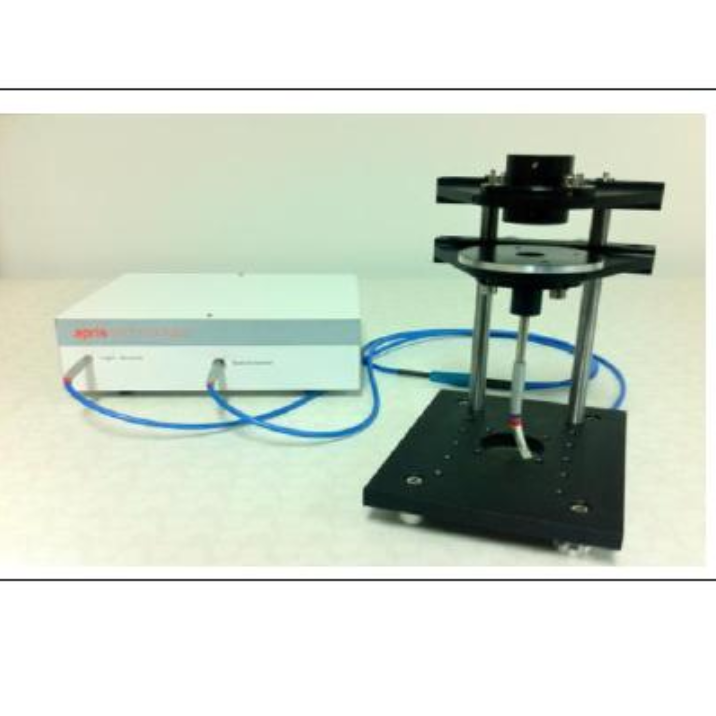
方案详情
文
应用立陶宛Ekspla公司的偏振分辨振动和频光谱测量系统(SFG-VS)对寡核苷酸对层状阳离子脂质双层膜的腹层吸附过程进行了实验研究和理论分析。
方案详情

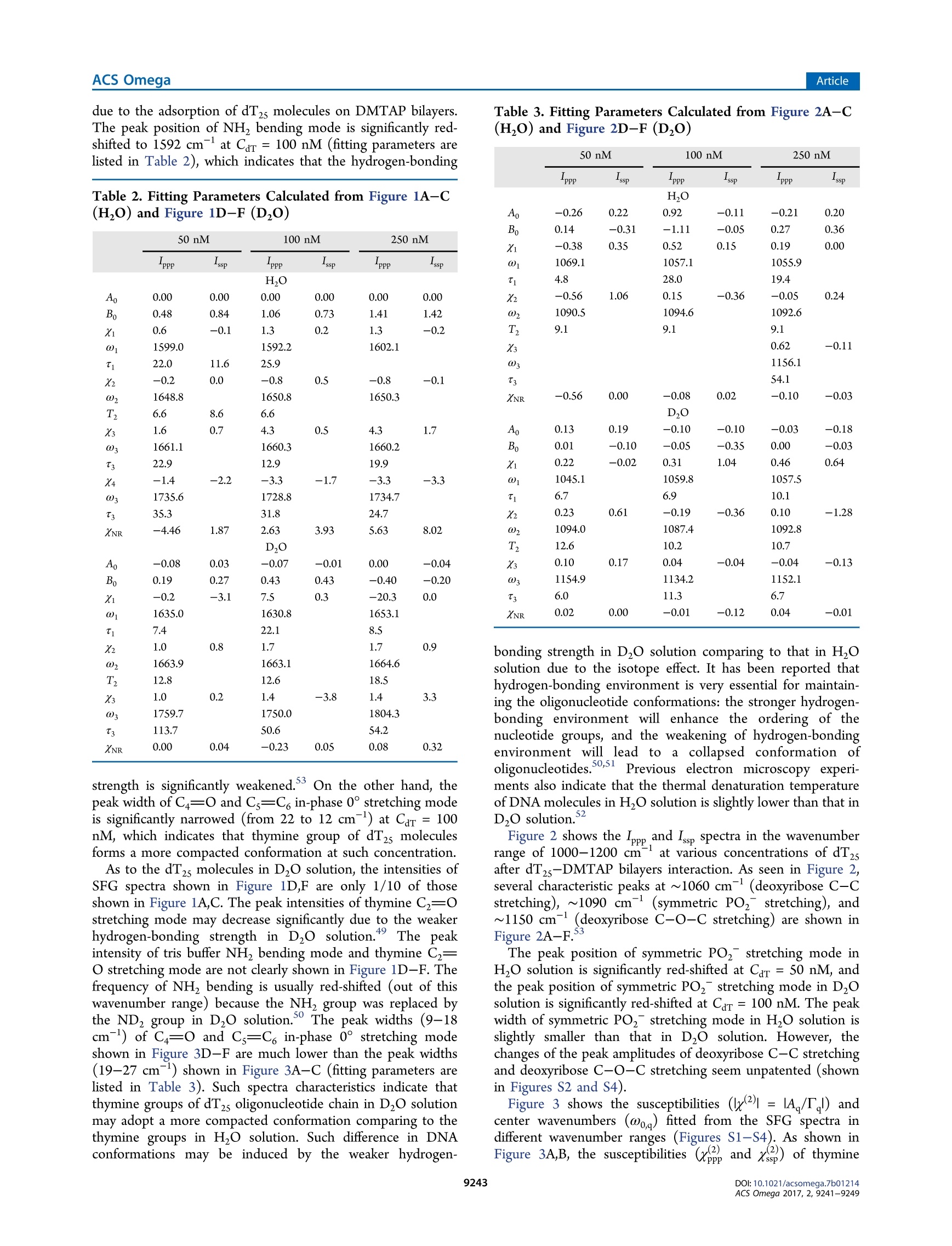
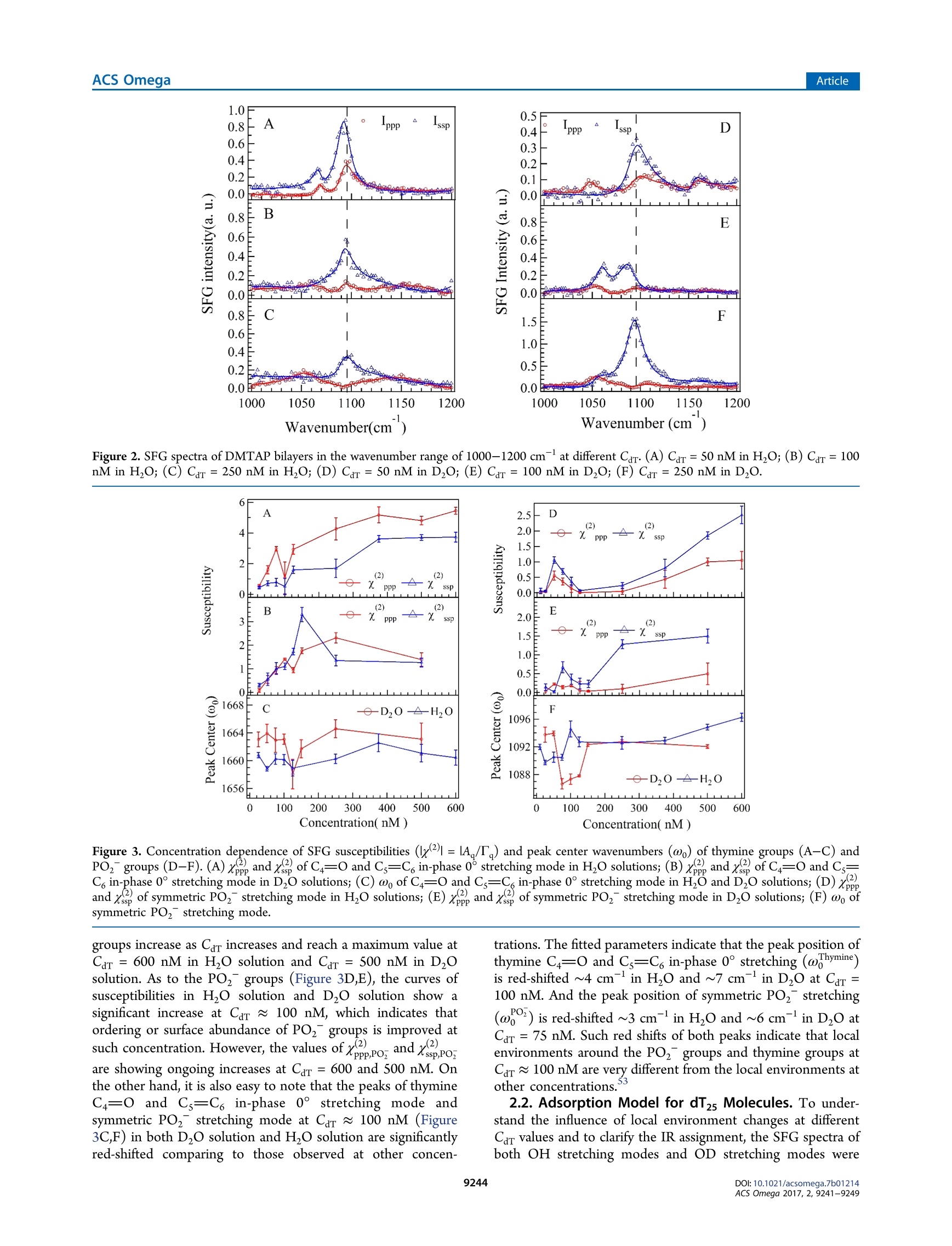
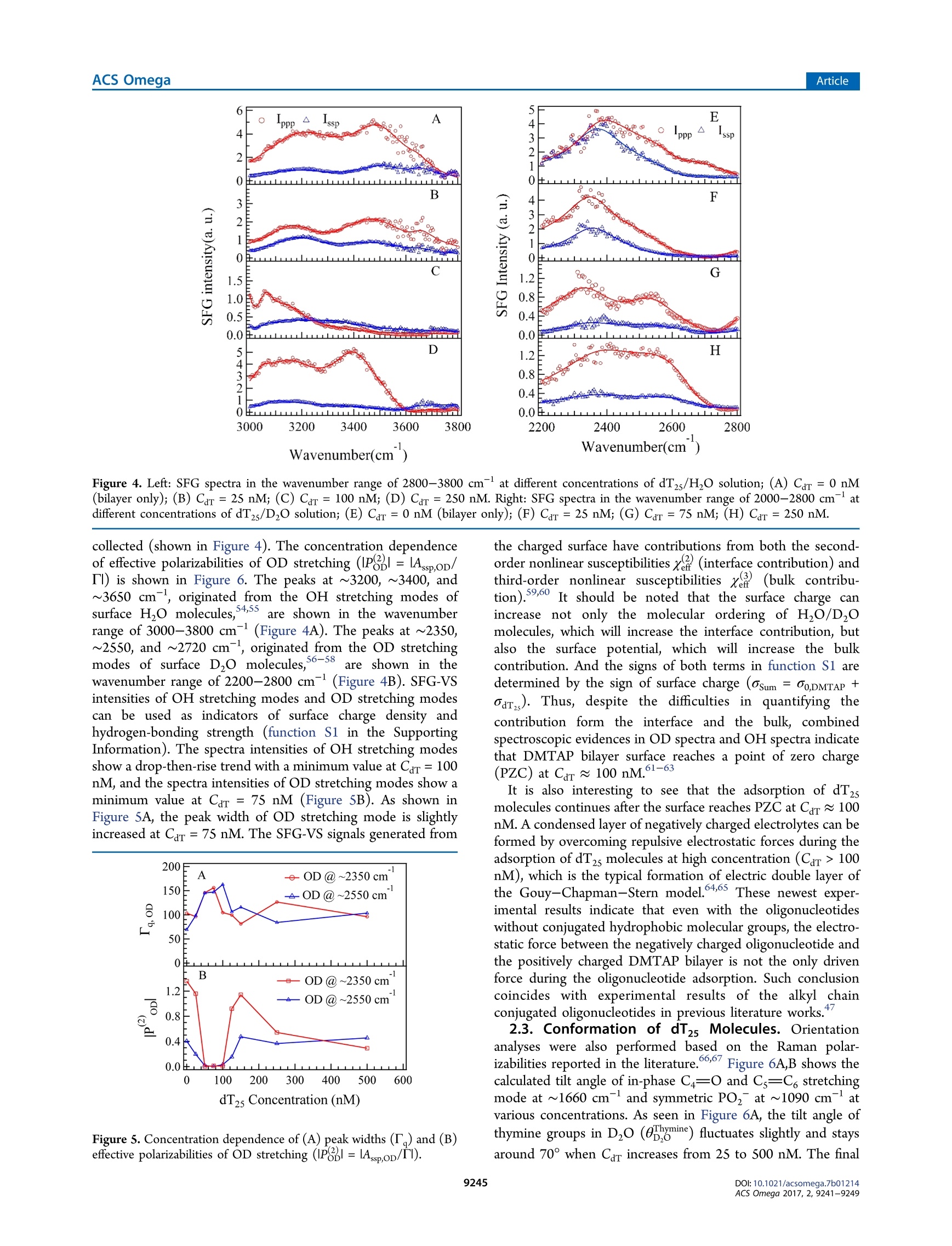
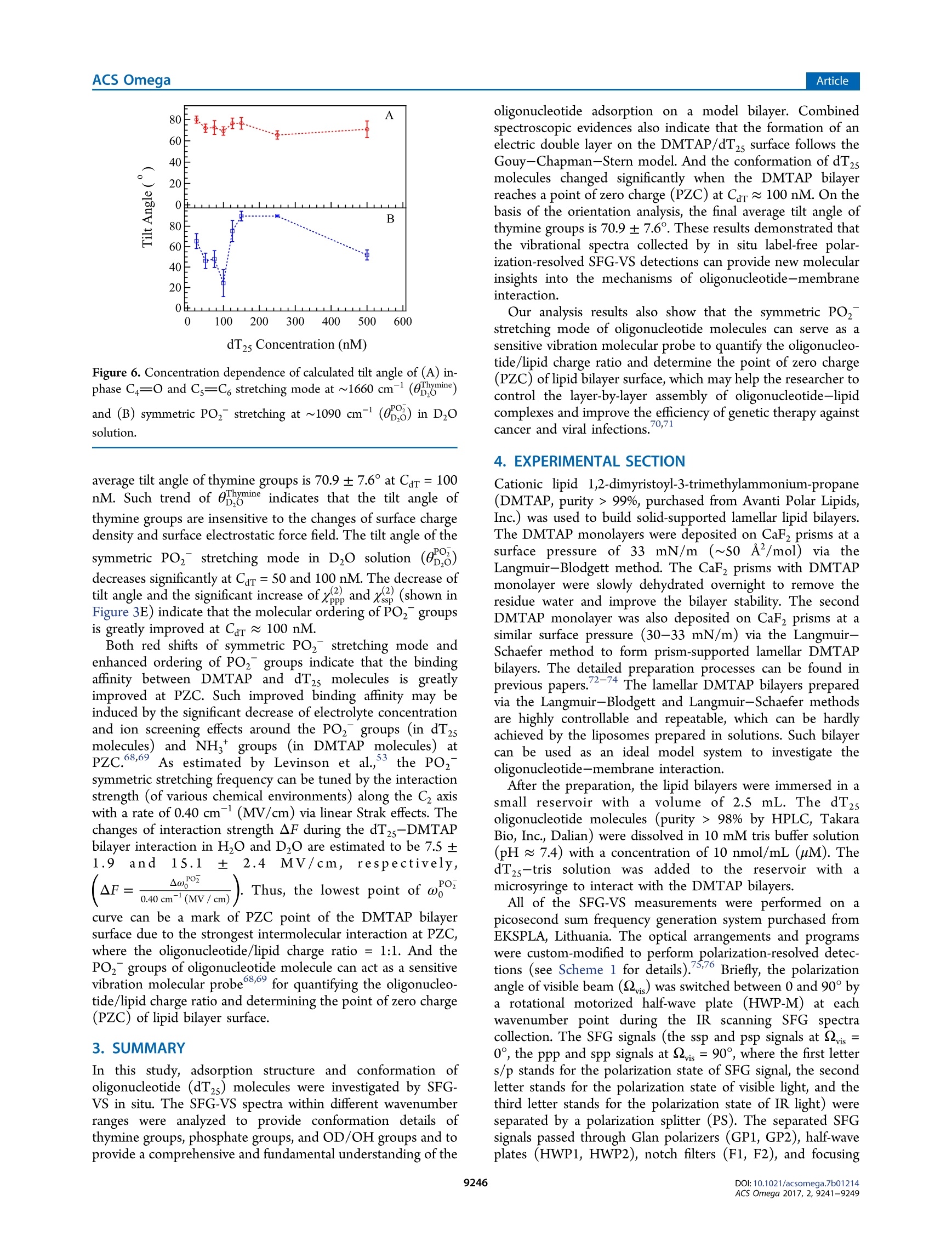
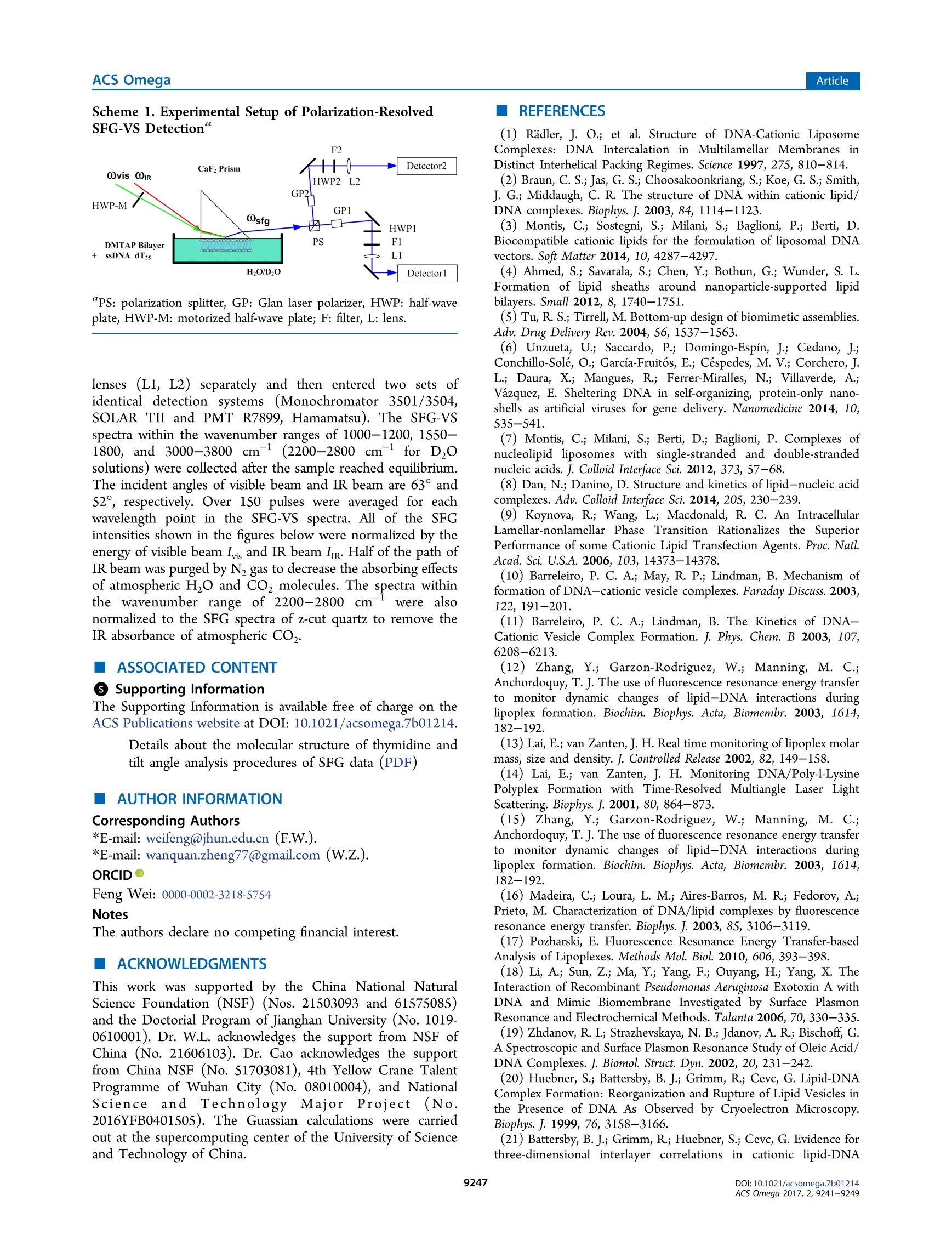
This is an open access article published under a Creative Commons Non-Commercial NoDerivative Works (CC-BY-NC-ND) Attribution License, which permits copying andredistribution of the article, and creation of adaptations, all for non-commercial purposes.Cite This: ACS Omega 2017, 2, 9241-9249 ArticleACS Omega Article Stern-Layer Adsorption of Oligonucleotides on Lamellar CationicLipid Bilayer Investigated by Polarization-Resolved SFG-VS Liqun Wang# Yang Shen,ts Yanbo Yang,* Wangting Lu, Wenhui Li, Feng Wei,*o Guang ZhengYouhua Zhou, Wanquan Zheng *t, and Yuancheng Cao 'Institution for Interdisciplinary Research, & Key Laboratory of Optoelectronic Chemical Materials and Devices of Ministry ofEducation, College of Life Science, School of Physics and Information Engineering, and School of Chemical and EnvironmentalEngineering, Jianghan University, 430056 Wuhan, Hubei, P. R. China -Institut des Sciences Moleculaires d'Orsay, Universite Paris-Sud, 91405 Orsay Cedex, France Supporting Information ABSTRACT: The molecular interaction between the oligonucleotides and lipidmembranes is the key to the functions of virus, aptamer, and various oligonucleotide-based materials. In this study, the conformational changes of oligonucleotides (dT2s)on lamellar cationic 1,2-dimyristoyl-3-trimethylammonium-propane (DMTAP) bilayerwere investigated by polarization-resolved sum frequency generation vibrationalspectroscopy (SFG-VS) in situ. The SFG-VS spectra within different wavenumberranges were analyzed to give conformation details of thymine groups, phosphategroups, and OD/OH groups and to provide a comprehensive and fundamentalunderstanding of the oligonucleotide adsorption on a model bilayer. It is shown thatthe adsorption of dT2s on DMTAP bilayer reaches maximum at Car ~ 500nM. Andthe conformation of dT2s molecules change significantly when surface charge ofDMTAP bilayer reaches the point of zero charge (PzC) at Car ~ 100 nM. Combinedspectroscopic evidences also indicate that the formation of electric double layer at theDMTAP/dT2s surface follows the Gouy-Chapman-Stern model. The analysis results also show that the symmetric PO, stretching mode of oligonucleotide molecules can serve as a sensitive vibration molecularprobe for quantifying the oligonucleotide/lipid charge ratio and determine the point of zero charge (PZC) of lipid bilayer surface,which may help researchers to control the layer-by-layer assembly of oligonucleotide-lipid complexes and to improve theefficiency genetic therapy against cancer and viral infections. 1. INTRODUCTION The complexes formed by cationic lipid membrane andoligonucleotides have attracted extensive interest because oftheir applications in gene delivery as nonviral carriers of geneticmaterial into living cells.The molecular interactions (forces)which assemble the oligonucleotides and lipid bilayers intocomplex but ordered lipoplexes are also the key to the functionsof virus, aptamer, and various oligonucleotide-based materi-als.2-6 The understanding of molecular interactions betweenlipid membrane and oligonucleotides will help researchersperform “bottom up”assemblies and build lipoplexes withequilibrium structures, which can ensure the safety andeffectiveness of oligonucleotide-based materials during thegene therapy against the cancer and viral infections. A number of experimental and theoretical studies have beenconducted to comprehend the mechanism of formation of lipidbilayer-oligonucleotide complexes. -9Small-angle X-rayscattering (SAXS),1 stopped-flow spectrofluorometry,10-12dynamic laser scattering (DLS),13,14 ffluorescence resonance18,19energy transfer,15-17surface plasmon resonance,atomicforce microscopy,cryotransmission electron microscopy,1,22Fourier transform infrared spectroscopy, and Raman vibrationalspectroscopy23-25were applied to investigate the lipoplex structures and kinetics of lipoplex formation. Theoreticalmodeling and simulation were also applied to understand themolecular driving force during the DN,26,27A adsorption.26.27 Ingeneral, the oligonucleotide-lipid bilayer complex formationcan be described in three steps: the accumulation of anionicoligonucleotides on the cationic bilayer, the reverse/rupture oflipid vesicles, and the formation of aggregated multilamellarstructure. It has been well established that the formation oflipoplex is driven by electrostatic attraction between theoppositely charged nucleic acid and lipids. Results of DLSand SAXS experiments also show that the size and5potentialof liposomes can be tuned by lipid composition and thecationic lipid/DNA charge ratio, which may improve thetransfection efficiency of the lipoplexes.28-31 Nevertheless, dueto the limitations of characterizing methods, the interface/surface specific information at the molecular level during thelipid-oligonucleotide interaction and lipoplex formation washard to be obtained. The insights of accumulation/adsorptionof oligonucleotide at molecular level are required to control the ( Re c e i v e d: A ugust 19, 2017 ) ( A ccepte d: N ovember 29, 2017 ) ( P ub li sh ed : December 27, 2017 ) Figure 1. SFG spectra collected in the wavenumber range of 1550-1800 