当前位置:
仪器信息网
>
行业应用
>
治疗类生物药品检测方案
>
抗体(biotherapeutic antibodies)中UHPLC-FLD with MS confirmation检测方案(液相色谱仪)
方案详情
文
This application note presents a step-by-step method for release, labeling, separation, and exoglycosidase-based structural elucidation of N-glycans from human serum IgG and commercial chimeric IgG1 mAb (infliximab) using Thermo Scientific™ Vanquish™ Horizon UHPLC-FLD and confirmation of the structures by a Thermo Scientific™ Q Exactive™ Plus Hybrid Quadrupole-Orbitrap™ mass spectrometer.
方案详情

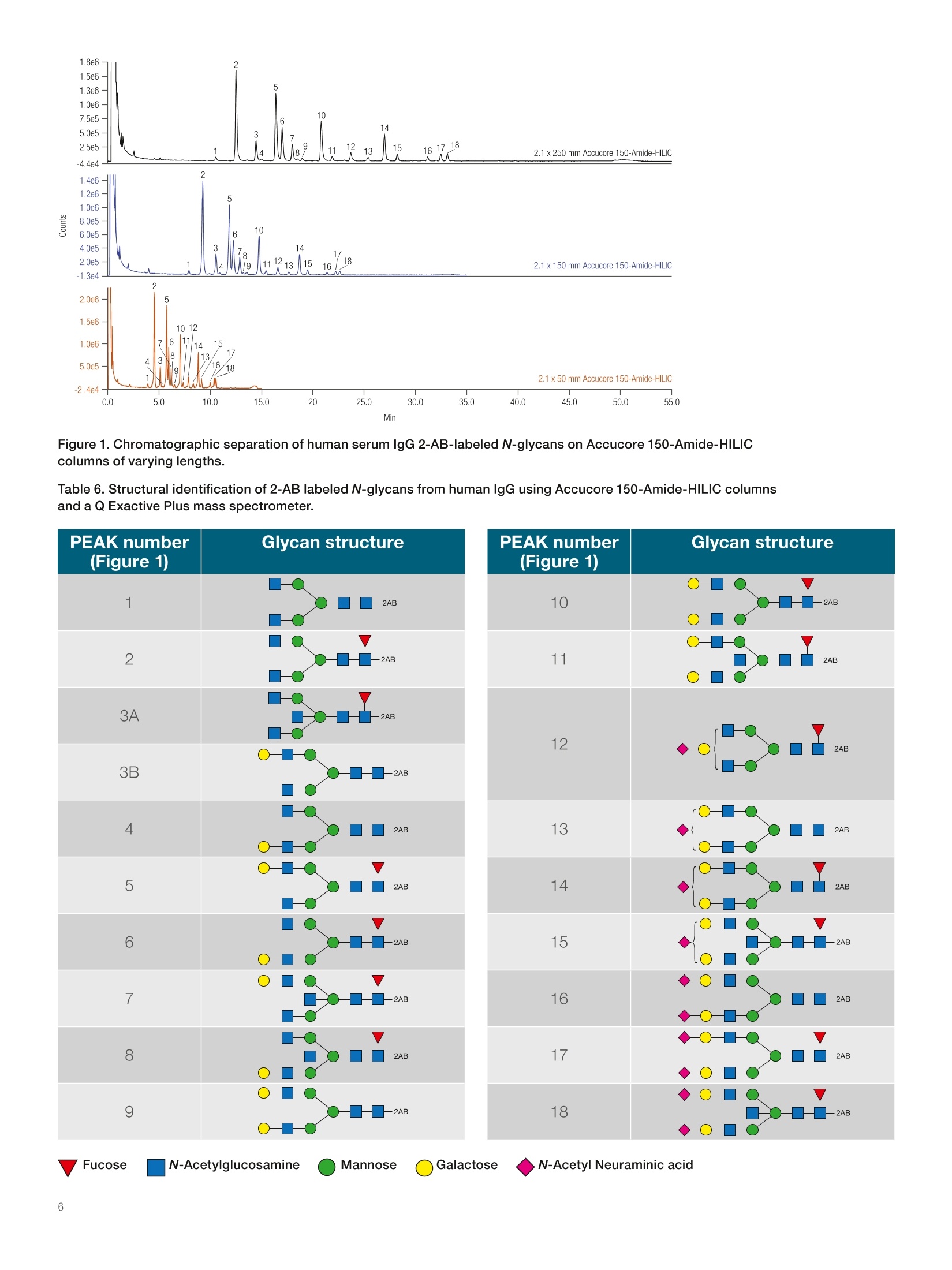
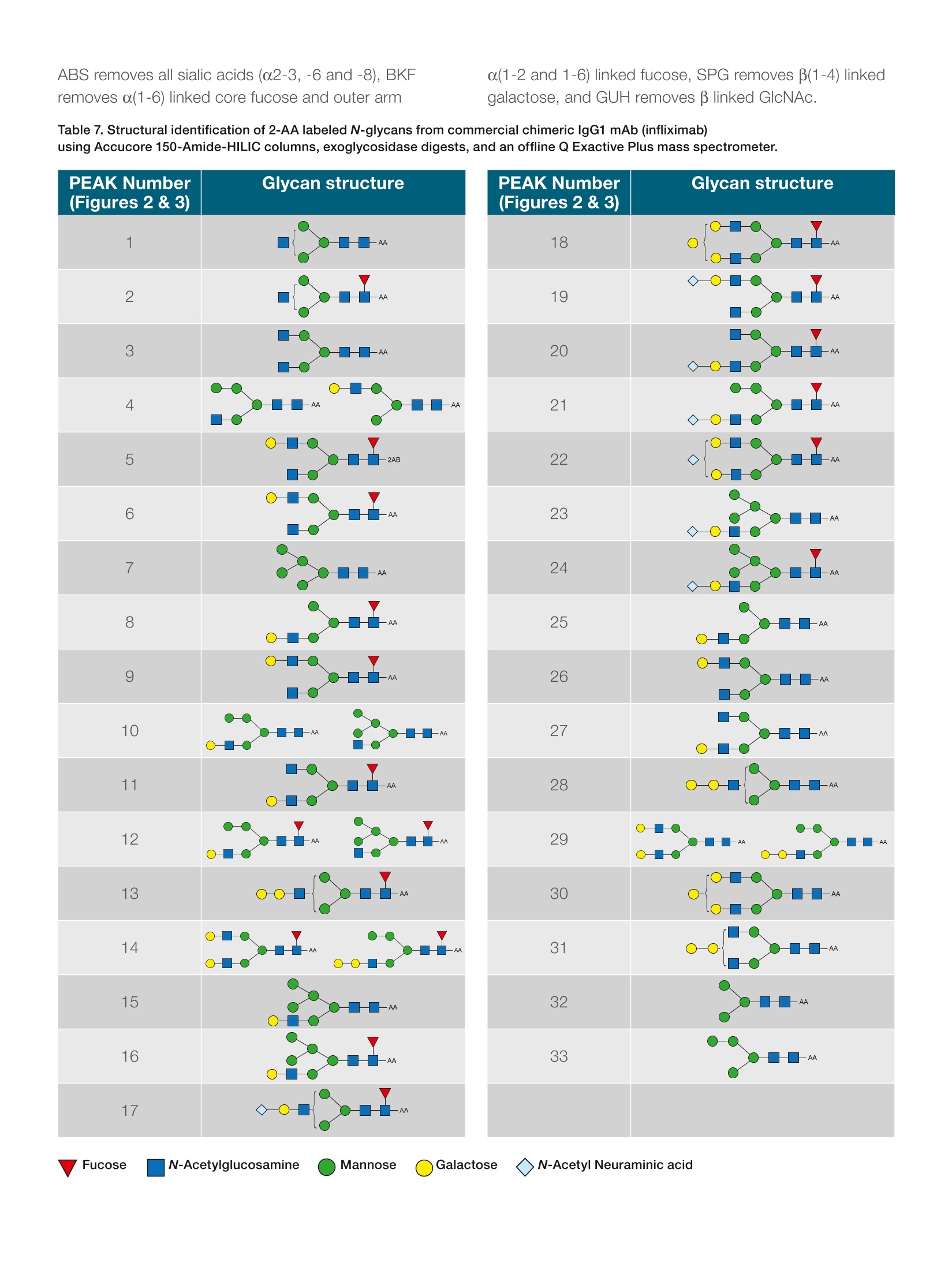
thermoscientific APPLICATION NOTE 21652 Fast profiling of the N-glycan population inbiotherapeutic antibodies by UHPLC-FLDwith MS confirmation Authors Silvia Millan, Stefan Mittermayr,Amy Farrell, and Jonathan BonesCharacterisation andComparability Laboratory,NIBRT- The National Institutefor Bioprocessing Research andTraining, Dublin, Ireland Keywords Glycosylation, Glycoprotein,Released N-glycan,Glycoform,Biosimilar, Innovator, MonoclonalAntibody, mAb, Biotherapeutics,lgG,Vanquish UHPLC, AccucoreAmide HILIC, Solid Core, 2-AA,2-AB, HRAMS MS, Q Exactive,Infliximab ·To develop a widely applicable UHPLC approach to fast, comprehensiveprofiling of 2-AA and 2-AB labeled glycans in lgG antibodies ·To validate the approach with a human serum lgG and a commercialchimeric lgG1 mAb (infliximab) · To confirm the glycan profiles by exoglycosidase enzyme digestion andhigh-resolution, accurate-mass mass spectrometry (HRAMS MS) ·To increase throughput with assay optimization Introduction Glycans play an essential role in many biological processes, including celldevelopment and differentiation, cell-cell or cell-matrix communication, andpathogen-host recognition. The glycan components of biotherapeutics canbe important determinants of biological activity and therapeutic efficacy1-3and hence characterization of the glycan profile of a biomolecule is requiredunder regulatory guidelines (ICH Q5E/Q6B and USP 129).4-5 Therapeuticantibodies must be demonstrated to meet applicable quality requirementsto ensure continued safety, purity, and potency of a drug product. Anintensive biochemical characterization of the antibody itself is required,which includes a thorough examination of glycan distribution and potential impacts of glycoforms on mAb function. On the otherhand, differences in glycan profiles between healthyand diseased states are