方案详情
文
双酚 A (BPA) 是用于制造塑料的主要化学物质之一。也大量用于生产各种类型的食品和饮料容器。因为已知 BPA 会从金属罐头食品的塑料衬里中浸出,所以暴露于 BPA 的潜在风险在过去几年一直是备受关注。
方案详情

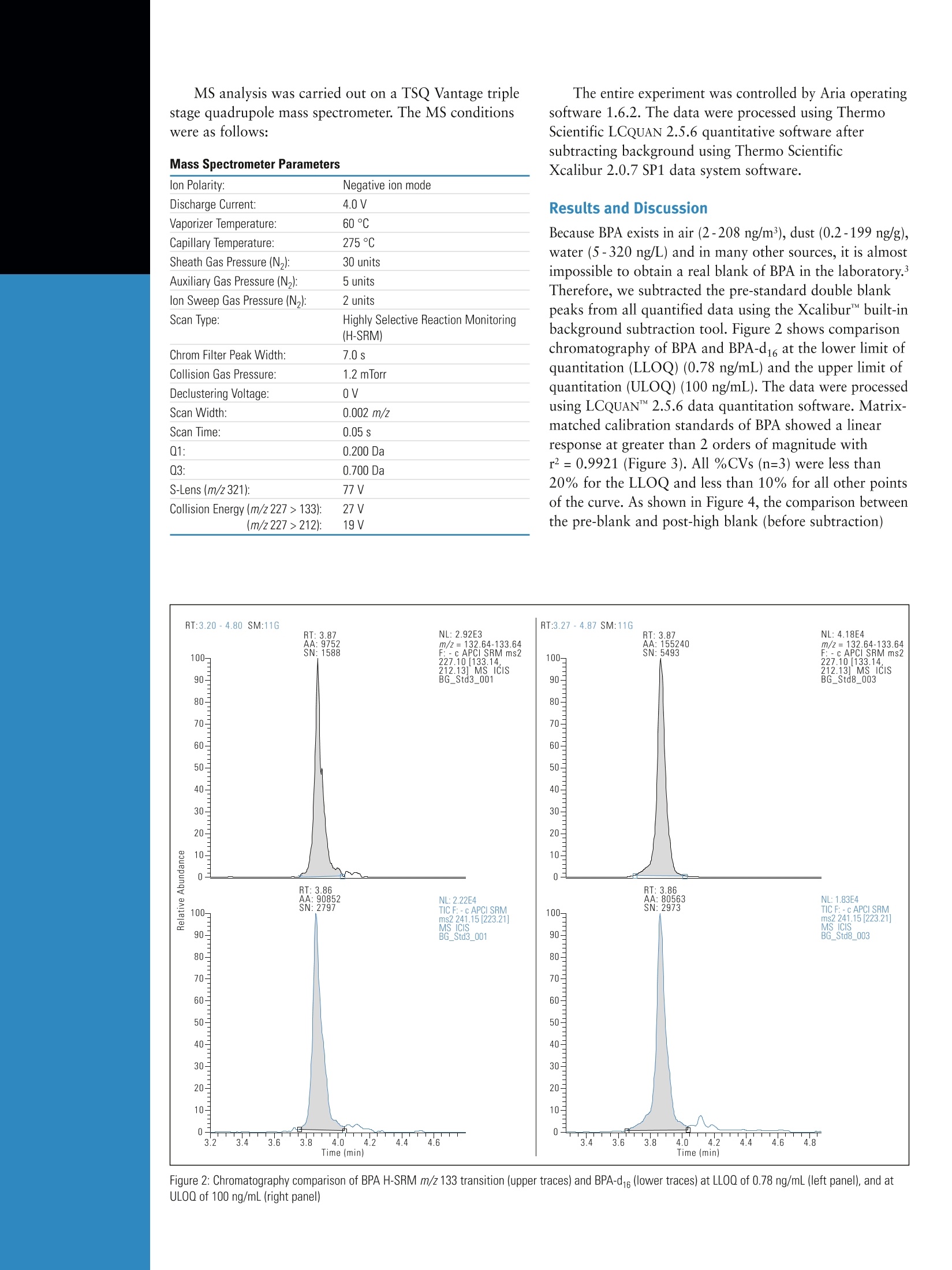
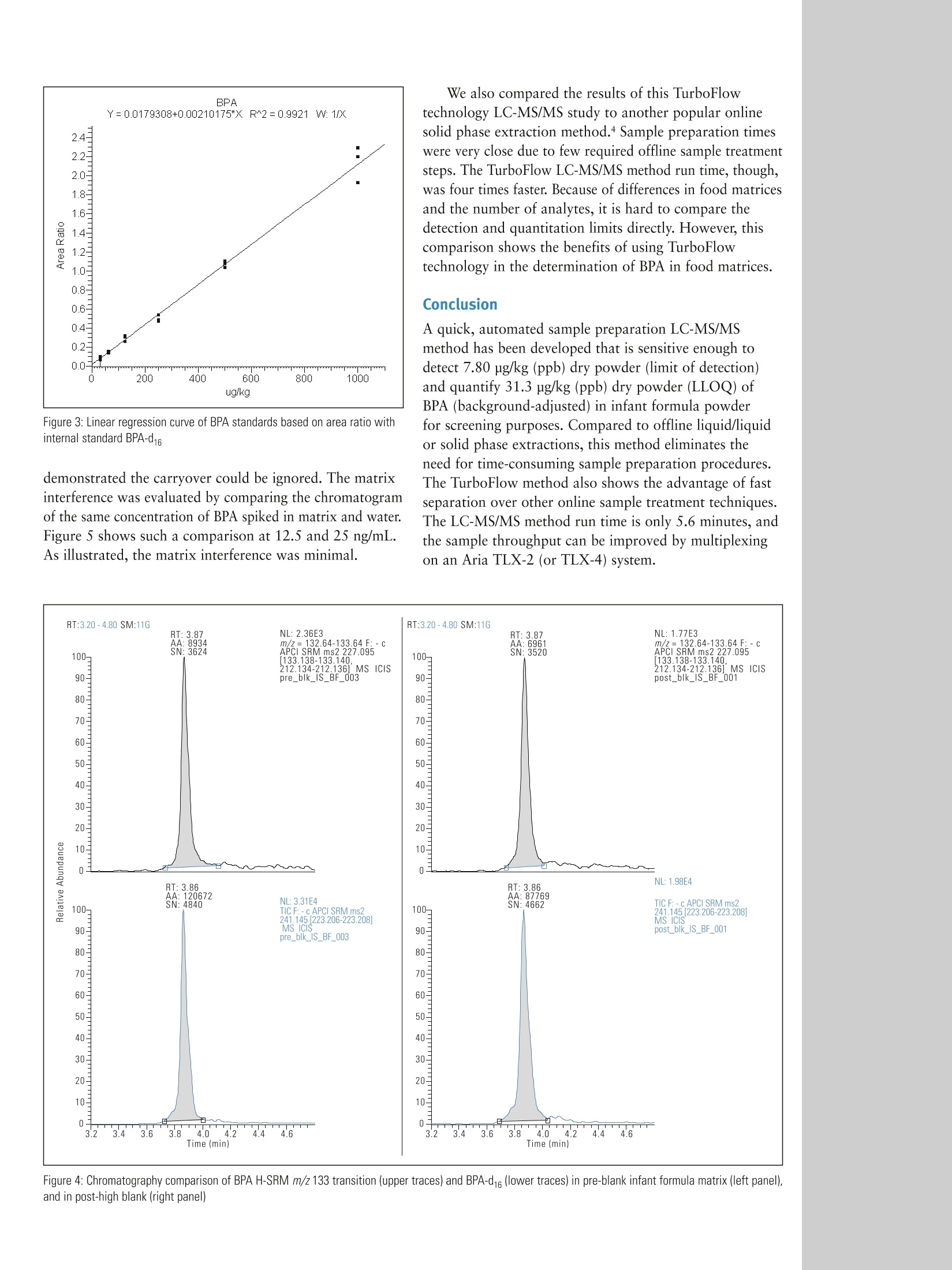
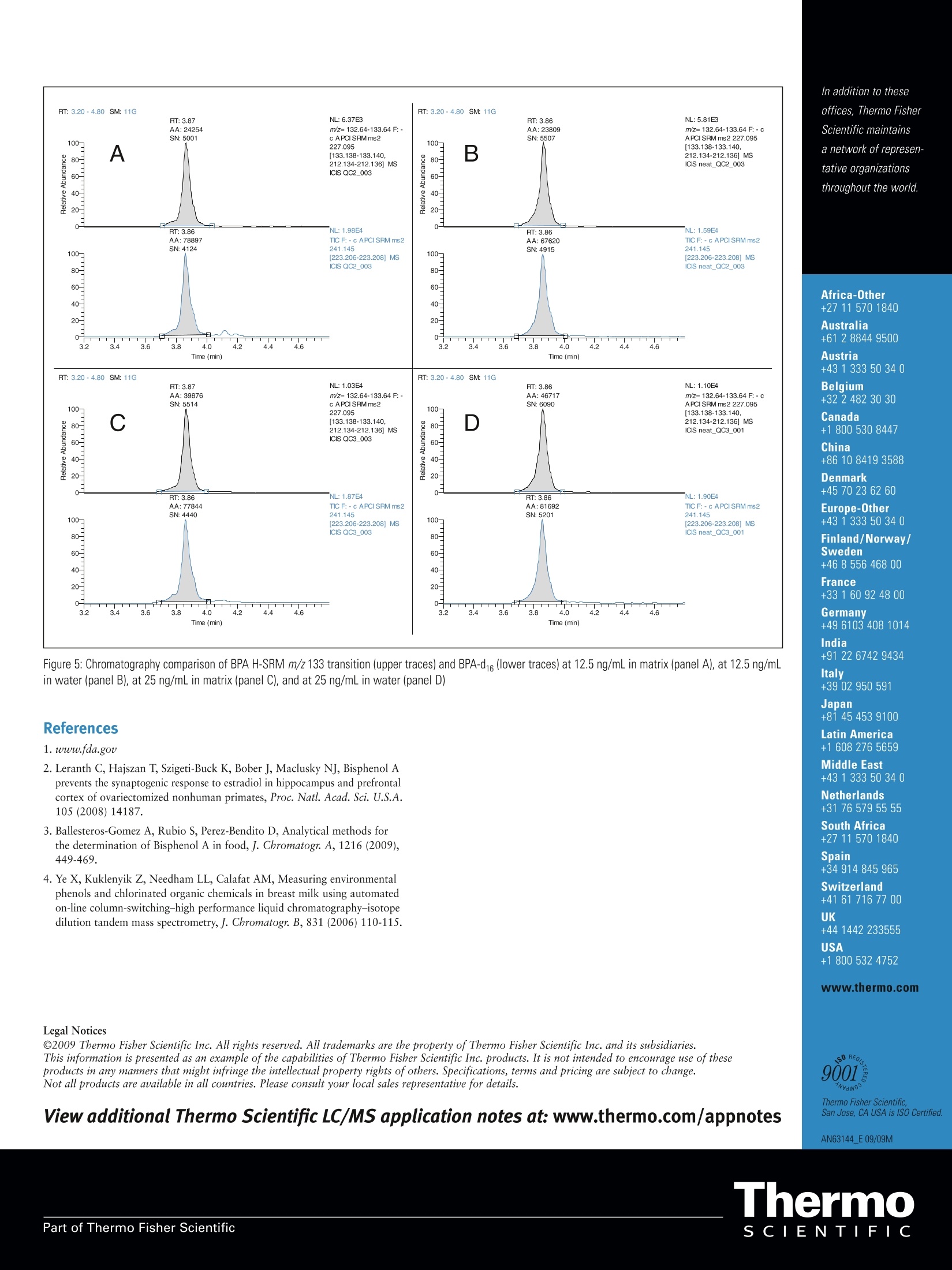
ThermoSCIENTIFICPart of Thermo Fisher Scientific Determination of Bisphenol A in Infant Formulaby Automated Sample Preparation and LiquidChromatography-Mass Spectrometry Yang Shi, Catherine Lafontaine, Matthew Berube, Francois Espourteille, Thermo Fisher Scientific, Franklin, MA, USA Introduction 2,2-bis(4-hydroxyphenyl) propane, commonly known asBisphenol A (BPA), is one of the primary chemicals usedto make plastics. It is also heavily used in the productionof various types of food and drink containers. BecauseBPA has been known to leach from the plastic lining ofmetal canned food, the potential risks of exposure to BPAhave been a great concern over the past few years. Higherbisphenol A levels are significantly associated with heartdisease, diabetes, and abnormally high levels of certainliver enzymes. There is a consensus that infants are at thegreatest risk of harm due to exposure to extremely lowlevels of BPA.1 The maximum acceptable or “reference” dose for BPA is50 pg/kg bodyweight/day, asestablished by theU.S. EnvironmentalProtection Agency.2 CH3 HO· OH CH3 Figure 1: Chemical Structure of Bisphenol A A liquid chromatography-mass spectrometry (LC-MS)technique has been recently described for the determinationof BPA in food.3 Current strategies for the detection ofBPA in canned infant formula employ sample preparationsthat involve complicated extraction steps such as solidphase extraction, solvent-based extraction, and somemicro-extraction techniques. All of these techniques requireadditional sample concentration and reconstitution in anappropriate solvent. Such sample preparation methods