cmat different concentrations of dTzs(Car). (A) Car=50 nM in HO;(B) Car = 100 nM in HO; (C) Car = 250 nM in H,O; (D) Car = 50 nM in DzO; (E) Car =100 nM in DzO; (F) Car=100 nM in D,O. successive events of charge reversing, vesicle rupturing, andmultilamellar lipoplex formation. Sum frequency generation vibrational spectroscopy (SFG-VS) has been successfully applied as an interface-specific probeto investigate the molecular structure and kinetics atbiointerfaces.32-39 Recent progresses prove that SFG-VS isalso very useful for abstracting the structural and conforma-tional information from the interfacial anchored oligonucleo-tides.40-47 The structure of single-stranded DNA monolayerson platinum substrate was investigated by Sartenaer et al. usingSFG-VS.40 The divalent metal ion-induced deformation ofDNA monolayer was also investigated by Asanuma et al. usingSFG-VS and X-ray photoelectron spectroscopy.10n-oligonucleotide interactions were also investigated extensivelyby Geiger’s group using multiple nonlinear optical techniques,such as SFG-VS and second harmonic generation.4243 Thestructural differences between oligonucleotide films in air andunder various aqueous situations were also characterized byHowell et a1.44,45 SFG-VS was also applied to investigate theinteractions between oligonucleotides and model lipid mem-branes. A pioneer work, which investigated the interactionbetween the cationic lipid monolayer and A-phage DNAmolecules by monitoring the OD signals within the wave-number range of 2000-2700 cm-,was carried out by Wurpelet al. By focusing on the SFG spectra in the wavenumberrange of 1200-1800 cm-, the interfacial conformation and G-quadruplex forming kinetics of G-rich oligonucleotides on 1,2-dimyristoyl-3-trimethylammonium-propane(DMTAP) lipidbilayers were also elucidated by Wei et al. In spite of theseprevious investigations, the conformational and kinetic proper-ties of oligonucleotides during the adsorption/interactionremain elusive. For instance, Wurpel et al.s results indicatesignificant reconstruction of surface water molecules during theDNA-lipid interaction, which can account for the Langmuiradsorption of DNA molecules.6 However, the conformationchanges of oligonucleotide molecules during such adsorptionare still unclear. In the current study, the spectra of dT2s-DMTAP bilayercomplexes were collected by the polarization-resolved SFG-VSsystem in situ. The SFG spectra detected within different wavenumber regions can provide conformational details ofdifferent molecular groups, such as phosphate, thymine, anddeoxyribose groups, thus facilitating the elucidation of themolecular mechanism of dT2s molecule-membrane interac-tion. 2. RESULTS AND DISCUSSION 2.1. SFG Spectra in H,0 and D,0 and Peak Assign-ments. Figure 1 shows the ppp and ssp spectra in thewavenumber range of 1550-1800 cm at various concen-tration of dT2s after the dT2s-DMTAP bilayer interaction.Several characteristic peaks at ~1600 cm(NH, bendingmode of tris buffer), ~1650 cm(thymine C4=0 and Cs=C6 out-of-phase 180°stretching mode),~1660 cm-(thymineC4=0 and Cs=C, in-phase 0° stretching mode),and ~1730cm(thymine C2=0 stretching mode) can be observed inFigure 1A-C (see Table 1 for details).48,49 As shown in Figure3A-C, the peak intensities of C4=0 and Cs=C6 in-phase 0°stretching mode increase gradually as Car increases to 250 nM peaks (cm-) assignments ~1060 ribose C-C stretching ~1090 PO, symmetric stretching ~1150 ribose C-O-C stretching ~1600 tris 8-NH, bending ~1650 dT C4=0 and C=C out-of phase ~1660 dT C4=0 and C=C in-phase ~1730 C=0 stretching ~2350 OD stretching of D2O ~2550 ~2720 ~3060 dT C6-H stretching ~3140 dT N-H stretching ~3200 OH stretching of H O, dT, and tris molecules ~3400 ~3600 due to the adsorption of dT2s molecules on DMTAP bilayers.The peak position of NH bending mode is significantly red-shifted to 1592 cm-at Car= 100 nM (fitting parameters arelisted in Table 2), which indicates that the hydrogen-bonding Table 2. Fitting Parameters Calculated from Figure 1A-C(H,O) and Figure 1D-F (Dz0) strength is significantly weakened.3 On the other hand, thepeak width of C4=0 and Cs=C6 in-phase 0°stretching modeis significantly narrowed (from 22 to 12 cm-) at Car = 100nM, which indicates that thymine group of dT2s moleculesforms a more compacted conformation at such concentration. As to the dT2s molecules in D,O solution, the intensities ofSFG spectra shown in Figure 1D,F are only 1/10 of thoseshown in Figure 1A,C. The peak intensities of thymine C2=0stretching mode may decrease significantly due to the weakerhydrogen-bonding strength in DzO solution. The peakintensity of tris buffer NH, bending mode and thymine C2=O stretching mode are not clearly shown in Figure 1D-F.Thefrequency of NH bending is usually red-shifted (out of thiswavenumber range) because the NH group was replaced bythe NDz group in D,O solution. The peak widths (9-18cm-) of C4=0 and Cs=C6 in-phase 0°stretching modeshown in Figure 3D-F are much lower than the peak widths(19-27 cm-) shown in Figure 3A-C (fitting parameters arelisted in Table 3). Such spectra characteristics indicate thatthymine groups of dT2s oligonucleotide chain in DzO solutionmay adopt a more compacted conformation comparing to thethymine groups in H O solution. Such difference in DNAconformations may be induced by the weaker hydrogen- Table3. Fitting Parameters Calculated from Figure 2A-C(H,O) and Figure 2D-F (Dz0) “PPP -ssp “PPP -ssp PPP -ssp H,O A -0.26 0.22 0.92 -0.11 -0.21 