utilized for clinical diagnosis8,providing targets for many novel classes of therapeuticsincluding cancer chemotherapy, diabetes treatment, andantibiotic and anti-viral medicine. The‘gold standard' for studying lgG glycosylationrelies on enzymatic N-glycan release, subsequentfluorescent labeling by reductive amination, and analysisof the labeled glycans by high-performance liquidchromatography with fluorescence detection (HPLC-FLD), using hydrophilic interaction liquid chromatography(HILIC) with fluorescence detection. In addition tocharacterization of the sugar sequence, the analysis mustelucidate linkages and separate all isomeric, charge, andbranching variations of glycans. HILIC columns commonly used for glycan analysisare based on amide, amine, or zwitterionic packingmaterials. These columns separate glycans mainly byhydrogen bonding, resulting in separations based onsize and composition. The Thermo ScientificAccucoreTM150-Amide-HILIC HPLC column is a solid particlecore phase designed for the separation of hydrophilicbiomolecules in HILIC mode, which offers an excellentchoice for glycan separation This application note presents a step-by-step method forrelease, labeling, separation, and exoglycosidase-basedstructural elucidation of N-glycans from human serumlgG and commercial chimeric lgG1 mAb (infliximab) usingThermo Scientific"Vanquish"Horizon UHPLC-FLD andconfirmation of the structures by a Thermo ScientificQ ExactivePlus Hybrid Quadrupole-Orbitrapmassspectrometer. .Chemicals and reagents · Deionized (DI) water, 18.2 MQcm resistivity · Fisher ScientificAcetonitrile, HPLC grade(P/N 10407440) · Fisher ScientificOptima" Formic acid, LC-MS(P/N 10596814) ( · Fish e r S c ienti f ic Ammon i um hyd r oxid e (P/N 1 05 08610) ) · Fisher Scientific Ammonium bicarbonate(P/N 10207183) ( ·T h ermo S cie n t if ic V i rtu o so V ia l k i t ( P/N 6 0 1 80- V T4 02 ) ) · PNGase F (New England Biolabs@, Ipswich,MA, USA) ·Amicon@ Ultra 0.5 mL centrifugal filters MWCO 10 kDa(purchased from a reputable supplier) ·Fisher Scientific Sodium cyanoborohydride(P/N10082110) · Fisher Scientific Glacial acetic acid (P/N A/0360/PB17) · Fisher Scientific Dimethylsulfoxide (DMSO)(P/N 10213810) ·Anthranilamide (2-AB) (purchased from a reputablesupplier) ·Anthranilic acid (2-AA) (purchased from a reputablesupplier) ·Fisher Scientific Tris(hydroxymethyl)methylaminehydrochloride, (P/N 10060390) ·Fisher Scientific Urea (P/N 