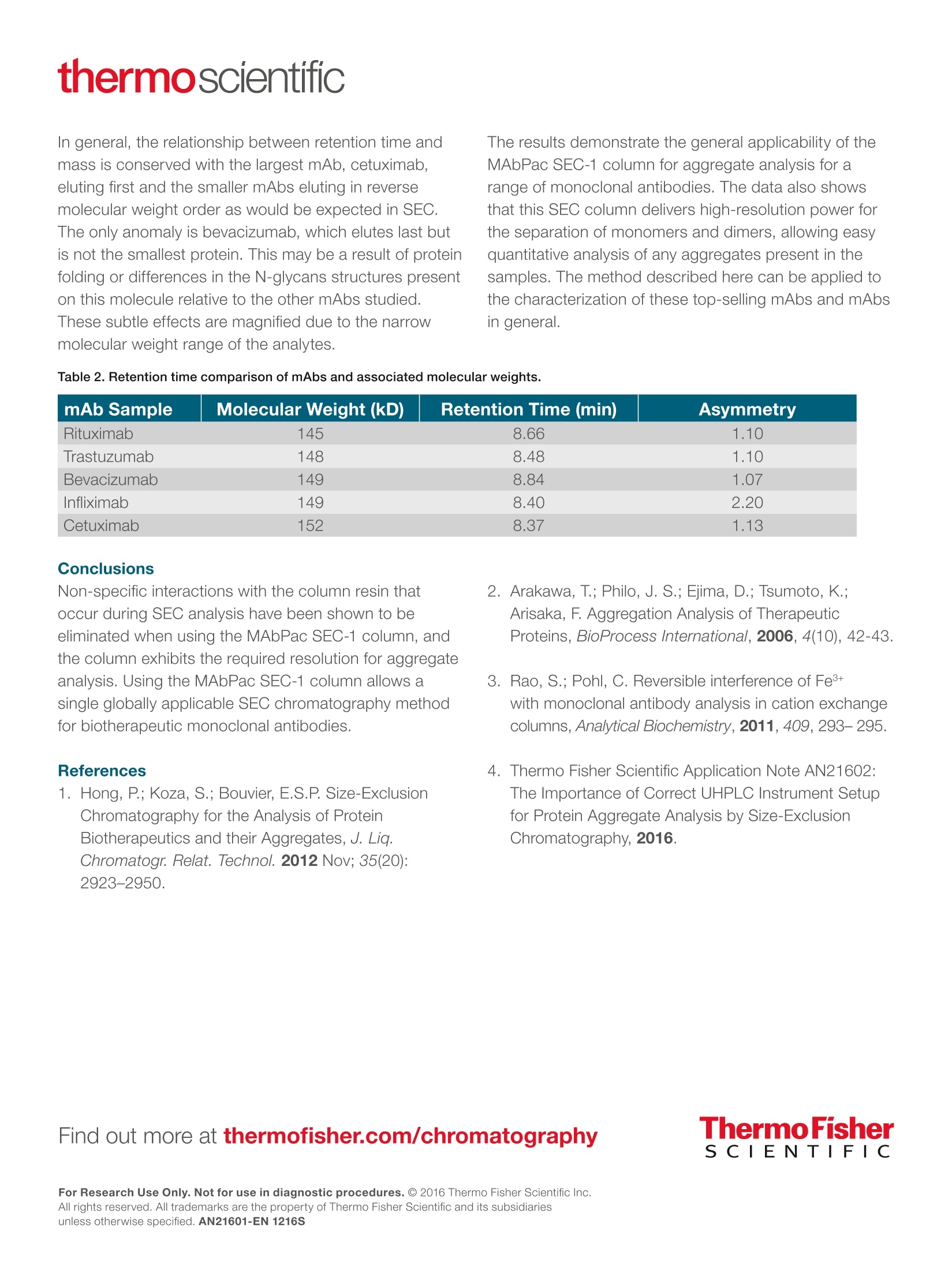
thermoscientificAPPLICATION NOTEFind out more at thermofisher.com/chromatographyAll rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiariesunless otherwise specified. AN21601-EN 1216S A universal chromatography methodfor aggregate analysis of monoclonalantibodies Amy Farrell1, Jonathan Bones , and Ken Cook2 1 NIBRT, Dublin, Ireland; 2 Thermo FisherScientific, Hemel Hempstead, UK Key words MAbPac SEC-1, monoclonal antibody,mAb,aggregation analysis, biotherapeutics, size-exclusionchromatography Goal Analysis of protein aggregation of five importantbiotherapeutic monoclonal antibodies (mAbs)by size-exclusion chromatography, showing theuniversal applicability of the Thermo Scientific"MAbPac SEC-1 column for aggregate analysis ofmAbs. The aim of this study was threefold: · Produce a single globally applicable SECchromatography method for the five mAb samples · Show peak symmetry (implying limited secondaryinteraction with the column) · Maintain the required monomer and dimerresolution Introduction The biopharmaceutical industry continues to developmAb-based biotherapeutics in increasing numbers.Due to the complexity of these biotherapeutics, thereare several key quality attributes (CQAs) that need to bemeasured and controlled to guarantee their safety andefficacy. The presence of aggregates in a formulateddrug product must be assessed to avoid potential issueswith immunogenicity. Aggregates are typically dimers, trimers, or larger orderstructures of antibody molecules. They are formed at thefollowing stages: · Product expression during fermentation · Product purification in downstream processing of thedrug substance ·Storage or mishandling of the drug prior to patientadministration. Protein aggregation has been implicated as the causeof adverse immunological reactions that result inserious safety and efficacy issues. Aggregation must bemonitored throughout the production process and duringstorage of the formulated biotherapeutic. MAb fragmentsthat are smaller than the expected molecular weight eluteafter the parent peak and can also be determined. Size-exclusion chromatography (SEC) is the standardmethod for this important analysis, but the compoundscan show non-specific binding to the columns, whichleads to retention time shifts, peak tailing, or evencomplete loss of protein peaks.12 The MAbPac SEC-1column is silica based with a proprietary, covalentlybonded diol hydrophilic layer to prevent secondaryinteractions. Even so, the mobile phase eluents usuallycontain high salt concentrations to prevent ionicinteractions, which can lead to corrosion of metalcomponents. For this reason, an inert UHPLC system isrecommended.sThe MAbPac SEC-1 column separatesby size and the pore size for this column (300 A) waschosen to give a good separation in the molecular weightrange of the monomer and dimers of a typical 150 kDamAb. This column therefore serves as a good, broadlyapplicable column for mAb aggregate analysis. Five important biotherapeutic mAbs (bevacizumab,.cetuximab, infliximab, rituximab, and trastuzumab)were selected to investigate column performance. Themonoclonal antibodies chosen are structurally diverseto investigate secondary interactions over a widerange of the physicochemical space. They cover a plrange between 7.6 and 8.7 and have widely differentglycosylation patterns from very simple (bevacizumab) tohighly complex (cetuximab). The MAbPac SEC-1 columnand Thermo ScientificVanquish"Flex QuaternaryUHPLC system were applied to the aggregate