aretime-consuming and are more vulnerable to variability dueto errors in manual preparation. To offer a high sensitivity(low ppb) BPA detection method and timely, automatedanalysis of multiple samples, our approach is to useThermo Scientific TurboFlow technology coupled to thedetection capabilities of a high-sensitivity Thermo ScientificTSQ Vantage triple stage quadrupole mass spectrometer. Goal Develop a six-minute LC-MS/MS method using automatedsample preparation for the assay of BPA in canned infantformula powder by negative ion atmospheric pressurechemical ionization (APCI) using a deuterated internalstandard (BPA-d16). Experimental Sample Preparation Canned infant formula powder, used in this analysis forpreparation of blanks, QCs, and standards, was obtainedfrom a local supermarket in Massachusetts. The lid lacquer is low-density polyethylene and the body is polyester. BPAand BPA-d16 were obtained from Sigma-Aldrich, US(St. Louis,MO). The diluent (AmAcACN solution) wasmade using 3% ammonium acetate in acetonitrile-water(70:30, v/v). A BPA working solution was prepared inAmAcACN solution at 10 pg/mL. The infant formulasolution was prepared by adding 10 mL of AmAcACNsolution to 1 g of infant formula powder and thencentrifuging at 10,000 RPM for 30 minutes. BPA standardsand QC standards were serially diluted to the targetconcentrations with the resulting supernatant containing25 ng/mL BPA-d16 as the internal standard. Target standardconcentrations ranged from 0.78 ng/mL to 1000 ng/mL.The injection volume was 25 pL. Method The extract clean-up was accomplished using a TurboFlow"Mmethod run on a Thermo Scientific Aria TLX-1 LC systemusing a TurboFlow Cyclone P polymer-based extractioncolumn. Large molecules were not retained and weremoved to waste during the loading step while the analyteof interest was retained on the