0.20 B 0.14 -0.31 -1.11 -0.05 0.27 0.36 Xi -0.38 0.35 0.52 0.15 0.19 0.00 01 1069.1 1057.1 1055.9 4.8 28.0 19.4 X2 -0.56 1.06 0.15 -0.36 -0.05 0.24 02 1090.5 1094.6 1092.6 T2 9.1 9.1 9.1 X3 0.62 -0.11 03 1156.1 T3 54.1 XNR -0.56 0.00 -0.08 0.02 -0.10 -0.03 D,O A 0.13 0.19 -0.10 -0.10 -0.03 -0.18 B 0.01 -0.10 -0.05 -0.35 0.00 -0.03 Xi 0.22 -0.02 0.31 1.04 0.46 0.64 1045.1 1059.8 1057.5 6.7 6.9 10.1 0.23 0.61 -0.19 -0.36 0.10 -1.28 1094.0 1087.4 1092.8 12.6 10.2 10.7 0.10 0.17 0.04 -0.04 -0.04 -0.13 1154.9 1134.2 1152.1 6.0 11.3 6.7 XNR 0.02 0.00 -0.01 -0.12 0.04 -0.01 bonding strength in DzO solution comparing to that in H Osolution due to the isotope effect. It has been reported thathydrogen-bonding environment is very essential for maintain-ing the oligonucleotide conformations: the stronger hydrogen-bonding environment will enhance the ordering of thenucleotide groups, and the weakening of hydrogen-bondingenvironment will lead to a collapsed conformation (O1oligonucleotides.50,511Previous electron microscopy experi-ments also indicate that the thermal denaturation temperatureof DNA molecules in H,O solution is slightly lower than that inDO solution. Figure 2 shows the Ippp and Iss spectra in the wavenumberrange of 1000-1200 cm-1at various concentrations of dT,after dTs-DMTAP bilayers interaction. As seen in Figure 2,several characteristic peaks at ~1060 cm(deoxyribose C-Cstretching), ~1090 cm (symmetric PO, stretching), and~1150 cm(deoxyribose C-O-C stretching) are shown inFigure 2A-F. The peak position of symmetric PO stretching mode inH O solution is significantly red-shifted at Car= 50 nM, andthe peak position of symmetric PO,stretching mode in DzOsolution is significantly red-shifted at Car=100 nM. The peakwidth of symmetric PO, stretching mode in HO solution isslightly smaller than that in DzO solution. However, thechanges of the peak amplitudes of deoxyribose C-C stretchingand deoxyribose C-O-C stretching seem unpatented (shownin Figures S2 and S4). Figure 3 shows the susceptibilities (x2)=IA./T,l) andcenter wavenumbers (@o,) fitted from the SFG spectra indifferent wavenumber ranges (Figures S1-S4). As shown inFigure 3A,B, the susceptibilities (,x, and ) of thymine Wavenumber(cm ) Figure 2. SFG spectra of DMTAP bilayers in the wavenumber range of 1000-1200 cmat different Car(A) Car=50 nM in H O; (B) Car=100nM in H O; (C) Car=250nM in H,O; (D) Car =50 nM in DzO; (E) Car= 100 nM in DzO; (F) Car =250 nM in D O. Figure 3. Concentration dependence of SFG susceptibilities (lx()=IA/T) and peak center wavenumbers (ω) of thymine groups (A-C) andPO, groups (D-F).(A)x, and xof C4=0 and Cs=C in-phase 0 stretching mode in H,O solutions; (B) x3, andx of C4=0 and Cs=C in-phase 0° stretching mode in D,O solutions; (C) w of C4=0 and Cs=C, in-phase 0° stretching mode in H,O and D,O solutions; (D) x.and x of symmetric PO, stretching mode in H,O solutions; (E) xand x of symmetric PO, stretching mode in D,O solutions; (F) ω ofsymmetric PO2 stretching mode. groups increase as Car increases and reach a maximum value atCar = 600 nM in H,O solution and Car=500 nM in D,Osolution. As to the PO, groups (Figure 3D,E), the curves ofSSsusceptibilities in H,O solution and D,O solution show asignificant increase at Car ~ 100 nM, which indicates thatordering or surface abundance of PO2 groups is improved atsuch concentration. However, the values ofxppPo, and xsspPoare showing ongoing increases at Car= 600 and 500 nM. Onthe other hand, it is also easy to note that the peaks of thymineC4=0 and Cs=C6 in-phase 0° stretching modeandsymmetric PO, stretching mode at Car ~ 100 nM (Figure3C,F) in both DzO solution and H,O solution are significantlyred-shifted comparing to those observed at other concen- trations. The fitted parameters indicate that the peak position ofthymine C4=0 and Cs=C in-phase 0° stretching (ωhymine)is red-shifted ~4 cmin HO and~7 cm-in D0 at Car =100 nM. And the peak position of symmetric POz stretching(a) is red-shifted ~3 cm- in H,O and ~6 cm-in DO atCar =75 nM. Such red shifts of both peaks indicate that localenvironments around the PO groups and thymine groups atCar~ 100 nM are very different from the local environments atother concentrations. 2.2. Adsorption Model for dT25 Molecules. To under-stand the influence of local environment changes at differentCar values and to clarify the IR assignment, the SFG spectra ofboth OH stretching modes and OD stretching modes were Figure 4. Left: SFG spectra in the wavenumber range of 2800-3800 cm-at different concentrations of dT2s/H,O solution; (A) Car =0 nM(bilayer only); (B) Car=25 nM; (C) Car=100 nM; (D) Car =250 nM. Right: SFG spectra in the wavenumber range of 2000-2800 cm-atdifferent concentrations of dTzs/D20 solution;(E) Car=0 nM (bilayer only); (F) Car=25 nM; (G) Car=75 nM; (H) Car=250 nM. collected (shown in Figure 4). The concentration dependenceof effective polarizabilities of OD stretching (P品1=lAssp,oD/Il) is shown in Figure 6. The peaks at ~3200, ~3400, and~3650 cm-, originated from the OH stretching modes ofsurface H O molecules,4,S5are shown in the wavenumberrange of 3000-3800 cm (Figure 4A). The peaks at ~2350,~2550, and ~2720 cm-, originated from the OD stretchingmodes of surface D,O molecules,56-58are shown in thewavenumber range of2200-2800 cm(Figure 4B). SFG-VSintensities of OH stretching modes and OD stretching modescan be used as indicators of surface charge density andhydrogen-bonding strength (function S1 in the SupportingInformation). The