analysis offive important biotherapeutic mAbs using a common highsalt buffer mobile phase at pH 6.8. Consumables ·Fisher Scientific" HPLC grade water (P/N 10449380) ·Deionized water, 18.2 MQ.cm resistivity · Fisher Scientific Sodium phosphate dibasic anhydrous(P/N 10440481) ·Fisher Scientific Sodium phosphate monobasicanhydrous (P/N 10751135) ·Fisher Scientific Sodium chloride (P/N 11964051) · Thermo Scientific"VirtuosoVial Identification System(P/N 60180-VT100) ·Virtuoso 9 mm Wide Opening SureStop Screw ThreadVial Convenience Kit (P/N 60180-VT405) Sample pre-treatment Samples were reconstituted in water for injection withgentle swirling to aid in mAb solubilization as directedfrom the manufacturer's product insert information. The following formulated drug products were injecteddirectly: ·Bevacizumab 25 mg/mL · Cetuximab 5 mg/mL · Infliximab 10 mg/mL · Rituximab 10 mg/mL ·Trastuzumab 21 mg/mL Separation conditions Instrumentation Vanquish Flex Quaternary UHPLC system, standardconfiguration, equipped with: ·System Base Vanquish Flex (P/NVF-S01-A) ·Quaternary Pump F (P/N VF-P20-A) · Split Sampler FT (P/N VF-A10-A) ·Column Compartment H (P/N VH-C10-A) ·Diode Array Detector HL (P/N VH-D10-A) · Thermo ScientificLightPipeFlow Cell, Standard,10 mm (P/N 6083.0100) Mobile phaseData processingComposition:0.2 M sodium chloride inThe Thermo ScientificChromeleon Chromatography100 mM phosphate bufferData System software, version 7.2 SR4, was used forpH 6.8data acquisition and analysis.Flow rate:0.3 mL/minColumn temperature:25 °℃Injection volume:1 pLUV:214 nm Results and discussion The separation profiles obtained from each mAb sample are represented in Figures 1A to 1E and normalized in anoverlay in Figure 2. 2.25- 1-8.48 400- 2.00 monomer 1.50· dimer 1.00- 0.50· tragment 0.00· -0.26- 6.116.507.0077.508.008.509.009.5010.00 10.50 11.0011.50 12.00 12.24 min min Figure 1A. Trastuzumab SEC separation, expanded view (left), full range chromatogram (right). Figure 1B. Rituximab SEC separation, expanded view (left), full range chromatogram (right). Figure 1C. Infliximab SEC separation, expanded view (left), full range chromatogram (right). Figure 1D. Cetuximab SEC separation, expanded view (left), full range chromatogram (right). 500- Figure 1E. Bevacizumab SEC separation, expanded view (left), full range chromatogram (right). 350- Table 1. Percentage of aggregates in each mAb. mAb Sample % Aggregates Trastuzumab 0.39 Rituximab 0.92 Infliximab 0.10 Cetuximab 0.29 Bevacizumab 2.99 Figure 2. All 5 mAb samples overlaid to show relative retention timeand peak shape (cetuximab, infliximab, trastuzumab, rituximab,bevacizumab). In terms of performance, despite structural differencesin the mAb samples, the MAbPac SEC-1 column givesresolution of the aggregates and fragments from themain monomer peak for all the samples tested. Thisresolution between monomer and dimer peaks was highenough to allow a good determination of the percentageaggregation. This was best demonstrated withtrastuzumab (Figure 1A) where the calculated resolutionwas 2.21, which compares favorably with other SECmethods. Each pharmaceutically available mAb drug productshows a different aggregation pattern and different levelsof aggregation (Figures 1A to 1E). Table 1 shows thepercentage of aggregates in each mAb. Trastuzumabhas a clear dimer peak that is well resolved from themonomer. Cetuximab and infliximab show the lowestlevel of aggregation and lowest fragment peaks.In contrast, bevacizumab exhibits a higher level ofaggregation with