10132740) ·Fisher Scientific Ethanol (P/N 10644795) · ABS Sialidase/NANase III (purchased from a reputablesupplier) ( ·BKF α ( 1- 2,3,4 ,6 ) Fuc o si d a se (bovine k i dn e y) ( p u rc ha sed fr o m a r e putable s u pp lie r ) ) ( · S PG bet a( 1- 4) -Gala ct osid ase ( Str e ptoc o ccuspneumon i a e) ( p u r cha s ed fr o m a r eput able s u pp lier) ) ·GUH B-N-Acetylhexosaminidase / Hexase l (purchasedfroma reputable supplier) Equipment ( Th e rmo Sci en t i f icU ltiMat e30 00 RS sy s tem , i ncl u d i ng: ) ( ·L PG - 3 400R S Ra p i d Sep a ration Quaternary Pump ( P /N 50 4 0.0036 ) ) ( WP S- 3000TRS Rapid Separation Therm os ta tte d W ellPla t e Autosampler ( P/N 5 8 40. 0 020 ) ) ( T CC -3000RS Ra p id S eparatio n T h ermostatt e dColu m n Compartm e nt (P/ N 5 730. 0 000 ) ) ( ●FL D -3 4 00 R S Fl uo res c en ce Det e ctor with D ua l -P M T (P/ N 5078.0025 ) ) ( · S R -3 000 Solvent R ack (P/ N 5 0 35.9200) ) ( · 2 pL Micro Flow C ell (P /N 6 078.4 3 30 ) ) ( Th e rmo S cien t i fi cVa n quis hH ori zon U HPLC syst e m,inclu d ing : ) ( · S ystem Ba s e Vanq u ish Ho ri zon ( P / N V H- S0 1 -A) ) · Binary Pump H (P/NVH-P10-A) ( ·C o lu m n Compar tm ent H (P / N V H - C1 0 -A) ) ( · Spli t S am p l e r HT ( P/N VH - A10-A) w ith 2 5 pL(V=50 u L) sample l oop ) ·Fluorescence Detector F (P/N VF-D50-A) Q Exactive Plus Hybrid Quadrupole-Orbitrap massspectrometer Thermo ScientificM SpeedVac"Concentrator(P/N SPD121p) Accucore 150-Amide-HILIC, 2.6 pm, 2.1×50 mm(P/N 16726-052130) Accucore 150-Amide-HILIC, 2.6um, 2.1×150 mm(P/N 16726-152130) Accucore 150-Amide-HILIC, 2.6 um, 2.1×250mm(P/N 16726-252130) Preparation of buffers ·Ammonium formate (50 mM, pH 4.4): 1.8g of formicacid was dissolved in 1 L of DI water. pH was adjustedpH to 4.4 with ammonium hydroxide solution. ·8 M urea in 0.1 M Tris-HCI, pH 8.5 (UA buffer):1.57 g of Tris-HCI was dissolved in 100 mL DI waterand pH adjusted to 8.5 with 1 M NaOH. 24 g of ureawas dissolved in 50 mL 0.1 M Tris-HCI, pH 8.5. ·Ammonium bicarbonate (50 mM, pH 7.8): 0.39 g ofammonium bicarbonate was dissolved in 100 mL of DIwater. Release of N-glycans from proteins 1. 100 pg of protein were denatured using 8 M urea in0.1 M tris buffer pH 8.0 (UA solution) and subsequentlyreduced and alkylated using 10 mM DTT and 50 mMIAA prepared in UA solution. 2. Following buffer exchange into 50 mM ammoniumbicarbonate using 10 kDa MWCO filters, N-glycanrelease was performed by incubation of the reducedand alkylated sample with 500 units of PNGase Fovernight at 37°℃. 