extraction column. Thiswas followed by organic elution to a Thermo ScientificHypersil GOLD aQ end-capped, silica-based C18 reversedphase analytical column and gradient elution to a TSQVantage MS with an APCI source. The BPA precursorm/z 227> 133 and 212 high-resolution selective reactionmonitoring (H-SRM) transitions were monitored in negativeionization mode. The 133 m/z product ion for BPA was usedfor quantitation, and the 212 m/z product ion was usedas confirmation. The precursor m/z 241 >223 H-SRMtransition was monitored for BPA-d16 because BPA-d16 istransformed into BPA-d14 (MW242) in water. The totalLC-MS/MS method run time was 5.6 minutes. Aria"TLX-1 System Parameters TurboFlow Cyclone P column (0.5 x50 mm) Hypersil GOLD"aQ (4 x50 mm, 3 pm particle size) Loading Pump Mobile Phases Mobile Phase A: 10 mM Ammonium bicarbonate pH 10 Mobile Phase B: 0.1% Formic acid in ACN Mobile Phase C: 20:40:40 Acetone: Acetonitrile: Isopropanol Elution Pump Mobile Phase A: H,0 Mobile Phase B: Methanol MS analysis was carried out on a TSQ Vantage triplestage quadrupole mass spectrometer. The MS conditionswere as follows: Mass Spectrometer Parameters The entire experiment was controlled by Aria operatingsoftware 1.6.2. The data were processed using ThermoScientific LCQUAN 2.5.6 quantitative software aftersubtracting background using Thermo ScientificXcalibur 2.0.7 SP1 data system software. lon Polarity: Negative ion mode Discharge Current: 4.0V Vaporizer Temperature: 60°℃ Capillary Temperature: 275°℃ Sheath Gas Pressure (N): 30 units Auxiliary Gas Pressure (N2): 5 units Ion Sweep Gas Pressure (N): 2 units Scan Type: Highly Selective Reaction Monitoring (H-SRM) Chrom Filter Peak Width: 7.0s Collision Gas Pressure: 1.2 mTorr Declustering Voltage: 0V Scan Width: 0.002 m/z Scan Time: 0.05s 01: 0.200 Da 03: 0.700 Da S-Lens (m/z 321): 77V Collision