a complex aggregation pattern, but hasa low level of fragments. Figure 2 shows the overlaid chromatograms for thefive mAbs. Molecular weights for these proteins are allrelatively close to each other, which is reflected by theirsimilar retention times. Given the retention times and thesymmetrical peaks observed (Table 2), this demonstratesa lack of secondary interactions with the column packingmaterial and hardware. Infliximab exhibits the worstasymmetry, which does not improve upon addition ofsolvent.4 In general, the relationship between retention time andmass is conserved with the largest mAb, cetuximab,eluting first and the smaller mAbs eluting in reversemolecular weight order as would be expected in SEC.The only anomaly is bevacizumab, which elutes last butis not the smallest protein. This may be a result of proteinfolding or differences in the N-glycans structures presenton this molecule relative to the other mAbs studied.These subtle effects are magnified due to the narrowmolecular weight range of the analytes. The results demonstrate the general applicability of theMAbPac SEC-1 column for aggregate analysis for arange of monoclonal antibodies. The data also showsthat this SEC column delivers high-resolution power forthe separation of monomers and dimers, allowing easyquantitative analysis of any aggregates present in thesamples. The method described here can be applied tothe characterization of these top-selling mAbs and mAbsin general. Table 2. Retention time comparison of mAbs and associated molecular weights. mAb Sample Molecular Weight (kD) Retention Time (min) Asymmetry Rituximab 145 8.66 1.10 Trastuzumab 148 8.48 1.10 Bevacizumab 149 8.84 1.07 Infliximab 149 8.40 2.20 Cetuximab 152 8.37 1.13 Non-specific interactions with the column resin thatoccur during SEC analysis have been shown to beeliminated when using the MAbPac SEC-1 column, andthe column exhibits the required resolution for aggregateanalysis. Using the MAbPac SEC-1 column allows asingle globally applicable SEC chromatography methodfor biotherapeutic monoclonal antibodies. 1. Hong, P.; Koza, S.; Bouvier, E.S.P. Size-ExclusionChromatography for the Analysis of ProteinBiotherapeutics and their Aggregates, J. Liq.Chromatogr. Relat. Technol.2012 Nov; 35(20):2923-2950. 2. Arakawa, T.;Philo, J. S.; Ejima, D.; Tsumoto, K.;Arisaka, F. Aggregation Analysis of TherapeuticProteins, BioProcess International, 2006,4(10),42-43. 3. Rao, S.; Pohl, C. Reversible interference of Fe3+with monoclonal antibody analysis in cation exchangecolumns, Analytical Biochemistry,2011,409,293-295. 4.Thermo Fisher Scientific Application Note AN21602:The Importance of Correct UHPLC Instrument Setupfor Protein Aggregate Analysis by Size-ExclusionChromatography, 2016. For Research Use Only. Not for use in diagnostic procedures. 2016 Thermo Fisher Scientific Inc. The biopharmaceutical industry continues to develop mAb-based biotherapeutics in increasing numbers. Due to the complexity of these biotherapeutics, there are several key quality attributes (CQAs) that need to be measured and controlled to guarantee their safety and efficacy. The presence of aggregates in a formulated drug product must be assessed to avoid potential issues with immunogenicity.
确定


还剩4页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《单克隆抗体(monoclonal antibodies)中聚合分析(aggregate analysis)检测方案(液相色谱仪)》,该方案主要用于其他中聚合分析(aggregate analysis)检测,参考标准--,《单克隆抗体(monoclonal antibodies)中聚合分析(aggregate analysis)检测方案(液相色谱仪)》用到的仪器有赛默飞 Vanquish Flex UHPLC系统
推荐专场
相关方案
更多
该厂商其他方案
更多