3. Released glycans were collected from deglycosylatedproteins by centrifugation through 10 kDa molecularweight cut-off (MWCO) filters and subsequentlyreduced to dryness via vacuum centrifugation. 4. Dried glycans were reconstituted in 50 pL of 1%(v/v)aqueous formic acid to ensure complete conversionto the reducing sugar form prior to derivatization andsubsequently reduced to dryness 2-AA labeling reaction of released N-glycans 1.2-AA labeling reagent (100 pL) was prepared bydissolving 2-aminobenzoic acid (5 mg) and sodiumcyanoborohydride(6 mg) in 70/30 DMSO/glacial aceticacid. 2.5 pL of 2-AA labeling reagent solution were addedto the mixture (dried N-glycans released from 100 pgglycoprotein). 3.The solution was incubated at 60 °C for 5 hours. 2-AB labeling reaction of released N-glycans 1.2-AB labeling reagent (100 pL) was prepared bydissolving 2-aminobenzamide (5 mg) and sodiumcyanoborohydride (6 mg) in 70/30 DMSO/glacial aceticacid. 2. 10 pL of 2-AB labeling reagent solution were addedto the mixture (dried N-glycans released from 100 ugglycoprotein). 3. The solution was incubated at 65 °C for 2 hours. Cleanup of 2-AB/2-AA labeled N-glycans 1.Excess labeling dye removal was carried out by HILICpurification using an UltiMate 3000RS system. 2. Samples were loaded in 85% acetonitrile, 15%50mM ammonium formate pH 4.4 (v/v) (for 2-AB labeledN-glycans) or in 80% acetonitrile, 20% 50 mMammonium formate pH 4.4 (v/v) (for 2-AA labeledN-glycans) onto an Accucore 150-Amide-HILIC2.1×50 mm column at 0.5 mL/min for 2.5 minutes. 3. Labeled glycans were eluted in 20% aqueousacetonitrile for 2.5 minutes,monitored by fluorescencedetection,_=330/420 nm (for 2-AB labeledN-glycans) or Nex/em,=350/425 nm (for 2-AB labeledN-glycans), and evaporated to dryness. Sample preparation for analysis using UHPLC-FLD 1. 5 pL of purified labeled N-glycans re-suspended in DIwater (at 2.5 ug/uL) were mixed with 20 uL ofacetonitrile. 2. The total solution was transferred to the auto-samplervial for analysis. Note: Store the standard at -20 ℃. Exoglycosidase digests All exoglycosidase digestions were performed in 50 mMammonium acetate buffer, pH 5.5 in a final volume of10 pL at 37C overnight. Amounts of enzyme areindicated in Table 1. 1. For each enzyme digestion, 5 pL of labeled N-glycanpool were placed in a 0.2 mL PCR tube (for GUHdigest, sample was dried in vacuum centrifuge andre-suspended in 3 pL water). 