spectra intensities of OH stretching modesshow a drop-then-rise trend with a minimum value at Car =100nM, and the spectra intensities of OD stretching modes show aminimum value at Car =75 nM (Figure 5B). As shown inFigure SA, the peak width of OD stretching mode is slightlyincreased at Car =75 nM. The SFG-VS signals generated from Figure 5. Concentration dependence of (A) peak widths (T) and (B)effective polarizabilities of OD stretching (IP品1=IAsspoD/Tl). the charged surface have contributions from both the second-order nonlinear susceptibilities x(interface contribution) andthird-order nonlinear susceptibilities x(bulk contribu-tion).59,6500It should be noted that the surface charge canincrease not only the molecular ordering of H,O/D0molecules, which will increase the interface contribution, butalso the surface potential, which will increase the bulkcontribution. And the signs of both terms in function S1 aredetermined by the sign of surface charge (osum =00,DMTAP +dar,s). Thus, despite the difficulties in quantifying thecontribution form the interface and the bulk, combinedspectroscopic evidences in OD spectra and OH spectra indicatethat DMTAP bilayer surface reaches a point of zero charge(PZC) at Car ~100 nM.1-63 It is also interesting to see that the adsorption of dT2smolecules continues after the surface reaches PZC at Car ~100nM. A condensed layer of negatively charged electrolytes can beformed by overcoming repulsive electrostatic forces during theadsorption of dT2s molecules at high concentration (Car >100nM), which is the typical formation of electric double layer ofthe Gouy-Chapman-Stern model.64,65 These newest exper-imental results indicate that even with the oligonucleotideswithout conjugated hydrophobic molecular groups, the electro-static force between the negatively charged oligonucleotide andthe positively charged DMTAP bilayer is not the only drivenforce during the oligonucleotide adsorption. Such conclusioncoincides with experimental results of the alkyl chainconjugated oligonucleotides in previous literature works. 2.3. Conformation of dT5 Molecules. Orientationanalyses were also performed based on the Raman polar-izabilities reported in the literature.66,67 Figure 6A,B shows thecalculated tilt angle of in-phase C4=O and Cs=C6 stretchingmode at ~1660 cm-and symmetric PO,at~1090 cmatvarious concentrations. As seen in Figure 6A, the tilt angle ofthymine groups in Dzo (0pgmine) fluctuates slightly and staysaround 70° when Car increases from 25 to 500 nM. The final Figure 6. Concentration dependence of calculated tilt angle of (A) in-phase C4=0 and Cs=C, stretching mode at ~1660 cm-(0b,gmine)and (B) symmetric PO, stretching at ~1090 cm- () in D,osolution. average tilt angle of thymine groups is 70.9 ±7.6° at Car = 100nM. Such trend of op,omine indicates that the tilt angle ofthymine groups are insensitive to the changes of surface chargedensity and surface electrostatic force field. The tilt angle of thesymmetric PO, stretching mode in D,O solution (0,)decreases significantly at Car=50 and 100 nM. The decrease oftilt angle and the significant increase of x and xs, (shown inFigure 3E) indicate that the molecular ordering of POz groupsis greatly improved at Car ~ 100 nM. Both red shifts of symmetric PO stretching mode andenhanced ordering of POz groups indicate that the bindingaffinity between DMTAP and dT2s molecules is greatlyimproved at PZC. Such improved binding affinity may beinduced by the significant decrease of electrolyte concentrationand ion screening effects around the PO groups (in dT2smolecules)and NH groups (in DMTAP molecules)) atPzc 68,699'As estimated by Levinson et al.,3 the PO,symmetric stretching frequency can be tuned by the interactionstrength (of various chemical environments) along the C2 axiswith a rate of 0.40 cm-(MV/cm) via linear Strak effects. Thechanges of interaction strength AF during the dT2s-DMTAPbilayer interaction in H,O and D20 are estimated to be 7.5±1.9 and 15.1 2.4 MV/cm,respectively, (AF=040 -). Thus, the lowest point ofωo 0.40cm(MV/cm)/curve can be a mark of PZC point of the DMTAP bilayersurface due to the strongest intermolecular interaction at PZC,where the oligonucleotide/lipid charge ratio =1:1. And thePO groups of oligonucleotide molecule can act as a sensitivevibration molecular probe68.69 for quantifying the oligonucleo-tide/lipid charge ratio and determining the point of zero charge(PZC) of lipid bilayer surface. In this study, adsorption structure and conformation ofoligonucleotide (dT2s) molecules were investigated by SFG-VS in situ. The SFG-VS spectra within different wavenumberranges were analyzed to provide conformation details ofthymine groups, phosphate groups, and OD/OH groups and toprovide a comprehensive and fundamental understanding of the oligonucleotide adsorption on a model bilayer. Combinedspectroscopic evidences also indicate that the formation of anelectric double layer on the DMTAP/dT25 surface follows theGouy-Chapman-Stern model. And the conformation of dT2smolecules changed significantly when the DMTAP bilayerreaches a point of zero charge(PZC) at Car~ 100 nM. On thebasis of the orientation analysis, the final average tilt angle ofthymine groups is 70.9±7.6°. These results demonstrated thatthe vibrational spectra collected by in situ label-free polar-ization-resolved SFG-VS detections can provide new molecularinsights into the mechanisms of oligonucleotide-membraneinteraction. Our analysis results also show that the