Energy (m/z 227>133): 27 V (m/z227>212): 19V Results and Discussion Because BPA exists in air (2-208 ng/m’), dust (0.2-199 ng/g),water (5-320 ng/L) and in many other sources, it is almostimpossible to obtain a real blank of BPA in the laboratory.’Therefore, we subtracted the pre-standard double blankpeaks from all quantified data using the Xcalibur"" built-inbackground subtraction tool. Figure 2 shows comparisonchromatography of BPA and BPA-d16 at the lower limit ofquantitation (LLOQ) (0.78 ng/mL) and the upper limit ofquantitation (ULOQ)(100 ng/mL). The data were processedusing LCQUAN" 2.5.6 data quantitation software. Matrix-matched calibration standards of BPA showed a linearresponse at greater than 2 orders of magnitude withr²=0.9921 (Figure 3). All %CVs(n=3) were less than20% for the LLOQ and less than 10% for all other pointsof the curve. As shown in Figure 4, the comparison betweenthe pre-blank and post-high blank (before subtraction) Figure 2: Chromatography comparison of BPA H-SRM m/z 133 transition (upper traces) and BPA-d16 (lower traces) at LLOQ of 0.78 ng/mL (left panel), and atULOQ of 100 ng/mL (right panel) We also compared the results of this TurboFlowtechnology LC-MS/MS study to another popular onlinesolid phase extraction method.4 Sample preparation timeswere very close due to few required offline sample treatmentsteps. The TurboFlow LC-MS/MS method run time, though,was four times faster. Because of differences in food matricesand the number of analytes, it is hard to compare thedetection and quantitation limits directly. However, thiscomparison shows the benefits of using TurboFlowtechnology in the determination of BPA in food matrices. Conclusion Figure 3: Linear regression curve of BPA standards based on area ratio withinternal standard BPA-d16 demonstrated the carryover could be ignored. The matrixinterference