2.Required volume of buffer (1 uL), water, and enzyme(s)(Table 1) were added to each tube mixing by pipetteafter each addition (10 pL final volume). 3. Samples were incubated at 37 °C overnight(16 hours). 4. Cleanup was performed by ethanol precipitation:90 pL ethanol (-30°C) were added to the sample tubesand mixed thoroughly. Samples were kept at -30 ℃for 30 min and spun for 10 min at 16.1 x 1000 rcf,10°C. Supernatant was placed into a new tube andsample was dried completely in a vacuum centrifuge. 5. Digested samples were re-suspended in 5 pL DI waterand 20 pL acetonitrile for UHPLC-FLD analysis. Table 1. Enzyme specificity and required volume perexoglycosidase digestion. Enzyme Specificity Volumeper Digest ABS Releases o(2-3), α(2-6) and 1pL α(2-8) lined non-reducing terminal sialic acids (NeuNAc BKF and NeuNGc) Releases α(1-2)and o(1-6) linked non-reducing terminal fucose residues more 1pL efficiently than o(1-3) and o(1-4) linked fucose. Used for release of core o(1-6) fucose residues, can also remove SPG α(1-3), but less efficiently. Hydrolyses non-reducing terminal β(1-4) linked 2pL GUH galactose residues Recombinantly expressed in E. coli. Releases B-linked GlcNAc but not bisecting 2 pL GlcNAc B(1-4) Man Table 2. Mobile phase gradient for column #1 (P/N 16726-252130). Time (min) %A %B Flow Curve (mL/min) 0 20 80 1.4 5 48.10 40 60 1.4 5 49.88 50 50 1.4 5 51.19 50 50 1.4 5 51.31 20 80 1.4 5 55.00 20 80 1.4 5 Table 3. Mobile phase gradient for column #2 (P/N 16726-152130). Time (min) %A %B Flow Curve (mL/min) 0 20 80 1.3 5 31.05 40 60 1.3 5 32.23 50 50 1.3 5 33.08 50 50 1.3 5 33.15 20 80 1.3 5 35.00 20 80 1.3 5 Table 4. Mobile phase gradient for column #3 (P/N 16726-052130). Time (min) %A %B Flow Curve (mL/min) 0 20 80 1 5 13.45 40 60 1 5 13.97 50 50 1 5 14.33 50 50 1 5 14.37 20 80 1 5 15.00 20 80 1 5 N-glycan analysis by LC-MS Glycan samples were injected on a Q Exactive PlusHybrid Quadrupole-Orbitrap MS equipped with a HESIsource. Samples were diluted in 75% acetonitrile prior toanalysis. Separation conditions Column:Accucore 150-Amide-HILIC2.6 pm, 2.1 ×150 mmMobile phase A:Ammonium formate 50 mM.pH 4.4Mobile phase B:AcetonitrileFlow rate:0.4 mL/minColumn temperature:50°℃Sample volume:11 pL Table 5. Mobile phase gradient for N-glycan analysis by LC-MS. Time (min) %A %B Flow Curve (mL/min) 0 25 75 0.4 5 30.0 50 50 0.4 5 30.5 55 45 0.4 