symmetric POstretching mode of oligonucleotide molecules can serve as asensitive vibration molecular probe to quantify the oligonucleo-tide/lipid charge ratio and determine the point of zero charge(PZC) of lipid bilayer surface, which may help the researcher tocontrol the layer-by-layer assembly of oligonucleotide-lipidcomplexes and improve the efficiency of genetic therapy againstcancer and viral infections.70,71 4. EXPERIMENTAL SECTION Cationic lipid 1,2-dimyristoyl-3-trimethylammonium-propane(DMTAP, purity>99%, purchased from Avanti Polar Lipids,Inc.) was used to build solid-supported lamellar lipid bilayers.The DMTAP monolayers were deposited on CaF2 prisms at asurface pressure of 33 mN/m (~50 A/mol)via theLangmuir-Blodgett method. The CaF2 prisms with DMTAPmonolayer were slowly dehydrated overnight to remove theresidue water and improve the bilayer stability. The secondDMTAP monolayer was also deposited on CaF2 prisms at asimilar surface pressure (30-33 mN/m) via the Langmuir-Schaefer method to form prism-supported lamellar DMTAPbilayers. The detailed preparation processes can be found inprevious papers. 2-74 The lamellar DMTAP bilayers preparedvia the Langmuir-Blodgett and Langmuir-Schaefer methodsare highly controllable and repeatable, which can be hardlyachieved by the liposomes prepared in solutions. Such bilayercan be used as an ideal model system to investigate theoligonucleotide-membrane interaction. After the preparation, the lipid bilayers were immersed in asmall reservoir with a volume of 2.5 mL. The dT,oligonucleotide molecules (purity > 98% by HPLC, TakaraBio, Inc., Dalian) were dissolved in 10 mM tris buffer solution(pH ~ 7.4) with a concentration of 10 nmol/mL (uM). ThedTs-tris solution wasadded to the reservoir withamicrosyringe to interact with the DMTAP bilayers. All of the SFG-VS measurements were performed on apicosecond sum frequency generation system purchased fromEKSPLA, Lithuania. The optical arrangements and programswere custom-modified to perform polarization-resolved detec-tions (see Scheme 1 for details).75,76 Briefly, the polarizationangle of visible beam (vis) was switched between 0 and 90° bya rotational motorized half-wave plate (HWP-M) at eachwavenumber point during the IR scanning SFG spectracollection. The SFG signals (the ssp and psp signals at vis =0°, the ppp and spp signals at vis =90°, where the first letters/p stands for the polarization state of SFG signal, the secondletter stands for the polarization state of visible light, and thethird letter stands for the polarization state of IR light) wereseparated by a polarization splitter (PS). The separated SFGsignals passed through Glan polarizers (GP1, GP2), half-waveplates (HWP1, HWP2), notch filters (F1, F2), and focusing Scheme 1. Experimental Setup of Polarization-ResolvedSFG-VS Detection" plate, HWP-M: motorized half-wave plate; F: filter, L: lens. lenses (L1, L2) separately and then entered two sets ofidentical detection systems (Monochromator 3501/3504,SOLAR TII and PMT R7899, Hamamatsu). The SFG-VSspectra within the wavenumber ranges of 1000-1200,1550-1800, and 3000-3800) cm- (2200-2800 cm- for D,Osolutions) were collected after the sample reached equilibrium.The incident angles of visible beam and IR beam are 63° and52°, respectively. Over 150 pulses were averaged for eachwavelength point in the SFG-VS spectra. All of the SFGintensities shown in the figures below were normalized by theenergy of visible beam Ivis and IR beam Im. Half of the path ofIR beam was purged by N2 gas to decrease the absorbing effectsof atmospheric H,O and CO molecules. The spectra withinthe wavenumber range of 2200-2800 cm-were alsonormalized to the SFG spectra of z-cut quartz to remove theIR absorbance of atmospheric CO. ASSOCIATED CONTENT ⑤ Supporting Information The Supporting Information is available free of charge on theACS Publications website at DOI: 10.1021/acsomega.7b01214. Details about the molecular structure of thymidine andtilt angle analysis procedures of SFG data (PDF) AUTHOR INFORMATION Corresponding Authors *E-mail: weifeng@jhun.edu.cn (F.W.). *E-mail: wanquan.zheng77@gmail.com (W.Z.). ORCIDD Feng Wei:0000-0002-3218-5754 Notes The authors declare no competing financial interest. ACKNOWLEDGMENTS This work was supported by the China National NaturalScience Foundation (NSF) (Nos. 21503093 and 61575085)and the Doctorial Program of Jianghan University (No. 1019-0610001). Dr. W.L. acknowledges the support from NSF ofChina (No. 21606103). Dr. Cao acknowledges the supportfrom China NSF (No. 51703081), 4th Yellow Crane TalentProgramme of Wuhan City (No. 08010004), and NationalScienceand Technology Major Projectt(No.2016YFB0401505). The Guassian calculations were carriedout at the supercomputing center of the University of Scienceand Technology of China. REFERENCES ( ( 1) R adler, J. O .; et a l. Structure of DNA-Cationic Li posomeComplexes: DNA In t ercalation in Multilamellar Membranes i n Distinct Interhelica l Packing Regimes. Science 1997, 27S, 810-814. ) ( ( 2) B raun, C. S.; Jas, G. S.; Choosakoonkriang, S.; Koe, G. S.; Smith,J. G.; Middaugh, C . R . T he s t ructure of DNA within cationic lipid/DNA c o mplexes. Biophys. J. 2003 , 84,1114-1123. ) ( ( 3) M ontis, C. ; So s tegni, S. ; Mil a ni, S.; Bag l ioni, P.; Ber t i , D. B iocompatible cationic l ipids for the formulation of liposomal DNA vectors. Soft Matte r 2014 , 10,4287-4297. ) ( (4) Ahmed, S . ; S a varala, S .; Chen, Y.; B othun, G.; W u nder, S. L.Formation