was evaluated by comparing the chromatogramof the same concentration of BPA spiked in matrix and water.Figure 5 shows such a comparison at 12.5 and 25 ng/mL.As illustrated, the matrix interference was minimal. A quick, automated sample preparation LC-MS/MSmethod has been developed that is sensitive enough todetect 7.80 pg/kg (ppb) dry powder (limit of detection)and quantify 31.3 ug/kg (ppb) dry powder (LLOQ) ofBPA (background-adjusted) in infant formula powderfor screening purposes. Compared to offline liquid/liquidor solid phase extractions, this method eliminates theneed for time-consuming sample preparation procedures.The TurboFlow method also shows the advantage of fastseparation over other online sample treatment techniques.The LC-MS/MS method run time is only 5.6 minutes, andthe sample throughput can be improved by multiplexingon an Aria TLX-2 (or TLX-4) system. Figure 4: Chromatography comparison of BPA H-SRM m/z 133 transition (upper traces) and BPA-d16 (lower traces) in pre-blank infant formula matrix (left panel),and in post-high blank (right panel) In addition to these offices, Thermo FisherScientific maintainsa network of represen-tative organizationsthroughout the world. Africa-Other+27 11 570 1840 Australia +612 8844 9500 Austria +431333 50 34 0 Belgium +322 482 30 30 Canada +1800 530 8447 China +86 10 84193588 Denmark +45 70 23 62 60 Europe-Other +431 333 50 340 Finland/Norway/ Sweden +46 8 556468 00 France +331 60 92 48 00 Germany +49 61034081014 India +91 22 67429434 Figure 5: Chromatography comparison of BPA H-SRM m/z 133 transition (upper traces) and BPA-d16 (lower traces) at 12.5 ng/mL in matrix (panel A), at 12.5 ng/mLin water (panel B), at 25 ng/mL in matrix (panel C), and at 25 ng/mL in water (panel D) Italy +39 02 950591 Japan References 1.www.fda.gov 2. Leranth C, Hajszan T, Szigeti-Buck K, Bober J, Maclusky NJ, Bisphenol Aprevents the synaptogenic response to estradiol in hippocampus and prefrontalcortex of ovariectomized nonhuman primates, Proc. Natl. Acad. Sci. U.S.A.105(2008)14187. 