5 32.0 55 45 0.4 5 32.5 25 75 0.4 5 40.0 25 75 0.4 5 MS conditions Results and discussion Separation of 2-AB-labeled glycan from human IgGA sample of human serum lgG glycans was analyzed onAccucore 150-Amide-HILIC HPLC columns of differentlengths. The glycan separation and elution is based onsize and polarity by HILIC interaction. Figure 1 showsthe separation of neutral and acidic 2-AB labeledN-glycans from human lgG on different column lengths.FLD chromatograms reveal 18 well-resolved N-glycanpeaks for 2.1 ×250 and 2.1x150 mm columns. Despitesome loss in peak resolution when column length isreduced to 50 mm, it may be sufficient for fast analysis ofless-complex glycan profiles, enabling high-throughputanalysis within 15 minutes. The structural assignment foreach identified peak was confirmed by accurate MS dataand listed in Table 6. The oligosaccharides present in thepolyclonal lgG, attached at Asn297, are of the complexbi-antennary type and are comprised of a chitobiosecore with variable addition of fucose, galactose, bisectingN-acetylglucosamine, and sialic acid. Sialylation ismodest, with < 15% of structures being monosialylatedor disialylated. 1.8e6 Figure 1. Chromatographic separation of human serum IgG 2-AB-labeled N-glycans on Accucore 150-Amide-HILICcolumns of varying lengths. Table 6. Structural identification of 2-AB labeled N-glycans from human lgG using Accucore 150-Amide-HILIC columnsand a Q Exactive Plus mass spectrometer. PEAK number(Figure 1) Glycan structure 1 -2AB 2 -2AB 3A -2AB 3B --2AB 4 --2AB 5 -一2AB 6 -2AB 7 8 -2AB ¥ 9 Separation of 2-AA-labeled glycan from commercialchimeric lgG1 A commercial chimeric lgG1 was also analyzed onAccucore 150-Amide-HILIC columns with the aim todemonstrate appropriate separation for a more complexglycan sample. Figure 2 shows the separation of neutraland acidic 2-AA labeled N-glycans from chimeric lgG1 ondifferent column lengths. FLD chromatograms reveal 24N-glycan peaks for 2.1 ×250 and 2.1 × 150 mm columns.Annotation of the N-glycans structures and glycosidic linkages present in each chromatographic peak werededuced using exoglycosidase arrays as detailed inFigure 3 and Table 7. The oligosaccharide compositionwas also confirmed by high-resolution, accurate-mass(HRAM) MS data and listed in Table 7.Thirty-eightN-glycan structures were annotated, including highmannose, hybrid and complex bi-antennary glycans withvariable degrees of core