o f lipid sheaths a r ound nan o particle-supported lip i d b ilayers. Small 2012, 8, 1740-1751. ) ( ( 5) T u, R. S.; Tirrell, M. Bottom-up design o f biomimetic assemblies. Adv. Drug D elivery Rev. 2004, S6 , 1537-1563. ) ( (6) U nzueta, U.; Saccardo, P.; D omingo-Espin, J; Cedano, J;Conchillo-Sole, O.; Garcia-Fruitos, E.; Cespedes, M .V.; Corchero, J.L.; D aura, X . ; Mangues, R. ; Ferrer-Miralles, N.; Vil l a verde, A.;Vazquez, E . S heltering DNA in self-organizing, p r otein-only nano-shells as artificial virus e s f o r gen e delivery. Nanomedicine 2014, 1 0, 535-541. ) (7) Montis, C.; Milani, S.; Berti, D.; Baglioni, P. Complexes ofnucleolipid liposomes with single-stranded and double-strandednucleic acids. J. Colloid Interface Sci. 2012, 373,S7-68. ( (8) D an, N.; D anino, D . S t ructure and kinetics of lipid-nucleic acid complexes. Adv. C o lloid Interface Sci. 2014, 20S, 230-239. ) ( ( 9) K oynova, R . ; W a ng, L. ; Macdonald, R. C. A n Intracellular Lamellar-nonlamellar Phase T ransition R ationalizes the S uperiorPerformance o f s o me Cationic Lipid Transfection Age n ts. Proc. Natl. Acad. Sci. U.S.A. 2006, 103,14373-14378. ) (10) Barreleiro, P. C. A.; May, R. P.; Lindman, B. Mechanism offormation of DNA-cationic vesicle complexes. Faraday Discuss. 2003,122,191-201. ( (11) B arreleiro, P . C. A.; Lindman, B. The K inetics of D NA-Cationic Vesicle Complex Formation. J. Phys. Chem. B 2 00 3 , 107, 6208-6213. ) ( (12) Zhang, Y .; Garzon-Rodriguez, W .; Manning, M. C.; Anchordoquy, T. J. T he use of fluorescence resonance energy transfer to monitor d ynamic c h anges of li p id-DNA int e ractions duringlipoplex formation. Biochim. B iophys. A cta, Biomembr. 2 0 03, 1614, 182-192. ) ( ( 13) Lai, E .; van Z anten, J. H. R eal time monitoring of lipoplex molar mass, s ize and d ensity. J. Controlled Releas e 2002, 82, 149-158. ) ( (14) Lai, E.; van Zanten, J. H. Monitoring DNA/Poly-l-LysinePolyplex F ormation w ith Time-Resolved Mu l tiangle Laser Li g htScattering. Biophys. J.2001, 8 0, 864-873. ) ( (15) Z hang, Y.; G arzon-Rodriguez, W.; Manning, M. C . ; Anchordoquy, T. J. The use of fluorescence resonance en e rgy transfer to monitor d ynamic c h anges of lipid-DNA int e ractions duringlipoplex formation. Biochim. B iophys. Acta, Biomembr. 2 0 03, 1614, 182-192. ) ( ( 16) M adeira, C . ; Loura, L. M.; A i res-B a rros, M. R . ; Fedorov, A.;Prieto, M. Characterization of DNA/lipid complexes b y fluorescence resonance energy transfer. Biophys. J. 2003, 85, 3106-3119. ) ( (17) P ozharski, E. Fluorescence Resonance E n ergy Transfer-based Analysis of Lipoplexes. Methods Mol. Biol . 2010, 606 , 393-398. ) ( ( 18) L i, A.; Sun, Z.; Ma, Y.; Yang, F. ; Ouyang, H.; Yang, X. The I nteraction of Recombinant P seudomonas Aeruginosa E xotoxin A with DNA a n d M i mic Bi o membrane Investigated b y Surface P lasmon R esonance a n d Electrochemical Methods. Talanta 2006, 70, 33 0 -335.(19) Z h danov, R. I .; Strazhevskaya, N. B.; Jdanov, A. R .; Bis c hoff, G. ) ( A Spectroscopic and Surface Plasmon Resonance Study of Oleic Acid/ D NA Co m plexes. J. Bi o mol. Struct. Dyn. 2002, 20, 231-242. ) (20) Huebner, S.; Battersby, B. J.; Grimm, R.; Cevc, G. Lipid-DNAComplex Formation: Reorganization and Rupture of Lipid Vesicles inthe Presence of DNA As Observed by Cryoelectron Microscopy.Biophys. J. 1999, 76, 3158-3166. (21) Battersby, B. J.; Grimm, R.; Huebner, S.; Cevc, G. Evidence forthree-dimensional interlayer correlations in cationic lipid-DNA complexes as observed by cryo-electron microscopy. Biochim. Biophys.Acta, Biomembr. 1998,1372,379-383. ( (22) C hoosakoonkriang, S.; Wiethoff, C. M.; Anchordoquy, T . J;Koe, G . S.; S mith, J . G .; M iddaugh, C. R . Infrared S p ectroscopic Characterization o f the Interaction of Cationic L i pids with Pl a smid DNA. J. B iol. Chem. 2001, 276 , 8037-8043. ) ( (23) C hen, Q; Kang, X. ; Li, R . ; Du, X . ; Shang, Y.; Liu, H .; H u, Y.Structure of the Complex Monolayer of Gemini Surfactant and DNA at the Air/Water Interface . Langmuir 2012, 28, 3429-3438. ) ( (24) B enevides, J. M.; Overman, S. A.; Thomas, G . J. Raman, Polarized Raman a nd U ltraviolet Resonance Raman Spectroscopy of Nucleic Acids a nd T heir Complexes. J . Raman Sp e ctrosc. 2005, 36,279-299. ) ( (25) O berle, V.; Bakowsky, U.; Z uhorn, I. S.; Hoekstra, D. Lipoplexformation u nder equilibrium conditions re v eals a three-step m e cha-nism. Biophys.J. 2000, 7 9, 1447-1454. ) ( (26) A ntipina, A. Y.; Gurtovenko, A. A. Molecular M echanism ofCalcium-Induced Adsorption of DNA on Zwitterionic Phospholipid Membranes. J. Phys. C hem. B 2015, 119, 6 6 38-6645. ) ( (27) S ushko, M . L.; S hluger, A. L. DLVO th e ory for like-chargedpolyelectrolyte and surface interactions. Mater. Sci. E ng., C 2007, 2 7 , 1090-1095. ) ( (28) R eimer, D . L.; K o ng, S.; Bally, M. B. Analysis of C at i onicLiposome-mediated Interactions of Plasmid DNA with Murine andHuman Melanoma Cells in Vitro. J. Biol . Chem. 1997, 272 , 19480- 19487. ) (29) Pires, P.; Simoes, S.; Nir, S.; Gaspar, R.; Diizgiines, N.; Pedrosode Lima, M. C. Interaction of cationic liposomes and their DNAcomplexes with monocytic leukemia cells. Biochim. Biophys. Acta,Biomembr. 1999,1418,71-84. (30) Pozzi,D.; Marchini, C.; Cardarelli, F.; Amenitsch, H.; Garulli,C.; Bifone, A.; Caracciolo, G. Transfection efficiency boost ofcholesterol-containing lipoplexes. Biochim. Biophys. Acta, Biomembr.2012,1818,2335-2343. (31) Henriques, A. M.; Madeira, C.; Fevereiro, M.; Prazeres, D.M.F.; Aires-Barros, M. R.; Monteiro, G. A. Effect of cationic liposomes/DNA charge ratio on gene expression and antibody response of acandidate DNA vaccine against Maedi Visna virus. Int. J. Pharm. 