3. Ballesteros-Gomez A, Rubio S, Perez-Bendito D, Analytical methods forthe determination of Bisphenol A in food, J. Chromatogr. A, 1216 (2009),449-469. ( 4. Ye X, Kuklenyik Z , N eedham LL, Calafat A M , Measuring environmental phenols and c h lorinated organic che m icals in br e ast mi l k using automated on- l ine column-switching-high performance liquid chromatography-isotopedilution tandem mass spectrometry, J. Chromatogr. B, 831 ( 2006) 1 10-115. ) +81 45 4539100Latin America+16082765659Middle East+43 1 333 50 34 0Netherlands+31 76 579 55 55South Africa+2711 570 1840Spain+34914 845 965Switzerland+41 6171677 00UK+44 1442 233555USA +1 8005324752 www.thermo.com Legal Notices @2009 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries.This information is presented as an example of the capabilities of Thermo Fisher Scientific Inc. products. It is not intended to encourage use oftheseproducts in any manners that might infringe the intellectual property rights ofothers. Specifications, terms and pricing are subject to change.Not all products are available in all countries. Please consult your local sales representative for details. 9001 ivan0 View additional Thermo Scientific LC/MS application notes at: www.thermo.com/appnotes Thermo Fisher Scientific,San Jose, CA USA is ISO Certified. AN63144 E 09/09M 双酚 A (BPA) 是用于制造塑料的主要化学物质之一。也大量用于生产各种类型的食品和饮料容器。因为已知 BPA 会从金属罐头食品的塑料衬里中浸出,所以暴露于 BPA 的潜在风险在过去几年一直是备受关注。
确定

还剩2页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《婴儿配方中双酚A检测方案(液质联用仪)》,该方案主要用于婴幼儿配方乳粉中环境污染物检测,参考标准--,《婴儿配方中双酚A检测方案(液质联用仪)》用到的仪器有赛默飞TSQ Fortis™ 三重四极杆质谱仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