fucosylation, galactosylation,sialylation, and galactose o1-3 linked galactose epitopes. Figure 2. Chromatographic separation of commercial chimeric lgG1 mAb (infliximab) 2-AA-labeled N-glycans on Accucore150-Amide-HILIC columns of varying lengths. Min Figure 3. HILIC chromatograms of the infliximab 2-AA labeled N-glycan pool (undigested, UND) and after digestion with a range ofexoglycosidase enzymes. Separations were performed on an Accucore 150-Amide-HILIC 2.1×150 mm column using the gradientconditions outlined in the figure legend table. ABS removes all sialic acids (o2-3, -6 and -8), BKFremoves α(1-6) linked core fucose and outer arm α(1-2 and 1-6) linked fucose, SPG removes β(1-4) linkedgalactose, and GUH removes B linked GlcNAc. Table 7. Structural identification of 2-AA labeled N-glycans from commercial chimeric IgG1 mAb (infliximab) using Accucore 150-Amide-HILIC columns, exoglycosidase digests, and an offline Q Exactive Plus mass spectrometer. PEAK Number(Figures 2 &3) Glycan structure 18 -AA 19 -AA 20 -AA 21 -AA -AA 22 23 -AA 24 -AA 25 -AA 26 -AA 27 --AA 28 -AA 29 -AA —AA 30 -AA 31 -C-AA 32 --AA 33 -C-AA Conclusions ·A fully integrated workflow for glycan profiling andstructural characterization of fluorescently labeledN-glycans released was demonstrated successfully. · The combination of the Vanquish Horizon UHPLCsystem equipped with fluorescence detection andthe Accucore 150-Amide-HILIC column provided theopportunity for assay speed up and associatedincrease in throughput without compromising thequality of the analytical data. The chromatographic resolution, reproducibility, andsensitivity enabled analysis of minor glycoforms, whichare otherwise challenging to assign. ·Structural confirmation of glycans with exoglycosidaseenzyme digestion followed by determination using theQ Exactive Plus Hybrid Quadrupole-Orbitrap MSallowed rapid and unambiguous profiling. 1. Jenkins,N.; Murphy, L.; Tyther, R. Post-translational Modifications of recombinantproteins: significance for Biopharmaceuticals. Molecular Biotechnology, 2008, 39,113-118. 2. Costa,A.R.; Rodrigues, M.E.; Henriques, M.; Oliveira, R.; Azeredo, J. Glycosylation:impact, control and improvement during therapeutic protein production. CriticalReviews in Biotechnology, 2014, 34,281-299. 3. Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. NatureReviews Drug Discovery, 2009,8,226-234. 4. Reusch, D.; Tejada, M.L. Fc glycans of therapeutic antibodies as critical qualityattributes. Glycobiology,2015,25(12),1325-1334. 