2009,377, 92-98. ( (32) S hen, Y. R . T h e Principles o f Nonlinear Optics, 1 s t e d .; Wiley:New York, 1 984. ) ( (33) Y e, S.; Wei, F.; L i , H. ; Tian, K.; L u o, Y. St r ucture an d Orientation of Interfaci a l Proteins Determin e d by Sum FrequencyGeneration Vibrational S pectroscopy: M ethod an d Application. Adv. Protein Chem. S truct. Biol . 2013 , 93 , 213-255. ) ( (34) L iu, J.; Conboy, J. C. Direct Measurement of the T r ansbilayer Movement of Phospholipids b y Sum Fr e quency Vibrational S p ectros-copy.J. Am. Chem. Soc. 2004, 126 , 8376-8377. ) ( (35) W ang, H . F.; Gan, W.; Lu, R.; Rao, Y.; Wu, B. H. Q uantitativeSpectral and Orientational A nalysis in Surface Sum Fr e quencyGeneration V ibrational S pectroscopy (SFG-VS). In t . Re v . Ph y s.Chem. 2005,24,191-256. ) ( (36) C astellana, E. T.; Cremer, P. S. Solid Supported L ipid B ilayers: From B i ophysical Studies to Sensor Design. Surf. Sci. R e p.2006, 61,429-444. ) ( (37) Wu, F. G.; Yang, P.; Zhang, C.; Li , B.; H a n, X.; Song, M.; Chen, Z. Molecular i nteraction s between amantadine and m odel cell membranes. L angmuir 2014, 30, 8491-4899. ) ( (38) W u, F. G.; Yang, P .; Zhang, C.; Han, X.; Song, M.; Chen, Z .Investigation of Drug-Model Cell Membrane Interactions Using Sum F requency G eneration Vibrational Spectroscopy: A C ase S tudy of Chlorpromazine. J. Phys. Chem. C 2014, 118, 1 7538-17548. ) ( (39) Li, B.; Wang, H.-Y.; F eng, P.; Han, X.; Chen, Z.; Lu, X.; Wu, F .G. Q ualitative a nd Q u antitative A n alyses o f th e Molecular-LevelInteraction between M emantine and Mo d el Cell Membranes. J. Phys. Chem. C 2015, 119, 17074-17083. ) ( (40) Sartenaer, Y .; Tourillon, G .; Dreesen, L.; L is, D.; Mani, A . A .;Thiry, P . A.; Peremans, A. Sum-Frequency Ge n eration Spectroscopyof DNA Monolayers. Biosens. B i oelectron. 2007, 22, 2179-2183. ) ( ( 41) Asanuma, H.; Noguchi, H.; Uos a lki, K.; Yu , H. Z. Me t al Cation-induced Deformation of DNA Sel f -assembled Monolayers on S i li c on: Vibrational Sum Frequency G eneration Spectroscopy. J. Am. Chem.Soc. 2008, 130,8016-8022. ) ( (42) Chen, E . H.; W a lter, S. R.; N guyen, S . T.; G e iger, F . M . Arylsilanated SiO, Surfaces for Mild a nd Simple Two-Step Click F unctionalization w ith S mall Molecules a n d Oligonucleotides. J. Phys. Chem. C 2012, 116, 19886-19892. ) ( ( 43) W alter, S. R.; Geiger , F . M. DNA o n Stage: S howcasing Oligonucleotides a t Surfaces and Interfaces w ith S econd H a rmonic and V ibrational Sum F r equency Generation. J. Ph y s. Chem. Lett. 2 0 10, 1,9-15. ) ( ( 44) Howell, C.; S chmidt, R.; Kurz, V.; Koelsch, P. Sum-Frequency- Generation Spectroscopy of DNA F ilms in Air a nd A q ueousEnvironments. B iointerphases 2008, 3 , FC47-FC51. ) ( ( 45) Howell, C . ; Zhao, J.; Koelsch, P.; Z harnikov, M. Hybridization in S SDNA Fi l ms-A Multi-Technique Spe c troscopy Study. Phys. Chem. Chem. Phys. 2011 , 13,15512-15522. ) ( ( 46) Wurpel, G . W . H.; Sovago, M.; Bonn, M. Sensitive Probing ofDNA Binding to a Cationic Lipid Monolayer. J. Am. Che m . Soc. 200 7 , 129,8420-8421. ) ( (47) Wei, F . ; Tian, K .; Z heng, W . In t erfacial S tructure a nd T ransformation of Guanine-Rich Oligonucleotides on Solid Supported Lipid Bilayer Investigated by Sum Frequency Generation Vibr a tionalSpectroscopy. J. Phys. Chem . C 2015, 119, 27038-27044. ) ( ( 48) Z hang, S . L .; Michaelian, K. H.; Loppnow, G. R. Vibrational Spectra and Experimental Assignments o f Thymine a n d Nine of Its Isotopomers. J. Phys . Chem. A 1998, 102, 461-470. ) ( ( 49) W eng, G . ; Chen, C. X.; B a logh-Nair, V . ; Callender, R.; Manor, D. Hydrogen bond interactions o f G proteins with the guanine ring moiety of guanine nucleotides. Protein Sci. 1 9 94, 3, 22-29. ) ( (50) S cheiner, S.; Cuma, M . R elative Stability o f Hydrogen and Deuterium Bonds. J. Am. C hem. Soc. 1 996, 1 18, 1511-1521. ) (51) Lucazeau, G.; Guemas, L.; Novak, A. Vibrational spectra andstructure of KZn(NH2)4 and Rb,(NH)4 amides. Inorg. Chim. Acta1976,20,11-18. (52) Izzo, V.; Fornili, S. L.; Cordone, L. Thermal denaturation of B.subtilis DNA in H,O and D2O observed by electron microscopy.Nucleic Acids Res. 1975, 2, 1805-1810. (53) Levinson, N. M.; Bolte, E. E.; Miller, C. S.; Corcelli, S. A.; Boxer,S. G. Phosphate vibrations probe local electric fields and hydration inbiomolecules. J. Am. Chem. Soc. 2011, 133,13236-13239. ( ( 54) Gan, W .; W u, D.; Zhang, Z. ; F e ng, R. R.; W a ng, H. F.Polarization and experimental configuration analyses of sum frequency g eneration vibrational spectra, structure, and o r ientational m o tion of 1vS. the air/water i nterface. J . Chem. Phys. 2006, 124, No. 1 14705. ) (SS) Gan, W.; Wu, D.; Zhang, Z.; Guo, Y.; Wang, H. F. Orientationand motion of water molecules at air/water interface. Chin. J. Chem.Phys. 2006, 19, 20-24. (56) Mondal, J. A.; Nihonyanagi, S.; Yamaguchi, S.; Tahara,T. J. Am.Chem. Soc.2010, 132, 10656-10657. (57) Mondal, J. A.; Nihonyanagi, S.; Yamaguchi, S.;Tahara, T. J. Am.Chem. Soc. 2012, 134, 7842-7850. (58) Sheu, S.-Y.; Schlag, E. W.; Selzle, H. L.; Yang, D.-Y. MolecularDynamics of Hydrogen Bonds in Protein-DO: The Solvent IsotopeEffect. J. Phys. Chem. A 2008, 112, 797-802. (59) Sovago, M.; Vartiainen, E.; Bonn, M. Observation of buriedwater molecules in phospholipid membranes by surface sum-frequencygeneration spectroscopy. J. Chem. Phys. 2009, 131, No. 161107. ( ( 60) Gonella, G.; Liitgebaucks, C.; de Beer, A. G. F.; R oke, S. SecondHarmonic and Sum-Frequency Gen e ration from Aqueous Interfaces Is Modulated by Interference. J. Phys. Chem. C 2016, 1 20,9165-9173. ) (61) Wang, H. F. Sum frequency generation vibrational spectroscopy(SFG-VS) for complex molecular surfaces and interfaces: Spectrallineshape measurement and analysis plus some controversial issues.Prog. Surf. Sci. 2016, 91, 155-182. ( ( 62) Ong, S . ; Z h ao, X.; Ei s enthal, K . B . Po l arization o f w a termolecules at a charged i n terface: second harmonic stu d ies of t he silica/ water i nterface. Chem. P hys. Lett. 1992, 191,327-335. ) ( (63) E f tekhari-Bafrooei, A.; Borguet, E. E f fect o f E l ectric F i elds on the U ltrafast Vibrational Relaxation of Water a t a C h arged Solid- Liquid Interface as Probed by Vibrational Sum Frequency Generation. J . Phys. Chem. L ett. 2011, 2, 1353-1358. ) ( (64) L is, D .; B ackus, E . H .; H unger, J . ; P arekh, S. H .; Bonn, M. L iquid f l ow al o ng a s o l i d sur f ace reversibly alters inte r facial chemistry. Science 2014, 344,1138-1142. ) ( (65) Oldham, K. B. A Gouy-Chapman-Stern model of the doublelayer a t a ( m etal)/(ionic l i quid) i n terface. J . Electroanal. C hem. 2 0 08, 613, 131-138. ) ( (66) Grahame, D. C. T he Electrical Double Layer and the Theory of Electrocapillarity. Chem. Rev. 1947, 41, 441-501. ) ( (67) Tsuboi, M.; Benevides, J . M. ; Thomas,J. G. J . Raman Tensors a nd their a p plication in st r uctural studies of b iological systems. Proc.Jpn. Acad., Ser. B 2009, 85 , 83-97. ) ( ( 68) K umakura, A.; T s uboi, M. ; Us h izawa, K.; Ueda, T. Pol a rized Raman spectrum of a single crystal of AZT. Bio s pectroscopy 1996, 2,233-242. ) ( (69) Fried, S . D.; B o xer, S. G . Me a suring electric fie l ds an d noncovalent interactions u s ing the vibrational stark effect. Acc. C hem. Res. 2015, 48,998-1006. ) ( (70) M a, J.; Pazos, I . M.; Z h ang, W.; Culik, R. M .; Gai, F. S i te - Specific Infrared Probes of Proteins . Annu.Rev. Phys. Chem. 2015, 66, 357-377. ) ( (71) K o ltover, I; Sa l ditt, T . ; S a finya, C. R. Ph a se Dia g ram, Stability, and Overcharging o f Lamellar Cationic Lipid-DNA Self-Assembled Complexes. Biophys. J. 1999, 77 , 915-924. ) ( (72) Alhakamy, N. A.; El a ndaloussi, I.; Ghazvini, S. ; Berkland, C. J.; Dhar, P. E ffect of lipid headgroup charge a nd pH on the stability and membrane insertion potential of calcium condensed gene complexes. Langmuir 2015, 31, 4232-4245. ) ( (73) W ei, F .; Ye, S.; Li , H. ; Luo, Y . Phosphate Ions PromotingAssociation between Peptide and Modeling Cell Membrane R evealed by S um F r equency Ge n eration Vi b rational Spectroscopy. J . Phys.Chem. C 2013, 117 , 11095-11103. ) ( (74) Wei, F . ; Li, H .; Ye, S. Specific I on Interaction Dominates overHydrophobic Matching Effects in Peptide-Lipid Bilayer Interactions: t he Case of Short Peptide. J. Phys. Chem. C 2 013, 117, 26190-26196. ) ( (75) W ei, F .; Xiong, W.; Li, W.; Lu, W.; Allen, H. C.; Zheng, W. fiongWbew Assembly and r e laxation b ehaviours o f p h osphatidylethanolamine monolayers investigated by polarization and f r equency resolved SF G - VS. Phys. Chem. Chem. Phys. 2015,17,25114-25122. ) ( (76) Wei, F . ; Xia, W. X . ; H u , Z. J.;Li, W. H . ; Z h ang, J. Y.; Z h eng, W.Q. Laser Linewidth a n d Spectral Resolution in I n frared Scanning Sum Frequency Generation Vibrational S p ectroscopy Sy s tem. Chi n . J.Chem. Phys. 2016, 29 , 171-178. ) American Chemical SocietyDOI:acsomega.bCS Omega , “Check Figure S for the corresponding atoms of thymidine molecule.CS Omega , The molecular interaction between the oligonucleotides and lipid membranes is the key to the functions of virus, aptamer, and various oligonucleotidebased materials. In this study, the conformational changes of oligonucleotides (dT25) on lamellar cationic 1,2-dimyristoyl-3-trimethylammonium-propane (DMTAP) bilayer were investigated by polarization-resolved sum frequency generation vibrational spectroscopy (SFG-VS) in situ. The SFG-VS spectra within different wavenumber ranges were analyzed to give conformation details of thymine groups, phosphate groups, and OD/OH groups and to provide a comprehensive and fundamentalunderstanding of the oligonucleotide adsorption on a model bilayer. It is shown that the adsorption of dT25 on DMTAP bilayer reaches maximum at CdT ≈ 500 nM. And the conformation of dT25 molecules change significantly when surface charge of DMTAP bilayer reaches the point of zero charge (PZC) at CdT ≈ 100 nM. Combined spectroscopic evidences also indicate that the formation of electric double layer at the DMTAP/dT25 surface follows the Gouy−Chapman−Stern model. The analysis results also show that the symmetric PO2− stretching mode of oligonucleotide molecules can serve as a sensitive vibration molecularprobe for quantifying the oligonucleotide/lipid charge ratio and determine the point of zero charge (PZC) of lipid bilayer surface,which may help researchers to control the layer-by-layer assembly of oligonucleotide−lipid complexes and to improve the efficiency genetic therapy against cancer and viral infections.
确定


还剩7页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《寡核苷酸和层状阳离子脂质双层膜中偏振分辨振动和频光谱(SFG-VS)检测方案(其它光谱仪)》,该方案主要用于其他中可靠性能检测,参考标准--,《寡核苷酸和层状阳离子脂质双层膜中偏振分辨振动和频光谱(SFG-VS)检测方案(其它光谱仪)》用到的仪器有Ekspla SFG 表面和频光谱分析系统
推荐专场
该厂商其他方案
更多