5. EMA, Guideline on Development, Production, Characterization and Specificationsfor Monoclonal Antibodies and Related Products, EMEA/CHMP BWP/157653/2007(December 2008). 6. ICH. (1999) Q6B: Specifications: Test Procedures and Acceptance Criteria forBiotechnological Biological Products. International Conference on Harmonisation ofTechnical Requirements for Registration of Pharmaceuticals for Human Use. Geneva,Switzerland. 7. Hundson, H.F. Genetic defects in the human glycome. Nature Reviews Genetics, 2006,7,537-551. 8. Adamczyk, B.; Tharmalingan,T.; Rudd, P.M. Glycans as cancer biomarkers. Biochimicaet Biophysica Acta, 2012,1820,1347-1353. Find out more at thermofisher.com ( 2017 Thermo F is h e r Sc ientif ic In c. All r i g ht s r e s er ved. Ami c on is a r e g is t ere d t r ad em ar k of M il lipore Corporatio n . Ne w Engla n dBiolab s is a r egis t ered t rad e mar k of Ne w Eng l a nd Bio l a bs, In c . All o th er t r ad e marks ar e the pro pe r t y of Th erm o F i s he r S c ie n t if i c a n d its s ub s i dia r ie s. T hi s in fo rma t i o n i s p resen t ed a s an e xa mpl e o f t h e c apa b ili t ie s of Th e rm o F is he r S c ient i fic pro du c ts. It i snot i nt end e d to en co ura g e us e of th ese pr o duc ts i n a n y m ann e rs that mig h t i nfr ing e t h e in t e llectual pr ope r ty r i gh t s o f o the r s.Sp e cif ic at io ns, t e rms and p r i ci ng are subject to c hange . N o t all p r o du ct s are a v ailab le i n a l l c o untri es . P le a se consul t your lo c a l sa l es r ep res en t a tive s for de tails. AN2165 2 -EN 0417S ) Fucose N-Acetylglucosamine Mannose Galactose N-Acetyl Neuraminic acid This application note presents a step-by-step method for release, labeling, separation, and exoglycosidase-based structural elucidation of N-glycans from human serum IgG and commercial chimeric IgG1 mAb (infliximab) using Thermo Scientific™ Vanquish™ Horizon UHPLC-FLD and confirmation of the structures by a Thermo Scientific™ Q Exactive™ Plus Hybrid Quadrupole-Orbitrap™ mass spectrometer.
确定





还剩7页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《抗体(biotherapeutic antibodies)中UHPLC-FLD with MS confirmation检测方案(液相色谱仪)》,该方案主要用于治疗类生物药品中UHPLC-FLD with MS confirmation检测,参考标准--,《抗体(biotherapeutic antibodies)中UHPLC-FLD with MS confirmation检测方案(液相色谱仪)》用到的仪器有Vanquish Horizon UHPLC 系统
推荐专场
相关方案
更多
该厂商其他方案
更多